Abstract
Factors that influence viability and function of cryopreserved peripheral blood mononuclear cells (PBMC) were identified on 54 samples from 27 AIDS Clinical Trial Units. PBMC viability ranged from 1 to 96% with a median of 70%, was higher in laboratories with experienced staff, and was not significantly associated with CD4 cell number. Function of cryopreserved PBMC, measured by lymphocyte proliferation, was associated with viability. Preparations with viability greater than or equal to 70% had consistent proliferative responses and were suitable for functional analyses.
Highly active antiretroviral therapy often results in potent and durable suppression of human immunodeficiency virus (HIV) replication, elevation of CD4 cell counts, decreased incidence of opportunistic infections, and increased survival in HIV-infected patients, including those with advanced disease (6, 7). Immunologic studies in these patients (1, 4) show improvement of some immune functions, but not others, raising the question of whether restoration of this immune function will be complete. Such studies are sometimes performed on cryopreserved peripheral blood mononuclear cells (PBMC). Our understanding of the extent, timing, and determinants of the immune reconstitution will be expanded if assays can be reliably performed on frozen and thawed cells from well-characterized patients.
To identify biological and/or technical factors that influence functional assays performed on cryopreserved PBMC, we analyzed the results of the cryopreservation quality control (QC) program established for the immunology component of protocol AIDS Clinical Trial Group (ACTG) 360, “A Longitudinal Study of the Predictive Value of Quantitative CMV Viremia Assays for CMV Disease in Persons with AIDS”. This is an ongoing study which enrolled 403 patients at 27 AIDS Clinical Trials Units (ACTU). PBMC from all subjects were cryopreserved every 4 months at ACTU sites. Personnel at the ACTU were asked to freeze the cells following the ACTG cryopreservation protocol, which included separation of PBMC on Ficoll-Hypaque gradients, washing the cells, and resuspending them at 107 PBMC/ml in cold fetal calf serum with 10% dimethyl sulfoxide. Working on ice, we aliquoted the cell suspension into cryovials at 0.5 ml/vial (5 × 106 cells/vial), gradually brought the suspension to a temperature less than or equal to −70°C over 24 h by using Mr. Frosty devices (Curtis Matheson Scientific) or controlled-rate freezers, and then transferred the frozen aliquots to liquid nitrogen tanks. For testing, the cryovials were shipped every 6 months on dry ice to the University of Colorado Health Sciences Center. The QC protocol was performed on randomly selected samples from each participating ACTU. Cells were thawed by quickly bringing them to 4°C followed by slow addition of cold RPMI medium containing 10% human AB serum. Cells were washed and counted in 0.5% trypan blue to assess numbers of viable cells. The total number of cells in each vial and the percentage and absolute number of viable cells were recorded; functional assays were performed if at least 2 × 106 viable cells were recovered.
Viability.
The first QC protocol analyzed 25 samples processed at 21 ACTU; these sites had variable experience with the cryopreservation of PBMC but had judged themselves capable of performing this task (Table 1). The peripheral blood CD4 cell numbers corresponding to the samples utilized in this QC protocol ranged from 8 to 564 cells/μl. The percentages of viable cells in these samples varied from 1 to 91% with a median of 76%. The number of viable cells per vial ranged from 1 × 106 to 17.5 × 106. In an effort to improve the subsequent quality of the frozen PBMC, the results of the first QC protocol were distributed to the ACTU and were discussed together with potential pitfalls of the cryopreservation procedure via conference calls.
TABLE 1.
Viability of cryopreserved PBMC obtained from HIV-infected patients enrolled in ACTG 360
| Category | QC 1a | QC 2 | |
|---|---|---|---|
| No. of samples | 25 | 29 | |
| Viability (%) | Range | 1–91 | 4–96 |
| Median | 76 | 68 | |
| 25th Percentile | 56 | 52 | |
| 75th Percentile | 88 | 82 |
QC 1, quality control experiment 1.
A second QC protocol, performed on specimens from the 6-month shipment that followed the conference calls, analyzed 29 samples from 22 laboratories. Peripheral blood CD4 cell numbers in these patients ranged between 7 and 1,192 cells/μl. Cell viability ranged from 4 to 96% with a median of 68%. The number of viable cells per vial varied between 0.3 × 106 and 16.2 × 106. The data combined from both QC protocols did not show a significant association between the number of CD4 cells and the percentage of viable cells in each sample with a P value of 0.4 (Spearman rank correlation), indicating that a low number of CD4 cells does not preclude successful cryopreservation of PBMC.
To explore the possibility that successful cryopreservation is related to technical expertise, we limited the analysis to the cells frozen at nine ACTG immunology core laboratories whose staff members self-reported high levels of expertise for immunological assays. All of these samples had viability greater than or equal to 70%. This was in accordance with previous reports (5), which found excellent viability in samples frozen at laboratories with experienced staff members. Repeat specimens were tested from four sites whose initial specimens had viability less than or equal to 2%. The results of the first and second samples were similar when repeated in three of these sites, but not for the remaining site, which had improved results. These findings suggest that the experience level of the laboratory staff members performing the cryopreservation is a major determinant of the viability of cryopreserved PBMC.
Functional assays.
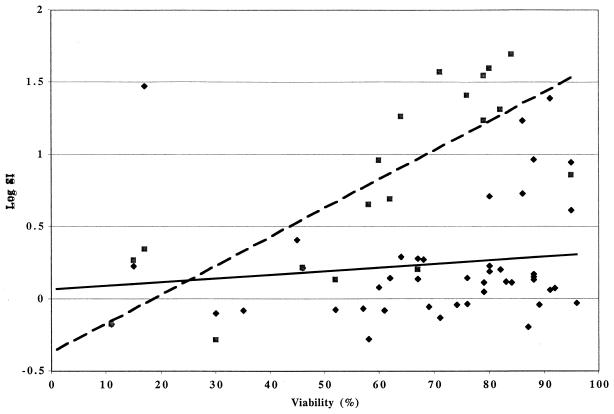
Functional assays were performed on 45 cryopreserved samples with ≥2 × 106 cells. These consisted of lymphocyte proliferation assays (LPAs) for cytomegalovirus (CMV) and pokeweed mitogen (PWM) and/or responder cell frequency (RCF) for CMV. The LPAs were performed as previously described (8): triplicate wells containing 105 cells each in RPMI medium containing 10% human AB serum were incubated for 6 days in the presence of CMV antigen and control, PWM, or plain medium. Proliferation was measured by [3H]thymidine incorporation during a 6-h pulse. Stimulation indices (SIs) were calculated by the ratio between median counts per minute in antigen- or mitogen-stimulated wells and median counts per minute of the appropriate controls, i.e., mock-infected cell control for CMV and plain medium for PWM. Positive responses were defined as SI greater than or equal to 3 for CMV and greater than or equal to 5 for PWM. The RCF measured the frequency of CMV-specific memory CD4 cells by adding a limiting dilution step to the LPA (9). Briefly, 24 replicate cultures containing 100,000, 50,000, 25,000, 12,500, and 6,250 PBMC per well were stimulated with CMV and mock-infected control antigens for 8 days (2). On the last day of culture, cells were pulsed with [3H]thymidine for 6 h and harvested, and incorporated radioactivity was counted in a scintillation counter. The RCF was calculated as described by Henry et al. (3): responder wells were defined as those whose counts per minute exceeded the mean counts per minute plus 3 standard deviations of the control cultures at the same cell concentration. The percentage of nonresponder wells was plotted on a log scale against the number of cells per well plotted on a linear scale, and the RCF was interpolated at the 37% nonresponder well frequency. An SI was also calculated by dividing mean counts per minute in CMV-stimulated wells by mean counts per minute in control wells at 105 cells/well. Among 45 specimens used in functional assays in either of the two QC protocols, positive proliferative responses to CMV or PWM were significantly associated with increased viability of PBMC in each sample (P = 0.05 and 0.002, respectively, Spearman rank correlation) (Fig. 1). Outlier results were confirmed by review of the raw data. The associations persisted when the data were analyzed excluding possible outliers.
FIG. 1.
CMV and PWM proliferative results as functions of viability. Data were derived from 44 CMV (⧫) and 19 PWM (■) assays. The continuous and dashed lines indicate smoothed regression curves (moving average) for CMV and PWM, respectively. The outlier result (CMV log SI of 1.48 with a viability of 17%) was confirmed as a valid result by analysis of the raw data.
At 70% viability, which is the median of the combined QC samples, as a discriminative value (Table 2), 100% of the samples above 70% viability proliferated in response to PWM, and 28% had a positive LPA or RCF after CMV-specific stimulation. In contrast, among samples with <70% viability, only 25% responded to PWM, and none of the samples responded to CMV stimulation.
TABLE 2.
Effect of cell viability on functional assays of cryopreserved PBMC obtained from HIV-infected patients
| Stimulant | Viability ≥70% | Viability <70% | P valuea |
|---|---|---|---|
| PWMb | 9/9 (100) | 3/12 (25) | 0.001 |
| CMVc | 7/25 (28) | 0/19 (0) | 0.03 |
Two-tailed Fisher's exact test.
Numbers represent positive assays/total number of performed assays; a positive assay was defined as a SI greater than or equal to 3 for CMV and SI greater than or equal to 5 for PWM. Numbers in parentheses represent percentages of positive assays.
More CMV-specific assays were performed because they were part of the planned analysis for ACTG 360.
These data indicate that functional assays on cryopreserved PBMC are associated with viability of the cells. Viability thresholds should be used in clinical trials in order to obtain reliable results of functional assays. Furthermore, to achieve consistently high viability, cryopreservation must be performed in laboratories whose staff members have proven proficiency in this technique. QC programs for laboratories undertaking cryopreservation of PBMC for immunological assays are necessary to ensure satisfactory performance.
Acknowledgments
This work was supported by the Adult AIDS Clinical Trials Group and WESTAT/NICHD.
REFERENCES
- 1.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Hayward A R, Levin M J, Wolf W, Gilden D. Varicella zoster virus specific immunity following herpes zoster. J Infect Dis. 1990;163:873–875. doi: 10.1093/infdis/163.4.873. [DOI] [PubMed] [Google Scholar]
- 3.Henry C, Marbrook J, Vann D C, Kodlin D C, Wojsy C. Limiting dilution analysis. In: Mishell B B, Shigii S M, editors. Selected methods in cell mediated immunity. San Francisco, Calif: Freeman Press; 1980. pp. 138–152. [Google Scholar]
- 4.Kellehrer A D, Carr A, Zaunders J, Cooper D A. Alterations in the immune response of human immunodeficiency virus-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J Infect Dis. 1996;173:321–329. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 5.Kleeberger C A, Lyles R H, Margolick J B, Rinaldo C R, Phair J P, Giorgi J V. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immunol. 1999;6:14–19. doi: 10.1128/cdli.6.1.14-19.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz M, Saag M, Powderly W, et al. Clinical evaluation of the safety and efficacy of ritonavir (ABT-538), an inhibitor of HIV-1 protease. N Engl J Med. 1995;333:1534. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 7.Mouton Y, Cartier C, Dellamonica P, Humbert G, Lang J M, Massip P, Micoud M, Modai J, Portier H. Proceedings of the 4th Conference on Retroviruses and Opportunistic Infections. Alexandria, Va: Foundation for Retrovirology and Human Health; 1997. Dramatic cut in AIDS defining events and hospitalization for patients under protease inhibitors and tritherapies in 9 AIDS reference centers and 7,391 patients; p. 208. [Google Scholar]
- 8.Weinberg A, Betensky R, Zhang L, Ray G. Effect of shipment, storage, anticoagulant and cell separation on lymphoproliferative responses in HIV-infected patients. Clin Diagn Lab Immunol. 1998;5:804–807. doi: 10.1128/cdli.5.6.804-807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasukawa M, Inatsuki A, Horiuchi T, Kobayashi Y. Functional heterogeneity among herpes simplex virus specific human CD4+ T cells. Immunology. 1991;146:1341–1347. [PubMed] [Google Scholar]