Abstract
The α-aminophosphonate UAMC-00050, a newly developed trypsin-like serine protease inhibitor, is a lead compound for the treatment of dry eye syndrome and ocular inflammation. The medicinal chemistry route developed at the University of Antwerp possessed several problems hampering the scale-up such as poor yields for some of the steps, hazardous reagents, and environmental footprint. Herein, we report an optimized route for the UAMC-00050, in which environmental unfriendly solvents were excluded, hazardous reagents were replaced with safer alternatives, and are more efficient in terms of atom economy. Every reaction step was optimized to reach a higher yield, and design of experiment was used to find the optimum conditions in the last step. Furthermore, all the flash chromatography purifications of intermediates were replaced with plug filtration, slurry purifications, or crystallization. The overall yield was increased from 3% in the medicinal chemistry route to 22% in the process development route.
Keywords: α-aminophosphonate, design of experiment, dry eye disease, yttrium catalysis, uPA
Introduction
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a multifactorial disease of the ocular surface1 that affects hundreds of millions of people worldwide.2 The disease is characterized by a dry, gritty, or burning feeling in the eye and excessive tearing and photosensitivity.3 Recently, a new pharmacologically active molecule, UAMC-00050 (Figure 1), was developed at the University of Antwerp (UA) for the treatment of DED.4,5 This compound is based on an α-aminophosphonate substructure mimicking amino acids where the carboxylic acid is swapped with a phosphonate ester. Compound 1 has shown good inhibitory potency against a urokinase plasminogen activator (uPA) and other trypsin-like serine proteases, which play a role in eye diseases.6 To continue pre-clinical investigation, we needed rapid access to reproducible multigram quantities (10–20 g per year) of compound 1. We optimized the discovery route to one suitable for a multigram scale with a potential for large-scale application.
Figure 1.

Structure of the α-aminophosphonate UAMC-00050.
Result and Discussion
Route Selection
The medicinal chemistry route started from the commercially available 4-aminophenethyl alcohol (4). After protection of the amine with Boc2O in the presence of triethylamine, alcohol 5 was oxidized to aldehyde 6 with Dess–Martin periodinane (DMP). The aminophosphonate intermediate 8 was assembled by the one-pot three-component Birum–Oleksyszyn reaction between aldehyde 6, benzyl carbamate (7), and phosphite 3, using copper triflate as the catalyst.7−9 Triarylphosphite 3 was prepared from paracetamol (2) and used as a crude reagent. Then, the Boc group in aminophosphonate 8 was removed with TFA in DCM (1:1 v/v) to generate salt 9. The guanidine moiety was inserted using N,N′-di-Boc-1H-pyrazole-1-carboxamidine (10), the two Boc groups were removed with TFA in DCM (1:1 v/v), and the trifluoroacetate counterion was exchanged with chloride after stirring compound 12 with a DOWEX 1X8 Cl resin to get 1 (Scheme 1).
Scheme 1. Medicinal Chemistry Route to UAMC-00050.
When performing the original process on a multigram scale, we noted reproducibility issues. In particular, the Birum–Oleksyszyn reaction represented a bottleneck for the overall yield and purity of the final material since the majority of side products were formed in this step. In previous studies, we optimized the preparation of 8, improving the yield and purity profile and finding yttrium triflate as an optimal catalyst.10 Further optimizations were necessary to prepare phosphite 3 due to its particular instability in the presence of oxygen and water. The purity of 3 was important to curb the generation of impurities in the Birum–Oleksyszyn reaction since the diarylphosphite can cleave the Boc group and lead to the formation of side products. Notably, purification by chromatography led to almost complete decomposition of the phosphite 3. This led us to remove the chromatographic separation from the preparation of phosphite 3 and focus on a careful synthetic protocol that yielded 3 with a purity above 90%.
Preparation of Phosphite 3
For the preparation of compound 3, the original conditions5 were successfully upscaled with minor modifications (Scheme 2). The reaction time was decreased from 105 to 60 min as longer times led to reduced product purity. Separating the triarylphosphite 3 from the main impurities (diarylphosphite 13 and paracetamol (2)) via chromatographic separation, precipitation, or crystallization proved challenging. Therefore, particular attention was paid to optimizing the reaction conditions leading to a minimum amount of side products.
Scheme 2. Synthesis of Compound 3 from Paracetamol (2).
The presence of water in the starting material was the main reason for the reduced purity of the triarylphosphite 3 as water can decompose PCl3 to H3PO3 and HCl. This changes the ratio of the reagents, increasing the amount of 2 and 13 in the crude product. Moreover, water can also decompose the triarylphosphite 3 generating paracetamol (2) and diarylphosphite 13 (Scheme 2).
Careful drying of the starting material 2 in a vacuum (5 mbar) for at least 24 h significantly improved the conversion and the purity of triarylphosphite 3. The water content in paracetamol (2) after drying was 0.030% (Karl Fischer titration). Once the reaction was completed, the product was separated from triethylammonium chloride by filtration of the reaction mixture under argon flow. To prevent thermal decomposition of 3, THF was then removed under reduced pressure at 20–25 °C. Phosphite 3 was obtained with yields of 98 and 92.3% area normalized (AN) by HPLC on a 44 mmol scale.
Boc-Protection of the Amino Group
To enable the oxidation of alcohol 5, protection of the amino group in 4 was required. In the medicinal chemistry procedure, this was achieved using 1.1 equiv of di-tert-butyl dicarbonate in the presence of 1.0 equiv of triethylamine in dioxane5 (Table 1, entry 1). The standard reaction protocol reported in the literature14 (which does not require triethylamine) was successfully used in our process development. Compound 4 was treated with 1.1 equiv of di-tert-butyl dicarbonate in EtOAc for 16 h (Table 1, entry 2). The purified product 5 was obtained from the reaction mixture after silica pad filtration (Table 1, entry 3). The amount of silica required in the filter pad was then reduced from 15.0 to 8.3 w/w, obtaining intermediate 5 (99% AN by HPLC) on a 73 mmol scale (Table 1, entry 4). In a later improvement, after the reaction, crude 5 was purified with crystallization. Among seven different conditions (see the Supporting Information), the mixture of MeCN/MTBE 1:1 v/v (solvent/5 = 1.5:1 v/w) was found able to provide the product with 99% AN by HPLC and 98% yield.
Table 1. Optimization of the Purification Process of Alcohol 5.

| entry | solvent | scale (mmol) | additive | method of purification | yield (%) | purity (%) |
|---|---|---|---|---|---|---|
| 1 | dioxane | 37.00 | TEA | flash chromatography | 97 | 99 |
| 2 | EtOAc | 37.00 | flash chromatography | 99 | 99 | |
| 3 | EtOAc | 37.00 | pad of silica (SiO2/5 = 15:1 w/w ) | 95 | 99 | |
| 4 | EtOAc | 73.00 | pad of silica (SiO2/5 = 8.3:1 w/w) | 99 | 99 | |
| 5 | EtOAc | 73.00 | crystallization (MeCN/MTBE 1:1/5 = 1.5:1 v/w) | 98 | 99 |
Preparation of Aldehyde 6
The medicinal chemistry synthesis of aldehyde 6 used 1.5 equiv of DMP in DCM at −78 to 23 °C, and the crude product was purified by flash chromatography. An attempt to apply the (bpy)CuI/TEMPO-catalyzed aerobic oxidation12 failed to provide conversion of substrate 5. Hydrogen peroxide with AlCl315 or KBr/TEMPO/pTsOH16 was also not successful under oxidation conditions. Using 1.5 equiv of sodium hypochlorite in the presence of a catalytic amount of TEMPO, KBr and nBu4Br13,17 provided a 69% conversion of substrate 5 (Table 2, entry 1). Gratifyingly, raising the equivalents of sodium hypochlorite to 1.8, we obtained a complete conversion of alcohol 5, after 15 min (Table 2, entry 2). Despite the complete consumption of 5, a poor isolated yield (31%) was obtained (Table 2, entry 3). Carboxylic acid 14 was noted as one of the main impurities of the desired compound 6. As reported by Lucio Anelli et al.,18 the presence of nBu4NBr catalyzes the oxidation of the aldehyde to carboxylic acid 14. Removal of the quaternary salt allowed us to increase the yield of aldehyde 6 to 59% (Table 2, entry 4). The yield was further increased to 71% when the amount of TEMPO was reduced from 0.05 to 0.01 equiv, and the reaction time was reduced from 60 to 30 min (Table 2, entry 5). However, upscaling the reaction to 37 mmol resulted in a decrease in the yield to 60% (Table 2, entry 6). This was fixed by further reducing the amount of NaClO at 1.6 equiv and the reaction time to 15 min, which allowed us to get aldehyde 6 in 66% yield (Table 2, entry 7).
Table 2. Optimization of Reaction Conditions and Purification of 6.
| entry | alcohol (mmol) | equiv NaClOa | equiv KBr | equiv nBu4NBr | equiv TEMPO | time (min) | bisulfite extractionb | conversion (%) | yield (%)c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.42 | 1.5 | 0.1 | 0.05 | 0.05 | 120 | 69 | ND | |
| 2 | 0.42 | 1.8 | 0.1 | 0.05 | 0.05 | 15 | 100 | ND | |
| 3 | 4.22 | 1.8 | 0.1 | 0.05 | 0.05 | 15 | 2 h r.t. | 100 | 31 |
| 4 | 4.22 | 1.7 | 0.1 | 0.05 | 60 | 2 h r.t. | 100 | 59 | |
| 5 | 4.22 | 1.7 | 0.1 | 0.01 | 30 | 2 h r.t. | 100 | 71 | |
| 6 | 37.00 | 1.7 | 0.1 | 0.01 | 30 | 2 h r.t. | 100 | 60 | |
| 7 | 37.00 | 1.6 | 0.1 | 0.01 | 15 | 2 h r.t. | 100 | 66 | |
| 8 | 73.00 | 1.6 | 0.1 | 0.01 | 15 | 2 h r.t. | 100 | 59 | |
| 9 | 73.00 | 1.6 | 0.1 | 0.01 | 15 | 16 h r.t. | 100 | 71 |
NaClO concentration (11–15%).
Stirred for 1 h at 0 °C before filtration.
Isolated yield.
To avoid the chromatographic column purification, several methods for the isolation of aldehyde 6 were investigated. An attempt to use a silica pad to purify the crude product failed to provide 6 with an acceptable purity (52% AN by HPLC %). On the other hand, the bisulfite adduct protocol19 provided 6 with 99% AN by HPLC. The crude material was reacted with NaHSO3, and the bisulfite adduct 15 was separated by filtration (Table 2). The aldehyde 6 was regenerated in good purity when treating 15 with aqueous Na2CO3 followed by extraction with EtOAc. When the bisulfite adduct purification method was applied to a 73 mmol scale preparation of 6, a decrease in yield (59%) was observed (Table 2, entry 8). Examination of the mother liquid indicated that it was caused by a problem with the formation of adduct 15 rather than with the catalytic oxidation. Extending the reaction time of the crude aldehyde with NaHSO3 from 2 to 16 h allowed the complete conversion of aldehyde 6 to the bisulfite adduct 15. The bisulfite derivative was then converted back to the aldehyde, providing 6 in 71% yield from 5 with 99.2% AN by HPLC (Table 2, entry 9).
Birum–Oleksyszyn Reaction
The one-pot three-component reaction between the aldehyde 6, the phosphite 3, and the benzyl carbamate (7) is a key step for the synthetic process of compound 1. Unfortunately, this reaction step suffered from a poor yield (11%) and a low selectivity toward the product even on a 1 g scale. Moreover, the impurities in the crude material made purification challenging. In a separate study, we investigated the role of the catalyst and found Y(OTf)3 as the most efficient in providing α-aminophosphonate 8 in an improved 42% yield10 (Table 3, entry 1).
Table 3. Screening of Birum–Oleksyszyn Conditions.
| entrya | solvent | additive | time (h) | conc (M) | yield of 8 (%)b |
|---|---|---|---|---|---|
| 1 | MeCN | 4 | 0.07 | 42c | |
| 2 | MeCN/THF 1:1 | 4 | 0.07 | 44 | |
| 3 | MeCN/THF 1:1 | 4 | 0.17 | 45 | |
| 4 | MeCN/THF 1:1 | 1.0 equiv Ac2O | 4 | 0.17 | 50 |
| 5 | MeCN/THF 1:1 | 1.0 equiv TFAA | 4 | 0.17 | 52 |
All reactions were carried out with 4.3 mmol of aldehyde 6.
Isolated yield after flash chromatography.
From ref (10).
With a good catalyst in hand, our attention moved to solvent selection. While running the reaction in acetonitrile, we noted the formation of a precipitate, identified as aminal 16. We then started to investigate a new medium for the α-aminophosphonate 8 preparation. Screening of seven anhydrous solvents and one solvent combination (see the Supporting Information) revealed the mixture THF/MeCN (1:1 v/v) as the most appropriate to improve the yield (44%) of aminophosphonate 8 (Table 3, entry 2). The higher yield, obtained with the mixture of THF/MeCN (1:1 v/v), is likely due to the capability of THF to solubilize aminal 16 and, therefore, increase the reaction rate. In addition, the yield was slightly raised to 45% when the Birum–Oleksyszyn reaction was run in THF/MeCN (1:1 v/v) with a concentration of 0.17 M (Table 3, entry 3).
A range of anhydrides was then screened as additives as these are known in literature21−24 to promote the reaction between intermediate aminals and alkylphosphonous acids. Equimolar amounts of both of acetic and trifluoroacetic anhydride were able to increase the yields of α-aminophosphonate 8 to 50 and 52% (Table 3, entries 4 and 5, respectively).
Among the range of side products, anilines resulting from Boc cleavage of the group were also observed during the reaction.10 We hypothesized that anilines get oxidized to form colored impurities, in which separation proved to be challenging. Based on these considerations, we decided to investigate the use of different aldehydes as intermediates. In the first case, the amino group in 4 was protected with a Fmoc group, and then, the alcohol 18 was converted to aldehyde 19 by oxidation with DMP. In the second case, 4-nitrophenethyl alcohol (21) was oxidized to the corresponding aldehyde 22 with DMP (Scheme 3). Unfortunately, Fmoc-protected aldehyde 19 failed to provide a good yield and a good purity profile to give aminophosphonate 20 in the Birum–Oleksyszyn reaction. 2-(4-Nitrophenyl)acetaldehyde (22) rapidly decomposed when in contact with air, a Lewis acid, or a base like Na2CO3, rendering it inappropriate for the synthesis of aminophosphonate 23.
Scheme 3. Alternative Aldehydes and Their Performance in Birum–Oleksyszyn Reaction.
The unsuccessful performance of aldehydes 19 and 22 prompted us to focus on the purification of aminophosphonate 8 derived from Boc-protected substrate 6. At the end of the reaction, the HPLC chromatogram of the reaction mixture showed paracetamol (2), diarylphosphite 13, monoaryl phosphonate 17, and aminal 16 as major impurities. The acidic impurities such as paracetamol (2), monoaryl phosphonate 17, and diarylphosphite 13 were almost completely removed after washing the organic phase with an 0.5 M aqueous NaOH. Most of the aminal 19 and other lipophilic impurities were separated from product 8 with silica pad filtration. These procedures provided the α-aminophosphonate 8 with an HPLC purity of 64.2%. After the basic wash, an 8.3% relative area percentage of paracetamol was still present in the crude material. Antisolvent precipitation in basic aqueous solution allowed us to remove the remaining 2: the crude product 8 was dissolved in EtOH and added dropwise to an aqueous solution of NaHCO3. The precipitated α-aminophosphonate 8 was collected with an HPLC purity of 74.1%. THF was also able to dissolve the crude compound 8; however, once the solution was added to aqueous NaHCO3, an oil was formed. Under the same conditions, acetone, as a solvent for crude compound 8, provided the precipitate as very fine particles that clogged the filter. Changing the mode of addition (i.e., adding the bicarbonate solution to the acetone solution) made compound 8 a better filterable solid with an HPLC purity of 82.3%.
Next, we focused on the removal of the yellow color. Charcoal was first tested as a standard treatment for the removal of colored impurities.27,28 A total of 11 different charcoal batches were tested (see the Supporting Information), but none of them were able to remove the yellow color from the crude material. Slurry conditions were also screened as a purification method. The crude material was stirred in EtOAc for 16 h at r.t., and then, the solid was filtered obtaining an off-white product with 92% HPLC purity. We were pleased to find that the target purity (98%) was reached after using a solution of EtOAc/acetone (19:1 v/v) instead of pure EtOAc. After 16 h of stirring, compound 8 was isolated from the mother liquor with an HPLC purity of 98%. The reaction and the purification protocols were then tested with 10.00 g (43 mmol) of aldehyde 6 as a starting material, providing the α-aminophosphonate 8 in 44% yield with a 98.2% AN by HPLC.
Boc-Cleavage for the Preparation of Aniline 24
In the medicinal chemistry procedure, the removal of the Boc-protecting group was carried out with TFA in DCM (1:1 v/v) at room temperature. HCl was investigated as a more economical alternative to TFA and also leading to less hygroscopic HCl salt (Scheme 4). The use of 4 N HCl in dioxane enabled the complete cleavage of the Boc group within 3 h despite the fact that the starting material 8 is poorly soluble in dioxane.
Scheme 4. Cleavage of the Boc Group in α-Aminophosphonate 8.
Crude aniline salt 24 was dissolved in 96% EtOH and was added dropwise to EtOAc while stirring at r.t. Unfortunately, the product formed clots that stuck to the walls of the flask. Using absolute ethanol instead of 96% ethanol prevented the formation of clots, and the solid was obtained as off-white flakes with an HPLC purity of 96.9%. The conditions of Boc-cleavage and work-up were applied for the upscale. Aniline 24 was obtained with yields of 99 and 97.9% AN by HPLC, when using 10.00 g (14 mmol) of α-aminophosphonate 8 as a starting material.
Preparation of Product 1
In the medicinal chemistry route, the final product 1 was prepared from aniline TFA salt 9 by inserting the guanyl group using N,N′-di-Boc-1H-pyrazole-1-carboxamidine (10). This was followed by removal of the Boc groups with TFA in DCM and salt exchange with DOWEX 1X8 Cl resin to convert intermediate 12 to product 1.
We investigated a direct way to convert aniline HCl salt 24 to product 1, reducing the step count, increasing the overall yield, and cutting the cost. A range of literature methods is available for the direct transformation of aniline to aryl guanidine.29−35 From these, guanylation with cyanamide was selected to develop the protocol with the best atom economy and costs.29,30 However, heating aniline HCl salt 24 with cyanamide in a protic solvent in the presence of a Brønsted or a Lewis acid led to a decomposition of the α-aminophosphonate. Therefore, we investigated the guanylation of aniline salt 24 with 1.2 equiv of cyanamide in the presence of 0.1 equiv of Sc(OTf)3 in a panel of solvents and solvent mixtures at room temperature (see the Supporting Information).
These studies revealed MeCN/iPrOH (1:1 v/v) as the most optimal reaction media to give 38% conversion of aniline salt 24 in 72 h (Table 4, entry 1). Then, we moved our focus to the reaction’s catalyst. Lewis acids like Bi(OTf)3 and Y(OTf)3 (Table 4, entries 2 and 3) and Brønsted acids like HCl, HNO3, and AcOH (Table 4, entries 4–6) failed to provide improved conversion compared to Sc(OTf)3.
Table 4. Catalyst Optimization for the Direct Guanylation of 24.
| entrya | catalyst | equiv catalyst | conversion (%) |
|---|---|---|---|
| 1 | Sc(OTf)3 | 0.1 | 38 |
| 2 | Bi(OTf)3 | 0.1 | 34 |
| 3 | Y(OTf)3 | 0.1 | 25 |
| 4 | HCl | 1.0 | 8 |
| 5 | HNO3 | 1.0 | 40 |
| 6 | AcOH | 1.0 | 11 |
Aniline 28 (0.08 mmol), 1.2 equiv of NH2CN, 1.0 M, 72 h, MeCN/iPrOH 1:1 v/v.
Before further optimization efforts, an isolation/purification method for product 1 was developed. The reaction mixture was first concentrated, and the residue was dissolved in abs-EtOH. Then, HCl 2.5 N in EtOH (HCl 2.5 N/1 = 1:2 v/w) was added to form a guanidine HCl salt, and the ethanol solution was dropped to an antisolvent (see the Supporting Information). iPrOAc was found to be the antisolvent of choice providing a solid that was easily filtrated.
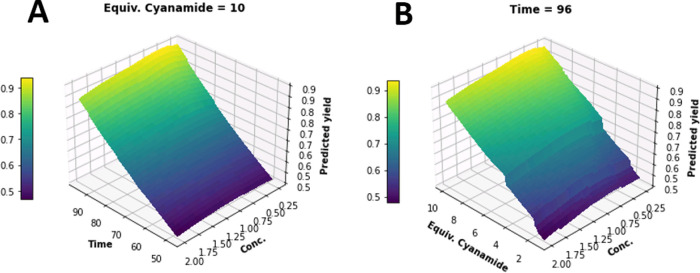
To increase the conversion, three variables were investigated: concentration, reaction time, and equivalents of cyanamide. A design of experiment (DoE) approach was selected to explore all the three variables at the same time and eventually identify any interaction between them. At first, we set the limits of the three variables: 0.1–2.0 M for the concentration, 1.2–10.0 for the cyanamide equivalents, and 48–96 h for the reaction time. The DoE was performed with the support of the artificial intelligence web-based software xT SAAM.36 The program uses stochastic optimization techniques to produce suggestions for the next experiments until an objective is satisfied. The objective was to maximize the purity and the yield of the final product as well as to create models for predicting purity and yield. Within this study, four consecutive iterations of parallel experiments were carried out, with a total of 22 experiments (see the Supporting Information). The results on purity and yield were collected, and the xT SAAM software uses an automated mechanism to produce a cross-validated ensemble modeling to create the final model. Ensemble modeling is a type of modeling that combines the results of multiple individual models to produce a more accurate final model. A multitude of non-linear features is produced from the input parameters and iteratively tested within the ensemble model using cross-validation; only parts of randomly selected data points are used at a time for training and fitting the model. Then, the average test-data R2 score is reported and the appropriate final model is selected. In our case, we used ensemble modeling to generate the response surface model (RSM). From the RSM (Figure 2), it was observed that the best yield and purity could be obtained when the concentration was 0.5 M with NH2CN equivalents and time maximized.
Figure 2.
Predicted yield RSM for the cyanamide guanylation of 1 in iPrOH/MeCN 1:1: (A) when cyanamide equivalents are fixed at 10 and (B) when time is fixed at 96 h. Yellow regions indicate the maximum predicted yield.
With a concentration of starting material 24 0.5 M, 10.0 equiv of cyanamide, and 96 h of reaction time, the conversion was improved to 95%, and the final product 1 was obtained on a small scale (0.08 mmol) with 86% yield and 89% HPLC purity (Table 5, entry 1). Upscaling the reaction to a 0.77 mmol scale, we noticed a drop in conversion and therefore in yield and purity. An increase in the equivalents of cyanamide to 15.0 was necessary to maintain 95% conversion of starting material 24 and a purity of final product 1 around 87–88% (Table 5, entries 2 and 3). Further optimization of the reaction conditions identified that the mixture of THF/EtOH (2:1 v/v) was also able to provide a 95% conversion when using 10 equiv of cyanamide (see the Supporting Information). Gratifyingly, when the reaction in THF/EtOH (2:1 v/v) was upscaled from 0.77 to 7.7 mmol, the conversion of aniline 24 to guanidine 1 was kept above 95% without needing to increase the equivalents of cyanamide (Table 5, entries 4 and 5).
Table 5. Medium Optimization for the Direct Guanylation of 24.
| entrya | scale (mmol) | solvent | equiv NH2CN | yield (%)b | purity (%) |
|---|---|---|---|---|---|
| 1 | 0.08 | MeCN/iPrOH 1:1 | 10 | 86 | 89 |
| 2 | 0.77 | MeCN/iPrOH 1:1 | 10 | 77 | 85 |
| 3 | 0.77 | MeCN/iPrOH 1:1 | 15 | 83 | 88 |
| 4 | 0.77 | THF/EtOH 2:1 | 10 | 89 | 91 |
| 5 | 7.7 | THF/EtOH 2:1 | 10 | 90 | 91 |
Sc(OTf)3 (0.1 equiv) as the catalyst, 96 h, 0.5 M.
Isolated yield.
On the 7.7 mmol scale, a direct guanylation of aniline 24 in THF/EtOH (2:1 v/v) with 10 equiv of NH2CN provided 1 in 90.0% yield with 91.0% AN by HPLC. Among the impurities in the final material, we noticed a small presence of monoarylguanidine 25 amounting to 0.4–1.1% RAP by HPLC.
Purification of the Final Compound 1
The first attempt was to crystallize the crude product 1; however, none of the 17 solvents screened were able to yield a pure 1 (see the Supporting Information).
With these results in hand, we focused on different methods of purification. In the work-up of 1, we noted that the antisolvent precipitation in iPrOAc was able to remove part of the impurities generated in the guanylation reaction. We decided to test precipitation with a series of antisolvents to see if it was possible to increase the purity. Crude 1 was dissolved in absolute ethanol, and the solution was added to eight different antisolvents (see the Supporting Information). Among five organic solvents and two aqueous solutions, only iPrOAc and EtOAc were able to slightly increase the HPLC purity by 0.9 and 1.5%, respectively, but not in a sufficient way to reach the 98% purity target.
Last, we investigated the reverse phase chromatography (RP) for the purification of final product 1. After a range of eluents screening, a gradient of premixed MeCN/EtOH (9:1 v/v) and water was selected. Compound 1 was successfully isolated with a C-18 RP column. The purification was tested on a 3.75 g scale obtaining 1 in two fractions, S1 with 98.1% AN by HPLC and S2 with 99.4% AN by HPLC. The pure material was recovered with 79% yield from the crude product, with a total yield of α-aminophosphonate 1 from aniline 24 of 72%.
Conclusions
In summary, an optimized process for the scalable preparation of the α-aminophosphonate UAMC-00050 has been developed (Scheme 5). The Anelli–Montanari protocol using TEMPO as the oxidation catalyst for the synthesis of aldehyde 6 proved to be superior to the DMP oxidation. The yield was increased from 65 to 71%, and the atom economy was improved from 33 to 66%. The key step of the route, the synthesis of α-aminophosphonate 8 by a three-component reaction between aldehyde 6, carbamate 7, and phosphite 3, was optimized. The use of Y(OTf)3 as the catalyst, TFAA as the additive, and THF/MeCN (1:1 v/v) as the reaction medium provided the product 8 in increased yield. For the preparation of product 1, N,N′-di-Boc-1H-pyrazole-1-carboxamidine was substituted with considerably less expensive cyanamide to introduce a guanidine moiety. Smart DoE was used to optimize the conditions for the guanylation step. The use of chlorinated solvents and purification of intermediates by flash chromatography were removed from the process. The only chromatographic purification was done for the final product to reach the target purity >98%. The new process improved the overall yield of compound 1 from 3 to 22% with a total of six steps. The improved route was executed on a multigram scale and is suitable for preclinical batch preparation of UAMC-00050.
Scheme 5. Optimized Synthetic Route to UAMC-00050.
Experimental Section
General
Unless otherwise specified, all commercially available reagents were used as received. 1H-, 13C-, and 31P-NMR spectra were obtained on a 400 MHz Bruker Avance 400 spectrometer at ambient temperatures at 400, 101, and 162 MHz, respectively. Chemical shifts (δ) are reported in parts per million (ppm) relative to a residual DMSO peak (s, δ 2.50 for 1H and t, δ 39.53 for 13C); for 31P-NMR, it was calibrated with the use of an external standard (H3PO4). Multiplicities are given as s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Complex splittings are described by a combination of these abbreviations, i.e., dd (doublet of doublets). Reaction conversion was estimated by LC–MS on a Waters Acquity UPLC H-class instrument, column Waters Acquity UPLC BEH-C18, 2.1 × 50 mm, 1.7 μm, eluent 5–95% MeCN in 0.1% aq. HCOOH; flow rate: 0.8 mL/min; detection Waters PDA Detector (200–300 nm). HPLC was recorded with a Waters Alliance instrument equipped with a 2695 separations module, consisting of a quaternary pump, degasser, autosampler, and column heater, and a Waters 2489 dual wavelength absorbance detector was used for detection of analytes or Shimadzu Prominence-I LC-2030C, column prevail organic acid or Apollo C18-13, 4.6 × 150 mm, eluent 25–95% or 40–95% MeCN in 0.1% aq. H3PO4: flow rate: 1.0 mL/min, temperature = 40 °C, detector at 254 nm. HRMS spectra were acquired on an electrospray ionization mass spectrometer with a TOF analyzer using the following parameters: positive ionization mode, drying gas (10 mL/min), 325 °C, and fragment or ionization (100 V).
Tris(4-acetamidophenyl) Phosphite (3)
To a dry 500 mL flask equipped with a magnetic stirrer were added, under argon, paracetamol (2) (10.00 g, 0.132 mol, 3.0 equiv) (water content <0.030%), previously dried in vacuum for 24 h, dry-THF (100 mL) (water content <0.005%), and dry-triethylamine (9.20 mL, 0.132 mol, 3.0 equiv) (water content <0.04%), the flask was placed in an ice bath, and after 10 min, phosphorus trichloride (1.92 mL, 0.044 mol, 1.0 equiv) was added dropwise. The mixture was stirred for 1 h at 0 °C and then filtered under an argon flow to remove the solid byproduct formed during the reaction. The filtrate cake was washed with dry-THF (50 mL), the liquid was poured into a 500 mL flask, and the solvent was removed under vacuum at 20–25 °C. Once a solid was formed in the flask, it was kept in the vacuum for 6 h to give a white foamy solid. Yield 98%. 92.3% AN by HPLC. HRMS (ESI+): m/z calculated for C24H25N3O6P [M + H]+:482.1481, found 482.1492 1H-NMR: (400 MHz, DMSO-d6) δ: 9.99 (s, 3H), 7.58 (d, J = 8 Hz, 6H), 7.09 (d, J = 8 Hz, 6H), 2.03 (s, 9H) 13C-NMR: (101 MHz, DMSO-d6) δ: 168.14, 146.06, 136.00, 120.75, 120.47, 23.89 31P-NMR: (162 MHz, DMSO-d6) δ: 129.32.
tert-Butyl (4-(2-Hydroxyethyl)phenyl)carbamate (5)
To a 500 mL flask equipped with a magnetic stirrer were added 4-aminophenetyl alcohol (4) (10.0 g, 0.073 mol, 1.0 equiv), ethyl acetate (200 mL), and di-tert-butyldicarbonate (17.50 g, 0.08 mol, 1.1 equiv). The mixture was stirred for 16 h at 20–25 °C (Caution: increase in pressure in the flask), and then, the solvent was removed to give an off-white product. To the flask containing the crude material was added 26 mL of MeCN/MTBE 1:1 v/v, and the mixture was warmed up until complete dissolution of the solid. The solution was left to cool down at 20–25 °C for 1 h; then, 10 mg of pure compound 5 was added. The solid was left at 20–25 °C for 4 h; then, it was filtered and washed with 85 mL of heptane. The filtrate was dried under vacuum (5 mbar) for 16 h to yield a white solid with a yield of 98%. 99.6% AN by HPLC. HRMS (ESI+) m/z calculated for C13H19NO3 [M + Na]+: 260.1263 found 260.1267. 1H-NMR (400 MHz, DMSO-d6) δ: 9.20 (s, 1H), 7.35 (d, J = 8 Hz, 2H), 7.09 (d, J = 8 Hz, 2H), 4.95 (s, 1H), 3.56 (q, J = 8 Hz, 2H), 2.65 (t, J = 8 Hz, 2H,), 1.47 (s, 9H). 13C-NMR (100 MHz, DMSO-d6) δ: 153.29, 137.87, 133.49, 129.40, 118.59, 79.24, 62.83, 38.88, 28.61.
Sodium 2-(4-((tert-Butoxycarbonyl)amino)phenyl)-1-hydroxyethane-1-sulfonate (15)
To a 500 mL flask equipped with a magnetic stirrer were added in this sequence: compound 5 (17.30 g, 0.073 mol, 1.0 equiv) dissolved in ethyl acetate (90 mL), TEMPO (114 mg, 0.73 mmol, 0.01 equiv) dissolved in toluene (90 mL), and then potassium bromide (869 mg, 7.3 mmol, 0.1 equiv) dissolved in NaHCO3 sat. (67 mL). The mixture was vigorously stirred for 10 min in an ice bath, and then, sodium hypochlorite 11–15% (67 mL) was added dropwise in 5 min. The reaction was vigorously stirred for 10 min and then was quenched with sodium thiosulfate 10% (250 mL), the reaction mixture was washed with ethyl acetate (3 × 200 mL), the combined organic layers were then washed with brine (500 mL) and dried with Na2SO4 (250 g), and the solvent was removed by rotary evaporation. The crude aldehyde was then dissolved in ethanol 96% (340 mL) in a 500 mL flask equipped with a magnetic stirrer. Sodium bisulfite (11.71 g, 0.113 mol, 1.5 equiv) dissolved in 20 mL of deionized water was added dropwise in 5 min, and the mixture was stirred for 18 h at 20–25 °C and 1 h at 0 °C. The solid was filtered, washed with cold ethanol 96% (300 mL), and dried in a vacuum (5 mbar) for 16 h to give a white solid with a yield of 80%. 97.8% AN by HPLC. HRMS (ESI−) m/z calculated for C13H18NO6S [M]−: 316.0861, found 316.0855. 1H-NMR (400 MHz, D2O) δ 7.30 (d, J = 2 Hz, 4H), 4.61 (dd, J = 11, 3 Hz, 1H), 4.32 (dd, J = 16, 4 Hz, 1H), 2.88 (dd, J = 12, 12 Hz, 1H), 1.50 (s, 9H). 13C-NMR (100 MHz, D2O) δ: 153.34, 134.15, 130.32, 127.68, 117.93, 82.43, 79.59, 34.45, 25.39.
tert-Butyl (4-(2-Oxoethyl)phenyl)carbamate (6)
To a 500 mL flask were added compound 15 (19.68 g, 0.085 mol, 1.0 equiv) dissolved in deionized water (260 mL), sodium carbonate (17.20 g, 0.162 mol, 2.2 equiv), and ethyl acetate (300 mL), and the mixture was stirred for 3 h at 20–25 °C. Then, the mixture was placed in a 1.0 L separation funnel and extracted with ethyl acetate (3 × 250 mL), the combined organic layers were washed with brine (400 mL) and dried on Na2SO4 (250 g), and the solvent was removed with vacuum to get a pale yellow solid with a yield of 89%. 99.0% AN by HPLC. HRMS (ESI+) m/z calculated for C13H17NO3Na [M + Na]+: 258.1106, found 258.1115. 1H-NMR (400 MHz, DMSO-d6) δ: 9.63 (t, J = 4 Hz, 1H), 9.32 (s, 1H), 7.43 (d, J = 8 Hz, 2H), 7.11 (d, J = 8 Hz, 2H), 3.66 (d, J = 4 Hz, 2H), 1.47 (s, 9H). 13C-NMR (101 MHz, DMSO-d6) δ: 200.52, 152.80, 138.44, 129.91, 126.04, 118.39, 78.99, 48.95, 28.13.
tert-Butyl(4-(2-(((benzyloxy)carbonyl)amino)2(bis(4acetamidophenoxy)phosphoryl)ethyl)phenyl)carbamate (8)
To a 500 mL flask equipped with a magnetic stirrer were added, under argon, compound 6 (10.0 g, 0.043 mol, 1.0 equiv), yttrium triflate (2.30 g, 4.3 mmol, 0.1 equiv) dissolved in dry-MeCN (130.0 mL) (water content <0.001%), benzyl carbamate (7) (6.50 g, 0.043 mol, 1.0 equiv), tris(4-acetamidophenyl)phosphite (3) (23.00 g, 0.043 mol, 1.0 equiv), dry-THF (130.0 mL) (water content <0.005%), and trifluoroacetic anhydride (5.98 mL, 0.043 mol, 1.0 equiv). The mixture was stirred for 4 h at 20–25 °C. The solvent was removed, and the residue was dissolved in a solution of ethyl acetate/ethanol (4:1 v/v) (500 mL). The organic phase was washed with NaOH 0.5 M (4 × 500 mL) and brine (500 mL). The organic layers were collected together and dried on Na2SO4 (300 g), and the solvent was removed with vacuum. A silica pad with silica gel (200 g) was packed in a 500 mL glass filter. The residue was dissolved in ethyl acetate/ethanol (3:1 v/v), celite (20 g) was added, and the solvent was removed. The solid mixture was placed on top of the filter and washed with ethyl acetate/heptane (2:1 v/v) (2000 mL) to collect fraction 1, the collection flask was changed, the silica pad was washed with ethyl acetate/ethanol (3:1 v/v) (1000 mL) to collect fraction 2, and the solvent was removed from fraction 2 to give a yellow foamy solid. The crude product was dissolved in acetone (100 mL), and a solution of NaHCO3 0.5% (200 mL) was added dropwise. The solid was filtered, washed with MTBE (100 mL), and dried in vacuum overnight. The dry solid was suspended in ethyl acetate/acetone (19:1 v/v) (300 mL), stirred for 24 h, filtered, washed with ethyl acetate (100 mL), and dried in vacuum (5 mbar) overnight to get a white solid with a yield of 44%. 98.2% AN by HPLC. HRMS (ESI+): m/z calculated for C37H41N4O9PNa [M + Na]+: 739.2509, found 739.2519. 1H-NMR (400 MHz, DMSO-d6) δ: 10.00 (s, 2H), 9.32 (s, 1H), 8.11 (d, J = 8 Hz, 1H), 7.57 ( m, 4H), 7.39 (d, J = 8 Hz, 2H,), 7.30 (m, 3H), 7.19 (d, J = 8 Hz, 2H), 7.12 (m, 6H), 4.97 (dd, J = 32, 12 Hz, 2H), 4.42 (q, J = 12 Hz, 1H), 3.18 (d, J = 12 Hz, 1H), 2.90 (m, 1H), 2.04 (s, 6H), 1.49 (s, 9H). 13C-NMR: (101 MHz, DMSO-d6) δ 168.70, 156.39, 153.27, 145.74, 145.46, 138.60, 137.45, 137.01, 130.92, 129.83, 128.71, 128.02, 127.59, 121.29, 121.03, 120.62, 118.36, 79.40, 65.87, 50.51, 34.04, 28.62, 24.37. 31P-NMR: (162 MHz, DMSO-d6) δ: 18.35.
4-(2-(((Benzyloxy)carbonyl)amino)-2-(bis(4-acetamidophenoxy)phosphoryl)ethyl)benzenaminium Chloride (24)
In a 500 mL flask equipped with a magnetic stirrer were added in this sequence: compound 8 (10.00 g, 0.014 mol) and 4 N HCl in dioxane (150 mL), and the solution was stirred for 3 h at 20–25 °C (Caution: increase in pressure in the flask). The solvent was removed with vacuum, then the residue was dissolved in absolute EtOH (100 mL) (water content <0.005%), and the solution was added dropwise to EtOAc (1000 mL). The mixture was stirred for 30 min at 20–25 °C, and the precipitate was filtered, washed with EtOAc (100 mL), and dried in vacuum (5 mbar) overnight. An off-white powder was obtained with a yield of 99%. 97.9% AN by HPLC. HRMS (ESI+) m/z calculated for C32H34N4O7P [M + H]+: 617.2165, found 617.2175 1H-NMR (400 MHz, DMSO-d6) δ: 10.09 (d, J = 4 Hz, 2H), 9.76 (s, 2H), 8.17 (d, J = 12 Hz, 1H), 7.658 (m, 4H), 7.38–7.28 (m, 5H), 7.21 (t, J = 8 Hz, 5H), 7.09 (m, 4H), 4.96 (dd, J = 20, 12 Hz, 2H), 4.45 (m, 1H), 3.25 (m, 1H), 2.99 (m, 1H), 2.03 (s, 6H). 13C-NMR (100 MHz, DMSO-d6) δ: 168.70, 156.35, 145.67, 145.40, 137.28, 137.07, 130.81, 128.82, 128.25, 127.84, 122.26, 121.24, 120.97, 120.62, 66.06, 50.29, 34.14, 31.17, 24.37. 31P-NMR (162 MHz, DMSO-d6) δ: 18.00.
1-(4-(2-(((Benzyloxy)carbonyl)amino)-2-(bis(4-acetamidophenoxy)phosphoryl)ethyl)phenyl) Guanidinium Chloride (1)
In a 50 mL flask, flushed with argon were added compound 24 (5.00 g, 7.7 mmol, 1.0 equiv), Sc(OTf)3 (377 mg, 0.77 mmol, 0.1 equiv) dissolved in 15.4 mL of dry-THF/abs-EtOH (2:1 v/v) (water content THF and EtOH <0.005%), and cyanamide (3.23 g, 77.0 mmol, 10.0 equiv). The solution was left to stir for 96 h, then the solvent was removed, and the crude product was dissolved in absolute EtOH (50 mL) (water content <0.005%), dropped in iPrOAc (500 mL) at r.t., and stirred for 1 h. The solid was filtered, washed with iPrOAc (250 mL), and dried in vacuum overnight. The crude material was dissolved in deionized water/EtOH (10:1 v/v) (500 mL) and pre-loaded on a 300 g YMC-DispoPack AT reverse phase column. The column was eluted with deionized water/(MeCN/EtOH 9:1 v/v) gradient 0–100%. The solid was collected from the selected tubes, and the solvent was removed with freeze-drying to get product 1: fraction 1 (S1) 98.1% AN by HPLC, fraction 2 (S2) 99.4% AN by HPLC, and a yield of 72%. HRMS (ESI+) m/z calculated for C33H37N6O7P [M + H]+: 659.2383, found 659.2398 1H-NMR (400 MHz, DMSO-d6) δ: 10.04 (s, 2H), 8.44 (s, 1H), 8.18 (d, J = 12 Hz, 1H), 7.97 (s, 3H), 7.57 (m, 4H), 7.35 (d, J = 8 Hz, 2H), 7.29 (m, 3H), 7.19 (m, 2H), 7.10 (m, 6H), 4.97 (dd, J = 20, 12 Hz, 2H), 4.46 (q, J = 8 Hz, 1H), 3.22 (m, 1H), 3.00 (m, 1H), 2.03 (s, 6H). 13C-NMR (100 MHz, DMSO-d6) δ: 168.72, 168.37, 156.60, 156.31, 145.70, 145.44, 137.29, 137.09, 135.34, 135.03, 134.85, 130.72, 128.77, 128.24, 127.90, 123.93, 121.26, 120.99, 120.64, 66.08, 50.19, 34.01, 24.37. 31P-NMR (162 MHz, DMSO-d6) δ: 17.39.
Acknowledgments
The authors would like to thank Emma Sarule, Hele̅na Kažoka, Ludmila Gridṅeva, Marina Gosteva, Toms Upmanis, Marina Petrova, Solveiga Gri̅nberga, Dace Hartmane, Vale̅rija Križanovska, Eduards Sevostjanovs, and Baiba Guka̅lova for analytical support. Rossella Castagna, Andrejs Pelšs, Giulio Mattedi, and Aigars Jirgensons are gratefully acknowledged for the revision of the manuscript.
Glossary
ABBREVIATIONS
- Boc
tert-butyloxycarbonyl protecting group
- DED
dry eye disease
- UA
University of Antwerp
- DMP
Dess–Martin periodinane
- Fmoc
fluorenylmethoxycarbonyl
- RSM
response surface model
- TEMPO
(2,2,6,6-tetramethylpiperidin-1-yl)oxyl
- TFAA
trifluoroacetic anhydride
- TFA
trifluoroacetic acid
- uPA
urokinase plasminogen activator
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.oprd.2c00244.
Preparation of aldehydes, supplementary screening, DoE experimental data, HPLC chromatograms, and 1H-, 13C-, and 31P-NMR spectra (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Initial Training Network (ITN) “ITDED3” (H2020-MSCA-ITN-2017) grant agreement no. 765608.
The authors declare no competing financial interest.
Supplementary Material
References
- Craig J. P.; Nichols K. K.; Akpek E. K.; Caffery B.; Dua H. S.; Joo C.-K.; Liu Z.; Nelson J. D.; Nichols J. J.; Tsubota K.; Stapleton F. TFOS DEWS II Definition and Classification Report. The Ocular Surface. 2017, 15, 276–283. 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Stapleton F.; Alves M.; Bunya V. Y.; Jalbert I.; Lekhanont K.; Malet F.; Na K.-S.; Schaumberg D.; Uchino M.; Vehof J.; Viso E.; Vitale S.; Jones L. TFOS DEWS II Epidemiology Report. The Ocular Surface. 2017, 15, 334–365. 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Paulsen A. J.; Cruickshanks K. J.; Fischer M. E.; Huang G.-H.; Klein B. E. K.; Klein R.; Dalton D. S. Dry Eye in the Beaver Dam Offspring Study: Prevalence, Risk Factors, and Health-Related Quality of Life. American Journal of Ophthalmology. 2014, 157, 799–806. 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joossens J.; Van der Veken P.; Surpateanu G.; Lambeir A.-M.; El-Sayed I.; Ali O. M.; Augustyns K.; Haemers A. Diphenyl Phosphonate Inhibitors for the Urokinase-Type Plasminogen Activator: Optimization of the P4 Position. J. Med. Chem. 2006, 49, 5785–5793. 10.1021/jm060622g. [DOI] [PubMed] [Google Scholar]
- Joossens J.; Ali O. M.; El-Sayed I.; Surpateanu G.; Van der Veken P.; Lambeir A.-M.; Setyono-Han B.; Foekens J. A.; Schneider A.; Schmalix W.; Haemers A.; Augustyns K. Small, Potent, and Selective Diaryl Phosphonate Inhibitors for Urokinase-Type Plasminogen Activator with In Vivo Antimetastatic Properties. J. Med. Chem. 2007, 50, 6638–6646. 10.1021/jm700962j. [DOI] [PubMed] [Google Scholar]
- Joossen C.; Baán A.; Moreno-Cinos C.; Joossens J.; Cools N.; Lanckacker E.; Moons L.; Lemmens K.; Lambeir A.-M.; Fransen E.; Delputte P.; Caljon G.; Van Der Veken P.; Maes L.; De Meester I.; Kiekens F.; Augustyns K.; Cos P. A Novel Serine Protease Inhibitor as Potential Treatment for Dry Eye Syndrome and Ocular Inflammation. Sci. Rep. 2020, 10, 17268. 10.1038/s41598-020-74159-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksyszyn J.; Subotkowska L.; Mastalerz P. Diphenyl 1-Aminoalkanephosphonates. Synthesis 1979, 12, 985–986. [Google Scholar]
- Birum G. H. Urylenediphosphonates. General Method for the Synthesis of.Alpha.-Ureidophosphonates and Related Structures. J. Org. Chem. 1974, 39, 209–213. 10.1021/jo00916a019. [DOI] [Google Scholar]
- der Veken P. V.; Sayed I. E.; Joossens J.; Stevens C. V.; Augustyns K.; Haemers A. Lewis Acid Catalyzed Synthesis of N-Protected Diphenyl 1-Aminoalkylphosphonates. Synthesis 2005, 4, 634–638. [Google Scholar]
- Ceradini D.; Shubin K. One-Pot Synthesis of α-Aminophosphonates by Yttrium-Catalyzed Birum–Oleksyszyn Reaction. RSC Adv. 2021, 11, 39147–39152. 10.1039/D1RA07718J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover J. M.; Steves J. E.; Stahl S. S. Copper(I)/TEMPO-Catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes with Ambient Air. Nat. Protoc. 2012, 7, 1161–1166. 10.1038/nprot.2012.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton P. D.; Whealon M. D.; Roberts L. M.; Yaeger A. A.; Boydson R. Continuous Organic Synthesis in a Spinning Tube-in-Tube Reactor: TEMPO-Catalyzed Oxidation of Alcohols by Hypochlorite. Org. Process Res. Dev. 2008, 12, 946–949. 10.1021/op800051t. [DOI] [Google Scholar]
- Pulido D.; Casadó-Anguera V.; Pérez-Benito L.; Moreno E.; Cordomí A.; López L.; Cortés A.; Ferré S.; Pardo L.; Casadó V.; Royo M. Design of a True Bivalent Ligand with Picomolar Binding Affinity for a G Protein-Coupled Receptor Homodimer. J. Med. Chem. 2018, 61, 9335–9346. 10.1021/acs.jmedchem.8b01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z.; Wang R. Oxidation of Alcohols Using H2O2 as Oxidant Catalyzed by AlCl3. Catal. Commun. 2008, 9, 740–742. 10.1016/j.catcom.2007.08.012. [DOI] [Google Scholar]
- Moriyama K.; Takemura M.; Togo H. Selective Oxidation of Alcohols with Alkali Metal Bromides as Bromide Catalysts: Experimental Study of the Reaction Mechanism. J. Org. Chem. 2014, 79, 6094–6104. 10.1021/jo5008064. [DOI] [PubMed] [Google Scholar]
- Dong Z.; Gao P.; Xiao Y. Efficient Selective Oxidation of Alcohols to Aldehydes Catalyzed by a Morpholinone Nitroxide. Synth. Commun. 2019, 49, 3380–3388. 10.1080/00397911.2019.1666284. [DOI] [Google Scholar]
- Lucio Anelli P.; Biffi C.; Montanari F.; Quici S. Fast and Selective Oxidation of Primary Alcohols to Aldehydes or to Carboxylic Acids and of Secondary Alcohols to Ketones Mediated by Oxoammonium Salts under Two-Phase Conditions. J. Org. Chem. 1987, 52, 2559–2562. 10.1021/jo00388a038. [DOI] [Google Scholar]
- Furigay M. H.; Boucher M. M.; Mizgier N. A.; Brindle C. S. Separation of Aldehydes and Reactive Ketones from Mixtures Using a Bisulfite Extraction Protocol. J. Visualized Exp. 2018, 134, e57639. 10.3791/57639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev M. E.; Ragulin V. V. New Opinions on the Amidoalkylation of Hydrophosphorylic Compounds. Tetrahedron Lett. 2010, 51, 2613–2616. 10.1016/j.tetlet.2010.03.020. [DOI] [Google Scholar]
- Dmitriev M. E.; Rossinets E. A.; Ragulin V. V. Amidoalkylation of Hydrophosphoryl Compounds. Russ. J. Gen. Chem. 2011, 81, 1092–1104. 10.1134/S1070363211060041. [DOI] [Google Scholar]
- Dmitriev M. E.; Ragulin V. V. Arbuzov-Type Reaction of Acylphosphonites and N-Alkoxycarbonylimine Cations Generated in Situ with Trifluoroacetic Anhydride. Tetrahedron Lett. 2012, 53, 1634–1636. 10.1016/j.tetlet.2012.01.094. [DOI] [Google Scholar]
- Vinyukov A. V.; Dmitriev M. E.; Ragulin V. V. One-Pot Synthesis of N-Cbz-α-Aminophosphonic Acids. Phosphorus, Sulfur Silicon Relat. Elem. 2017, 192, 437–441. 10.1080/10426507.2016.1248289. [DOI] [Google Scholar]
- Welch C. J.; Leonard W. R.; Henderson D. W.; Dorner B.; Childers K. G.; Chung J. Y. L.; Hartner F. W.; Albaneze-Walker J.; Sajonz P. Adsorbent Screening Using Microplate Spectroscopy for Selective Removal of Colored Impurities from Active Pharmaceutical Intermediates. Org. Process Res. Dev. 2008, 12, 81–87. 10.1021/op700191z. [DOI] [Google Scholar]
- Crini G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085. 10.1016/j.biortech.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Tsubokura K.; Iwata T.; Taichi M.; Kurbangalieva A.; Fukase K.; Nakao Y.; Tanaka K. Direct Guanylation of Amino Groups by Cyanamide in Water: Catalytic Generation and Activation of Unsubstituted Carbodiimide by Scandium(III) Triflate. Synlett 2014, 25, 1302–1306. 10.1055/s-0033-1341080. [DOI] [Google Scholar]
- Diab S.; Teo T.; Kumarasiri M.; Li P.; Yu M.; Lam F.; Basnet S. K. C.; Sykes M. J.; Albrecht H.; Milne R.; Wang S. Discovery of 5-(2-(Phenylamino)Pyrimidin-4-Yl)Thiazol-2(3H)-One Derivatives as Potent Mnk2 Inhibitors: Synthesis, SAR Analysis and Biological Evaluation. ChemMedChem 2014, 9, 962–972. 10.1002/cmdc.201300552. [DOI] [PubMed] [Google Scholar]
- Kondraganti L.; Manabolu S. B.; Dittakavi R. Synthesis of Benzimidazoles evia Domino Intra and Intermolecular C-N Cross-Coupling Reaction. ChemistrySelect 2018, 3, 11744–11748. 10.1002/slct.201802754. [DOI] [Google Scholar]
- An T.; Kang B.; Kang S.; Pac J.; Youk J.; Lin D.; Lee Y. Guanidine Cyclic Diimides and Their Polymers. Chem. Commun. 2019, 55, 10222–10225. 10.1039/C9CC04522H. [DOI] [PubMed] [Google Scholar]
- Kim K.; Lin Y.-T.; Mosher H. S. Monosubstituted Guanidines from Primary Amines and Aminoiminomethanesulfonic Acid. Tetrahedron Lett. 1988, 29, 3183–3186. 10.1016/0040-4039(88)85116-5. [DOI] [Google Scholar]
- Armitage I.; Fu M.; Hicks F.; Kattuboina A.; Li J. S. N.; McCarron A.; Zhu L. The Use of Chloroformamidine Hydrochloride as a Reagent for the Synthesis of Guanidines from Electron Deficient Aromatic Amines. J. Heterocyclic Chem. 2017, 54, 728–734. 10.1002/jhet.2567. [DOI] [Google Scholar]
- GoswamiShyamaprosad; HazraAnita; JanaSubrata One-Pot Two-Step Solvent-Free Rapid and Clean Synthesis of 2-(Substituted Amino)Pyrimidines by Microwave Irradiation. Bull. Chem. Soc. Jpn. 2009, 82, 1175–1181. [Google Scholar]
- https://www.x-t.ai/xt-saam/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.