Purpose of review
Activation of the type 1 interferon (T1 IFN) pathway has been implicated in the pathogenesis of systemic sclerosis (SSc) by an increasing number of studies, most of which share key findings with similar studies in systemic lupus erythematosus (SLE). Here we will focus on the evidence for T1 IFN activation and dysregulation in SSc, and the rationale behind targeting the pathway going forward.
Recent findings
An increased expression and activation of T1 IFN-regulated genes has been shown to be present in a significant proportion of SSc patients. TI IFN activation markers have been found to predict and correlate with response to immunosuppressive treatment as well as severity of organ involvement. As inhibition of the IFN-α receptor has been proven to be effective in active SLE, benefit may be seen in targeting the IFN pathway in SSc.
Summary
The role played by T1 IFN and its regulatory genes in SSc is becoming increasingly evident and strikingly similar to the role observed in SLE. This observation, together with the benefit of type 1 IFN targeting in SLE, supports the notion of a potential therapeutic benefit in targeting T1 IFN in SSc.
Keywords: interferon regulatory factors, plasmacytoid dendritic cells, systemic sclerosis, Toll-like receptors, type 1 interferon
INTRODUCTION
Systemic sclerosis (SSc) is a progressive, heterogenous multisystem autoimmune disease, which is characterized by autoimmune activation as well as a pathognomonic tissue and vascular fibrosis [1,2]. It has the greatest mortality amongst the major rheumatic diseases [1,3,4▪▪]. Genetic predisposition combined with triggers activating a persistent immune response at the level of the tissue is thought to drive the pathogenetic process in SSc. Type 1 interferons (T1 IFNs) are a family of cytokines playing a key role in response to viruses and a variety of danger and damage signals, triggering innate immune activation. The dysregulation in T1 IFN signalling has now been implicated in the pathogenesis of certain autoimmune diseases, including SSc and systemic lupus erythematosus (SLE) [4▪▪,5,6]. Clinical evidence of the harmful effects of T1 IFN in SSc, is provided by a randomized, placebo-controlled trial of IFN-α, in patients with early diffuse SSc, where the trial had to be stopped early because of a deleterious effect seen in the lung function of the treatment group. The withdrawal and serious adverse event rates were also greater in the treatment group than in the placebo group [7].
Here we will focus on the evidence for T1 IFN activation in SSc, the potential mechanisms leading to its dysregulation, the predictive role on disease progression and the rationale to target the pathway going forward.
Box 1.
no caption available
FROM DANGER SENSORS TO INTERFERON-STIMULATED GENES: A MULTIFACETED DEFENSE MACHINERY THAT CAN LEAD TO IMMUNE-MEDIATED TISSUE DAMAGE
T1 IFNs are a heterogenous family of cytokines, which provide a robust first line of antiviral defence. Type 2 and 3 interferons have partially different roles, which are outside the scope of this review and have been summarized elsewhere [8,9].
T1 IFN can be divided into five classes in humans: α, β, ω, ε and κ. All five, T1 IFN classes signal through the same type 1 IFN heterodimeric receptor complex constituting IFN-α receptor 1 (IFNAR1) and IFNAR2 subunits.
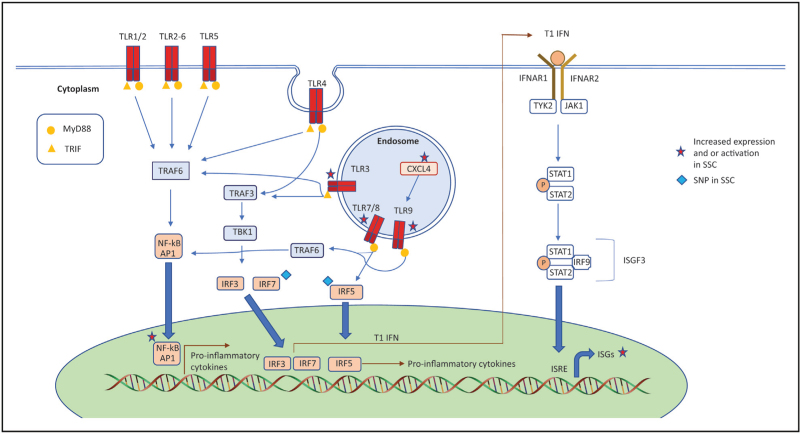
Secretion of T1 IFN in the extracellular space is the terminal event of an ‘innate’ response mechanism to a variety of danger and damage stimuli. The detection of repetitive molecular patterns displayed by a pathogen (pathogen-associated molecular patterns or PAMPs) is one of the stimuli, which is ‘sensed’ by the pattern recognition receptors (PRRs) [10]. There are four classes of PRRs – the Toll-like receptors (TLRs), the nucleotide-binding oligomerization domain-like receptors (NLR), the retinoic acid inducible gene I (RIG-I) and the C-type lectin receptors [11]. They all differ in ligand recognition, signal transduction and cell localization.
TLRs are the most extensively studied class of PRRs and consist of 10 types (TLR 1–10) [10,12,13]. TLRs are expressed on most nucleated cells, and once they are engaged with their ligand, they lead to T1 IFN pathway activation. While this is true in most cells, plasmacytoid dendritic cells (pDCs) are the cells that are ‘professionally’ differentiated to secrete vast amounts of T1 IFN in response to TLR engagement. For this reason, they are believed to play a central part in the T1 IFN-mediated immune response and their role has been implicated both in the pathogenesis of SLE [14] and SSc [15▪,16].
The first indirect evidence of a putative involvement of pDC in the aberrant T1 IFN activation in SSc was suggested by a proteome-wide analysis showing that CXCL4 in the plasma of SSc patients was substantially higher than healthy controls, and it predicted the presence and worsening of lung fibrosis and pulmonary hypertension. In the same study, the authors implicated pDC as one of the potential sources of CXCL4 [17]. More recently, CXCL4 has been found to function as a Damage Associated Molecular Pattern (DAMP) sensor. Lande et al. observed that CXCL4 organiszd microbial and self-DNA into liquid crystalline complexes that amplified TLR9-mediated IFN-α production in pDCs. Importantly, CXCL4-DNA complexes were present in vivo, and correlated with T1 IFN in SSc blood and skin, revealing a direct link between CXCL4 overexpression and T1 IFN production in patients with SSc [18]. Another study also indicated the infiltration of SSc skin by pDCs, where they were chronically activated, producing high levels of IFN-α and CXCL4. CXCL4 was under the control of phosphatidylinositol 3-kinase δ, which was linked to the aberrant presence of TLR8 on pDCs in SSc patients. CXCL4 was also found to potentiate the activities of TLR8-induced and TLR9-induced IFN production in SSc pDCs [16].
Importantly, Ross et al.[15▪] have shown that functional inhibition of pDCs was effective in preventing skin activation and fibrosis in preclinical models of SSc, similar to what has been observed in SLE [14].
In another study, anti-CXCL4 antibodies were shown to be present in at least half of SSc patients and correlated with serum/plasma IFN-α levels. Recently, CXCL4 itself was found to behave as a self-antigen, maintaining a vicious cycle by promoting T1 IFN activation via pDCs and anti-CXCL4 antibodies by B cells, sustaining the SSc IFN signature [19]. Further work with CXCL4 has interestingly shown that the anti-CXCL4 antibodies were present in patients with VEDOSS (very early diagnosis of systemic sclerosis), suggesting that this mechanism can intervene very early in the pathogenesis of disease, before clinically apparent tissue damage [20].
Activation of TLRs have also been found to play a role in interstitial lung disease (ILD). TLR3 activation by poly I:C has been reported to increase lung inflammatory proteins including the cytokines CCL3, CCL5 and CXCL10, in airway epithelial cells. Importantly, TLR3 knockout mice showed protection against the inflammatory response [21]. TLR4 has also been implicated in pulmonary and skin fibrosis, with the ability to activate IRF5 [22].
Pathogens have long been proposed as a trigger for autoimmune illnesses, and one mechanism for this is ‘molecular mimicry’ between self-derived and pathogen-derived molecules. Another mechanism, occurs through the inability to clear the pathogen, resulting in infection persistence, and repeated stimulation of the innate immune cells via TLRs [23,24]. Farina and colleagues have shown evidence of infectious Epstein–Barr virus (EBV) in monocytes triggering SSc. Induction of EBV viral lytic genes resulted in the induction of TLR8 expression in both healthy control and SSc monocytes infected with EBV [25]. Further, Farina et al.[26] have shown that EBV can infect endothelial cells and fibroblasts in SSc skin, leading to an aberrant TLR activation. A novel mechanism has also now been demonstrated by which human monocytes bound to EBV recombinant virus are capable to transfer EBV to the endothelial cells. In the same study, EBV lytic antigens in scleroderma dermal vessels were detected, suggesting EBV could target endothelial cells in SSc skin, activating TLR 9 in the process and possibly contributing to the vascular injury seen in SSc [27].
Beyond classic pathogens, there is increasing evidence for an important role played by mitochondria, in the events driving T1 IFN activation and subsequent autoimmunity. It is widely accepted that fragmentation in mitochondrial DNA (mtDNA), can lead to the activation of T1 IFN pathway, through cGAS (cytosolic cyclic GMP-AMP synthase), a specific cytosolic receptor for free DNA, which, in turn, activates the endoplasmic reticulum membrane protein, stimulator of interferon genes (STING). cGAS-STING activation by mtDNA was shown to be positively associated with T1 IFN and IL-6 expression in SSc as well as in SLE [28,29]. Consistent with these findings, mtDNA has been found to be at increased concentration in SSc plasma, with the ability to function as DAMPs and interact with PRRs [30]. This is one of the putative mechanisms by which necrotic cells or those under stress have been found to activate TLR 9 and the double-stranded DNA sensor, cGAS.
Interestingly, it has been also proposed that mtDNA could be damaged as a consequence of oxidative stress because of high exposure to reactive oxidative species (ROS) produced by the mitochondria itself [28].
Regardless of the source of its secretion, T1IFN signal through IFNAR1 and IFNAR2, which in turn activate Janus kinase (JAK)-signalling pathway downstream [4▪▪,8]. This consists initially with phosphorylation of pre-associated JAK1 and tyrosine kinase 2 (TYK2), which triggers kinase activity of signal transducers and activators of transcriptions 1 and 2 (STAT1 and 2) via cross-phosphorylation. This leads, in turn, to the recruitment of IFN-regulatory factor 9 (IRF9), a member of the family of transcription factors called IFN Regulatory Factors (IRFs), for their ability to regulate the expression of T1 IFN and its effects on target gene expression. IRF9 together with STAT1 and 2 form a complex known as the IFN-stimulated gene factor 3 (ISGF3). This complex translocates to the nucleus to bind to IFN-stimulated response elements (ISRE) in order to induce a family of genes that for this reason are called interferon-stimulated genes – ISGs [4▪▪,8]. A summary of the different pathways and key factors mentioned above leading to T1 IFN activation is shown in Fig. 1.
FIGURE 1.
Different pathways and key factors leading to T1 IFN activation and ISG release.
GENETICS AND EPIGENETICS OF TYPE 1 INTERFERON DYSREGULATION IN SYSTEMIC SCLEROSIS
Familial association studies have previously shown that family history appears to be the strongest known risk factor for SSc. It was found that amongst first-degree relatives of SSc patients, the prevalence of the disease was 0.33%, with a relative risk factor of 13 when compared with the general United States population, which had a prevalence of 0.026% [31]. A twin study including 42 twin pairs (24 monozygotic and 18 dizygotic), found that the overall concordance of SSc was only 4.2% (1 out of 24) in monozygotic twins and 5.6% in dizygotic twins. The concordance, however, of antinuclear antibodies (ANAs) was significantly higher in monozygotic twins vs. dizygotic twins (90% vs 40%), suggesting that concordance for autoimmunity was much higher than the one for clinical disease phenotype. Consistent with these findings, a study in 4612 first-degree relatives of 1071 probands revealed an increased risk for familial autoimmunity among subtypes of SSc, with thyroid diseases and SLE showing the most significant increased prevalence when compared with control families, together with Raynaud's phenomenon and ILD [32].
The most frequent form of genetic variation in humans is the single-nucleotide polymorphism (SNP), which influences protein function and is key to personalized medicine [33]. In a recent meta-analysis of Genome-Wide Association Studies (Meta-GWAS), which included 26 679 individuals, 27 independent genome-wide associated signals were identified, which included 13 new-risk loci, and nearly doubled the number of genome-wide hits previously reported in SSc [34]. This meta-analysis has suggested a variety of IFN-signalling loci, including T1 IFN regulatory factors IRF4 [35], IRF5 [36,37], IRF7 [34,38] and IRF8 [34,39,40]. (Fig. 1) Interestingly, apart from SSc, the genes have also shown an association with SLE [41–44]. Tyrosine kinase 2 (TYK2) [45], and STAT4 [34,46] are genes that have also been linked to SSc genetic susceptibility.
A shared genetic background of autoimmune diseases is clearly seen in GWAS, but additionally a vital role played by environmental factors (air pollution, infection and chemical substances, such as silicon) [47], and epigenetic influences in the pathogenesis of SSc has been suggested. Links to the pathogenesis of SSc have been previously reported for all the major epigenetic alterations, including DNA methylation [48–50], histone modifications [51,52], noncoding small (miRNA) and long (lncRNA) RNA transcript expression [53–56]. For instance, MiR-618 was found to be significantly overexpressed in SSc pDCs, causing an IRF8-dependent inhibition of pDC differentiation and activation, as well as increased production in IFN-α upon TLR9 stimulation [57]. LncRNAs are a larger class of transcribed RNA molecules, that are not translated but regulate gene expression [58]. It has recently been shown that a group of lncRNAs were modulated in a T1 IFN-dependent manner in human monocytes in response to TLR4 activation [59]. Among the lncRNAs, the negative regulator of the IFN response (NRIR) was found significantly upregulated in-vivo in SSc monocytes, and affected the expression of the ISGs, CXCL10 and CXCL11. Therefore, dysregulation of NRIR in SSc monocytes may play a part in contributing to the aberrant IFN response present in SSc patients [59].
EVIDENCE OF INCREASED TYPE 1 INTERFERON ACTIVATION IN SYSTEMIC SCLEROSIS
Due to the difficulty of directly measuring T1 IFN levels from human samples, an ‘interferon signature’ including the levels of expression of the transcript levels of multiple known ISGs has been widely used for this purpose. This method established the presence of increased T1 IFN in SLE, and more recently in other rheumatic diseases [60]. The first reported finding of an IFN signature in SSc dates back to 2006 [61]. Since then, it has been shown that an IFN signature in blood is found in a large proportion of SSc patients [5,62,63]. It has been also shown that activated monocytes and macrophages can be a potent source of T1 IFN and other profibrotic factors, stimulating the proliferation of fibroblasts and extracellular matrix accumulation [64]. An IFN signature in monocytes has even been found at the earliest phases of SSc, before overt fibrosis, suggesting of this being an early event in SSc pathogenesis [10].
A higher IFN signature in SSc whole blood or plasma has been found to correlate with the antibody profiling, where antitopoisomerase and anti-U1-RNP antibodies were associated with a higher IFN signature [5,65]. Correlation of this higher IFN signature was also seen in more severe vascular manifestations and lung involvement [65–68]. Organs known to be targeted in SSc such as the skin and lung, have also demonstrated an overexpression of ISGs in SSc patients [69,70].
Upregulation of ISGs in the skin of SSc patients was also demonstrated in skin biopsy gene expression studies [70,71]. A study performing microarrays from lung tissue revealed upregulation of ISGs in addition to TGF-β-regulated genes in SSc patients with ILD, with an increased expression of ISGs, associated with a higher rate of progression in ILD [69]. Interestingly, a recent multiomic comparative analysis of the serum profile, peripheral blood cells and skin ISG expression in SSc patients showed that the serum protein profile correlated more closely with the transcriptome of the skin than that of the PBMCs. This may be because of a spill-over effect from diseased end organs and suggests that IFN-inducible chemokine concentration may be a better predictor of tissue IFN activity than PBMC ISG expression levels [72,73▪].
Apart from the trial in IFN-α mentioned in the introduction of this review, case reports have been documented of the development of SSc in individuals treated with T1 IFN for other conditions. Interestingly, Anifrolumab (anti-IFNAR1 monoclonal antibody) in a phase 1 trial of SSc patients led to the suppression of the IFN signature and TGFβ signalling in SSc skin [74]. Additionally, in a graft-versus-host disease (GVHD) mouse model of SSc, neutralization of IFNAR1, and consequent normalization in the overexpression of T1 IFN-inducible genes, led to a marked reduction in the dermal fibrosis [75]. Consistent with these findings, in SSc patients treated with high-dose cyclophosphamide followed by rescue autologous hematopoietic stem cell transplantation, clinical response strongly correlated with normalization in T1 IFN module by RNAseq of peripheral blood cells [76].
The close mirroring of disease activity of T1 IFN activation has also been shown in the analysis of the SLS2 trial. Assassi et al.[77▪▪] have shown that higher serum IFN-inducible chemokine score predicted a better clinical response in both the cyclophosphamide and the mycophenalate mofetil arms. Importantly during the second year of the study, higher serum IFN score predicted worse clinical course in patients put on placebo, supporting the notion that IFN activation in SSc is deleterious, unless immunosuppressive treatment is initiated.
Vascular injury plays an important role in organ dysfunction in SSc, and it is the main driver of disease in patients with the limited cutaneous subset (LcSSc) of SSc. T1 IFN has been implicated in the dysregulation of the vascular remodelling process in SSc. Myxovirus-resistance protein A (MxA), which is induced by T1 IFN, was found to correlate with digital ulcerations and lower pulmonary forced vital capacity in SSc [78]. T1 IFN has also been shown to contribute to the increased vascular permeability in SSc through downregulation of Fli1 (friend leukemia integration 1 transcription factor) and vascular endothelial cadherin (VE-cadherin) in endothelial cells and fibroblasts [79]. Features of SSc vasculopathy were also seen in mice with conditional deletion of Fli1 in endothelial cells confirming that T1 IFN-mediated downregulation of Fli1 enhanced the development of SSc [80].
Consistent with these observations, IFN-inducible chemokines were found to predict progression of patients with LcSSc as far as a multi-morbidity score including skin, lung, vascular and gastrointestinal progression [81▪].
Taken together, these observations suggest that T1 IFN is involved in both tissue and vascular fibrosis in SSc, strongly supporting the rationale for a direct therapeutic approach targeting the pathway.
CURRENT EXPERIENCE IN TYPE 1 INTERFERON TARGETING FOR DISEASE MODIFICATION
Dysregulation in the T1 IFN response has been shown to contribute to the development of autoimmunity. Although the clinical manifestations vary amongst the different types of autoimmune diseases, T1 IFN protein or transcript signatures have now been identified in many of them (SSc, SLE, dermatomyositis and Sjogren's disease) [5,10,82–85].
In SLE, up to 80% of patients were shown to have a T1 IFN signature, with around 50% having chronically elevated T1 IFN levels, detectable in blood [86,87]. SLE patients with high T1 IFN activity, also tend to have higher disease activity scores with a greater tendency to relapse whilst in remission and a lower response rate to placebo medication [88–90]. Similarly to what has been observed in SSc, deranged pDC activation also occurs in SLE, and monoclonal antibodies against pDC have recently shown benefit on cutaneous and musculoskeletal lupus [91–93].
The effectiveness of blocking IFNAR, which plays a critical role in T1 IFN signalling, has now been concretely demonstrated in SLE patients with the monoclonal antibody Anifrolumab. The phase III Tulip-2 trial met its primary end-point, with an improvement in overall disease activity vs. placebo [94], leading to Food and Drug Administration (FDA) and European Medicine Agency (EMA) approval for treatment in SLE.
The similarities of T1 IFN activation in SSc, therefore, informs the rationale to block IFNAR in SSc and determine its therapeutic effectiveness [4▪▪]. As mentioned above in this review, early phase 1 study of 34 SSc patients, showed that anifrolumab was well tolerated and showed peak inhibition of the T1 IFN signature in blood [95]. A follow-up mechanistic study showed that treatment with anifrolumab led to the reduction of the T1 IFN signature in whole blood and skin biopsy samples, demonstrating the suppressive effects of the anti-IFNAR1 antibody [74]. These findings provide further support for future larger double-blind, placebo-controlled trials of Anifrolumab in early SSc.
CONCLUSION
Over the past few years, substantial progress has been made in deconvoluting the immune complexity of SSc, which has led to identify key molecular and cellular components of T1 IFN signalling involved in disease pathogenesis. In spite of the progress made, many unanswered questions in the pathogenesis of SSc remain. The origin and triggers of T1 IFN, and the interactions played between genetic and environmental factors, leading to dysfunction in the T1 IFN response still remains a grey area. However, newly discovered function of molecules such as CXCL4, start to lead towards a better understanding of the connections between pDCs, the IFN continuum and the fibrotic process. Further studies are also needed to elucidate downstream processes linking the T1 IFN activation to the exaggerated fibrotic response in fibroblasts and other key effector cells implicated in SSc pathogenesis.
Specifically, the identification of specific ligands and signalling pathways driving T1 IFN signalling in SSc will need further investigation with in-vivo and in-vitro studies. This will improve our understanding of SSc pathogenesis, and will increase the armamentarium of the therapeutic targets that could be exploited to improve patient outcome.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
S.A. has received grants to his institution from Momenta, Janssen and Boehringer Ingelheim and consultancy fees from Novarits, AstraZeneca, Boehringer Ingelheim, CSL Behring and Abbvie. Y.A. received consulting honorarium and/or research grants from Alpine ImmunoSciences, Astra-Zeneca, Bayer, Boehringer, Janssen, Medsenic, Prometheus, Roche, Sanofi and Topadur with regards to the management and treatment of systemic sclerosis.
C.P.D. reports personal fees or research grants to his institution from GlaxoSmithKline, Galapagos, Boehringer Ingelheim, Roche, CSL Behring, Corbus, Horizon, Capella Bioscience and Arxx Therapeutics; all outside the submitted work.
M.K. received consulting honorarium and/or research grants from Astra-Zeneca, Boehringer-Ingelheim, Chugai, GSK and Horizon with regards to the management and treatment of systemic sclerosis.
D.K.: Consultant/Advisor: Actelion; Boehringer Ingelheim International GmbH; Bristol Myers Squibb Company; CSL Behring; Horizon Therapeutics USA, Inc.; Janssen Global Services, LLC; Prometheus Biosciences; Mitsubishi Tanabe Pharma Corporation, Genentech/Roche. Grant/Research Support: Bristol Myers Squibb Company; Horizon Therapeutics USA, Inc.; Pfizer Inc.
F.D.G.: consultancies and research support from Abbvie, AstraZeneca, Boehringer-Ingelheim, Capella Biosciences, Chemomab Therapeutics, Janssen, Kymab ltd, Mitsubishi-Tanabe.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet 2017; 390:1685–1699. [DOI] [PubMed] [Google Scholar]
- 2.Allanore Y, Simms R, Distler O, et al. Systemic sclerosis. Nat Rev Dis Primers 2015; 1:15002. [DOI] [PubMed] [Google Scholar]
- 3.Lescoat A, Roofeh D, Kuwana M, et al. Therapeutic approaches to systemic sclerosis: recent approvals and future candidate therapies. Clin Rev Allergy Immunol 2021; doi: 10.1007/s12016-021-08891-0 [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪▪.Wu M, Assassi S. Dysregulation of type 1 interferon signaling in systemic sclerosis: a promising therapeutic target? Curr Treatm Opt Rheumatol 2021; 7:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review covering the key druggable therapeutic targets of the T1 IFN pathway that can be pursued in future randomized clinical trials, in order to develop more effective therapeutic options for SSc.
- 5.Skaug B, Assassi S. Type I interferon dysregulation in systemic sclerosis. Cytokine 2020; 132:154635. [DOI] [PubMed] [Google Scholar]
- 6.Northcott M, Jones S, Koelmeyer R, et al. Type 1 interferon status in systemic lupus erythematosus: a longitudinal analysis. Lupus Sci Med 2022; 9:e000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black CM, Silman AJ, Herrick AI, et al. Interferon-alpha does not improve outcome at one year in patients with diffuse cutaneous scleroderma: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 1999; 42:299–305. [DOI] [PubMed] [Google Scholar]
- 8.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 2014; 32:513–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanifer ML, Guo C, Doldan P, Boulant S. Importance of type I and III interferons at respiratory and intestinal barrier surfaces. Front Immunol 2020; 11:608645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brkic Z, van Bon L, Cossu M, et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann Rheum Dis 2016; 75:1567–1573. [DOI] [PubMed] [Google Scholar]
- 11.Amarante-Mendes GP, Adjemian S, Branco LM, et al. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol 2018; 9:2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frasca L, Lande R. Toll-like receptors in mediating pathogenesis in systemic sclerosis. Clin Exp Immunol 2020; 201:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhagwani A, Thompson AAR, Farkas L. When innate immunity meets angiogenesis-the role of toll-like receptors in endothelial cells and pulmonary hypertension. Front Med (Lausanne) 2020; 7:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowland SL, Riggs JM, Gilfillan S, et al. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med 2014; 211:1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Ross RL, Corinaldesi C, Wasson CW, et al. Targeting human plasmacytoid dendritic cells through BDCA2 prevents skin inflammation and fibrosis in a novel xenotransplant mouse model of scleroderma. Ann Rheum Dis 2021; 80:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrated pDCs and their cytokine production as a key cell type in the pathogenesis of SSc
- 16.Ah Kioon MD, Tripodo C, Fernandez D, et al. Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci Transl Med 2018; 10:eaam8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Bon L, Affandi AJ, Broen J, et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med 2014; 370:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lande R, Lee EY, Palazzo R, et al. CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9-mediated interferon-α production in systemic sclerosis. Nat Commun 2019; 10:1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lande R, Mennella A, Palazzo R, et al. Anti-CXCL4 antibody reactivity is present in systemic sclerosis (SSc) and correlates with the SSc type i interferon signature. Int J Mol Sci 2020; 21:5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lande R, Palazzo R, Mennella A, et al. New autoantibody specificities in systemic sclerosis and very early systemic sclerosis. Antibodies (Basel) 2021; 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stowell NC, Seideman J, Raymond HA, et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir Res 2009; 10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saigusa R, Asano Y, Taniguchi T, et al. Multifaceted contribution of the TLR4-activated IRF5 transcription factor in systemic sclerosis. Proc Natl Acad Sci U S A 2015; 112:15136–15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rashid T, Ebringer A. Autoimmunity in rheumatic diseases is induced by microbial infections via crossreactivity or molecular mimicry. Autoimmune Dis 2012; 2012:539282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randone SB, Guiducci S, Cerinic MM. Systemic sclerosis and infections. Autoimmun Rev 2008; 8:36–40. [DOI] [PubMed] [Google Scholar]
- 25.Farina A, Peruzzi G, Lacconi V, et al. Epstein-Barr virus lytic infection promotes activation of Toll-like receptor 8 innate immune response in systemic sclerosis monocytes. Arthritis Res Ther 2017; 19:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farina A, Cirone M, York M, et al. Epstein-Barr virus infection induces aberrant TLR activation pathway and fibroblast-myofibroblast conversion in scleroderma. J Invest Dermatol 2014; 134:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farina A, Rosato E, York M, et al. Innate immune modulation induced by EBV lytic infection promotes endothelial cell inflammation and vascular injury in scleroderma. Front Immunol 2021; 12:651013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu C, Walia A, Ortiz V, et al. Bioactive plasma mitochondrial DNA is associated with disease progression in scleroderma-associated interstitial lung disease. Arthritis Rheumatol 2020; 72:1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crow MK. Mitochondrial DNA promotes autoimmunity. Science 2019; 366:1445–1446. [DOI] [PubMed] [Google Scholar]
- 30.West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol 2017; 17:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnett FC, Cho M, Chatterjee S, et al. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis Rheum 2001; 44:1359–1362. [DOI] [PubMed] [Google Scholar]
- 32.Arora-Singh RK, Assassi S, del Junco DJ, et al. Autoimmune diseases and autoantibodies in the first degree relatives of patients with systemic sclerosis. J Autoimmun 2010; 35:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azizzadeh-Roodpish S, Garzon MH, Mainali S. Classifying single nucleotide polymorphisms in humans. Mol Genet Genomics 2021; 296:1161–1173. [DOI] [PubMed] [Google Scholar]
- 34.López-Isac E, Acosta-Herrera M, Kerick M, et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. Nat Commun 2019; 10:4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López-Isac E, Martín JE, Assassi S, et al. Brief Report: IRF4 newly identified as a common susceptibility locus for systemic sclerosis and rheumatoid arthritis in a cross-disease meta-analysis of genome-wide association studies. Arthritis Rheumatol 2016; 68:2338–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dieudé P, Guedj M, Wipff J, et al. Association between the IRF5 rs2004640 functional polymorphism and systemic sclerosis: a new perspective for pulmonary fibrosis. Arthritis Rheum 2009; 60:225–233. [DOI] [PubMed] [Google Scholar]
- 37.Radstake TR, Gorlova O, Rueda B, et al. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nat Genet 2010; 42:426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmona FD, Gutala R, Simeón CP, et al. Novel identification of the IRF7 region as an anticentromere autoantibody propensity locus in systemic sclerosis. Ann Rheum Dis 2012; 71:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorlova O, Martin JE, Rueda B, et al. Identification of novel genetic markers associated with clinical phenotypes of systemic sclerosis through a genome-wide association strategy. PLoS Genet 2011; 7:e1002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arismendi M, Giraud M, Ruzehaji N, et al. Identification of NF-κB and PLCL2 as new susceptibility genes and highlights on a potential role of IRF8 through interferon signature modulation in systemic sclerosis. Arthritis Res Ther 2015; 17:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham RR, Kozyrev SV, Baechler EC, et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet 2006; 38:550–555. [DOI] [PubMed] [Google Scholar]
- 42.Dieguez-Gonzalez R, Calaza M, Perez-Pampin E, et al. Association of interferon regulatory factor 5 haplotypes, similar to that found in systemic lupus erythematosus, in a large subgroup of patients with rheumatoid arthritis. Arthritis Rheum 2008; 58:1264–1274. [DOI] [PubMed] [Google Scholar]
- 43.Fu Q, Zhao J, Qian X, Wong JL, et al. Association of a functional IRF7 variant with systemic lupus erythematosus. Arthritis Rheum 2011; 63:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunninghame Graham DS, Morris DL, Bhangale TR, et al. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet 2011; 7:e1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López-Isac E, Campillo-Davo D, Bossini-Castillo L, et al. Influence of TYK2 in systemic sclerosis susceptibility: a new locus in the IL-12 pathway. Ann Rheum Dis 2016; 75:1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orvain C, Assassi S, Avouac J, Allanore Y. Systemic sclerosis pathogenesis: contribution of recent advances in genetics. Curr Opin Rheumatol 2020; 32:505–514. [DOI] [PubMed] [Google Scholar]
- 47.Ferri C, Arcangeletti MC, Caselli E, et al. Insights into the knowledge of complex diseases: environmental infectious/toxic agents as potential etiopathogenetic factors of systemic sclerosis. J Autoimmun 2021; 124:102727. [DOI] [PubMed] [Google Scholar]
- 48.Ding W, Pu W, Wang L, et al. Genome-wide DNA methylation analysis in systemic sclerosis reveals hypomethylation of IFN-associated genes in CD4(+) and CD8(+) T cells. J Invest Dermatol 2018; 138:1069–1077. [DOI] [PubMed] [Google Scholar]
- 49.Dees C, Schlottmann I, Funke R, et al. The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Ann Rheum Dis 2014; 73:1232–1239. [DOI] [PubMed] [Google Scholar]
- 50.Altorok N, Tsou PS, Coit P, et al. Genome-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann Rheum Dis 2015; 74:1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krämer M, Dees C, Huang J, et al. Inhibition of H3K27 histone trimethylation activates fibroblasts and induces fibrosis. Ann Rheum Dis 2013; 72:614–620. [DOI] [PubMed] [Google Scholar]
- 52.Van der Kroef M, Castellucci M, Mokry M, et al. Histone modifications underlie monocyte dysregulation in patients with systemic sclerosis, underlining the treatment potential of epigenetic targeting. Ann Rheum Dis 2019; 78:529–538. [DOI] [PubMed] [Google Scholar]
- 53.Henry TW, Mendoza FA, Jimenez SA. Role of microRNA in the pathogenesis of systemic sclerosis tissue fibrosis and vasculopathy. Autoimmun Rev 2019; 18:102396. [DOI] [PubMed] [Google Scholar]
- 54.Fioretto BS, Rosa I, Romano E, et al. The contribution of epigenetics to the pathogenesis and gender dimorphism of systemic sclerosis: a comprehensive overview. Ther Adv Musculoskelet Dis 2020; 12:1759720x20918456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramahi A, Altorok N, Kahaleh B. Epigenetics and systemic sclerosis: an answer to disease onset and evolution? Eur J Rheumatol 2020; 7: (Suppl 3): S147–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasson CW, Ross RL, Wells R, et al. Long noncoding RNA HOTAIR induces GLI2 expression through Notch signalling in systemic sclerosis dermal fibroblasts. Arthritis Res Ther 2020; 22:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossato M, Affandi AJ, Thordardottir S, et al. Association of microRNA-618 expression with altered frequency and activation of plasmacytoid dendritic cells in patients with systemic sclerosis. Arthritis Rheumatol 2017; 69:1891–1902. [DOI] [PubMed] [Google Scholar]
- 58.Mazzone R, Zwergel C, Artico M, et al. The emerging role of epigenetics in human autoimmune disorders. Clin Epigenet 2019; 11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mariotti B, Servaas NH, Rossato M, et al. The long noncoding RNA NRIR Drives IFN-response in monocytes: implication for systemic sclerosis. Front Immunol 2019; 10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muskardin TLW, Niewold TB. Type I interferon in rheumatic diseases. Nat Rev Rheumatol 2018; 14:214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan FK, Zhou X, Mayes MD, et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 2006; 45:694–702. [DOI] [PubMed] [Google Scholar]
- 62.Ciechomska M, Skalska U. Targeting interferons as a strategy for systemic sclerosis treatment. Immunol Lett 2018; 195:45–54. [DOI] [PubMed] [Google Scholar]
- 63.de Oliveira DB, Almeida GM, Guedes AC, et al. Basal activation of type i interferons (Alpha2 and Beta) and 2’5’OAS genes: insights into differential expression profiles of interferon system components in systemic sclerosis. Int J Rheumatol 2011; 2011:275617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuschiotti P. Current perspectives on the immunopathogenesis of systemic sclerosis. Immunotargets Ther 2016; 5:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D, Peck A, Santer D, et al. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum 2008; 58:2163–2173. [DOI] [PubMed] [Google Scholar]
- 66.Eloranta ML, Franck-Larsson K, Lövgren T, et al. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann Rheum Dis 2010; 69:1396–1402. [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Mayes MD, Tan FK, et al. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheum 2013; 65:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George PM, Oliver E, Dorfmuller P, et al. Evidence for the involvement of type I interferon in pulmonary arterial hypertension. Circ Res 2014; 114:677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christmann RB, Sampaio-Barros P, Stifano G, et al. Association of interferon- and transforming growth factor β-regulated genes and macrophage activation with systemic sclerosis–related progressive lung fibrosis. Arthritis Rheumatol 2014; 66:714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum 2010; 62:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Assassi S, Swindell WR, Wu M, et al. Dissecting the heterogeneity of skin gene expression patterns in systemic sclerosis. Arthritis Rheumatol 2015; 67:3016–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farutin V, Kurtagic E, Pradines JR, et al. Multiomic study of skin, peripheral blood, and serum: is serum proteome a reflection of disease process at the end-organ level in systemic sclerosis? Arthritis Res Ther 2021; 23:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73▪.Assassi S, Volkmann ER, Zheng WJ, et al. Peripheral blood gene expression profiling shows predictive significance for response to mycophenolate in systemic sclerosis-related interstitial lung disease. Ann Rheum Dis 2022; 81:854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]; A multiomic comparative analysis of the serum profile, peripheral blood cells and skin ISG expression in SSc patients showing the serum proteome correlated more closely with the transcriptome of the skin than that of the PBMCs.
- 74.Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol 2015; 135:2402–2409. [DOI] [PubMed] [Google Scholar]
- 75.Delaney TA, Morehouse C, Brohawn PZ, et al. Type I IFNs regulate inflammation, vasculopathy, and fibrosis in chronic cutaneous graft-versus-host disease. J Immunol 2016; 197:42–50. [DOI] [PubMed] [Google Scholar]
- 76.Assassi S, Wang X, Chen G, et al. Myeloablation followed by autologous stem cell transplantation normalises systemic sclerosis molecular signatures. Ann Rheum Dis 2019; 78:1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77▪▪.Assassi S, Li N, Volkmann ER, et al. Predictive significance of serum interferon-inducible protein score for response to treatment in systemic sclerosis-related interstitial lung disease. Arthritis Rheumatol 2021; 73:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates a composite serum IFN-inducible protein score exhibiting a predictive significance in the response to immunosuppression in SSc-ILD.
- 78.Airò P, Ghidini C, Zanotti C, et al. Upregulation of myxovirus-resistance protein A: a possible marker of type I interferon induction in systemic sclerosis. J Rheumatol 2008; 35:2192–2200. [DOI] [PubMed] [Google Scholar]
- 79.Chrobak I, Lenna S, Stawski L, Trojanowska M. Interferon-γ promotes vascular remodeling in human microvascular endothelial cells by upregulating endothelin (ET)-1 and transforming growth factor (TGF) β2. J Cell Physiol 2013; 228:1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asano Y, Stawski L, Hant F, et al. Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol 2010; 176:1983–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81▪.Karanth RAG, Kakkar V, Ross R, et al. ABSTRACT NUMBER: 1856 - aerum IFN score predicts long term outcome in limited cutaneous SSc. ACR Convergence 2021; 2021:2021. [Google Scholar]; This abstract further shows T1 IFN dysregulation in a subset of SSc, with a novel composite IFN score being shown to be able to stratify a subset of SSc patients.
- 82.Smith MA, Henault J, Karnell JL, et al. SLE plasma profiling identifies unique signatures of lupus nephritis and discoid lupus. Sci Rep 2019; 9:14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Higgs BW, Liu Z, White B, et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis 2011; 70:2029–2036. [DOI] [PubMed] [Google Scholar]
- 84.Smith MA, Chiang C-C, Zerrouki K, et al. Using the circulating proteome to assess type I interferon activity in systemic lupus erythematosus. Sci Rep 2020; 10:4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao Y, Liu Z, Jallal B, et al. Type I interferons in Sjögren's syndrome. Autoimmun Rev 2013; 12:558–566. [DOI] [PubMed] [Google Scholar]
- 86.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 2003; 100:2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weckerle CE, Franek BS, Kelly JA, et al. Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum 2011; 63:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kirou KA, Lee C, George S, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum 2005; 52:1491–1503. [DOI] [PubMed] [Google Scholar]
- 89.Mathian A, Mouries-Martin S, Dorgham K, et al. Ultrasensitive serum interferon-α quantification during SLE remission identifies patients at risk for relapse. Ann Rheum Dis 2019; 78:1669–1676. [DOI] [PubMed] [Google Scholar]
- 90.Furie R, Khamashta M, Merrill JT, et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol 2017; 69:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mavragani CP, Sagalovskiy I, Guo Q, et al. Expression of long interspersed nuclear element 1 retroelements and induction of type i interferon in patients with systemic autoimmune disease. Arthritis Rheumatol 2016; 68:2686–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munroe ME, Lu R, Zhao YD, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis 2016; 75:2014–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niewold TB, Hua J, Lehman TJ, et al. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun 2007; 8:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. New Engl J Med 2019; 382:211–221. [DOI] [PubMed] [Google Scholar]
- 95.Goldberg A, Geppert T, Schiopu E, et al. Dose-escalation of human antiinterferon-α receptor monoclonal antibody MEDI-546 in subjects with systemic sclerosis: a phase 1, multicenter, open label study. Arthritis Res Ther 2014; 16:R57. [DOI] [PMC free article] [PubMed] [Google Scholar]