Highlights
-
•
Amandin (AMP) binding to EGCG changed protein structure.
-
•
AMP bound to EGCG primarily through glutamate and cysteine residues.
-
•
Alkaline and free radical methods dented AMP allergenic, but the principles differed.
Keywords: Amandin, EGCG, Structure, Allergenicity, LC-MS/MS
Abstract
Potential allergenicity of amandin was reduced by binding amandin with (−)-epigallocatechin gallate (EGCG) via alkaline, free radical, ultrasound-assisted alkaline, and ultrasound-assisted free radical methods. These results of total phenol content, free sulfhydryl group, free amino group, surface hydrophobicity, and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) indicated that amandin might be covalently bound to EGCG through reactive groups such as sulfhydryl and amino groups, or non-covalently through hydrophobic interactions. Fourier transformed infrared (FT-IR) spectroscopy and fluorescence spectroscopy revealed structural changes of amandin-EGCG conjugate, which also caused significant reduction in potential allergenicity of amandin. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) found that amandin bound to EGCG mainly through cysteine and glutamate residues, and linear epitope for amandin was reduced. This provided a new method and theoretical basis of hypoallergenic almond food.
1. Introduction
Amandin is a central storage protein in almonds, accounting for 65 % of the soluble protein (Zhang et al., 2018), which is an oligomeric protein containing prunin monomers, with the three protomers forming a doughnut-shaped trimer connected by a non-crystalline 3-fold axis. Almonds, one of the eight major food allergens, caused allergic reactions that were persistent and even severe to life-threatening (Bezerra et al., 2021).
The diversity of food made it exist in the market in a unitary form and various forms, such as pastries, drinks, and snack foods. This condition significantly increases the risk of food allergies for consumers. Currently, the methods for reducing food allergenicity are mainly heat and non-heat treatments. Heat treatment included dry heat, boiling, autoclaving, roasting, and ohmic heating (Zaffran and Sathe, 2018, Zhang et al., 2016, Pereira et al., 2021). Non-heat treatment included radiation, ultrasound, Maillard reaction, fermentation, ultrahigh pressure, and enzymatic hydrolysis (Deng Han, 2017, Zhao et al., 2017). Some proteins had high thermal stability during food processing, so reducing the potential allergenicity of protein through heat treatment was prohibitively expensive.
(−)-Epigallocatechin gallate (EGCG) is the main constituent of green tea polyphenol. Phenols are mainly non-covalently and covalently bound to proteins through hydrogen bonding, p-bonding, and hydrophobic, ionic, and covalent linkages (Wang et al., 2014). EGCG oxidized to o-quinone under alkaline conditions and reacted nucleophilically with sulfhydryl groups in proteins, resulting in Ces bonds forming covalent crosslinks. The free radical method mainly involved the interaction of redox components in generating hydroxyl radicals, which attacked the reaction residues on the protein side chain and caused the protein residues that react with EGCG (Wang et al., 2014, Zhou et al., 2020). As previously described, protein allergens, such as ovalbumin and whey proteins, significantly reduce the potential allergenicity of proteins when combined with EGCG (He et al., 2019, Pessato et al., 2018). The determination of total phenol content in protein-EGCG conjugates was mainly performed by the Folin-Ciocalteu method (Wang et al., 2014, Wei et al., 2015). Polyphenols react specifically with Folin-Ciocalteu reagent, and their reaction products have maximum absorption at specific wavelengths. The absorption value is linearly related to the number of polyphenols in a particular range.
In this paper, amandin-EGCG conjugate was prepared by alkaline and free radical methods. The effects of EGCG on potential allergenicity of amandin were examined by enzyme linked immunosorbent assay (ELISA) and Western blot, and the changes of EGCG on the physicochemical property and structure of amandin were studied by free sulfhydryl content, free amino content, Fourier transformed infrared (FT-IR) spectroscopy, and fluorescence spectroscopy. Further, liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to explore the sites where amandin could bind to EGCG and the alteration of amandin linear epitopes. These results provide a new idea and theoretical basis for reducing the potential allergenicity of almond-related foods in processing.
2. Material and methods
2.1. Materials
The stone almonds were purchased from the almond farm in Shache County, Kashgar Prefecture (Xinjiang, China), and the harvest date was usually around August 20. Almond ELISA kit (EKT-A10) was purchased from Qingdao Pribolab Bioengineering Co. (Qingdao, China). EGCG, ascorbic acid, Folin–Ciocalteu reagent, Ellman reagent, ortho-phthaldialdehyde (OPA), bromophenol blue, and potassium bromide were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). SDS-PAGE gel preparation kit, loading buffer, and Coomassie Brilliant Blue rapid dyeing solution were purchased from Beijing Solarbio Technology Co., Ltd (Beijing, China). Other analytical reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All solutions were prepared with deionized water.
2.2. The extraction of amandin
The method of extracting amandin was referred to Xin et al. (2021). Distilled water containing 0.02 % sodium azide was added to almond defatted powder. Almond defatted powder was extracted by magnetic stirring at room temperature (RT, 25 ℃) for 1 h. The extracted mixture after extraction was centrifuged at 4 ℃ for 10 min, and the above extraction operation was repeated. The supernatant was filtrated by brucellofer funnel and stood at 4 ℃ for 12–18 h. Precipitation was collected after centrifugation (8000g, 20 min, 4 ℃) of supernatant. The extracted amandin was freeze-dried and stored in the refrigerator at −20 ℃ for later use.
2.3. Preparation of amandin-EGCG conjugate
The preparation of amandin-EGCG conjugate was based on the method of He et al. (2019) and slightly modified, including alkaline, free radical, ultrasound-assisted alkaline, and ultrasound-assisted free radical method.
Alkaline method: The pH of amandin (5 mg/mL) was adjusted to 9.0, and then 0.2 mmol EGCG was added to the reaction at RT and 130 rpm for 24 h. After the reaction, unreacted EGCG was removed by dialysis with deionized water (4 ℃, 48 h).
Free radical method: The 0.25 g ascorbic acid and 1 mL hydrogen peroxide (5.0 mol/L) were added to amandin (5 mg/mL) and responded at RT and 130 rpm for 2 h. Then 0.2 mmol EGCG was added and reacted at RT and 130 rmp for 24 h. After the reaction, the unreacted EGCG was removed by dialysis with deionized water (4 ℃, 48 h).
Ultrasound-assisted alkaline method: Amandin (5 mg/mL) was ultrasonicated at 400 W for 15 min, and then subsequent operations were the same as alkaline method.
Ultrasound-assisted free radical method: Amandin (5 mg/mL) was ultrasonicated at 400 W for 15 min, and subsequent preparation steps were the same as free radical method.
2.4. Determination of total phenol content
The determination of total phenol content was based on the method of Jing et al. (2020). About 1 mg/mL amanidn was mixed with 2.5 mL Folin–Ciocalteu reagent at 25 ℃ for 5 min, then 2 mL anhydrous sodium carbonate (7.5 %) was added and reacted at RT for 2 h under dark condition. The absorbance was measured at 760 nm by ultraviolet–visible spectrophotometer (UV-1780PC, Shimadzu Co. Ltd, Suzhou, China), and the EGCG content was calculated using EGCG standard curve.
2.5. Determination of free sulfhydryl group content
The free sulfhydryl group content of amandin and amandin-EGCG conjugate was determined by referring to the method of Luo et al. (2022). Amandin was mixed with 8 mL Tris-glycine solution and centrifuged for 15 min to remove the residue. The 4.5 mL supernatant and 0.5 mL Ellman reagent (10 mmol/L) were thoroughly mixed and reacted for 30 min under dark. The absorbance value was measured at 412 nm, and the molar extinction coefficient was 13,600 L/(mole cm).
2.6. Determination of free amino content
The determination of free amino content was slightly modified according to the method of Wu et al. (2022). The 200 µL amandin was added to 4 mL OPA reagent, and the absorbance was measured at 340 nm. Free amino content of sample was determined by Lys standard curve, and the absorbance value of sample was substituted into a standard curve to calculate free amino content.
2.7. Determination of surface hydrophobicity
About 200 μL bromophenol blue solution was added to 1 mL amandin (1 mg/mL) and then reacted at RT for 10 min. The mixture was centrifuged (5000g, 10 min), and the absorbance of supernatant was measured at 595 nm.
2.8. Determination of solubility
Solubility was determined by referring to the method of Huang et al. (2021a). The protein concentration was determined before and after centrifugation (10,000g, 10 min). Protein solubility was the percentage of protein content in protein supernatant to total protein content after centrifugation.
2.9. SDS-PAGe
The SDS-PAGE operation method referring to Huang et al. (2021b) was carried out and slightly modified. Amandin (8 mg/mL) was mixed with the sample loading buffer (containing β -mercaptoethanol) in a ratio of 4:1 (V/V), and then the protein was bathed in boiling water for 10 min. The cooled sample solution was centrifuged at 4 ℃ and 10,000g for 10 min. About 5 μL supernatant was added to the gel (separate 12 % and concentrate 4 %) and electrophoresis was performed at 100 V for 1–2 h. After electrophoresis, Coomassie Brilliant Blue rapid dyeing solution was used for rapid staining and decolorization until the bands were clear.
2.10. Fourier transform infrared (FT-IR) spectroscopy
About 4 mg of freeze-dried protein was mixed with 400 mg of dried potassium bromide for milling and tablet pressing. FT-IR spectrometer (Shimadzu Corporation, Tokyo, Japan) was used for measurement, with a wave number range of 4000–400 cm−1 and resolution of 4.0 cm−1. The number of scans was 32 (Li et al., 2021).
2.11. Fluorescence spectroscopy
Fluorescence spectroscopy was determined according to the method described by Geng et al. (2021), with some modifications. The excitation wavelength of protein solution was 280 nm, and the emission wavelength was 300–450 nm. The measurement was carried out with fluorescence spectrometer (RF-5301, Shimadzu Crop., Kyoto, Japan) under the narrow slit width of 5 nm.
2.12. Western blot
The operation method of Western blot was modified according to Zhang et al. (2018). The target area of protein glue was soaked in methanol for 5 min and then transferred to a membrane transfer solution. The filter paper was laid flat on the sponge. The bond was spread on filter paper and membrane transfer solution was added to drive away bubbles again. Electrophoresis tank was placed in the ice water mixture at a constant flow of 150 mA for 1.5 h. PVDF membrane was immersed in sealing solution and slowly rocked in a horizontal shaker at RT for 1 h. Then it was soaked in the corresponding primary antibody and incubated gently at RT for 1 h or overnight at 4 ℃. After primary antibody incubation, PVDF membrane was washed with TBST five times. PVDF membrane was infiltrated into the corresponding secondary antibody and incubated gently at RT for 1 h. After the second antibody incubation, PVDF membrane was rewashed. Horseradish peroxidase HRP-ECL luminescence method was used. ECL chemiluminescence substrate A and B luminescence solutions were diluted and mixed at 1:1 (V/V) ratio. Carton was covered with plastic wrap, and then plastic wrap was placed on filter paper angle of the PVDF film. Mixed solutions A and B were dropped on PVDF membrane and photographed by GelView 6000 M (Guangzhou Boluteng Instrument Co., Guangzhou, China).
2.13. ELISa
The almond ELISA kit determined IgE-binding capacity of amandin and amdnin-EGCG conjugate. The 100 μL sample dilution was added to an enzyme-labeled plate containing antibodies and incubated at RT for 20 min. The unbound antibody samples were removed by washing solution three times and then incubated with 100 μL peroxidase-marzipan second antibody conjugate. After washing again, about 100 μL chromogenic solution was added and reacted at RT for 20 min under dark conditions. Finally, the 100 μL stop solution was added, and the absorbance value was measured at 450 nm.
2.14. LC-MS/Ms
The target rubber strip was treated with trypsin and digested with the enzyme at 37 ℃ for 16 h. Extraction solution in water bath for 1 h was treated by ultrasound and centrifugation (5 min), then transferred into a new EP tube and repeated. Extract was combined and dried by vacuum centrifugation. After enzymatic digestion, the peptide was desalted by self-filling desalting column and dried in 45 ℃ vacuum centrifuge concentrator. The analytical column was 150 μm i.d. × 150 mm, packed with Acclaim PepMap RPLCC18, 1.9 μm, 100 Å. Mobile phase A was 0.1 % formic acid; mobile phase B was 0.1 % formic acid and 80 % ACN. The flow rate was controlled at 600 nL/min and analysis time for each component was 60 min. The resolution of the primary mass spectrometry was 60,000, AGCtarget was 3e6, MaximumIT was 100 ms, and the scan range was 400 to 1200 m/z. The parameters of the secondary mass spectrometry were 15,000 resolution, AGCtarget was 1e6, MaximumIT was 50 ms, TopN was 20, and NCE was 30.
2.14. Statistical analysis
The test results were expressed as mean ± standard deviation, and the number of sample replicates n = 3. The different letters (a, b) indicated significant differences (p < 0.05) between samples. SPSS 25.0.0 software (SPSS Inc., Chicago, IL, USA) was used for one-way ANOVA, and Origin 2018 software (OriginLab, Northampton, MA, USA) was used for plotting.
3. Results and discussion
3.1. Total phenols content
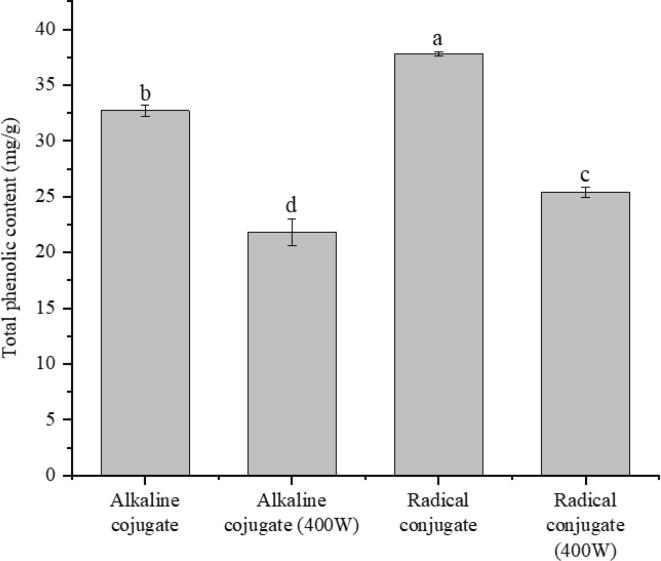
Total phenols content of amandin-EGCG conjugate could be used to assess the degree of amandin binding to EGCG. As shown in Fig. 1, total phenol content of the conjugate prepared by free radical method was the highest at 37.8 mg/g, and the lowest was 21.8 mg/g for the conjugate prepared by ultrasound-assisted alkaline method. Total phenol content of the conjugate prepared by free radical method was significantly greater than that of alkaline method. He et al found that the EGCG content in ovalbumin-EGCG conjugate prepared by free radical and alkaline methods were 33.24 and 11.31 mg/g, respectively. The binding efficiency of free radical method was higher than that of alkaline method (He et al., 2019). This result was consistent with trend of this paper. However, Jing et al showed that total phenolic content in ovalbumin-tea polyphenol conjugate prepared by alkaline method was greater than that of free radical method (Jing et al., 2020). The reason for this difference might not only be due to the different molecular mechanisms of protein–phenolic conjugate prepared by free radical and alkaline methods, but also due to the differences in amino acid composition and structure of different proteins. Total phenolic content of the conjugates prepared by ultrasound-alkaline method and ultrasound-assisted free radical method was lower than that of alkaline and free radical methods, respectively. However, the reaction time of ultrasound-assisted methods was 60 min compared to 24 h for the free radical and alkaline methods. Ultrasound assistance promoted protein unfolding and increased the amount of reactive amino groups in the grafting reaction, thus greatly reducing the reaction time (Jing et al., 2020).
Fig. 1.
Total phenolic content of amandin-EGCG conjugate prepared by different methods.
3.2. Free sulfhydryl group content
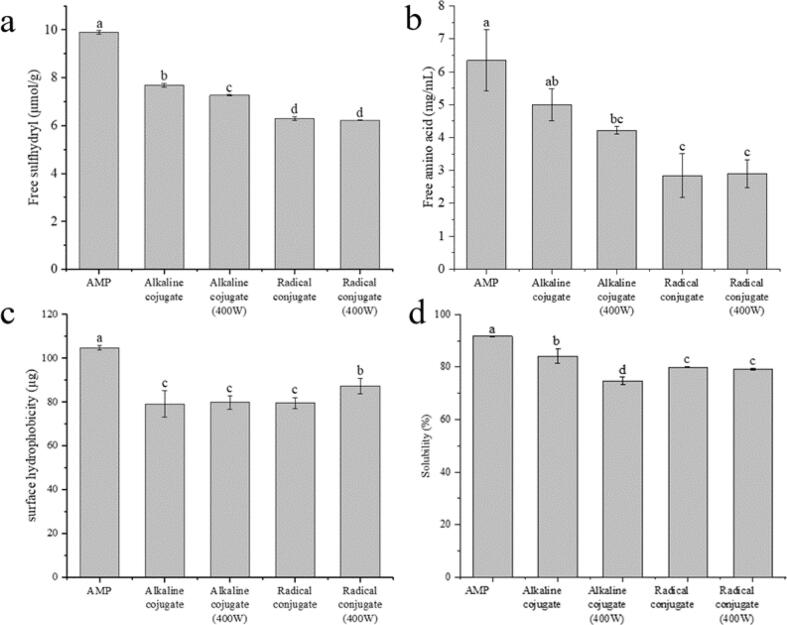
Sulfhydryl groups are reactive chemical groups in proteins, which are easily affected by phenols, with reducing ability. Free sulfhydryl content of amandin-EGCG conjugate prepared by different methods was shown in Fig. 2a. Free sulfhydryl content of amandin-EGCG conjugate was lower than that of amandin, and free sulfhydryl content of amandin-EGCG conjugate prepared by free-radical and ultrasound-assisted free-radical methods was significantly lower than that prepared by alkaline method. The presence of urea in the test avoided conversion of sulfhydryl groups to disulfide bonds. This could indicate that the decrease in sulfhydryl content of amandin-EGCG conjugate was due to the reaction of amandin with EGCG. EGCG oxidized to o-quinone under alkaline conditions and reacted nucleophilically with sulfhydryl groups in proteins, resulting in Ces bonds to form covalent crosslinks. Zhou et al found that free sulfhydryl groups of soybean isolate protein-EGCG conjugates prepared by alkaline method were significantly lower than those of soybean isolate (21.35 μmol/g), which was mainly because the covalent reaction of sulfhydryl groups in soybean isolate with EGCG to form Ces bonds (Zhou et al., 2020a). Free radical method mainly involved the interaction of redox components to generate hydroxyl radicals, which attacked the reaction residues on the protein side chain and generated the protein residues that react with EGCG. Free sulfhydryl content of amandin-EGCG conjugate prepared by free radical and ultrasound-assisted free radical methods was significantly lower than that prepared by alkaline method (Fig. 2a). Amandin exposed more sulfhydryl groups that could bind to EGCG in alkaline method than in free radical method.
Fig. 2.
Contents of free sulfhydryl group (a), free amino group (b), surface hydrophobicity (c), solubility (d) in amandin-EGCG conjugate prepared by different methods.
3.3. Free amino content
The OPA method can be used to determine free amino content of protein and can provide some information on the lysine side chain (Church et al., 1983). Amandin had free amino content of 6.34 mg/mL. Free amino content of amandin-EGCG conjugate prepared by different methods was lower than that of amandin, and the trend was consistent with that of free sulfhydryl groups (Fig. 2b). Fan et al showed that free amino group content of conjugates from reaction of a-lactalbumin, b-lactoglobulin, lactoferrin and sodium caseinate with EGCG was lower than that of single protein (Fan et al., 2015). Both the sulfhydryl and amino groups of protein were reactive groups in proteins. When polyphenols were converted to quinones, the hydroxyl group in EGCG could act as an intermolecular cross-linker and the quinones form CeS and CeN bonds with the reactive groups in proteins via Schiff base and Michael addition reactions (Wu et al., 2018).
3.4. Surface hydrophobicity
Surface hydrophobicity was the number of hydrophobic groups on the surface of protein in contact with water and was often expressed as the amount of bromophenol blue binding (Luo et al., 2022). The more the protein bound to bromophenol blue, the greater the surface hydrophobicity. Surface hydrophobicity of amandin-EGCG conjugate prepared by different methods was shown in Fig. 2c. Surface hydrophobicity of amandin was 104.67 μg. Surface hydrophobicity of amandin-EGCG conjugate prepared by different methods was lower than that of amandin, with the lowest surface hydrophobicity (79.07 μg) of amandin-EGCG conjugates prepared by alkaline method. Amandin and EGCG might react through hydrophobic groups. Hydrophobic interactions were mainly between the non-polar aromatic rings of phenolic compounds and hydrophobic sites of protein (Zhang et al., 2020). β-Lactoglobulin bound to EGCG mainly through hydrophobic interactions, and surface hydrophobicity of its conjugate was much lower than that of β-lactoglobulin (Zhang et al., 2020). Surface hydrophobicity of conjugates prepared by free radical method and ultrasound-assisted free radical method differed significantly, with surface hydrophobicity of ultrasound-assisted free radical method being significantly greater than that of free radical method. The ultrasonic treatment opened the structure of amandin and exposed the hydrophobic groups partially buried in amandin. However, there was no significant difference between alkaline method and ultrasound-assisted alkaline method. However, there was no significant difference between alkaline method and ultrasound-assisted alkaline method. This was consistent with the effect of sonication assistance on the surface hydrophobicity of prepared egg white protein-EGCG (EWP-EGCG) conjugate (Jing et al., 2020).
3.5. Solubility
Solubility could be used to measure the degree of protein denaturation and aggregation, which affected functional properties and immunoreactivity of proteins (Dong et al., 2019). Chen and Ma found that solubility of EWP-EGCG conjugate prepared under neutral conditions was lower than that of egg white protein (Chen & Ma, 2020). Combined with FT-IR spectroscopy, the decrease in solubility might be due to the lower polar microenvironment in which the conjugates were located and stable formation of egg-white protein-EGCG insoluble conjugates. Fig. 2d showed solubility of amandin-EGCG conjugate prepared by different methods. Solubility of amandin-EGCG conjugate prepared by different methods was less than that of amandin, among which solubility of amandin-EGCG conjugate prepared by ultrasound-assisted alkaline method was the lowest at 74.74 %. However, there was no significant difference between amandin-EGCG conjugate prepared by free radical method and ultrasound-assisted free radical method. Protein precipitation by phenolics might result from two different mechanisms. The phenolics acted as multisite ligands interacting with multiple sites or multiple proteins, resulting in the formation of protein–phenolic dimer or multimers. In addition, coverage of the proteins with a less hydrophilic 10 Q also led to reduced solubility of the bound material (Zhang et al., 2020).
3.6. SDS-PAGe
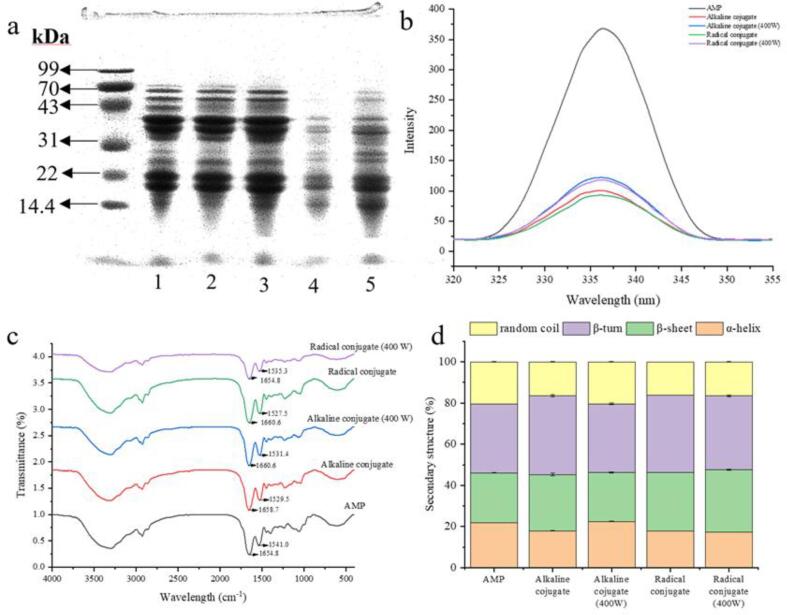
Amandin was the main storage protein in almonds, which mainly consisted of two polypeptide chains at 38–41 kDa and 20–22 kDa (Zhang et al., 2020). As shown in Fig. 3a, the extracted amandin mainly had bands at 70, 60, 45, 40, 35, 30, and 22 kDa. The bands at 40 and 30 kDa of amandin-EGCG conjugate prepared by alkaline method and ultrasound-assisted alkaline method were slightly shifted upward. Amandin-EGCG conjugate prepared by free radical and ultrasound-assisted free radical methods showed significant upward shift of the bands at 30 kDa, and the bands were both lighter than those of alkaline and ultrasound-assisted alkaline methods. Plundrich et al found the SDS-PAGE bands became blurred or even disappeared when polyphenols from green tea or fruit juice were added to peanut proteins, which might be due to complexation with phenolics that interfere with protein staining (Plundrich et al., 2014). The loading buffer contained β-mercaptoethanol, which could break the disulfide bond in the sample. The upward shift of the bands indicated that amandin bound to EGCG through non-disulfide bonds.
Fig. 3.
SDS-PAGE profile (a), fluorescence intensity (b), FT-IR spectra (c), contents of secondary structures (α-helix, β-folding, β-turning and random coil) (d) of amandin-EGCG conjugate prepared by different methods.
3.7. FT-IR spectroscopy
The secondary structure of amandin and amandin-EGCG conjugate were determined using FT-IR spectroscopy, and Fig. 3c showed the main spectral peaks of the samples. The absorption bands at 1600–1700 cm−1 and 1450–1500 cm−1 correspond to the groups in the amide I and II bands, respectively. The spectral peaks at 1541 cm−1 in amandin-EGCG conjugate underwent a shift to lower wave number, while the spectral peak at 1654.8 cm−1 underwent a shift to higher wave number. In addition, no new non-covalent bonds were generated in amandin-EGCG conjugate. The shift of the spectral peak to higher wave numbers might be a strong stretching vibration of the newly formed C—N covalent bond in conjugate, suggesting that amandin might be covalently bound to EGCG through the C—N bond. Jing et al found that the FT-IR spectroscopic results indicated that tea polyphenol (TP) and EWP in both free radical and alkaline methods might be covalently bound through C—N bonds, which was consistent with the results of this paper (Jing et al., 2020).
The amandin I band at 1600–1700 cm−1 was subjected to deconvolution and other processes to obtain the content of protein secondary structures (α-helix, β-sheet, β-turn and random coil). Fig. 3d represented secondary structures of amandin as well as amandin-EGCG conjugate. The amandin-EGCG conjugate prepared by alkaline and free radical methods showed reduced content of α-helix and random coil and increased content of β-sheet and β-turn relative to amandin. This might also be responsible for the reduced amide I band amplitude. Secondary structure within protein was associated with specific hydrogen bonding pattern (Jia et al., 2019), and protein structure was changed after amandin binding to EGCG, mainly from ordered to disordered structure.
3.8. Fluorescence spectroscopy
The endogenous fluorescence of proteins was mainly caused by chromophore groups tryptophan and tyrosine residues, of which tryptophan played a major role. The chromophores of proteins were susceptible to polarity of surrounding microenvironment, and small molecule ligands could modify the fluorescence intensity of proteins by altering surrounding microenvironment or binding to chromophores of proteins (Chen & Ma, 2020). Fig. 3d showed fluorescence intensity of amandin-EGCG conjugate prepared by different methods. Amandin had the strongest fluorescence intensity, and fluorescence intensity of amandin-EGCG prepared by different methods was lower than that of amandin, but the maximum emission wavelength did not change. Combined with total phenolic content of amandin-EGCG conjugate, the higher total phenolic content of amandin-EGCG conjugate, the lower its fluorescence intensity. This was consistent with the trend of results of previous studies (Jing et al., 2020, You et al., 2014). These phenomena suggested that the binding of EGCG to amandin led to the quenching of tryptophan and tyrosine or formation of shielding region around tryptophan residuesa. The highest degree of EGCG grafting on amandin, the more significant the quenching of fluorescence intensity.
3.9. Western blot
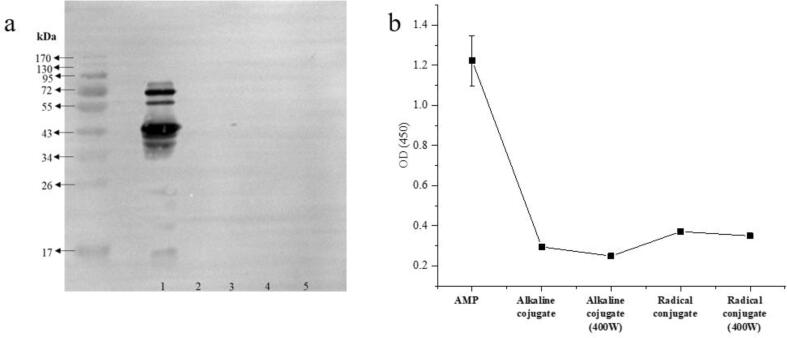
Western blot detected antigenicity in samples based primarily on specific binding of antigenic antibodies and was often used to assess potential allergenicity of proteins. As shown in Fig. 4a, amandin showed bands at 72, 60, 43, and 38 kDa, which indicated that amandin could bind to IgE-antibodies and was potential allergenicity. Amandin-EGCG conjugate did not show bands binding to IgE-antibodies, and EGCG binding significantly reduced the potential allergenicity of amandin. Combined with solubility and structural characterization, amandin-EGCG conjugate showed significant decrease in solubility and changed in protein structure. The antigenic epitope of amandin was destroyed or buried, and its potential allergenicity was reduced. Structural and conformational changes caused by processes such as food processing alter the immunoreactivity of proteins (Fotschki et al., 2020). The reduced potential allergenicity of amandin might be mainly due to the absence of disulfide bonds, decreased solubility such as formation of protein–polyphenol insoluble conjugates, and burial of antigenic epitopes (Chhabra et al., 2017).
Fig. 4.
Immunoreactivity data of amandin-EGCG conjugate prepared by different methods. Where A is the western blot of amandin-EGCG conjuage, 1, 2, 3, 4, and 5 are amandidn, amandin-EGCG conjugate prepared by alkaline method, amandin-EGCG conjugate prepared by ultrasound-assisted alkaline method, amandin-EGCG conjugate prepared by free radical method and amandin-EGCG conjugate prepared by ultrasound-assisted free radical method, respectively. B is the ELISA data of amandin-EGCG conjugate prepared by different methods.
3.10. ELISa
The effect of EGCG binding on the IgE-binding capacity of amandin was shown in Fig. 4b. The higher absorbance value of ELISA assay, the stronger binding capacity of protein to antibody. amandin had the OD (4 5 0) value of 1.23, and the OD (4 5 0) value of amandin-EGCG conjugate decreased significantly. The OD (4 5 0) value of amandin-EGCG conjugate prepared by ultrasound-assisted alkaline method was the lowest at 0.25, which was 79.67 % lower compared to the OD (4 5 0) value of amandin. Combined with Western blotting, EGCG binding significantly reduced potential sensitization of amandin. EGCG has been documented to reduce the potential sensitization of some proteins such as egg white proteins, soy isolates and lactoglobulins. Among them (Sen & Marina, 2018) showed that minor structural modifications of ovomucoid significantly decreased the IgE-binding capacity of egg whites. Soybean isolated protein was modified by EGCG through cysteine, histidine, lysine and arginine residues, thus reducing IgE-binding capacity (Zhou et al., 2020a). The results of this paper also confirmed that EGCG modification could alter the potential allergenicity of amandin. In addition, protein degradation and cross-linking between proteins and peptides might occur after ultrahigh-pressure treatment, leading to significant reduction in allergenicity. The degraded protein fragments or released peptides might also disrupt the linear epitopes of the antigen, thus reducing the IgE/IgG-binding capacity (Quirós et al., 2007, Zhang et al., 2019, Zhang et al., 2017).
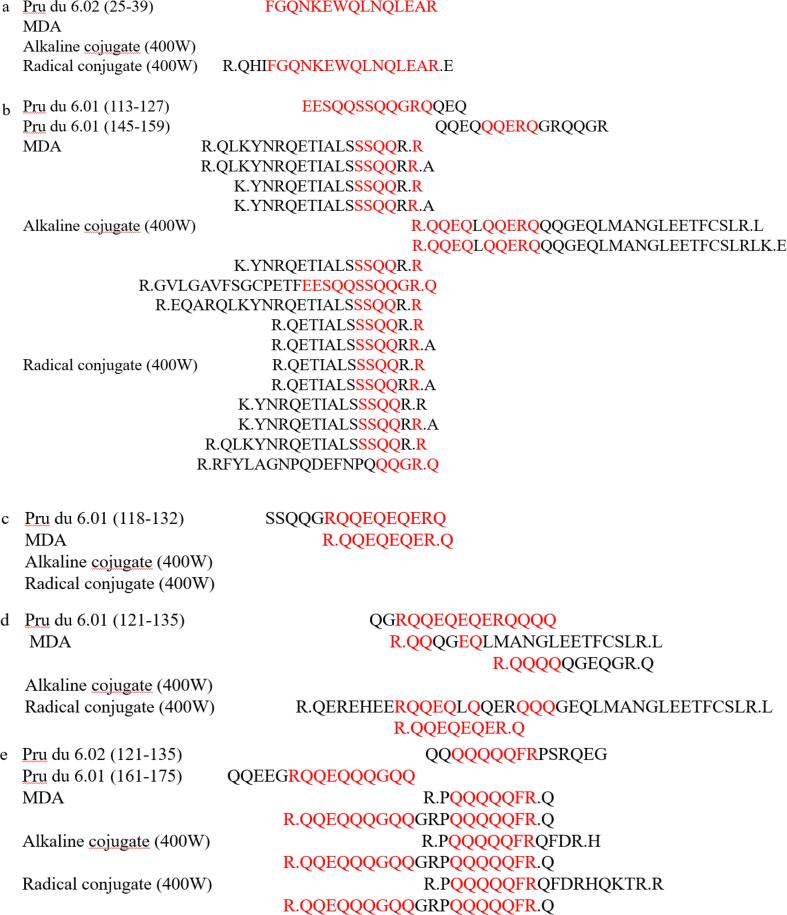
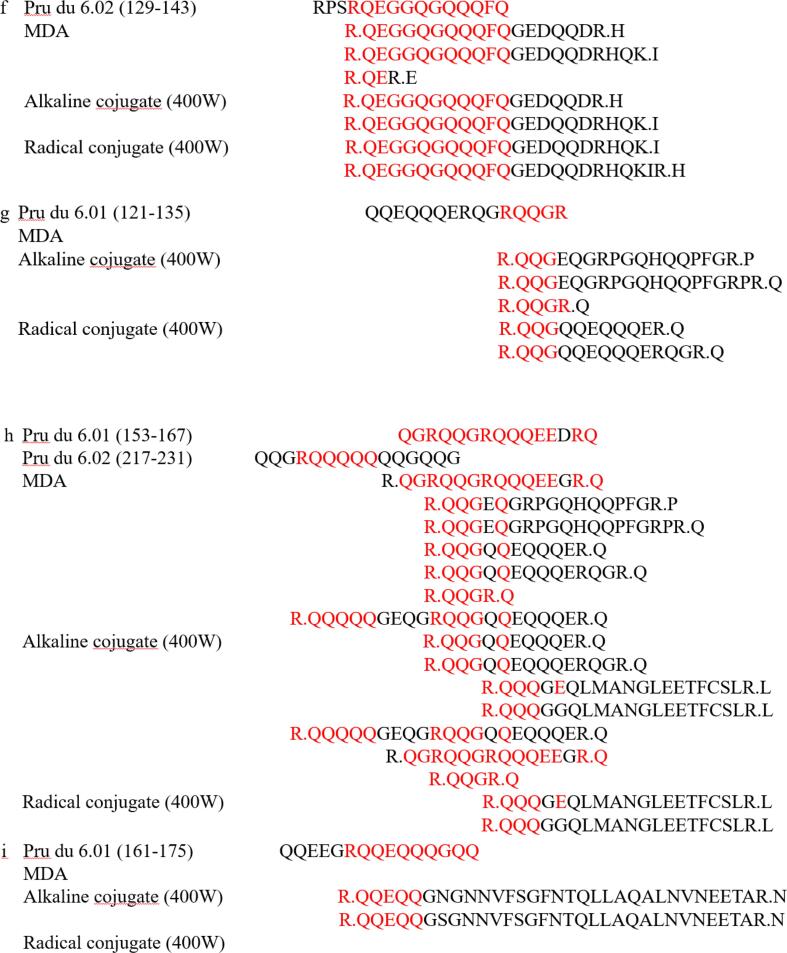
3.11. LC-MS/Ms
The main cause of food allergy was presence of linear and conformational epitopes in food. Conformational epitopes were mainly unique three-dimensional structure of allergen, which was more complex and rarely measured by more intuitive detection methods. Linear epitopes were composed of simple primary structures of a few amino acids and were often identified using LC-MS/MS. Han et al identified peptide sequence of novel oyster allergen Sarcoplasmic Calcium-Binding Protein by LC-MS/MS and found that its gene sequence cross-reacted with both mollusks and aroids (Han et al., 2020). Combined with the SDS-PAGE and immunoreactivity data analysis, the SDS-PAGE bands of ultrasound-assisted alkaline method and ultrasound-assisted free radical method were clear and their immunoreactivity was significantly reduced, so the amandin-EGCG conjugate was prepared by ultrasound-assisted alkaline method and ultrasound-assisted free radical method for subsequent LC-MS/MS analysis. The peptide sequences of amandin detected by LC/MS-MS after trypsin digestion were compared with the reported amandin-sensitizing peptide sequences (Fig. 5). The results showed that amandin contained 37 peptide sequences with linear epitopes, and amandin-EGCG conjugate prepared by the ultrasound-assisted alkaline method identified 41 peptide sequences with linear epitopes, while amandin-EGCG conjugate prepared by the ultrasound-assisted free radical method had only 28 peptide sequences with linear epitopes. The aim of ultrasound assistance was to open the structure of amandin and expose more binding sites. Whereas the mechanism of amandin-EGCG conjugate preparation by free radical method and alkaline method were different, the reason for reduced potential allergenicity of their conjugates might also be different. The conjugates prepared by the ultrasound-assisted free radical method contained the least number of linear epitopes, which might be due to hydroxyl radicals attacking the active residues of amandin, resulting in the disruption of linear epitopes. In addition, shielding of certain epitopes by covalent attachment of amino acid residues and EGCG might be responsible for the reduced IgE-binding capacity (Wu et al., 2018).
Fig. 5.
Linear epitopes contained in amandin-EGCG conjugate prepared by amandin, ultrasonic-assisted alkaline method and ultrasonic-assisted free radical method.
Combined with structural characterization and immunoreactivity related data, total phenol content of the conjugates prepared by free radical and ultrasound-assisted free radical methods was higher than that of alkaline and ultrasound-assisted alkaline methods. Free amino group and free sulfhydryl group of amandin-EGCG conjugate were significantly reduced compared with amandin, indicating that EGCG bound to amandin through covalent bonds. The conformational epitopes of protein antigens were closely related to their secondary or tertiary structures. Secondary structure of amandin-EGCG conjugate was significantly altered and its fluorescence intensity was significantly reduced. The binding of EGCG significantly altered the protein conformation of amandin, thus significantly reducing the potential allergenicity of amandin by disrupting its conformational epitopes. Furthermore, the potential allergenicity of sensitized proteins was determined by both linear and conformational epitopes. Although the amandin-EGCG conjugate prepared by alkaline method had more linear epitopes than amandin, its immunoreactivity was significantly reduced. This might be due to the dominance of disruption or burial of conformational epitopes of amandin during ultrasound-assisted alkaline method.
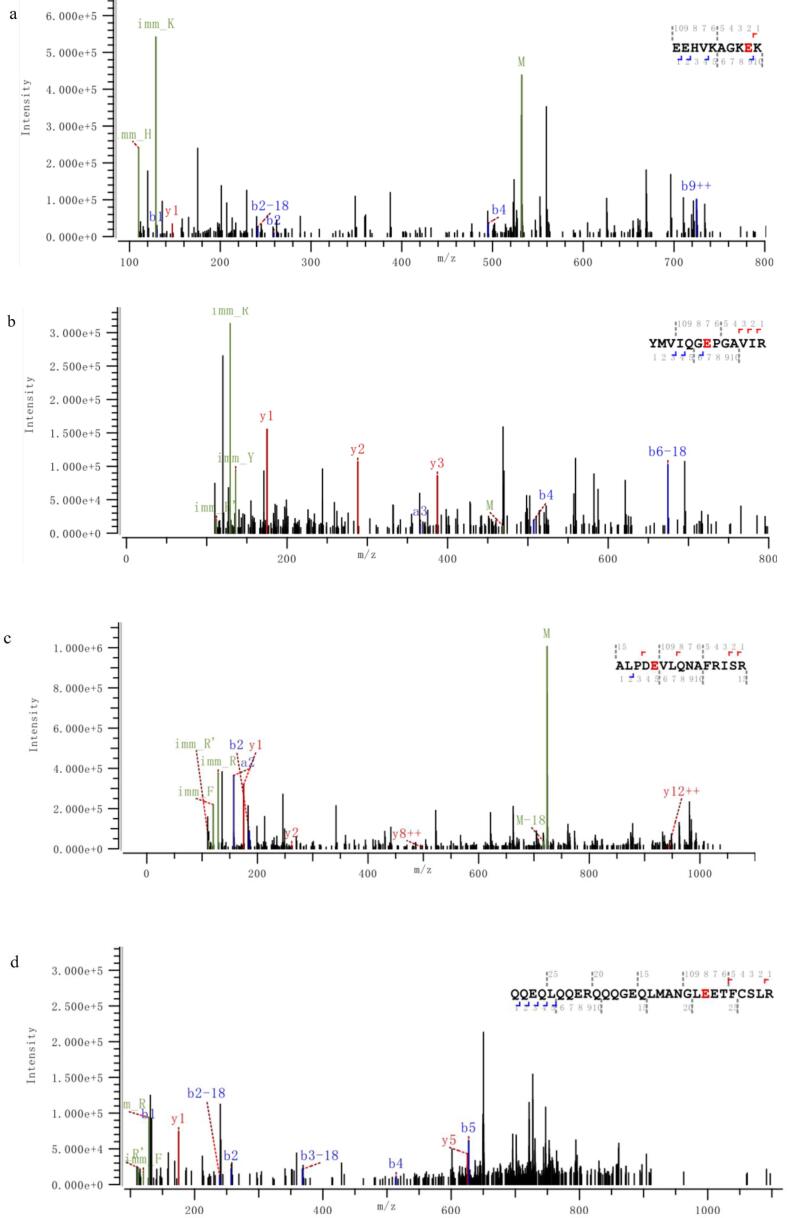
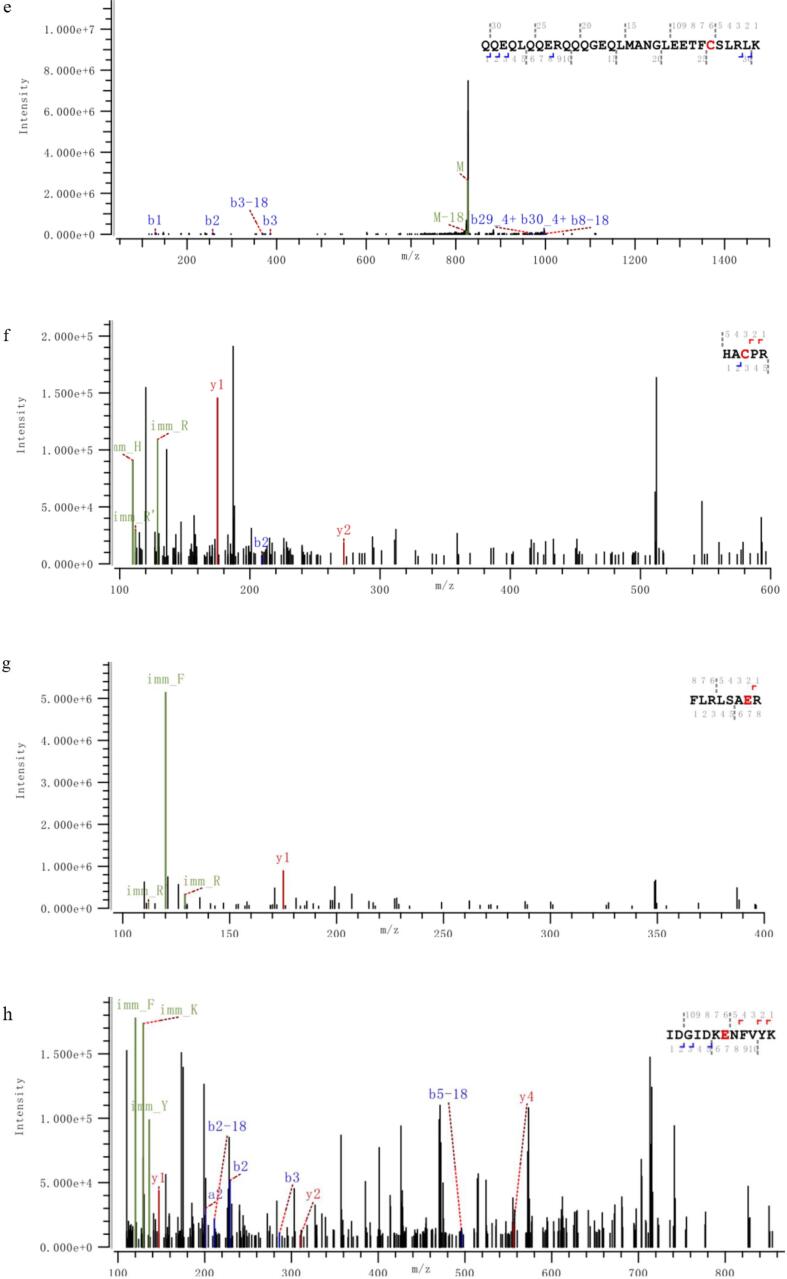
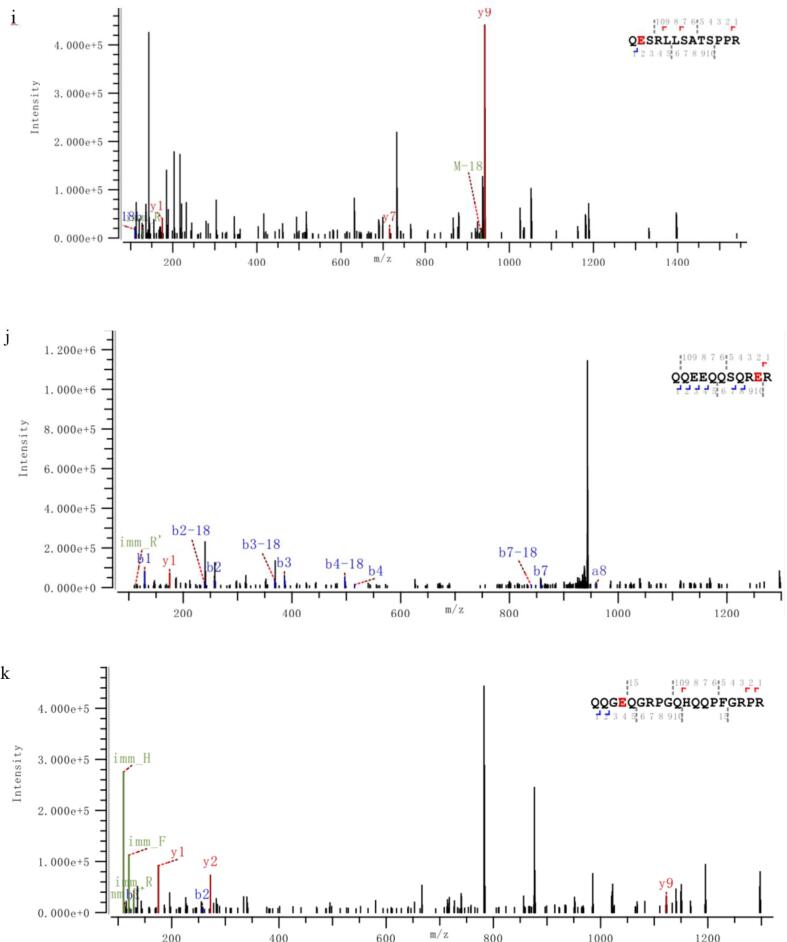
Information on modification sites of amandin was obtained by MS/MS scanning after trypsin digestion. Five peptides of amandin-EGCG conjugate prepared by alkaline method were detected to be covalently bound to EGCG, while six peptides of conjugate prepared by free radical method were detected to be covalently bound to EGCG (Fig. 6). The peptides of amandin covalently bound to EGCG in amandin-EGCG conjugate prepared by alkaline and free radical methods were different, and these peptides were mainly bound to EGCG via glutamate and cysteine. Alkaline and free radical methods were two commonly used non-enzymatic protein-phenol covalent coupling methods. He et al found that EGCG reacted with Cys74 and Glu347 in ovalbumin in free radical reaction and reacted with Cys74 in alkaline reaction (He et al., 2019). Related studies found that hydroxyl radicals in free radical reactions, generated by interaction of hydrogen peroxide and ascorbic acid as redox components, attacked the reactive residues on protein side chain, generating protein residues that then reacted with EGCG (Spizzirri et al., 2009, Zhang et al., 2020). From the LC-MS/MS results, amandin was mainly covalently bound to EGCG via glutamate and cysteine residues. Phenols were sensitive to oxidation under alkali conditions and could be oxidized to semi-quinones, which were then rearranged to o-quinones. These highly reactive intermediates could react with nucleophilic amino acid residues on protein side chains (Kroll et al., 2003). Amandin-EGCG conjugate were formed by quinones generated by EGCG under alkali conditions, which could covalently bind to cysteine and glutamate residues. He et al and Ishii et al both found that EGCG oxidized under alkali conditions generates quinones that covalently bound to the cysteine residues of ovalbumin and glyceraldehyde-3-phosphate dehydrogenase (He et al., 2019, Ishii et al., 2008). Jing et al suggested that protein, glutamate and cysteine residues were the main cross-linking sites of egg white proteins with theophylline (Jing et al., 2020).
Fig. 6.
The M/Z spectrum of the LC-MS /MS of the peptides hydrolyzed by the amandin-EGCG conjuage in the ultrasound-assisted alkaline method and the ultrasound-assisted free radical method, in which A–E was the ultrasound-assisted alkaline method and F–K was the ultrasound-assisted free radical method. The peptide sequences marked in red are the amino acid residues that AMP and EGCG may bind to detected by LC-MS/MS. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
Protein binding with phenolics might reduce allergenicity of proteins and might prepare hypoallergenic protein foods. In this paper, amandin-EGCG conjugates were prepared by different methods aiming to reduce allergenicity of amandin. Although the ultrasound-assisted alkaline method and ultrasound-assisted free radical method had lower phenolic content than alkaline and free radical methods for the preparation of amandin-EGCG conjugate, their reaction time was 23 h less. The potential allergenicity of amandin-EGCG conjugate tended to be significantly reduced compared to amandin as evidenced by immunoblotting reactions and ELISA. This was mainly due to reduction of linear epitope of amandin-EGCG conjugate and change in conformation of amandin that might mask some of conformational epitopes. This study provided a new idea as well as a theoretical basis for the preparation of hypoallergenic foods from almonds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was financially supported through grants from the National Natural Science Foundation of China (No. 31860431), the Key Project of Natural Science Foundation of Guizhou Province (No. KY [2022] key 036) and the Foundation of Guizhou Educational Committee (No. KY [2021] 008).
Contributor Information
Qun Huang, Email: huangqunlaoshi@126.com.
Lei Chen, Email: chenlei841114@hotmail.com.
Shaofeng Wei, Email: shaofenggy@163.com.
Data availability
The authors do not have permission to share data.
References
- Bezerra M., Ribeiro M., Igrejas G. An updated overview of almond allergens. Nutrients. 2021;13(8) doi: 10.3390/nu13082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Ma M. Foam and conformational changes of egg white as affected by ultrasonic pretreatment and phenolic binding at neutral pH. Food Hydrocolloids. 2020;102 doi: 10.1016/j.foodhyd.2019.105568. [DOI] [Google Scholar]
- Chhabra G.S., Liu C., Su M., Venkatachalam M., Roux K.H., Sathe S.K. Effects of the maillard reaction on the immunoreactivity of amandin in food matrices. Journal of Food Science. 2017;82(10):2495–2503. doi: 10.1111/1750-3841.13839. [DOI] [PubMed] [Google Scholar]
- Church F.C., Swaisgood H.E., Porter D.H., Catignani G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins1. Journal of Dairy Science. 1983;66(6):1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. [DOI] [Google Scholar]
- Deng Han Z.U.Q.Z.H.U.J.Y.A.C.H. Effect of ultrasonic treatment on the potential allergenicity of soybean 7S globulin. Food Science. 2017;38(5):32–37. doi: 10.7506/spkx1002-6630-201705006. [DOI] [Google Scholar]
- Dong Z.Y., Li M.Y., Tian G., Zhang T.H., Ren H., Quek S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food Chemistry. 2019;299 doi: 10.1016/j.foodchem.2019.125103. [DOI] [PubMed] [Google Scholar]
- Fan R., Yuan F., Yang W., Yanxiang Evaluation of structural and functional properties of protein-EGCG complexes and their ability of stabilizing a model beta-carotene emulsion. Food Hydrocolloids. 2015;45:337–350. doi: 10.1016/j.foodhyd.2014.12.008. [DOI] [Google Scholar]
- Fotschki J., Wróblewska B., Fotschki B., Kalicki B., Rigby N., Mackie A. Microbial transglutaminase alters the immunogenic potential and cross-reactivity of horse and cow milk proteins. Journal of Dairy Science. 2020;103(3):2153–2166. doi: 10.3168/jds.2019-17264. [DOI] [PubMed] [Google Scholar]
- Geng F., Xie Y., Wang Y., Wang J. Depolymerization of chicken egg yolk granules induced by high-intensity ultrasound. Food Chemistry. 2021;354 doi: 10.1016/j.foodchem.2021.129580. [DOI] [PubMed] [Google Scholar]
- Han T.-J., Liu M., Huan F., Li M.-S., Xia F., Chen Y.-Y., et al. Identification and cross-reactivity analysis of sarcoplasmic-calcium-binding protein: A novel allergen in Crassostrea angulata. Journal of Agricultural and Food Chemistry. 2020;68(18):5221–5231. doi: 10.1021/acs.jafc.0c01543. [DOI] [PubMed] [Google Scholar]
- He, W., Xu, H., Lu, Y., Zhang, T., Li, S., Lin, X., . . . Wu, X. (2019). Function, digestibility and allergenicity assessment of ovalbumin–EGCG conjugates. Journal of Functional Foods, 61, 103490. https://doi.org/10.1016/j.jff.2019.103490.
- Huang Q., Huang X., Liu L., Song H., Geng F., Wu W., et al. Nano eggshell calcium enhanced gel properties of Nemipterus virgatus surimi sausage: Gel strength, water retention and microstructure. International Journal of Food Science & Technology. 2021;56(11):5738–5752. doi: 10.1111/ijfs.15142. [DOI] [Google Scholar]
- Huang Q., Huang X., Liu L., Wang G., Song H., Geng F., et al. Effect of nano eggshell calcium on the structure, physicochemical, and gel properties of threadfin bream (Nemipterus virgatus) actomyosin. LWT. 2021;150 doi: 10.1016/j.lwt.2021.112047. [DOI] [Google Scholar]
- Ishii T., Mori T., Tanaka T., Mizuno D., Yamaji R., Kumazawa S., et al. Covalent modification of proteins by green tea polyphenol (–)-epigallocatechin-3-gallate through autoxidation. Free Radical Biology and Medicine. 2008;45(10):1384–1394. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Jia X., Ning X., Li W., Deng Q., Yu X., Cheng C., et al. Non-covalent interaction between almond protein and sinapic acid: Impact on protein structure and antioxidantactivity. Oil Crop Science-Chinese Journal of Oil Crops. 2019;4(4):10. [Google Scholar]
- Jing H., Sun J., Mu Y., Obadi M., McClements D., Xu B. Sonochemical effects on the structure and antioxidant activity of egg white protein–tea polyphenol conjugates. Food & Function. 2020;11 doi: 10.1039/D0FO01636E. [DOI] [PubMed] [Google Scholar]
- Kroll J., Rawel H.M., Rohn S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Science and Technology Research. 2003;51(7):371–375. doi: 10.3136/nskkk.51.371. [DOI] [Google Scholar]
- Li S., Wang K., Huang Q., Geng F. Microwave pretreatment enhanced the properties of ovalbumin-inulin-oil emulsion gels and improved the storage stability of pomegranate seed oil. Food Hydrocolloids. 2021;113 doi: 10.1016/j.foodhyd.2020.106548. [DOI] [Google Scholar]
- Luo X., Wang Q., Wu Y., Duan W., Zhang Y., Geng F., et al. Mechanism of effect of heating temperature on functional characteristics of thick egg white. LWT. 2022;154 doi: 10.1016/j.lwt.2021.112807. [DOI] [Google Scholar]
- Pessato T.B., Morais F.P.R.D., de Carvalho N.C., et al. Protein structure modification and allergenic properties of whey proteins upon interaction with tea and coffee phenolic compounds. Journal of Functional Foods. 2018;51:121–129. doi: 10.1016/j.jff.2018.10.019. [DOI] [Google Scholar]
- Pereira R., Rodrigues R.M., Machado L., Ferreira S., Costa J., Villa C., et al. Influence of ohmic heating on the structural and immunoreactive properties of soybean proteins. LWT. 2021;148 doi: 10.1016/j.lwt.2021.111710. [DOI] [Google Scholar]
- Plundrich N.J., Kulis M., White B.L., Grace M.H., Guo R., Burks A.W., et al. Novel strategy to create hypoallergenic peanut protein-polyphenol edible matrices for oral immunotherapy. Journal of Agricultural and Food Chemistry. 2014;62(29):7010–7021. doi: 10.1021/jf405773b. [DOI] [PubMed] [Google Scholar]
- Quirós A., Chichón R., Recio I., López-Fandiño R. The use of high hydrostatic pressure to promote the proteolysis and release of bioactive peptides from ovalbumin. Food Chemistry. 2007;104(4):1734–1739. doi: 10.1016/j.foodchem.2006.10.050. [DOI] [Google Scholar]
- Sen, LI, Marina, OFFENGENDEN, Michael, G?Nzle, G., . . . WU. (2018). Aspergillus oryzae reduces IgE binding ability of allergenic egg white proteins. Frontiers of Agricultural Science & Engineering, 2018, 5(3): 373–381. https://doi.org/10.15302/J-FASE-2018210.
- Spizzirri U.G., Iemma F., Puoci F., Cirillo G., Curcio M., Parisi O.I., et al. Synthesis of antioxidant polymers by grafting of gallic acid and catechin on gelatin. Biomacromolecules. 2009;10(7):1923–1930. doi: 10.1021/bm900325t. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang J., Lei F., Liang C., Yuan F., Gao Y. Covalent complexation and functional evaluation of (-)-epigallocatechin gallate and alpha-lactalbumin. Food Chemistry. 2014;150:341–347. doi: 10.1016/j.foodchem. [DOI] [PubMed] [Google Scholar]
- Wei Z., Yang W., Fan R., et al. Evaluation of structural and functional properties of protein–EGCG complexes and their ability of stabilizing a model β-carotene emulsion. Food Hydrocolloids. 2015;45:337–350. [Google Scholar]
- Wu X., Lu Y., Xu H., Lin D., He Z., Wu H., et al. Reducing the allergenic capacity of β-lactoglobulin by covalent conjugation with dietary polyphenols. Food Chemistry. 2018;256:427–434. doi: 10.1016/j.foodchem. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang Y., Duan W., Wang Q., An F., Luo P., et al. Ball-milling is an effective pretreatment of glycosylation modified the foaming and gel properties of egg white protein. Journal of Food Engineering. 2022;319 doi: 10.1016/j.jfoodeng.2021.110908. [DOI] [Google Scholar]
- Xin L., Zhang Y., Duan W., Ai M., Song H., Huang Q., et al. Effect of malondialdehyde oxidation on structure and physicochemical properties of amandin. International Journal of Food Science & Technology. 2021 doi: 10.1111/ijfs.15213. [DOI] [Google Scholar]
- You J., Luo Y., Wu J. Conjugation of ovotransferrin with catechin shows improved antioxidant activity. Journal of Agricultural and Food Chemistry. 2014;62(12):2581–2587. doi: 10.1021/jf405635q. [DOI] [PubMed] [Google Scholar]
- Zaffran V.D., Sathe S.K. Immunoreactivity of biochemically purified amandin from thermally processed almonds (Prunus dulcis L.) Journal of Food Science. 2018;83(7):1805–1809. doi: 10.1111/1750-3841.14206. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Cheng Z., Wang Y., Fu L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Critical Reviews in Food Science and Nutrition. 2020 doi: 10.1080/10408398.2020.1803199. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Bi Y., Wang Q., Cheng K.-W., Chen F. Application of high pressure processing to improve digestibility, reduce allergenicity, and avoid protein oxidation in cod (Gadus morhua) Food Chemistry. 2019;298 doi: 10.1016/j.foodchem.2019.125087. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Deng Y., Zhao Y. Structure-based modelling of hemocyanin allergenicity in squid and its response to high hydrostatic pressure. Scientific Reports. 2017;7(1):40021. doi: 10.1038/srep40021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu C., Su M., Roux K.H., Sathe S.K. Effect of phenolics on amandin immunoreactivity. Food Science and Technology. 2018;98:515–523. [Google Scholar]
- Zhang Y., Zhang J., Sheng W., Wang S., Fu T.-J. Effects of heat and high-pressure treatments on the solubility and immunoreactivity of almond proteins. Food Chemistry. 2016;199:856–861. doi: 10.1016/j.foodchem.2015.12.063. [DOI] [PubMed] [Google Scholar]
- Zhao Y.-J., Cai Q.-F., Jin T.-C., Zhang L.-J., Fei D.-X., Liu G.-M., et al. Effect of Maillard reaction on the structural and immunological properties of recombinant silver carp parvalbumin. LWT. 2017;75:25–33. doi: 10.1016/j.lwt.2016.08.049. [DOI] [Google Scholar]
- Zhou S.-D., Huang L., Meng L., Lin Y.-F., Xu X., Dong M.-S. Soy protein isolate-(-)-epigallocatechin gallate conjugate: Covalent binding sites identification and IgE binding ability evaluation. Food Chemistry. 2020;333 doi: 10.1016/j.foodchem.2020.127400. [DOI] [PubMed] [Google Scholar]
- Zhou S.D., Lin Y.F., Xu X., Meng L., Dong M.S. Effect of non-covalent and covalent complexation of (-)-epigallocatechin gallate with soybean protein isolate on protein structure and in vitro digestion characteristics. Food Chemistry. 2020;309 doi: 10.1016/j.foodchem.2019.125718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.