Abstract
Objective
To assess the feasibility of the procedures of EPI-ASTHMA. EPI-ASTHMA is a population-based multicentre stepwise study about the prevalence and characterisation of patients with asthma based on disease severity in Portugal.
Methods
A pilot study of EPI-ASTHMA was conducted with adults from three primary care centres. We followed a stepwise approach comprising 4 stages: stage 0—invitation phone call (n ~1316); stage 1—telephone interview (n ~658); stage 2—clinical assessment with physical examination, diagnostic tests, and patient-reported outcome measures, to confirm the diagnosis of those with possible asthma at stage 1 (n ~160); stage 3—characterization of a subgroup of asthma patients by collecting data through a telephone interview, patient file review and CARATm app (n ~40), after 3-months. The frequency of asthma was calculated in relation to the entire study population (stage 1) and the frequency of difficult-to-treat/severe asthma in relation to the number of asthma patients (stage 3).
Results
From 1305 adults invited, 892 (68%) accepted to participate (stage 0) and 574 (64%; 53[42–67] y; 43% male) were interviewed (stage 1). From those, 148 (26%; 60[46–68] y; 43% male) were assessed at stage 2, and 46 (31%; 51[39–67] y; 44% male) were diagnosed with asthma. Half of these patients (n = 23) accepted to install the app. Stage 3 was completed by 41 (93%) patients, of whom 31 (83%) had asthma confirmed by their file review. A total of 8% of participants had asthma, of those 17% had difficult-to-treat and 5% severe asthma.
Conclusion
Attained recruitment rates and the quality of the results confirmed the feasibility of the EPI-ASTHMA stepwise approach. This pilot study provided insight into the improvement of the procedures to be generalized across the country.
Keywords: asthma, prevalence, epidemiology, severe asthma, difficult-to-treat asthma
Introduction
Asthma is among all chronic respiratory diseases, the second leading cause of morbidity and death, estimated to affect 5 to 10% of people worldwide.1 In relation to asthma severity, it has been estimated that up to 17% of patients have difficult-to-treat asthma and 3.7% have severe asthma.2 Both asthma subgroups account for a large proportion of the morbidity, mortality, and health care costs associated with asthma. However, these estimates may not represent the prevalence of asthma and its subgroups in Portugal as significant evidence exists on the wide variation across countries and regions.3
To date, the majority of the Portuguese epidemiological studies used non-standardised questionnaires applied to limited age groups which are subject to recall bias limitations inherent from self-reported health measurements.4 In 2011, the Portuguese National Asthma Survey, based on telephone interviews, indicated a disease prevalence of 6.8%,5 whereas previous studies have estimated a prevalence of 3.3–15%.6 Similarly, to the US and Denmark National Asthma Surveys, the 2005–2006 Portuguese Health Survey, also suggested an underdiagnosis of asthma, particularly in the male population and specifically in the country’s southern regions.6 Asthma overdiagnosis can also be of concern,7 although asthma diagnostic accuracy has been previously addressed in a study conducted in Portugal that concluded that the asthma diagnoses made by the family physicians in the Portuguese Sentinel Practice Network were accurate in 84% of all reported cases with reference to the diagnostic criteria used.8 Based on these facts, the Portuguese National Program for Respiratory Diseases maintains strategic research objectives in epidemiological surveillance both in asthma prevalence and its correct diagnosis,9 which is in line with the recommendations from the Global Initiative for Asthma (GINA).10
The prevalence of disease severity in Portugal is yet to be investigated. Difficult-to-treat asthma is an uncontrolled asthma that requires GINA Step 4 and 5 treatment to obtain symptom control and reduce exacerbation risk.10 In many cases, difficult-to-treat asthma is attributed to modifiable factors including, incorrect inhaler technique, poor adherence, smoking and other comorbidities. Alternatively, severe asthma is a subset of difficult-to-treat asthma uncontrolled despite maximal optimized therapy (GINA Step 4 and 5) adherence, treatment and other contributory factors that worsen after high-dose treatment is decreased.10 Both difficult-to-treat and severe asthma patients are more likely to experience life-threatening exacerbations and consume additional healthcare resources. But while difficult-to-treat asthma can be managed by a structured approach aiming to identify and treat modifiable factors, severe asthma may need additional targeted treatments and referral to specialist asthma centers.11–13 For that reason, it is important to differentiate between difficult-to-treat asthma and severe asthma, as the former might be manageable in primary health care, whereas the latter requires a specialized second or tertiary care team approach.14 Further knowledge on the distribution of severity and characteristics of patients in each group is needed to better support clinical management of the disease and inform personalized health policies for a smarter allocation of the needed healthcare resources.
To provide a more complete picture of the prevalence of asthma, difficult-to-treat and severe asthma in Portugal and to better understand patient’s characteristics, treatment profile and healthcare resources use, we designed EPI-ASTHMA, a population-based multicentre stepwise study.15 The stepwise approach, divided in 4 stages, aimed to simulate the clinical practice diagnostic pathway, which commonly initiates with the evaluation of symptom patterns and clinical features, and then evolves to a clinical decision by using different diagnostic tests. EPI-ASTHMA combines different data collection methods, including screening interviews, self-reported questionnaires, diagnostic tests, clinical interview with physical examination and access to the patient’s electronic health record (EHR). Also, considering that electronic sharing of information between patients and healthcare professionals using interoperable digital technologies effectively engages patients in the self-management of their disease and improves health outcomes, we also incorporated the use of a mobile Health (mHealth) solution for completeness of data collection. Thus, the aim of this study was to pilot test the feasibility of all the procedures of the EPI-ASTHMA study before being extended nationwide.
Materials and Methods
Study Design and Participants
This is a pilot study of the EPI-ASTHMA population-based, nationwide, prevalence protocol. This pilot study was conducted during May–October 2021 in three primary health care centers of the Matosinhos Local Health Unit (Unidade Local de Saúde de Matosinhos – ULSM), in the North Region of Portugal. The inclusion criteria were individuals, registered in the database of the three participating primary health care centers, who had at least 18 years of age and provided voluntary oral and written informed consent. Exclusion criteria were individuals with any specific physical and/or cognitive disabilities that prevented them from cooperating with the study procedures (eg, lung function tests) and/or understanding/answering questionnaires. The study followed the tenets of the Declaration of Helsinki and was approved by the Ethics Board Committee of the ULSM (38/CES/JAS, March 12, 2021). This study is reported according to STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology Statement: guidelines for reporting observational studies).16
Data Collection
EPI-ASTHMA used a stepwise approach, sequentially comprising 4 stages, that has been described in detail elsewhere.15 A brief summary of each stage is provided below and Table 1 specifies the data collected at each stage.
Table 1.
Brief Summary of the Data Collection Across Each Stage of the Pilot Study
| Stage 1 | Stage 2 | Stage 3 | |
|---|---|---|---|
| Sociodemographic and anthropometric characteristics | × | × | |
| Brief PA assessment | × | × | |
| Respiratory symptoms (wheeze, breathlessness) | × | × | × |
| Diagnosis of chronic respiratory disease | × | × | × |
| Comorbidities and allergies | × | × | × |
| Smoking habits and ETS | × | × | |
| A2 Score | × | ||
| Inhaler prescription/use | × | ||
| Signs of asthma | × | × | |
| CARAT | × | × | |
| Asthma pharmacological treatment | × | ×* | |
| Inhalation technique | × | ||
| Adherence to inhaled medication | × | ×* | |
| Other treatments | × | × | |
| Number of exacerbations | × | ×* | |
| Number of hospital admissions and length of hospital stay | × | ×* | |
| Number of unscheduled consultations | × | ×* | |
| Referral for specialist care | × | ×* | |
| Standard measurements (blood pressure, height, weight) | × | ||
| Pre-BD and post-BD lung function | × | ||
| Pulmonary diagnostic tests (previously performed) | × | ||
| FeNO | × | ||
| Peripheral blood eosinophil and neutrophil counts | × | ||
| Use of health and fitness apps | × | × | |
| CARATm app use and opinion | × |
Notes: *In the previous 3 and 12 months.
Stage 0—Study Enrolment
Telephone call invitation for enrolment of participants fulfilling the eligibility criteria, around 1316 participants were expected to be included. Invitations were conducted using an unstructured guide by 3 pre-trained clinical secretaries.
Stage 1—Screening Interview
Telephone screening interview to assess respiratory symptoms and confirmation of eligibility criteria. Around 658 participants were expected to be included. Interviews were performed by a team of experienced interviewers using a Computer Assisted Telephone Interviews system (CATI). Participants were considered eligible if they had at least one positive response in the Adult Asthma Epidemiological Score (A2 score),17 in that case they were invited to participate in stage 2 and the diagnostic visit was scheduled. A2 score comprises 8 questions (including “Did a physician confirm you had asthma?”) with very good properties to rule in/rule out asthma. This validated screening tool is used in adult asthma epidemiological studies and clinical screening/triage settings.17 Each interview lasted around 15 minutes.
Stage 2—Diagnostic Visit
Stage 2 include clinical assessment, diagnostic confirmation and patient characterization. At this stage, we expected to include around 160 (~20% of the eligible participants and for quality control reasons an additional 5–10% of those considered not eligible). Participants were clinically assessed in a mobile outpatient clinic, parked nearby two of the three primary health care centers and 2 km away from the third center. Thirteen general practitioners (GP), four clinical physiologists and one clinical researcher participated at this stage, after receiving on-site training on the study procedures. Clinical assessment included diagnostic tests, patient-reported outcome measures, and a clinical interview complemented by a physical examination. All participants were provided with a letter addressed to their primary care physician with the results of their clinical evaluation after 2–4 weeks of the visit. Patients with asthma were invited to use the CARATm app during the study (optional). CARATm is a mhealth app developed to collect clinical data from patients with asthma.
Stage 3—Sub-Group Asthma Characterization
Follow-up assessment 3 months after stage 2 for the patients with asthma. At this stage, we expected to include 40 patients. The same 13 physicians from stage 2 were responsible for data collection: i) follow-up telephone call to assess asthma control and symptoms; ii) review of patients’ EHR in the Portuguese National Health Service (NHS) database. In addition, asthma control data extracted from the CARATm app was aimed (only for patients who have used the app).
Selection, Blinding, and Minimization of Bias
Participants were randomly selected based on age (≥18 years) from the NHS database of the three primary care centers using the RAND function from Excel. The IT department of ULSM was responsible for extracting, randomizing and sending the patients list to the clinical secretaries, responsible for stage 0. At stage 1, participants were identified with a unique participant ID. In order to minimize diagnostic bias at stage 2, researchers, data collectors and participants were blinded to patient eligibility at stage 1. Over the stages, data (primary and secondary sources) were collected for a specific electronic case report form (e-CRF). All procedures and documentation were closely monitored to identify any issues during the pilot study. We also combined traditional management methodologies with an “agile” approach.18 The agile project management, originated in software development, is used in a growing number of areas.19 The agile approach is useful in complex projects, that are subject to environmental change and a wide array of uncertainties.19 This flexibility is essential to adjust procedures to the distinct realities encountered in each region/primary care center. Specifically, we use the scrum framework on Jira software, to document and time the duration and dependencies of issues within our study as well as to assign roles and responsibilities to the entire team.
Diagnosis Criteria and Definitions
All patients were given a diagnosis at stage 2. The possible diagnoses were asthma, chronic obstructive pulmonary disease (COPD), asthma-COPD overlap (ACO), other respiratory diseases, undefined diagnosis (when the diagnosis of asthma was dubious) and no respiratory diseases. Asthma diagnosis was determined according to GINA criteria10 at stage 2. Additionally, for quality control reasons, the authors JCS, DB and MJB (GPs) and JAF (allergist) revised the data available for all patients classified with undefined diagnosis (n = 14) by the end of the study.
Underdiagnosis cases were defined as participants that self-reported no previous diagnosis of asthma but were diagnosed at stage 2. Misdiagnosis cases were defined as participants that stated having a prior diagnosis of asthma, but the diagnosis could not be confirmed at stage 2.
Uncontrolled asthma was defined based on poor symptom control (based on Control of Allergic Rhinitis and Asthma Test (CARAT) ≥ 24)20 or one of the following, frequent exacerbations (≤2/year) or serious exacerbations (≤1/year) that required hospitalizations (information from both stages 2 and 3).12
Difficult-to-treat asthma was defined as uncontrolled asthma, despite high-intensity treatment (GINA step treatment 4–5).12 Severe asthma was defined as uncontrolled asthma despite high-intensity treatment (GINA step treatment 4–5),12 and good treatment adherence (visual analogue scale (VAS) ≤ 50)21,22 and good inhaler technique (number of critical errors = [0–1]).23
Data Analysis
The minimum initial sample size estimation for this pilot study was found to be 1316 participants based on an estimated prevalence described elsewhere.15 The characteristics of participants were described with mean and standard deviation (SD) for normally distributed variables; for skewed distributions we used median and interquartile range (IQR; range from 25th to 75th percentile). Categorical variables were described with absolute frequencies, proportions and 95% confidence intervals (95% CI). The frequency of asthma was calculated in relation to the entire study population (stage 1) and the frequency of difficult-to-treat/severe asthma in relation to the number of asthma patients (stage 3). Associations between two categorical variables were compared using a Χ2 test; continuous variables between the two groups were compared with a t-test for independent samples or Mann–Whitney test. All analyses were performed using R (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). A p-value ≤0.05 was considered statistically significant.
Results
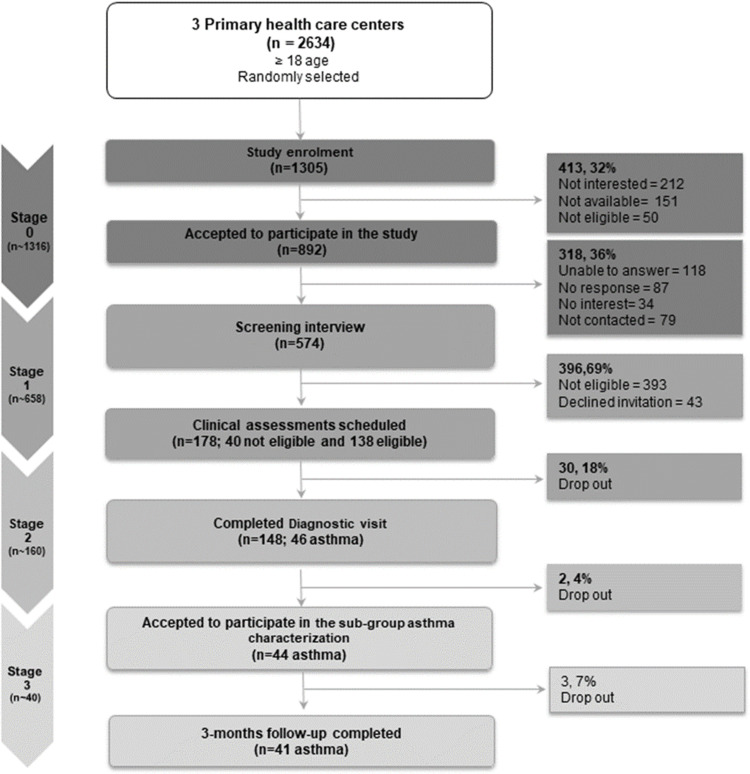
Figure 1 shows the flow of the participants across the study stages. Recruitment targets were overall met in relation to the sample size estimation across all stages: 99% at stage 0 (1305 of 1316); 87% at stage 1 (574 of 658), 93% at stage 2 (148 of 160) and >100% at stage 3 (41 of 40).
Figure 1.
Flow of participants through study.
Stages 0 and 1
From a total of 1305 participants invited at stage 0, 892 (68%; 95% CI 66–70) accepted to participate in the study. During stage 1, 574 (64%; 95% CI 61–67) participants were screened, resulting in 181 (32%; 95% CI 25–38) eligible participants and 40 (10%; 95% CI 8–12) not eligible participants. The demographics of the eligible and not eligible participants are summarized in Table 2. Overall, groups had similar characteristics and only differed with regard to their body mass index (Median (IQR) 26 (23–30) kg/m2 vs 25 (23–29) kg/m2, p = 0.04).
Table 2.
Socio-Demographic and Clinical Characteristics of the Participants at Stage 1
| Total (N = 574) | Eligible (N = 181) | Not Eligible (N = 393) | P-value | |
|---|---|---|---|---|
| Age, median (IQR, range) | 53 (42–67) [18–89] | 50 (39–66) [19–89] | 54 (43–67)a [18–87] | 0.15 |
| Male, N (%) | 246 (42.9) | 69 (38.1) | 177 (45.0)a | 0.13 |
| Marital status, N (%) | 0.36 | |||
| Married/partnership | 353 (61.5) | 109 (60.2) | 244 (62.1) | |
| Single | 115 (20.0) | 39 (21.5) | 76 (19.3) | |
| Divorced/separated | 64 (11.1) | 16 (8.8) | 48 (12.2) | |
| Widowed | 42 (7.3) | 17 (9.4) | 25 (6.4) | |
| Education, grade, N (%) | 0.34 | |||
| ≤9th | 174 (30.3) | 59 (32.6) | 115 (29.3) | |
| 10th–12th | 232 (40.4) | 79 (43.6) | 153 (38.9) | |
| >12th | 168 (29.3) | 43 (23.8) | 125 (31.8) | |
| Occupational status, N (%) | 0.54 | |||
| Employee | 320 (55.7) | 100 (55.2) | 220 (56.0) | |
| Retired | 174 (30.3) | 49 (27.1) | 125 (31.8) | |
| Unemployed | 56 (9.8) | 23 (12.7) | 33 (8.4) | |
| Student | 18 (3.1) | 7 (3.9) | 11 (2.8) | |
| Other | 6 (1.0) | 2 (1.1) | 4 (1.0) | |
| BMI, kg/m2 median (IQR) | 26 (23–29) | 26 (23–30)a | 25 (23–29)b | 0.04* |
| BMI, classification N (%) | 0.09 | |||
| Underweight (<18.5) | 5 (0.9) | 4 (2.2) | 1 (0.3) | |
| Normal weight (18.5–24.9) | 242 (42.2) | 65 (35.9) | 177 (45.0) | |
| Overweight (25–29.9) | 210 (36.6) | 67 (37.0) | 143 (36.4) | |
| Obese (>30) | 114 (19.9) | 44 (24.3) | 70 (17.8) | |
| Insufficiently active, N (%) | 358 (62.4) | 112 (61.9) | 246 (62.6)c | 0.31 |
| Smoking status, N (%) | 0.06 | |||
| Former smoker | 163 (28.4) | 45 (24.9)a | 118 (30.0)b | |
| Current smoker | 125 (21.8) | 50 (27.6) | 75 (19.1) | |
| Never-smoked | 282 (49.1) | 86 (47.5) | 196 (49.9) | |
| ETS, N (%) | 121 (21.1) | 53 (29.3) | 68 (17.3)d | 0.44 |
| Asthma family history, N (%) | 247 (43.0) | 105 (58.0) | 142 (36.1) | 0.43 |
| Self-reported Asthma, N (%) | 95 (16.6) | 95 (16.6) | - | - |
| Self-reported inhaler use, N (%) | 72 (12.5) | 72 (12.5) | - | - |
Notes: *p < 0.05; a1 missing value; b2 missing values; c4 missing values; d5 missing values.
Abbreviations: BMI, body mass index; ETS, environmental tobacco smoke.
Stage 2
At stage 2, 148 (26%; 95% CI 23–29) participants underwent clinical diagnosis; of those 46 (31%; 95% CI 20–42) were diagnosed with asthma. Table 3 shows the demographics and characteristics of the participants with and without asthma. The characteristics were overall similar between groups, but the median (IQR) age of the participants with asthma was lower (51 (39–67) years vs 63 (48–68) years, p = 0.02).
Table 3.
Socio-Demographic and Clinical Characteristics of the Participants at Stage 2
| Total (N = 148) | Asthma (N = 46) | No Asthma (N = 102) | P-value | |
|---|---|---|---|---|
| Age, median (IQR, range) | 60 (46–68) [19–89] | 51 (39–67) [19–82] | 63 (48–68) [19–89] | 0.02* |
| Male, N (%) | 64 (43.2) | 20 (43.4) | 44 (43.1) | 0.31 |
| Marital status, N (%) | 0.46 | |||
| Married/partnership | 105 (71.0) | 29 (63.0) | 76 (74.5) | |
| Single | 20 (13.5) | 9 (19.6) | 11 (10.8) | |
| Divorced/separated | 11 (7.4) | 4 (8.7) | 7 (6.8) | |
| Widowed | 12 (8.1) | 4 (8.7) | 8 (7.8) | |
| Education, grade, N (%) | 0.47 | |||
| ≤9th | 56 (37.8) | 14 (30.4) | 42 (41.2) | |
| 10th–12th | 58 (39.2) | 18 (39.1) | 39 (38.2) | |
| >12th | 34 (23.0) | 14 (30.4) | 20 (19.6) | |
| Occupational status, N (%) | 0.18 | |||
| Employee | 68 (45.9) | 26 (56.5) | 42 (41.1) | |
| Retired | 58 (39.2) | 15 (32.6) | 43 (42.2) | |
| Unemployed | 17 (11.5) | 2 (4.3) | 13 (13.0) | |
| Student | 3 (2.0) | 2 (4.3) | 1 (1.0) | |
| Other | 2 (1.5) | 1 (2.2) | 3 (3.0) | |
| BMI, kg/m2 median (IQR) | 27 (24–30) | 28 (24–30) | 26 (24–30) | 0.48 |
| BMI, classification N (%) | 0.74 | |||
| Underweight (<18.5) | 1 (0.7) | - | 1 (1.0) | |
| Normal weight (18.5–24.9) | 50 (33.8) | 17 (37) | 33 (32.4) | |
| Overweight (25–29.9) | 58 (39.2) | 15 (32.6) | 43 (42.2) | |
| Obese (>30) | 39 (26.4) | 14 (30.4) | 25 (24.5) | |
| Insufficiently active, N (%) | 90 (60.8) | 25 (54.3) | 65 (64.0) | 0.28 |
| Smoking status, N (%) | 0.26 | |||
| Former smoker | 45 (30.4) | 14 (30.4) | 31 (30.3) | |
| Current smoker | 34 (23.0) | 5 (10.9) | 29 (28.4) | |
| Never-smoked | 69 (46.6) | 27 (58.7) | 42 (41.2) | |
| ETS, N (%) | 39 (26.4) | 9 (19.6) | 30 (29.4) | 0.23 |
| Asthma family History, N (%) | 79 (53.4) | 31 (67.4) | 48 (47.0) | 0.39 |
| Comorbidities, N (%) | ||||
| Atopic dermatitis | 19 (12.8) | 6 (13.0) | 13 (12.7) | 0.31 |
| OSA | 10 (6.8) | 2 (4.3) | 8 (7.8) | 0.20 |
| GERD | 39 (26.4) | 9 (19.6) | 30 (29.4) | 0.23 |
| Heart failure | 10 (6.8) | 1 (2.2) | 9 (8.8) | 0.10 |
| Anxiety/depression | 60 (40.5) | 16 (34.8) | 44 (43.1) | 0.26 |
| Others | 98 (66.2) | 31 (67.4) | 67 (66.0) | 0.34 |
| Childhood respiratory symptoms, N (%) | 33 (22.3) | 19 (41.3) | 14 (13.7) | 0.58 |
| FeNO (ppb), N (%)a | <0.01* | |||
| <25 | 87 (58.8) | 14 (30.4) | 73 (71.6) | |
| [25–50] | 41 (27.7) | 20 (43.5) | 21 (20.6) | |
| >50 | 12 (8.1) | 9 (19.6) | 3 (2.9) | |
| Eosinophils, N (%)b | <0.01* | |||
| >0.3 × 109/L | 12 (8.1) | 9 (19.6) | 3 (2.9) | |
| ≤0.3 × 109/L | 88 (59.5) | 25 (54.3) | 63 (61.8) | |
| Spirometry parametersc | ||||
| Pre-bronchodilator, mean (SD) | ||||
| FEV1% predicted | 90 (20.0) | 86 (23.9) | 91 (17.9) | 0.25 |
| FVC % predicted | 90 (20.1) | 91 (24.2) | 90 (16.2) | 0.84 |
| FEV1/FVC (%) | 79 (7.8) | 77 (8.7) | 79 (7.7) | 0.13 |
| Post-bronchodilator, mean (SD) | ||||
| FEV1% predicted | 93 (19.0) | 92 (23.7) | 93 (16.6) | 0.79 |
| FVC % predicted | 92 (18.1) | 93 (24.7) | 91 (14.4) | 0.57 |
| FEV1/FVC (%) | 80 (8.0) | 79 (8.9) | 81 (7.5) | 0.32 |
| Positive bronchodilator response, N (%) | 10 (6.8.) | 6 (13.0) | 4 (3.9) | 0.60 |
Notes: *p<0.05; aParticipant refused test or experienced test fatigue (N = 8); bEquipment failure did not record value (N = 48); cParticipants did not meet the absolute contraindications for performing spirometry (N = 5). Positive bronchodilator response was defined as post-bronchodilator increase of ≥12% and an absolute change of >200 mL from the baseline FEV1.
Abbreviations: BMI, body mass index; ETS, environmental tobacco smoke; OSA, obstructive sleep apnoea; GERD, gastroesophageal reflux disease; FeNO, concentration of exhaled nitric oxide; FEV1, forced exhalation volume in one second; FVC, forced vital capacity.
Regarding those with asthma (n = 46), 22 (48%; 95% CI 36–60) had their disease not controlled (CARAT ≤24), 7 (15%; 95% CI 6–24) had at least 2 exacerbations in the past year and 8(17%; 95% CI 8–26) had at least 1 unscheduled consultation in primary/secondary care, also in the past year. No hospitalizations were reported. Regarding treatment, 6 participants (13%; 95% CI 5–21) were on GINA treatment step 4–5. Seventeen (37%; 95% CI 25–49) participants reported good inhaler treatment adherence (VAS adherence ≤ 50), and a good inhaler technique (Number of critical errors = [0–1]) was observed in 33 (80%; 95% CI 19–41). Regarding the CARATm app, 23 (50%) accepted to install it; these patients were considerably younger than those who refused the app invite (Median (IQR) 45 (34–54) years vs 67 (46–71) years, p < 0.01).
Stage 3
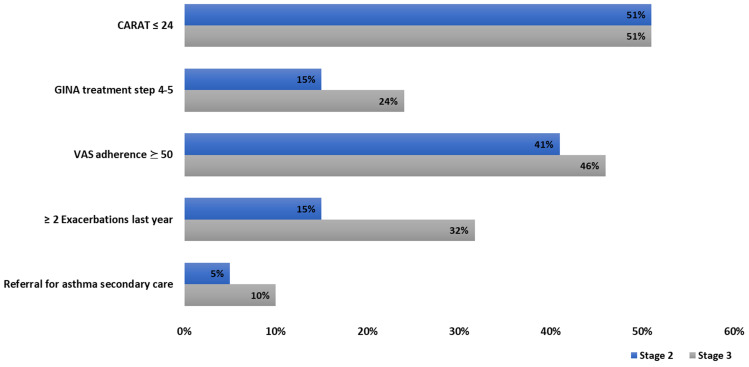
From the proportion of participants with asthma, 44 (96%; 95% CI 91–100) accepted to participate in stage 3 and 41 (52(39–67) years, 44% male) completed the 3-month follow-up. From those patients, 10 (24%; 95% CI 13–35) did not have their asthma diagnosis stated in their patient file. Figure 2 shows the clinical characteristics of these patients. According to patients’ EHR, 24% (95% CI 13–35) of patients were on GINA treatment steps 4–5, but it is of note that this information was missing in 33% (95% CI 21–45) of the participants. Regarding the CARATm app, 7 patients stated to have completed the installation, of those 3 patients reported to continue to use the app, 5 would recommend the app to other patients and 4 reported being willing to include the app in their daily routine.
Figure 2.
Clinical characteristics of patients with asthma, at stage 2 and 3 (N = 41).
Abbreviations: VAS, Visual Analogue Scale; GINA, Global Initiative for Asthma; CARAT, Control of Allergic Rhinitis and Asthma Test.
Proportion of Asthma and of Asthma Subgroups
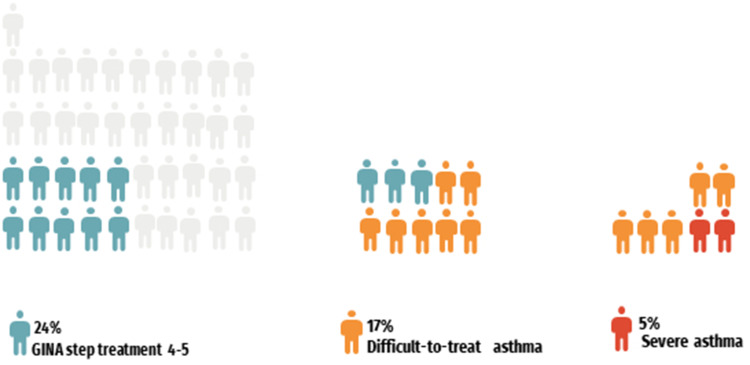
The proportion of asthma in this study was 8% (95% CI 6–10). The frequency of uncontrolled asthma was 61% (95% CI 48–64). The proportion of difficult-to-treat asthma was 17% (95% CI 7–27) based on those who had uncontrolled asthma and were on GINA treatment step 4–5 (10 (24%; 95% CI 13–35)). By considering the patients with difficult-to-treat asthma despite having good treatment adherence and inhaler technique at stage 2 and 3 (9(22%; 95% CI 11–33)), we found a proportion of severe asthma of 5% (95% CI 0–10). Figure 3 shows the proportion of participants per each asthma subgroup.
Figure 3.
Waffle chart with the proportion of asthma patients per subset of asthma severity.
Abbreviation: GINA, Global Initiative for Asthma.
Quality Control
For quality control of the screening interview (stage 1), we also invited 40 (10%; 95% CI 8–13) of the total of the not eligible participants (control population, n = 393) to proceed to stage 2. From those, 1 (2%; 95% CI 0–6) was diagnosed with asthma.
At stage 2, we found that the proportion of underdiagnosed and misdiagnosed asthma cases was 2% (95% CI 0.7–3, n = 10) and 3% (95% CI 2–5, n = 20), respectively.
For quality control of stage 2, we invited the 14 (2%; 95% CI 1–4) cases classified as undefined diagnoses to participate in stage 3. From those, 6 accepted to continue participating. After the 3-month follow-up, 3 of those undefined diagnoses had asthma stated in their patient file, 2 were classified with other respiratory disease and 1 kept an undefined diagnosis. According to the external experts reviewing, from the 14 who were not given a definitive diagnosis, 43% (n=6) had asthma, which would give a total asthma frequency of 8.7% (vs 8%, p = 0.74).
Discussion
This pilot study demonstrated the feasibility of the stepwise approach of EPI-ASTHMA, the first study that will assess the prevalence of asthma, difficult-to-treat, and severe asthma in mainland Portugal. Rates of acceptance to participate (stage 0, 68%) and of participants with respiratory symptoms (stage 1, 32%) were higher than anticipated (50 and 20%, respectively). We found that 8% of participants had asthma, of those 17% had difficult-to-treat and 5% severe asthma. Additionally, the nature and quality of the data collected allowed the characterization of patients with asthma, mainly in terms of severity and control. We believe that our procedures are therefore possible to be generalized to other regions of Portugal.
In our study, from 574 screened participants for respiratory symptoms, 8% were diagnosed with asthma. This proportion was within the estimate of 5 to 10% of people having asthma worldwide.1 A previous study conducted in 2009 in the same municipality found a prevalence of 10.24% (95% CI 8.16 to 12.32) in the overall population (including those under 18).6 This study used a combination of a medically verified asthma diagnosis using medical records and the use of self-reported symptoms by patients.6 With regard to the Portuguese population, our results are in agreement with the most recent population-based, telephone interview survey that showed a prevalence of “Current asthma” of 6.8% (95% CI 6.0–7.7) and of “Lifetime asthma” of 10.5% (95% CI 9.5–11.6).5 Previous studies in Portugal found a prevalence of current asthma ranging from 3% to 15%;6,24–26 nonetheless, in these studies, school-attending children and teenagers from selected cities/regions were enrolled, and in our study, only adults were considered. Therefore, the prevalence of asthma reported in our study is not easily compared to other estimates of asthma prevalence in Portugal due to (1) the differences in the age-groups, time periods and geographical settings studied and (2) the differences in the methods and operational definitions used. Regarding asthma severity, it has been estimated that up to 17% of patients have difficult-to-treat and 3.7% have severe asthma worldwide.2 Despite the proportion of severe asthma being slightly lower than our results, it needs to be noted that we conducted this pilot study in one single municipality of the northern region of Portugal, which may not be representative of the nationwide reality as a significant wide variation across regions and countries has been previously reported.3 Regarding patients’ characteristics, we observed that patients with asthma were younger, had higher levels of FeNO and higher blood eosinophil count in comparison with the other eligible participants. This was somewhat expected as asthma is more common to start in early life, in comparison with other respiratory diseases, such as COPD, which develops from the middle-age onwards. Asthma is also a disease characterized by inflammation of the airways.10 In fact, inflammatory markers, such as blood eosinophil and FeNO, are not only useful to support asthma diagnosis but also to determine response to treatment and underlying severity.27 Half of the patients with asthma had their disease not controlled. These results are not surprising as they are in agreement with previous observational studies conducted with Portuguese patients with asthma.4,28 The knowledge on the distribution of asthma severity and characteristics of patients in each asthma subgroup, after having completed the national picture, will surely better support the clinical management of the disease and will inform health policies for a future and smarter allocation of healthcare resources.
One of the strengths of our study is that it is the first epidemiological study to combine different data collection methods with specific pulmonary function assessment and to also include a mHealth tool to assess and characterize patients with asthma. Whilst we have undoubtedly made progress in addressing important knowledge gaps previously identified, there are, however, a number of limitations that need to be considered. For example, the definition of difficult-to-treat and severe asthma was mainly based on the GINA guidelines for diagnosis and management of difficult-to-treat and severe asthma in adult patients.12 However, the real-world application of those criteria in an observational study brought up some challenges that may be a source of error in the estimation of the prevalence of these subgroups. First, the definition of the GINA treatment steps was based on the medication registered in the patient EHR (stage 3), yet this information was missing in 33% of the participants. Also, even though we assessed asthma control during the study stages 2 and 3 to define uncontrolled disease considering two time-points, these assessments were only 3-months apart instead of 6 months as recommended.12 Comorbidity management during the follow-up period was also not controlled, which is also part of the GINA criteria to distinguish these asthma subgroups.12 This will probably be a source of overestimation of the asthma subgroups, linked to the observational and the real-world nature of this study. Another limitation is that we had a considerable number of interviewers (Stage 1) and GPs (Stage 2 and 3) collaborating in the study, which may be seen as a source of heterogeneity in data collection. Nevertheless, we believe this was mitigated by the fact that both interviewers and physicians were previously trained and continuously supervised by the management team, which was available to answer any doubts on a 24/7 basis. The use of self-report data is a potential criticism; however, discussions on this topic by other authors suggest that so long as data is consistently obtained from the same informant, any biases from such an approach should be minimized.
During this pilot study, we faced a few challenges across all the study stages that should be acknowledged and were extremely helpful to anticipate problems for the next 35 participating centers. The biggest issue from stage 0 was the difficulties regarding the telephone screening interview of patients’ eligibility, which led us to reinforce and refine the confirmation of the eligibility criteria at stage 1. In stage 1, the main challenge was the reduction of interviewer bias as well as proper articulation with the stage 2 team members in terms of scheduling and rescheduling participants, while data collection was ongoing. To ensure the quality of the data collected and the control of interviewer bias during stage 1, all interviewers and the quality of the interviews underwent rigorous daily training and assessment, respectively. The use of a mobile outpatient clinic to conduct the diagnosis visits, in stage 2, also had some shortcomings, mainly related to weather conditions (such as rain, wind, hot temperature) to ensure participants were comfortable and protected and staff had proper working conditions. Additionally, due to spatial limitations for equipment and number of patients served, it prolonged data collection. Half of patients with asthma accepted to install the CARATm app, being frequently younger than those refusing to use. Nevertheless, the persistence of app use was very low (3 patients at 3 months). EPI-ASTHMA will allow a better understanding of patients’ experiences and expectations with respect to the CARATm app, which will inform a better design of the app in the future.
During stage 3 we realized that the review of the patient’s EHR solely generated gaps in data collection, mainly related to lack of medication reports, as some of this information was not accessible in the EHR but only available in the specific database of Medical Electronic Prescription tool, an additional database that we have not previously considered needed. Therefore, for the next centers, whenever information is not found directly on patients’ EHR, this database will be also reviewed. Additionally, some GPs reported the need to consult the patient process on paper, as sometimes diagnostic information was not available in the EHR.
We have combined traditional management methodologies with an “agile” approach18 in which all issues, documentation, timelines and task distributions have been reported. This management combination is expected to fulfill the purpose of making the study adaptable and to anticipate future needs, preventing omissions forgetting and to avoid previous flaws in the future that are important and can have an impact on the outcomes due to the dimension of the study.
Conclusions
Our pilot study demonstrated the feasibility of the stepwise approach of EPI-ASTHMA, the first study that is currently assessing the prevalence of asthma, difficult-to-treat asthma, and severe asthma in Portugal. Furthermore, this pilot study provided insight into the improvement of the study’s procedures to be generalized across the other country regions. Thus, we believe we gather all necessary tools to produce the most comprehensive national study ever undertaken to estimate the prevalence, disease characterization, and healthcare utilization of asthma in Portugal.
Acknowledgments
The authors thank the Sociedade Portuguesa de Alergologia e Imunologia Clínica (SPAIC) for the 1st place poster in Asthma/Rhinitis awarded at the 42nd annual meeting in which part of this work was presented. We would also like to thank all the participants of this pilot study and the Matosinhos Local Health Unit, specifically the primary health care units of Oceanos, Horizonte and Lagoa for hosting and collaborating with us.
Funding Statement
This study was sponsored by AstraZeneca without influence in the preparation of data nor in the writing of the paper. The authors took final responsibility in the decision to submit for publication.
Pilot Study EPI-ASTHMA Group
Ana Noronha, Ana Sá-Sousa; Ana Teresa Frois; Carmo Novais; Catarina João, Catarina Rebelo; Clara Ferreira; Claúdia Bulhões, Cristina Jácome, Diana Gomes; Diana Pacheco; Dinis Brito, Filipa Bernardo, Filipa Lopes, Francisca Amorim; Helena Fonseca; Inês Freitas; Jaime Correia de Sousa, Janete Quelhas-Santos, João Almeida Fonseca, João Sousa; Leonor Carrapatoso; Leonor Duarte; Liliana Amorim, Liliana Silva; Maria Inês Ferreira; Maria João Barbosa, Marisa Pardal, Pedro Costa Dias; Pedro Couto Soares; Pedro Teixeira, Tânia Rodrigues; Tiago Jacinto; Viviana Barreira.
Ethics Approval
The study followed the tenets of the Declaration of Helsinki and was approved by the ethics board Committee of the ULSM (38/CES/JAS, March 12, 2021).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
CJ, CJ, DB, PT, JQS, LA, MJB, CB, and FL have no conflicts of interest to declare. MP and FB are employees of AstraZeneca, Produtos Farmacêuticos SA. JAF declares grants from or research agreements with AstraZeneca, Mundipharma, Sanofi Regeneron and Novartis. Personal fees for lectures and attending advisory boards from AstraZeneca, GSK, Mundipharma, Novartis, Sanofi Regeneron and TEVA. JCS reports Advisory Board from Boehringer Ingelheim, personal fees and Advisory Board from GSK, grants, personal fees and Advisory Board from AstraZeneca, personal fees and Advisory Board from Bial, non-financial support from Mundipharma, personal fees from Sanofi and Advisory Board from Novartis, outside the submitted work.
References
- 1.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 2.Narasimhan K. Difficult to treat and severe asthma: management strategies. Am Fam Physician. 2021;103(5):286–290. [PubMed] [Google Scholar]
- 3.Asher MI, García-Marcos L, Pearce NE, Strachan DP. Trends in worldwide asthma prevalence. Eur Respir J. 2020;56(6):2002094. doi: 10.1183/13993003.02094-2020 [DOI] [PubMed] [Google Scholar]
- 4.Sá-Sousa A, Amaral R, Morais-Almeida M, et al. Asthma control in the Portuguese national asthma survey. Pulmonology. 2015;21(4):209–213. [DOI] [PubMed] [Google Scholar]
- 5.Sa-Sousa A, Morais-Almeida M, Azevedo LF, et al. Prevalence of asthma in Portugal – the Portuguese national asthma survey. Clin Transl Allergy. 2012;2(1):15. doi: 10.1186/2045-7022-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sousa JC, Santo ME, Colaço T, Almada-Lobo F, Yaphe J. Asthma in an urban population in Portugal: a prevalence study. BMC Public Health. 2011;11:347. doi: 10.1186/1471-2458-11-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. 2018;198(8):1012–1020. doi: 10.1164/rccm.201804-0682CI [DOI] [PubMed] [Google Scholar]
- 8.Correia de Sousa J, Luciano Silva M, Lobo FA, Yaphe J. Asthma incidence and accuracy of diagnosis in the Portuguese sentinel practice network. Prim Care Respir J. 2010;19(4):352–357. doi: 10.4104/pcrj.2010.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Direcção-Geral da Saúde. Programa Nacional para as Doenças Respiratórias. Lisboa: Direção-Geral da Saúde; 2017. [Google Scholar]
- 10.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma; 2022. [Google Scholar]
- 11.Yılmaz I. Confusing terminology: difficult asthma, difficult-to-treat asthma, difficult-to-control asthma, therapy-resistant asthma, severe asthma, and refractory asthma. Which one is truly severe asthma? Turk Thorac J. 2018;19(4):235–236. doi: 10.5152/TurkThoracJ.2018.18026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Initiative for Asthma. GINA difficult-to-treat & severe asthma in adolescent and adult patients diagnosis and management: a GINA pocket guide for health professionals v2.0; 2019.
- 13.Yoo J, Meyers J, Reddel H. Difficult-to-treat and severe asthma in adults: towards a new treatment paradigm. Aust J Gen Pract. 2019;48(4):188–192. doi: 10.31128/AJGP-10-18-4750 [DOI] [PubMed] [Google Scholar]
- 14.Ryan D, Murphy A, Ställberg B, Baxter N, Heaney LG. ‘SIMPLES’: a structured primary care approach to adults with difficult asthma. Prim Care Respir J. 2013;22(3):365–373. doi: 10.4104/pcrj.2013.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jácome C, Brito D, João C, et al. EPI-ASTHMA study protocol – a population-based multicentre stepwise study on the prevalence and characterisation of patients with asthma according to disease severity in Portugal. BMJ Open. 2022;12:e064538. doi: 10.1136/bmjopen-2022-064538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sá-Sousa A, Pereira AM, Almeida R, et al. Adult asthma scores-development and validation of multivariable scores to identify asthma in surveys. J Allergy Clin Immunol Pract. 2019;7(1):183–190.e186. doi: 10.1016/j.jaip.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 18.Pavlović K, Berić I, Berezljev L. Agile transformation in clinical research. Eur Manag J. 2018;8(1):65–70. [Google Scholar]
- 19.Thesing T, Feldmann C, Burchardt M. Agile versus waterfall project management: decision model for selecting the appropriate approach to a project. Procedia Comput Sci. 2021;181:746–756. doi: 10.1016/j.procs.2021.01.227 [DOI] [Google Scholar]
- 20.Fonseca JA, Nogueira-Silva L, Morais-Almeida M, et al. Control of Allergic Rhinitis and Asthma Test (CARAT) can be used to assess individual patients over time. Clin Transl Allergy. 2012;2(1):16. doi: 10.1186/2045-7022-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jácome C, Pereira AM, Almeida R, et al. Patient-physician discordance in assessment of adherence to inhaled controller medication: a cross-sectional analysis of two cohorts. BMJ Open. 2019;9(11):e031732. doi: 10.1136/bmjopen-2019-031732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta K, Jean Bousquet P, Akiyama K, et al. Visual analog scale as a predictor of GINA-defined asthma control. The SACRA study in Japan. J Asthma. 2013;50(5):514–521. doi: 10.3109/02770903.2013.786726 [DOI] [PubMed] [Google Scholar]
- 23.Bosnic-Anticevich SZ, Cvetkovski B, Azzi EA, Srour P, Tan R, Kritikos V. Identifying critical errors: addressing inhaler technique in the context of asthma management. Pulm Ther. 2018;4(1):1–12. doi: 10.1007/s41030-018-0051-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos JM. Aspectos epidemiológicos da asma pediátrica numa comunidade portuguesa [Epidemiological aspects of pediatric asthma in a Portuguese community]. In: Rosado-Pinto J, editor. A Criança Asmática. Lisboa; 1993. [Google Scholar]
- 25.Barros H, Pereira C, Mateus P. Asma em crianças dos 6 aos 9 anos. Um estudo populacional em duas cidades portuguesas (Porto e Viseu). Rev Port Imunoalergol. 1999;7(1):9–18. [Google Scholar]
- 26.Vicente O, Rodrigues T, Silva A, Tzer T, Barros H. Prevalência de asma em estudantes das escolas secundárias portuguesas. Arq Med. 1995;9(2):90–92. [Google Scholar]
- 27.Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide (FeNO) in severe asthma management. Eur Respir J. 2020;55(3):1901633. doi: 10.1183/13993003.01633-2019 [DOI] [PubMed] [Google Scholar]
- 28.Neves AL, Jácome C, Taveira-Gomes T, et al. Determinants of the use of health and fitness mobile apps by patients with asthma: secondary analysis of observational studies. J Med Internet Res. 2021;23(9):e25472. doi: 10.2196/25472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz J, Jácome C, Oliveira A, et al. Construct validity of the brief physical activity assessment tool for clinical use in COPD. Clin Respir J. 2021;15(5):530–539. doi: 10.1111/crj.13333 [DOI] [PubMed] [Google Scholar]
- 30.Marshall AL, Smith BJ, Bauman AE, Kaur S. Reliability and validity of a brief physical activity assessment for use by family doctors. Br J Sports Med. 2005;39(5):294–297;discussion 294–297. doi: 10.1136/bjsm.2004.013771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]