One of the most prevalent uses of avidin-biotin technology in recent years has been their use in immunoassays. In many cases, the signal is enhanced 10-fold or more, owing to the four biotin-binding sites of avidin and the multiple biotinyl groups on the derivatized antibody (4, 5). This provides the possibility of cross-reactions when biotin-avidin systems are used in immunoassays. Unwanted binding of avidin conjugate may be induced by (i) the presence in the antigen of endogenous biotin-containing proteins which are found in different mammalian tissues (kidney, liver, pancreas, and brain) (2, 6) where its principal role is to serve as a prosthetic group for a set of enzymes involved in carboxylation, transcarboxylation, and decarboxylation (7). Bound biotin molecules are also present in other organisms (bacteria, plants, and insects) (1), and they have been found in helminths: Ascaris suum, Toxocara canis, Anisakis simplex, Hysterothylacium aduncum, Trichuris muris, and the cestode Bothriocephalus scorpii (3). Unwanted binding of avidin conjugate may also be induced by (ii) endogenous lectins, as many antigens contain associated lectins, mainly mannose-specific lectins which react with avidin, and (iii) macromolecules through nonspecific ionic or hydrophobic interactions (1).
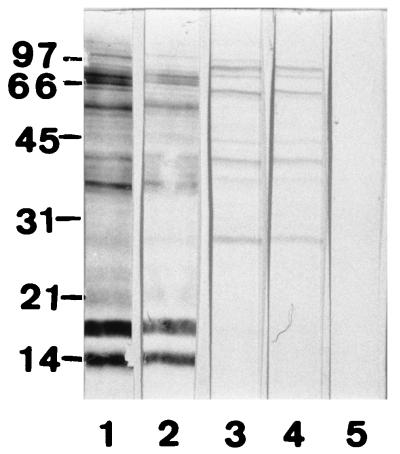
In our immunoblot (WB) experiments, avidin binds to several polypeptides in the Leishmania infantum and Leishmania panamensis antigens with molecular masses ranging from 28 to 96 kDa for L. infantum (Fig. 1) and from 14 to >100 kDa for L. panamensis in a 15% polyacrylamide gel. This nonspecific binding is blocked by diluting the avidin conjugate in 200 mM α-methyl-d-mannoside (1) but not by raising the ionic strength of dilution buffers by the addition of 0.3 M sodium chloride (NaCl), which suggests that the nonspecific binding observed is due to the presence of lectins in the Leishmania antigen.
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of an L. infantum antigen lysate transblotted to a nitrocellulose membrane. Lane 1, serum from a patient with leishmaniasis (1/100 in 20 mM Tris–0.13 mM NaCl–1% nonfat dry milk [TS-M]) and protein A-peroxidase (Sigma) (1/1,000 in TS-M); lane 2, serum from a patient with leishmaniasis (1/100 in TS-M), monoclonal anti-human immunoglobulin G1 biotin conjugate (Sigma), and avidin peroxidase (Sigma) (2.5 μg/ml in TS-M); lane 3, avidin peroxidase at 2.5 μg/ml in TS-M; lane 4, avidin peroxidase at 2.5 μg/ml in 0.3 M NaCl; lane 5, avidin peroxidase at 2.5 μg/ml in 200 mM α-methyl-d-mannoside. Color was developed with 4-chloro-1-naphthol (Sigma).
In the enzyme-linked immunosorbent assay (ELISA), we have observed different degrees of avidin nonspecific binding to L. infantum antigen, depending on the composition of the buffer in which avidin is diluted. The presence of nonfat dry milk or bovine serum albumin, Tween 20, and 0.3 M NaCl in the buffer (20 mM Tris, 0.13 M NaCl) influences the background, which may be as strong as the immunospecific reaction when a solution of 2.5 μg of avidin-peroxidase per ml is used. This nonspecific binding of avidin to Leishmania antigen in ELISA is mainly suppressed by raising the ionic strength of the buffer by adding 0.3 M NaCl. In contrast, the addition of 200 mM α-methyl-d-mannoside does not reduce the background.
The reasons for such differences in nonspecific binding of avidin to Leishmania antigen in WB and ELISA may be found in the different antigen treatments in the two immunoassays. Ionic binding is mainly responsible for background in microplate ELISA, in which a sonicated promastigote suspension is used. The presence of 5% 2-mercaptoethanol and 5% sodium dodecyl sulfate in antigen buffer in WB suppresses the nonspecific ionic binding of avidin but renders endogenous lectins accessible.
As this nonspecific binding may be a source of false-positive results in immunoenzymatic assays for leishmaniasis using avidin-biotin systems, we recommend the addition of α-methyl-d-mannoside to the avidin-enzyme conjugate in WB assays.
Acknowledgments
This study was financed through projects FIS97-2004-01 (from the Spanish government) and SGR97 (from Generalitat de Catalunya) and a grant from BID-COLCIENCIAS (Colombian government) to S. Agudelo.
We thank R. Rycroft for correcting the English version of the manuscript and S. Calle for technical support.
REFERENCES
- 1.Duhamel R C, Whitehead J S. Prevention of nonspecific binding of avidin. Methods Enzymol. 1990;184:201–207. doi: 10.1016/0076-6879(90)84275-l. [DOI] [PubMed] [Google Scholar]
- 2.Levine S M, Macklin W B. Biotin enrichment in oligodendrocytes in the rat brain. Brain Res. 1988;144:199–203. doi: 10.1016/0006-8993(88)90930-4. [DOI] [PubMed] [Google Scholar]
- 3.Romaris F, Iglesias R, García L O, Leiro J, Santamarina M T, Paniagua E, Ubeira F M. Free and bound biotin molecules in helminths: a source of artifacts for avidin biotin-based immunoassays. Parasitol Res. 1996;82:617–622. doi: 10.1007/s004360050174. [DOI] [PubMed] [Google Scholar]
- 4.Ternynck T, Avrameas S. Avidin-biotin system in enzyme immunoassays. Methods Enzymol. 1990;184:469–481. [PubMed] [Google Scholar]
- 5.Wilcheck M, Bayer E A. Applications of avidin-biotin technology: literature survey. Methods Enzymol. 1990;184:14–45. doi: 10.1016/0076-6879(90)84257-h. [DOI] [PubMed] [Google Scholar]
- 6.Wood G S, Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection system. J Histochem Cytochem. 1981;29:1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]
- 7.Wood H G, Barden R E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]