Abstract
Cytomegalovirus-stimulated CD4+ lymphocytes from seropositive but not seronegative donors suppressed viral gene expression in primary human astrocytes. This suppressive activity was mediated through soluble factors. These findings suggest that CD4+ lymphocytes play a role in defense of the brain against cytomegalovirus.
Between 60 and 90% of the world population is infected with human cytomegalovirus (CMV). Yet, despite this high prevalence of infection, CMV brain disease is restricted to those with severely impaired or underdeveloped immune systems (e.g., AIDS patients and the developing fetus). In human immunodeficiency virus-infected patients, the clinical outcome of CMV retinal disease is related to both CD4+ (29) and CD8+ (27) lymphocyte counts. CMV encephalitis, however, is observed only in advanced stages of AIDS, when CD4+ T-cell counts fall below 50 per mm3 (5).
Various model systems have been used to demonstrate that T lymphocytes, both CD4+ and CD8+, are important immune effectors responsible for protection against CMV (2, 6, 22, 26, 30). Control of CMV is not exclusive to any one lymphocyte subset, and it appears that there is a hierarchical control by distinct compartments of the immune system in different organs (24). CMV is known to productively infect astrocytes (11, 13, 17, 23). However, little is known about the role of lymphocytes in host defense against CMV in the central nervous system (CNS).
Cytotoxic T-cell (CTL) responses are important in controlling the spread of CMV (2, 22, 26, 30), but when operating within the CNS, they may be destructive rather than protective. CD4+ T lymphocytes have been shown to play a crucial role in tissue sites like the salivary gland, where CTLs have limited or no antiviral effect (15). Hence in this study, we explored the hypothesis that CD4+ T lymphocytes possess the ability to inhibit CMV gene expression in human astrocytes.
Peripheral blood mononuclear cells (PBMC) from CMV-seropositive and CMV-seronegative healthy donors were stimulated for 72 h in vitro with CMV strain AD 169 (21). To determine if T lymphocytes possess antiviral properties, CD4+ and CD8+ T cells were isolated from stimulated PBMC cultures using anti-CD4 and anti-CD8 antibody-coated immunomagnetic beads (Dynabeads; Dynal, Inc., Lake Success, N.Y.) yielding lymphocyte populations with >95% purity as determined by flow cytometry. Purified CD4+ or CD8+ lymphocytes were added to human fetal astrocytes, which were prepared as described previously (3). Seventy-two hours after the lymphocyte-astrocyte cocultures were constituted, the cultures were infected at a multiplicity of infection of 2.5 50% tissue culture infective doses per cell with a recombinant CMV strain, RC256 (25), expressing β-galactosidase from a viral β-promoter. Infected cells were harvested 72 h postinfection, resuspended in phosphate-buffered saline (100 μl), and subjected to three freeze-thaw cycles. The cell lysates were analyzed for β-galactosidase activity using CPRG (1 mg/ml; 5-bromo-4-chloro-3-indolyl-β-d-galactoside; Boehringer Mannheim, Indianapolis, Ind.) as a substrate (13). Optical density values at 595 nm (OD595) were used to determine differences in viral gene expression in cultures with and without added lymphocytes.
Lymphocytes from seropositive donors suppress CMV gene expression in human astrocytes.
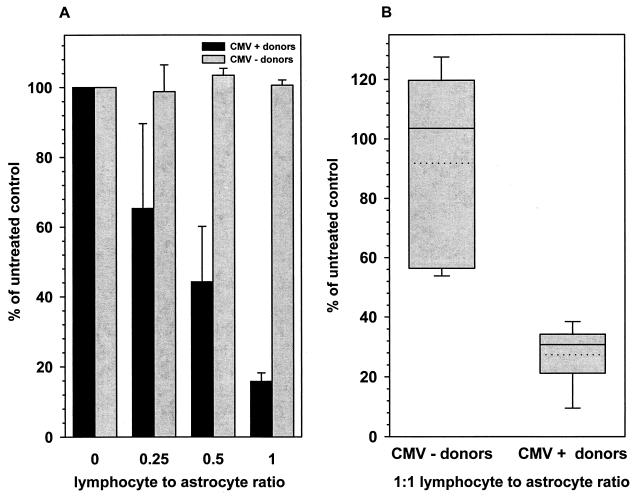
CMV gene expression was markedly reduced in cocultures containing astrocytes and lymphocytes isolated from CMV-seropositive donors compared to cultures with untreated astrocytes (Fig. 1). CD4+ lymphocytes obtained from four seropositive donors dose-dependently suppressed viral gene expression as measured by β-galactosidase activity (Fig. 1A). Lymphocyte-to-astrocyte ratios of 0.25:1, 0.5:1, and 1:1 suppressed viral expression by (34.6 ± 24.3)%, (55.8 ± 15.9)%, and (84.2 ± 2.4)%, respectively (n = 5 experiments). In comparison, CD8+ lymphocytes from seropositive donors suppressed CMV gene expression by (72.7 ± 4.94)% at a ratio of 1:1 (Fig. 1B). However, neither CD4+ nor CD8+ lymphocytes from five seronegative donors, cocultured with astrocytes at the same ratios, suppressed CMV gene expression in astrocytes (Fig. 1).
FIG. 1.
Effect of lymphocytes on CMV gene expression in astrocytes. CD4+ and CD8+ T cells were isolated from PBMC obtained from CMV-seropositive as well as seronegative donors and stimulated in vitro with CMV antigen. CD4+ (A) and CD8+ (B) lymphocytes were cocultured with astrocytes at the indicated ratios for 72 h prior to infection with the recombinant CMV strain RC256. Cultures were collected 72 h postinfection and assayed for β-galactosidase activity. Data, expressed as the percentages of viral expression compared to those in untreated infected controls (means ± standard errors), were obtained from five independent experiments with lymphocytes from seropositive and seronegative donors using astrocytes from different brain specimens.
To rule out the possibility that CD4+ lymphocyte-mediated viral suppression was associated with cytotoxicity in cocultures, MTT [3-(4,5-dimethylthizol-2-yl)-2,5-diphenyltetrazolium bromide] uptake assays (9) were performed at all lymphocyte/astrocyte ratios for the duration of the experiment (6 days). MTT (1 mg/ml) was added to the cultures for 4 h at 37°C. The cultures were then treated with lysis buffer (20% sodium dodecyl sulfate and 50% dimethyl formamide, pH 4.7) overnight at 37°C. OD570 readings, representing the conversion of MTT to formazan in living cells by dehydrogenases, were similar at all coculture ratios, with no appreciable difference between control and lymphocyte-treated astrocyte cultures. OD570 readings for cocultures containing astrocytes (105 cells) with CD4+ T cells (ratio, 1:1) or CD8+ T cells (ratio, 1:1) were 2.8 ± 0.10 and 2.4 ± 0.16, respectively. OD570 readings for astrocytes (105 cells) and lymphocytes (105 cells) cultured separately, representing baseline assay values, were 2.6 ± 0.13 and 0.4 ± 0.01, respectively. These results support the hypothesis that CD4+ lymphocytes possess the ability to confer upon astrocytes noncytotoxic protection against CMV. This antiviral property is dependent on prior exposure of the lymphocyte donor to CMV antigen in vivo. The ability of seropositive donor lymphocytes to suppress CMV gene expression may reflect an effective CD4+ T-cell memory response (7) capable of generating a unique set of cytokines on subsequent stimulation with CMV antigen (12, 16).
Soluble factors mediate antiviral effect of CD4+ lymphocytes.
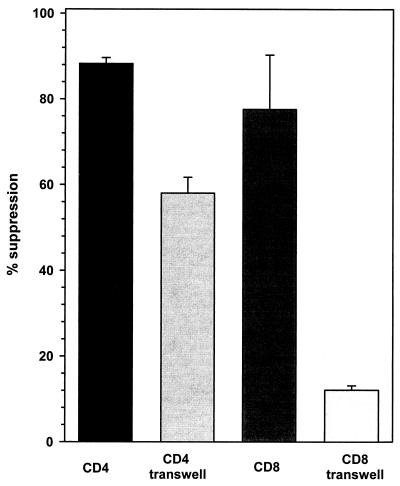
To determine if the noncytotoxic antiviral effect of CD4+ lymphocytes was mediated by soluble factors, transwell tissue culture inserts (Becton Dickinson, Franklin Lakes, N.J.) were used to physically separate the CMV-stimulated lymphocytes from astrocyte monolayers. Following viral infection, CMV expression was suppressed by (58.05 ± 3.67)% (n = 3) when CMV-stimulated CD4+ T cells from seropositive donors were added to one side of the transwell culture system (Fig. 2). CD4+ T cells from seronegative donors, however, had no antiviral effect in this system (data not shown). On the other hand, CD8+ lymphocytes from seropositive donors did not suppress CMV gene expression ([12.73 ± 1.02]%; n = 3) when separated from astrocytes by a porous membrane (Fig. 2), suggesting that effective viral suppression with these cells requires cellular contact.
FIG. 2.
Soluble factors mediate the induction of an antiviral state in astrocytes. CD4+ and CD8+ T cells were maintained in culture with primary human astrocytes across a transwell membrane for 72 h. The cultures were infected with CMV (RC256) and assayed for β-galactosidase activity 72 h postinfection. The data, expressed as percents suppression (means ± standard errors) compared with untreated infected controls, were obtained from three independent experiments using astrocytes and T cells from different donors.
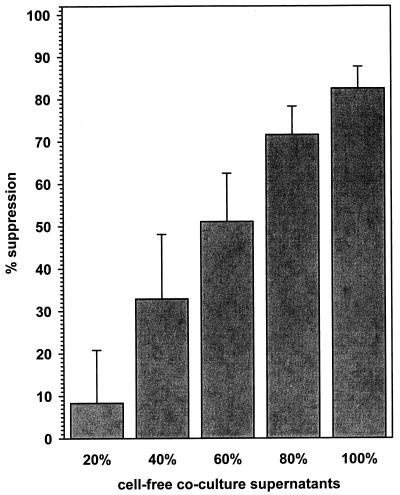
Since the antiviral effects of CD4+ T cells could be mediated across a porous membrane, we tested the ability of cell-free coculture supernatants to impart an antiviral state to astrocyte cultures. Serial dilutions of cell-free supernatants from seropositive lymphocyte-astrocyte cocultures, when added to fresh astrocytes, suppressed CMV gene expression in a concentration-dependent manner (Fig. 3). The decrease in CMV gene expression from four seropositive donor CD4+ lymphocyte-astrocyte cocultures, when compared to untreated controls, ranged from (8.33 ± 12.49)% (20% concentration) to (82.49 ± 5.17)% (100% cell-free supernatants). However, cell-free supernatants from uninfected lymphocyte-astrocyte cocultures, using CD4+ lymphocytes from seronegative donors and CD8+ lymphocytes from both seropositive and seronegative donors, did not suppress viral gene expression (data not shown).
FIG. 3.
Cell-free coculture supernatants confer an antiviral state upon primary human astrocytes. Dilutions of supernatants from CD4+ lymphocyte-astrocyte cocultures were applied to fresh astrocyte cultures for 72 h and then infected with CMV (RC256). Freeze-thawed lysates of infected cells were assayed for β-galactosidase activity 72 h postinfection. The data, expressed as percents suppression compared to untreated infected astrocyte cultures (means ± standard errors), were obtained from independent experiments using CD4+ T cells from four different seropositive donors.
We have attempted to inhibit the antiviral effects of coculture supernatants using neutralizing antibodies to tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ), two known antiviral cytokines. Cell-free supernatants from CD4+ lymphocyte-astrocyte cocultures were incubated with specific antibodies to TNF-α and/or IFN-γ (10 μg/ml) at room temperature for 30 min prior to applying them to astrocyte cultures. This treatment with cytokine-specific antibodies did not significantly abrogate the antiviral activity of the coculture supernatants. These data demonstrate that the soluble factors mediating this antiviral activity are complex and not restricted to TNF-α and IFN-γ.
Host defense mechanisms against viral infections of the CNS are shaped by the brain's limited capacity for antigen presentation and functional modulation of immune responses, designed to prevent extensive damage in this vital nonregenerating tissue (for reviews, see references 8 and 20). Activated lymphocytes routinely enter the CNS, in an antigen-independent manner, during immune stimulation (10) without associated neuropathology (18). Published studies provide evidence that lymphocytes also mediate clearance of viral infections from the CNS without conventional major histocompatibility complex expression on target cells and without massive cell death (1). We show here that CD4+ and CD8+ T lymphocytes from seropositive donors suppressed CMV gene expression in astrocytes. The suppression was not mediated by cytotoxic damage of infected cells but by soluble factors induced in the CD4+ lymphocyte-astrocyte cocultures. Similar experiments have indicated that the soluble factors derived from CMV-stimulated PBMC, which mediate anti-CMV effects on human fibroblasts, are IFNs and TNF (28). CD4+ T-cell clones specific to CMV immediate-early proteins produce TNF-α and IFN-γ, which can inhibit viral replication in U373MG, an astrocyte cell line (6). We have also shown in our laboratory that these proinflammatory cytokines inhibit CMV replication in primary human astrocytes (4). In addition, experiments performed in vivo suggest that TNF-α and IFN-γ may be responsible for control of CMV (14, 19), particularly in areas where CTLs have limited function (15).
This report indicates that lymphocytes mediate suppression of CMV in primary human brain cells. The use of primary astrocytes, in this study, enabled us to evaluate the antiviral effects of lymphocytes in a relevant tissue type. Although the precise mechanisms of viral suppression are unknown, it is likely that one or more cytokines are involved in mediating this noncytotoxic antiviral effect.
In conclusion, the results of this study suggest that CD4+ lymphocytes may play a role in host defense of the brain against CMV infection. The lack of host defense in advanced AIDS, normally provided by CD4+ lymphocytes, may allow viral replication in astrocytes, resulting ultimately in the necrotizing lesions seen during CMV ventriculoencephalitis. These findings also have implications in developing immune-based therapies for CMV brain infection. However, successful development of adoptive immunotherapies for CNS infections will require a much greater understanding of both viral pathogenesis and neuroimmune responses to the virus.
Acknowledgments
This study was supported in part by the United States Public Health Service grants MH-57617, T-32-DA-07239, and NS-38836.
REFERENCES
- 1.Armstrong W S, Lampson L A. Direct cell-mediated responses in the nervous system: CTL vs. NK activity, and their dependence upon MHC expression and modulation. In: Keane R W, Hickey W F, editors. Immunology of the nervous system. New York, N.Y: Oxford University Press; 1997. pp. 493–547. [Google Scholar]
- 2.Bigger J E, Tanigawa M, Thomas C A, 3rd, Atherton S S. Protection against murine cytomegalovirus retinitis by adoptive transfer of virus-specific CD8+ T cells. Investig Ophthalmol Vis Sci. 1999;40:2608–2613. [PubMed] [Google Scholar]
- 3.Chao C C, Hu S, Sheng W S, Bu D, Bukrinsky M I, Peterson P K. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia. 1996;16:276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AID-GLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Cheeran M C-J, Hu S, Gekker G, Lokensgard J R. Decreased cytomegalovirus expression following proinflammatory cytokine treatment of primary human astrocytes. J Immunol. 2000;164:926–933. doi: 10.4049/jimmunol.164.2.926. [DOI] [PubMed] [Google Scholar]
- 5.Cinque P, Marenzi R, Ceresa D. Cytomegalovirus infections of the nervous system. Intervirology. 1997;40:85–97. doi: 10.1159/000150536. [DOI] [PubMed] [Google Scholar]
- 6.Davignon J L, Castanie P, Yorke J A, Gautier N, Clement D, Davrinche C. Anti-human cytomegalovirus activity of cytokines produced by CD4+ T-cell clones specifically activated by IE1 peptides in vitro. J Virol. 1996;70:2162–2169. doi: 10.1128/jvi.70.4.2162-2169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davignon J L, Clement D, Alriquet J, Michelson S, Davrinche C. Analysis of the proliferative T cell response to human cytomegalovirus major immediate-early protein (IE1): phenotype, frequency and variability. Scand J Immunol. 1995;41:247–255. doi: 10.1111/j.1365-3083.1995.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 8.Griffin D E. Cytokines in the brain during viral infection: clues to HIV-associated dementia. J Clin Investig. 1997;100:2948–2951. doi: 10.1172/JCI119847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 10.Hickey W F. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1:97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 11.Ho W Z, Song L, Douglas S D. Human cytomegalovirus infection and trans-activation of HIV-1 LTR in human brain-derived cells. J Acquir Immune Defic Syndr. 1991;4:1098–1106. [PubMed] [Google Scholar]
- 12.Kallas E G, Reynolds K, Andrews J, Fitzgerald T, Kasper M, Menegus M, Evans T G. Cytomegalovirus-specific IFN-gamma and IL-4 are produced by antigen expanded human blood lymphocytes from seropositive volunteers. Immunol Lett. 1998;64:63–69. doi: 10.1016/s0165-2478(98)00080-7. [DOI] [PubMed] [Google Scholar]
- 13.Lokensgard J R, Cheeran M C, Gekker G, Hu S, Chao C C, Peterson P K. Human cytomegalovirus replication and modulation of apoptosis in astrocytes. J Hum Virol. 1999;2:91–101. [PubMed] [Google Scholar]
- 14.Lucin P, Jonjic S, Messerle M, Polic B, Hengel H, Koszinowski U H. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumour necrosis factor. J Gen Virol. 1994;75:101–110. doi: 10.1099/0022-1317-75-1-101. [DOI] [PubMed] [Google Scholar]
- 15.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski U H. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maino V C. Rapid assessment of antigen induced cytokine expression in memory T cells by flow cytometry. Vet Immunol Immunopathol. 1998;63:199–207. doi: 10.1016/s0165-2427(98)00096-8. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy M, Wood C, Fedoseyeva L, Whittemore S R. Media components influence viral gene expression assays in human fetal astrocyte cultures. J Neurovirol. 1995;1:275–285. doi: 10.3109/13550289509114024. [DOI] [PubMed] [Google Scholar]
- 18.Merrill J E, Benveniste E N. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 19.Pavic I, Polic B, Crnkovic I, Lucin P, Jonjic S, Koszinowski U H. Participation of endogenous tumour necrosis factor alpha in host resistance to cytomegalovirus infection. J Gen Virol. 1993;74:2215–2223. doi: 10.1099/0022-1317-74-10-2215. [DOI] [PubMed] [Google Scholar]
- 20.Perry V H, Bell M D, Brown H C, Matyszak M K. Inflammation in the nervous system. Curr Opin Neurobiol. 1995;5:636–641. doi: 10.1016/0959-4388(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 21.Peterson P K, Gekker G, Chao C C, Schut R, Verhoef J, Edelman C K, Erice A, Balfour H H., Jr Cocaine amplifies HIV-1 replication in cytomegalovirus-stimulated peripheral blood mononuclear cell cocultures. J Immunol. 1992;149:676–680. [PubMed] [Google Scholar]
- 22.Podlech J, Holtappels R, Wirtz N, Steffens H P, Reddehase M J. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol. 1998;79:2099–2104. doi: 10.1099/0022-1317-79-9-2099. [DOI] [PubMed] [Google Scholar]
- 23.Poland S D, Costello P, Dekaban G A, Rice G P. Cytomegalovirus in the brain: in vitro infection of human brain-derived cells. J Infect Dis. 1990;162:1252–1262. doi: 10.1093/infdis/162.6.1252. [DOI] [PubMed] [Google Scholar]
- 24.Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Luccaronin P, Jonjic S, Koszinowski U H. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med. 1998;188:1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaete R R, Mocarski E S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci USA. 1987;84:7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffens H P, Kurz S, Holtappels R, Reddehase M J. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J Virol. 1998;72:1797–1804. doi: 10.1128/jvi.72.3.1797-1804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tay-Kearney M L, Enger C, Semba R D, Royal W, 3rd, Dunn J P, Jabs D A. T cell subsets and cytomegalovirus retinitis in human immunodeficiency virus-infected patients. J Infect Dis. 1997;176:790–794. doi: 10.1086/517303. [DOI] [PubMed] [Google Scholar]
- 28.Torigoe S, Campbell D E, Starr S E. Cytokines released by human peripheral blood mononuclear cells inhibit the production of early and late cytomegalovirus proteins. Microbiol Immunol. 1997;41:403–413. doi: 10.1111/j.1348-0421.1997.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 29.van den Horn G J, Meenken C, Danner S A, Reiss P, de Smet M D. Effects of protease inhibitors on the course of CMV retinitis in relation to CD4+ lymphocyte responses in HIV+ patients. Br J Ophthalmol. 1998;82:988–990. doi: 10.1136/bjo.82.9.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weekes M P, Wills M R, Mynard K, Carmichael A J, Sissons J G. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J Virol. 1999;73:2099–2108. doi: 10.1128/jvi.73.3.2099-2108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]