Abstract
Purpose:
Cilengitide is a potent and selective inhibitor of the integrins αvβ3 and αvβ5. The primary objective of this phase I clinical trial was to establish the maximum tolerated dose and determine safety/tolerability of cilengitide in combination with paclitaxel in patients with advanced solid tumors. Secondary objectives included the evaluation of the preliminary clinical outcomes.
Patients and Methods:
Patients with advanced solid tumors experiencing disease progression on standard treatment were assigned to two different dose levels of cilengitide (2000 mg intravenously once or twice weekly) in combination with fixed-dose, weekly paclitaxel (90 mg/m2 intravenously).
Results:
Twelve evaluable patients were treated per protocol. A single dose limiting toxicity (DLT) of grade 4 neutropenia was observed at the starting dose level of once weekly cilengitide. There were no grade ≥3 adverse events that occurred with >10% frequency. One patient achieved a partial response to therapy. Five patients experienced stable disease as best response, 3 of which discontinued study participation due to progressive, peripheral neuropathy.
Conclusions:
Cilengitide in combination with paclitaxel was well-tolerated. Antitumor activity was observed. The recommended phase II dose is twice weekly cilengitide (2000 mg) with weekly paclitaxel (90 mg/m2). Further studies evaluating drugs that target this pathway are warranted.
Keywords: Cilengitide, αvβ3 and αvβ5 integrins, paclitaxel, solid tumors
Introduction:
Integrins have diverse functions. They mediate cell attachment and migration, and they interact directly and indirectly with growth factor receptors to control passage through the cell cycle and to regulate survival of normal cells[[1–4]]. Furthermore, they support survival of transformed cells under stress due to hypoxia, chemotherapy, and radiation[[5–7]].
The integrins αvβ3 and αvβ5 appear to be particularly important in the process of angiogenesis, and they are expressed in a variety of malignancies, including melanoma, breast cancer, prostate cancer, colon cancer, and gliomas[[8, 9]]. Intratumoral expression of integrins has been associated with tumor progression and metastasis in melanoma, glioblastoma, breast cancer, and prostate cancer[[10–12]]. For example, αvβ3 is highly expressed on malignant breast tumor vasculature, and it is a prognostic indicator of relapse-free survival in breast cancer[[12–14]]. The critical role of integrins in angiogenesis and their association with tumor progression and metastases make them an attractive target for cancer therapy.
The expression and activities of αvβ3 integrin are integrally related to one of its ligands, Cyr61, a pro-angiogenic factor belonging to the CCN family[15]. Cyr61 is a cysteine-rich, heparin binding protein that is secreted and associated with the cell surface and extracellular matrix (ECM)[16]. In in vitro studies, Cyr61 has been reported to mediate cell adhesion and migration, foster cell survival, and enhance angiogenesis[17–19], in part by binding with v 3 integrin which mediates the cell-ECM interactions[20]. Studies from the Lupu laboratory have demonstrated that forced expression of Cyr61 promotes the upregulation of αvβ3 integrin expression.[21] They and others have furthermore demonstrated similar findings regarding the role of Cyr61 in tumor growth, angiogenesis, and metastasis in vivo [18, 22, 23]. In translational studies, Cyr61 expression in breast tumors has been shown to correlate with stage, tumour size and nodal status[17–19], and high levels of expression are furthermore associated with locoregional relapse, metastasis, and breast cancer mortality[20].
Cyr61 expression is also associated with tumor resistance to anti-neoplastic therapy. Overexpression of Cyr61 in luminal MCF-7 breast cancer cells induces anchorage-independent growth, estrogen receptor signaling independent of ligand binding, and resistance to endocrine therapy[18]. Breast carcinoma cell lines over-expressing Cyr61 have furthermore been found to be more resistant to paclitaxel-induced cytotoxicity; and in xenograft models, the use of αvβ3-antagonists resulted in tumor growth inhibition and synergy with paclitaxel (unpublished data, Ruth Lupu laboratory).
Cilengitide is a potent and selective antagonist of αvβ3 and αvβ5 integrin. It is a pentapeptide with a terminal half-life of ~3–5 hours. It is predominantly renally cleared, and the minimum washout is estimated to be 24 hours. In preclinical studies, cilengitide is associated with anti-angiogenesis activity, suppression of tumor growth and progression, and enhancement of chemotherapy and radiotherapy efficacy[21–23]. As a single agent in a phase I trial, the maximum tolerated dose (MTD) was not reached[24]. In this study, we sought to combine cilengitide with paclitaxel in patients with locally advanced solid tumors who have experienced cancer progression with standard therapies.
Methods
Patients:
Eligible patients were aged ≥18 years and had histologically-confirmed metastatic, unresectable, solid tumor malignancy (excluding lymphoma) that had progressed on standard therapy; an Eastern Cooperative Oncology Group (ECOG) performance status ≤1; life expectancy of ≥12 weeks; and adequate hematologic, hepatic and renal function. All patients provided written informed consent.
Study design:
In this Phase I, open-label, single institution study, patients received cilengitide (2000 mg intravenously once or twice weekly according to dosing assignment), in combination with paclitaxel 90 mg/m2 on day 1, 8 and 15 of each 21-day treatment cycle (Table 1). The standard cohort 3+3 design was applied to determine the maximum tolerated dose.
Table 1: Study Drug Schedules and Dose Levels and for Cilengitide and Paclitaxel.
Study drug dosing, route of administration, schedule, and retreatment interval.
| Agent | Dose | Route | Schedule | Retreatment |
|---|---|---|---|---|
| Cilengitide | As assigned by Randomization Center | IV over 1 hour |
Levels −4, −3, −2, −1, 1*: Days 1, 8 and 15 |
Every 21 days +/− 3 days |
|
Level 2: Days 1, 2, 8, 9, 15, 16 | ||||
| Paclitaxel | As assigned by Randomization Center | IV over 1 hour | Days 1, 8 and 15 | Every 21 days +/− 3 days |
| Dose Level | Cilengitide (mg) | Paclitaxel (mg/m2) | |
|---|---|---|---|
| Dose | Frequency | ||
| −4 | 1500 | Once weekly | 60 |
| −3 | 2000 | Once weekly | 60 |
| −2 | 2000 | Once weekly | 70 |
| −1 | 2000 | Once weekly | 80 |
| 1* | 2000 | Once weekly | 90 |
| 2 | 2000 | Twice weekly | 90 |
Starting Dose Level
DLTs included the following study adverse events, attributed (definitely, probably, or possibly) to the study treatment, occurring during cycle 1: grade 4 anemia; febrile neutropenia; grade 3 neutrophil count decreased lasting ≥ 7 days; grade 4 neutrophil count decreased; grade 4 platelet count decreased; grade 3 platelet count decreased with bleeding; treatment delay of >2 weeks for any drug-related adverse event; any other grade 3 non-hematologic toxicities, except for grade 3 nausea, vomiting, or diarrhea with maximal supportive treatment(s).
The study was approved by the Mayo Clinic Institutional Review Board and performed in accordance with the Declaration of Helsinki and Good Clinical Practice.
Study endpoints and assessments:
The primary objective was to determine the safety and tolerability of cilengitide in combination with paclitaxel and to define the MTD for this treatment combination. Adverse events (AEs) and laboratory parameters were recorded and graded according to the National Cancer Institute CTCAE (version 4.0). Secondary objectives were to characterize the pharmacokinetics (PK) of cilengitide and paclitaxel with the proposed schedule, and to report the observed antitumor activity of cilengitide in combination with paclitaxel. As an exploratory objective, patient-reported outcomes (PRO) version of the CTCAE was piloted in this phase I study.
Tumor assessment was determined by CT and/or MRI scans at baseline and every 6 weeks thereafter. Tumor response and disease progression (PD) were defined by the modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria (version 1.1). Up to 2 target lesions per organ and a total of 3 target lesions were permissible for tumor assessments. Best tumor response is defined to be the best objective status (CR, PR, SD or PD) recorded from the start of the treatment until disease progression/recurrence (taking as reference for progressive disease the smallest measurements recorded since the treatment started).
Selected PRO-CTCAE items (21 items measuring 12 symptomatic adverse events) corresponding to the major adverse events required to be graded clinically were collected. PRO-CTCAE was administered in a paper booklet by a clinical research associate prior to treatment on days 1, 8 and 15 of each cycle during their regular clinical visits.
Statistical Analysis:
MTD was defined as the dose level below the lowest dose that induces DLT in at least one-third of patients (at least 2 of a maximum of 6 new patients).
The number and severity of all adverse events (overall, by dose-level, and by tumor group) were tabulated and summarized in this patient population. This provides an indication of the level of tolerance for this treatment combination in this patient group. The term toxicity is defined as adverse events that are classified as either possibly, probably, or definitely related to study treatment. The safety and tolerability will be assessed for all patients who receive at least one dose of the study medication.
Best tumor responses were summarized by simple descriptive summary statistics. A confirmed tumor response is defined to be a complete response (CR) or partial response (PR) noted as the objective status on 2 consecutive evaluations at least 6 weeks apart.
The PRO-CTCAE was not used for the determination of DLT or for dose-escalation. Instead, they measured toxicity from the patient point of view. The PRO-CTCAE data are summarized descriptively. Informal comparison and correlation of the PRO-CTCAE symptoms with their corresponding items in clinician reported CTCAE will be conducted in an exploratory manner.
Results
Study Population:
Thirteen patients enrolled between November 7, 2011, and August 22, 2012. Patient demographics and baseline characteristics are reported in Table 2. One patient was excluded from this analysis due to drop-out prior to receiving any treatment. Over half of the patients had either breast (n=4) or esophageal cancer (n=3), and the remaining 5 patients had other distinct solid tumor malignancies (lung, thyroid, bladder, neuroendocrine pancreas, sarcoma). Overall, 11 (91.7%) patients had visceral metastases (lung and/or liver). Nine of 12 (75.0%) patients had received prior taxane-based chemotherapy, and 5 of these 9 patients (55.6%) had experienced prior progression on taxane-based chemotherapy.
Table 2: Patient Baseline Characteristics.
The demographic, clinical, and pathologic characteristics for all evaluable patients at baseline are reported.
| Baseline Characteristic | All Evaluable Patients (n=12) |
|---|---|
| Age, Median Years (Range) | 56 (36, 67) |
| Gender | |
| Female | 6 (50%) |
| Male | 6 (50%) |
| Race | |
| White | 10 (83.3%) |
| Black or African American | 2 (16.7%) |
| Months Since Metastatic Diagnosis | 27.5 (0, 98) |
| Median (Range) | |
| Performance Score | |
| 0 | 2 (16.7%) |
| 1 | 10 (83.3%) |
| Tumor Types | |
| Breast | 4 (33.3%) |
| Esophageal | 3 (25%) |
| Other solid tumor, misc. | 5 (41.7%) |
| Sites of Metastasis | |
| Bone | 3 (25%) |
| Liver | 6 (50%) |
| Lung | 7 (58.3%) |
| Lymph Node | 5 (41.7%) |
| Pleura | 3 (25%) |
| Skin | 1 (8.3%) |
| Prior Treatments | |
| Chemotherapy | 11 (91.7%) |
| Taxane-based chemotherapy | 9 (75.0%) |
| Radiation Therapy | 7 (58.3%) |
| Surgery | 12 (100%) |
Dose-escalation and MTD determination:
Cilengitide dosing was started at 2000 mg intravenously once weekly (on day 1, 8, 15 every 21-day cycle) with standard weekly dosing of paclitaxel at 90 mg/m2. Dose escalation was structured per Table 1. A single DLT was experienced by an individual in the first cohort of 3 patients at the starting dose level (grade 4 neutrophil count decreased). There were no DLTs observed in the subsequent 9 evaluable patients. As such, the MTD for cilengitide when combined with standard weekly paclitaxel was not reached, and the recommended dose of cilengitide for subsequent study was determined to be 2000 mg twice weekly (on days 1, 2, 8, 9, 15, 16 every 21 days).
Paclitaxel dose reductions occurred in 5 patients. Two experienced their first dose reduction during the first 2 cycles of therapy, and 3 patients experienced their first dose reduction after the second cycle.
Safety:
The combination of cilengitide and paclitaxel was well tolerated (Table 3). The most commonly experienced adverse events (any grade, all cycles) were: nausea (75%), fatigue (66.7%), diarrhea (50%), alopecia (50%), sensory peripheral neuropathy (41.7%), neutropenia (33.3%), hypocalcemia (33.3%), and hyponatremia (33.3%). Of those, only nausea, fatigue, neutropenia, and alopecia occurred in one-third or more of patients in the first cycle of therapy.
Table 3: Cycle 1 and All Cycles Adverse Events.
Adverse Events (AEs), at least possibly related to treatment, are reported per CTCAE version 4.0 in all subjects at both dose levels. AEs that occurred in cycle 1 and in all cycles are further reported by severity.
| Adverse Events n (% of dose level total) |
Cycle 1 | All Cycles | |||
|---|---|---|---|---|---|
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | ||
| Body System | Type | 6 (50.0) | 1 (8.3) | 8 (66.7) | 3 (25.0) |
| Hematology | Anemia | ||||
| Lymphocyte count decreased | 1 (8.3) | 2 (16.7) | 1 (8.3) | ||
| Neutrophil count decreased | 4 (33.3) | 1 (8.3)* | 4 (33.3) | 1 (8.3) | |
| White blood cell count decreased | 2 (16.7) | 3 (25.0) | |||
| Hemorrhage | Hemorrhage nasal | 1 (8.3) | |||
| Hepatic | Alanine aminotransferase increased | 2 (16.7) | |||
| Alkaline phosphatase increased | 3 (25.0) | 1 (8.3) | |||
| Aspartate aminotransferase increased | 3 (25.0) | ||||
| Serum albumin decreased | 1 (8.3) | ||||
| Lymphatics | Edema limbs | 1 (8.3) | |||
| Metabolic/Laboratory | Serum calcium decreased | 1 (8.3) | 4 (33.3) | ||
| Serum potassium decreased | 3 (25.0) | ||||
| Serum magnesium decreased | 1 (8.3) | ||||
| Serum sodium decreased | 4 (33.3) | 1 (8.3) | |||
| Neurology | Dizziness | 1 (8.3) | |||
| Peripheral sensory neuropathy | 5 (41.7) | ||||
| Pain | Abdominal pain | 1 (8.3) | |||
| Joint pain | 1 (8.3) | 2 (16.7) | |||
| Bone pain | 2 (16.7) | ||||
| Myalgia | 1 (8.3) | 2 (16.7) | |||
| Pain | 1 (8.3) | 1 (8.3) | |||
| Pain in extremity | 2 (16.7) | 2 (16.7) | |||
| Pulmonary | Dyspnea | 3 (25.0) | |||
| Renal/Genitourinary | Creatinine increased | 1 (8.3) | 2 (16.7) | ||
| Cardiovascular | Hypotension | 1 (8.3) | |||
| Sinus tachycardia | 3 (25.0) | ||||
| Coagulation | Activated partial thromboplastin time prolonged | 1 (8.3) | |||
| Constitutional Symptoms | Chills | 1 (8.3) | |||
| Fatigue | 4 (33.3) | 8 (66.7) | |||
| Fever | 1 (8.3) | 2 (16.7) | |||
| Weight gain | 1 (8.3) | ||||
| Dermatology/Skin | Alopecia | 4 (33.3) | 6 (50.0) | ||
| Pruritus | 1 (8.3) | 2 (16.7) | |||
| Rash acneiform | 1 (8.3) | ||||
| Endocrine | Hot flashes | 1 (8.3) | 1 (8.3) | ||
| Hypothyroidism | 1 (8.3) | 1 (8.3) | |||
| Gastrointestinal | Anorexia | 3 (25.0) | 3 (25.0) | ||
| Constipation | 1 (8.3) | 1 (8.3) | |||
| Dehydration | 2 (16.7) | ||||
| Diarrhea | 3 (25.0) | 1 (8.3) | 6 (50.0) | 1 (8.3) | |
| Mucositis oral | 2 (16.7) | 2 (16.7) | |||
| Nausea | 9 (75.0) | 9 (75.0) | |||
| Vomiting | 2 (16.7) | 3 (25.0) | |||
Dose Limiting Toxicity
Of the 5 patients with any grade chemotherapy-induced peripheral neuropathy (CIPN), only 2 had received taxane-based chemotherapy for prior treatment of their advanced malignancy. As it relates to grade 3 and 4 toxicities, for the 6 patients enrolled on dose level 1, there was one other grade 4 event (decreased lymphocytes), but it was considered unrelated to treatment. Grade 3 events from dose level 1 that were considered related to treatment, included: anemia (3 patients), decreased lymphocytes (1 patient), hyponatremia (1 patient), increased alkaline phosphatase (1 patient), and decreased neutrophil count (1 patient). Grade 3 neutropenia, lymphopenia, diarrhea, hyponatremia, and increased alkaline phosphatase all occurred in a single patient. Other grade 3 events considered unrelated to treatment include hypophosphatemia, decreased lymphocytes, headache, dizziness, and increased alkaline phosphatase. New onset brain metastasis requiring hospitalization occurred in 1 patient.
For the 6 patients enrolled on dose level 2, there were no grade 4 events. One patient experienced grade 3 diarrhea considered possibly related to treatment. Other grade 3 events considered unrelated to treatment include pruritus, rash, and allergic reaction.
Tumor Assessments:
All patients were evaluable for best response as determined by investigator review (Figure 1). One of 12 patients (8.3%) achieved a partial response (PR) to therapy, a young woman with newly diagnosed metastatic triple negative breast cancer who had prior receipt of adjuvant paclitaxel >1 year prior to enrollment. Five of 12 patients (41.7%) achieved a best response of stable disease (SD). Of these 6 patients with clinical benefit, 2 (33.3%) had experienced prior progression on taxane-based chemotherapy, including a patient with metastatic leiomyosarcoma who maintained SD for 11 cycles and discontinued treatment due to toxicity (CIPN). Three patients had SD at the time they terminated study participation due to intolerable CIPN attributed to the paclitaxel. Two of these patients had received 4–6 cycles of prior taxane-based chemotherapy and the other patient had no prior taxane exposure. Six of 12 (50%) patients had PD after the first 2 cycles of therapy.
Figure 1: Duration of Therapy and Best Response.
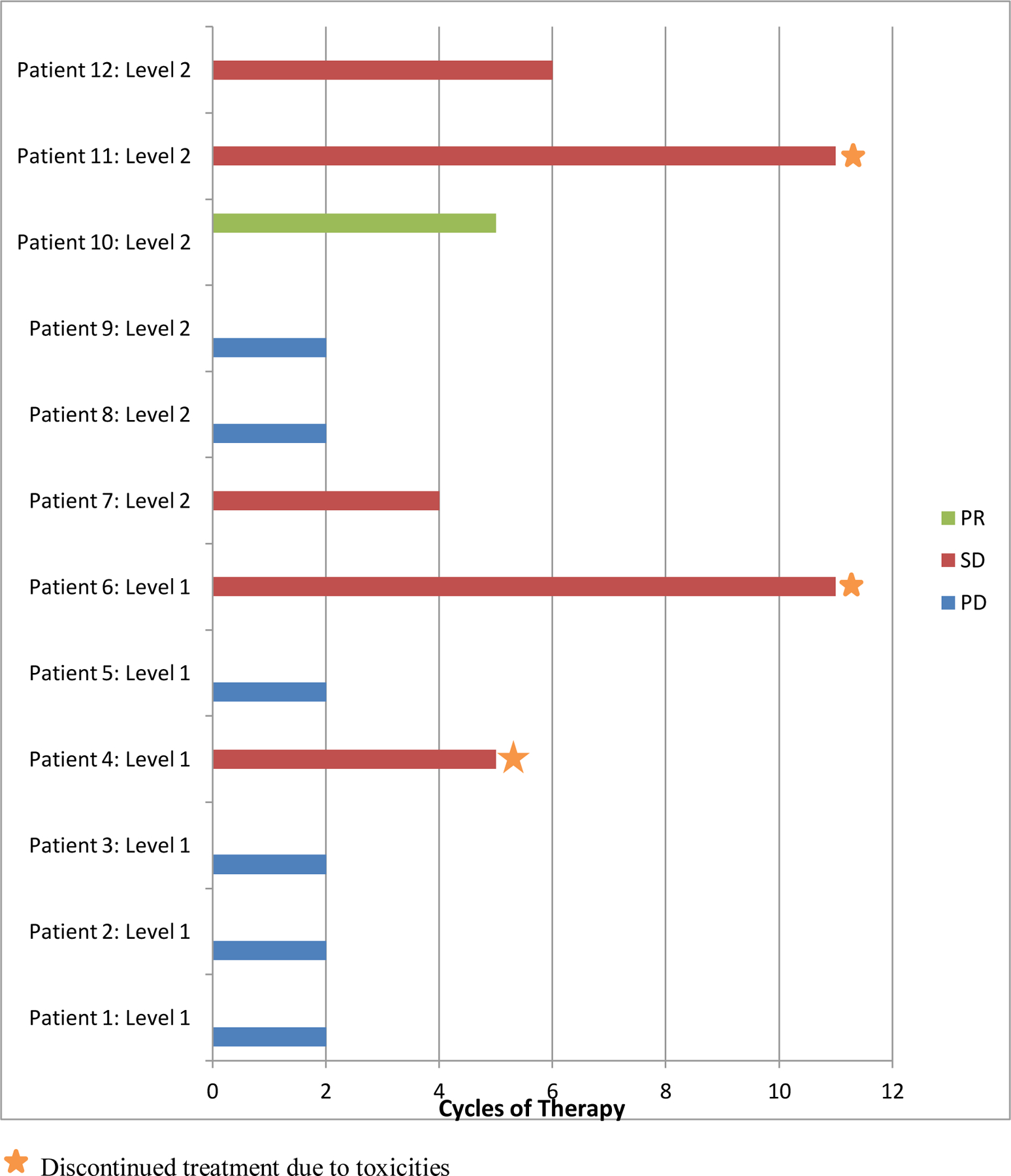
Treatment duration (in 21-day cycles) for patients exposed to standard weekly dose of paclitaxel (90 mg/m2) in combination with cilengitide at two dose levels and corresponding best radiographic response to therapy (Partial Response, PR = Green; Stable Disease, SD = Red; Progressive Disease, PD = Blue). The star indicates those patients who discontinued therapy due to toxicities.
PRO-CTCAE:
The PRO-CTCAE items were summarized descriptively in comparison to clinician-assessed CTCAE version 4.0 (NCI, 2009) during the first cycle. All but one patient completed weekly PRO-CTCAE. PRO-CTCAE captured most of the symptomatic adverse events reflected in clinician-assessed CTCAE. Some symptomatic adverse events were not reported clinically by CTCAE but were reported by patients by PRO-CTCAE. Overall, PRO-CTCAE items indicated slightly more severe degree of symptoms experienced by patients than those reported in CTCAE.
Discussion
The primary aim of this study was to determine the MTD of cilengitide administered concurrent with standard weekly paclitaxel so this combination could proceed to an expansion cohort in triple negative breast cancer and subsequent phase II trials. Standard 3+3 study design with dose escalation was utilized. As the MTD for single-agent cilengitide was not reached and there were no overlapping toxicities with paclitaxel, only 2 dose levels were planned for evaluation.
The MTD of cilengitide in combination with continuous, weekly paclitaxel (90mg/m2) was not established. A single DLT of grade 4 neutropenia was observed in the first cohort of 3 patients on cycle 1 day 15, and it was managed by dose reduction. There were no further DLTs in the remaining 9 evaluable patients. A cilengitide dose of 2000 mg IV on days 1 and 2 every 7 days in combination with weekly paclitaxel was deemed tolerable and recommended for further evaluation in subsequent trials.
The only grade 3 AE attributed to therapy in more than one patient was anemia. The most common grade 1–2 AEs include nausea, diarrhea, alopecia, peripheral neuropathy, hypocalcemia and hyponatremia.
One objective response was observed in a patient with taxane-sensitive, triple negative breast cancer achieved a partial response to therapy that was maintained for 5 cycles. Disease stabilization at first tumor assessment (after 2 cycles of therapy) was achieved in 5 of 12 patients (41.7%). Of these 5 patients, 3 maintained SD beyond the second tumor assessment (after 4 cycles of therapy), including one patient with taxane-resistant leiomyosarcoma who received 11 cycles of therapy; unfortunately these 3 patients subsequently discontinued study treatment after a mean of 9 cycles (range 5–11) due to progressive CIPN and neuropathic pain.
The combination of paclitaxel and cilengitide was associated with clinical benefit (best response PR or SD) in 6 (50.0%) of the 12 evaluable patients. Notably, of the 5 patients who had experienced prior progression on taxane-based chemotherapy, 2 (40.0%) achieved clinical benefit. As this phase I trial included patients who were taxane-naïve and taxane-sensitive, a phase II single-arm study of the combination therapy in a taxane-resistant study population or a randomized study of paclitaxel alone or combined with cilengitide would be necessary to better define the clinical activity of cilengitide.
It is unlikely that prior cumulative exposure to taxane-based chemotherapy predisposed patients to the CIPN observed in this study given that 3 of 9 (33.3%) patients with prior taxane exposure developed any grade CIPN. The collective safety and clinical outcomes data suggest that an alternative dose and schedule for paclitaxel should be explored in combination with cilengitide to help mitigate the risk or early onset of CIPN. This may prevent early discontinuation and improve the overall tolerability of the combination therapy.
In March 2013, the results of the CENTRIC trial were released[25]. In this phase III study patients with a new diagnosis of glioblastoma were randomized to receive temozolomide chemoradiotherapy alone or combined with cilengitide. As the addition of cilengitide did not improve overall survival, further development of cilengitide as an anticancer drug was discontinued. As a result of this, the planned triple negative breast cancer dose expansion cohort did not open to enrollment, and the PK studies were not completed. The PRO-CTCAE findings will be reported separately.
Acknowledgements:
Protocol administration, data management and statistical analysis efforts were supported under U01 CA69912 [PI: CE]. Merck KGaA manufactured cilengitide and provided the drug to the NCI Cancer Therapy Evaluation Program. Cilengitide was sponsored and supplied by the NCI. This publication was supported by the NIH Grant K12 CA90628 [TCH] and by the CTSA Grant UL1 TR000135 [TCH] from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Research Support:
NIH Grant U01 CA69912, NIH Grant K12 CA90628, CTSA Grant UL1 TR000135
Footnotes
Compliance with ethical standards:
Conflicts of Interest: Dr. Qin owns stock in Regeneron Pharmaceuticals. Dr. Goetz is a consultant for Eli Lilly.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References:
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110(6):673–87. [DOI] [PubMed] [Google Scholar]
- 2.De S, Razorenova O, McCabe NP, et al. VEGF-integrin interplay controls tumor growth and vascularization. Proceedings of the National Academy of Sciences of the United States of America 2005;102(21):7589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker JL, Fournier AK, Assoian RK. Regulation of growth factor signaling and cell cycle progression by cell adhesion and adhesion-dependent changes in cellular tension. Cytokine & growth factor reviews 2005;16(4–5):395–405. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. Journal of cell science 2001;114(Pt 14):2553–60. [DOI] [PubMed] [Google Scholar]
- 5.Cordes N Integrin-mediated cell-matrix interactions for prosurvival and antiapoptotic signaling after genotoxic injury. Cancer letters 2006;242(1):11–9. [DOI] [PubMed] [Google Scholar]
- 6.Monnier Y, Farmer P, Bieler G, et al. CYR61 and alphaVbeta5 integrin cooperate to promote invasion and metastasis of tumors growing in preirradiated stroma. Cancer research 2008;68(18):7323–31. [DOI] [PubMed] [Google Scholar]
- 7.Zutter MM. Integrin-mediated adhesion: tipping the balance between chemosensitivity and chemoresistance. Advances in experimental medicine and biology 2007;608:87–100. [DOI] [PubMed] [Google Scholar]
- 8.Max R, Gerritsen RR, Nooijen PT, et al. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. International journal of cancer. Journal international du cancer 1997;71(3):320–4. [DOI] [PubMed] [Google Scholar]
- 9.Sipos B, Kalthoff H, Kloppel G, et al. Expression of abv3, avb5, laminin-5, vitronectin and EGF-R in human colorectal, lung, breast, and ovarian carcinomas Merck KGaA, Darmstadt: 2006:EMD 121974. [Google Scholar]
- 10.Albelda SM, Mette SA, Elder DE, et al. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer research 1990;50(20):6757–64. [PubMed] [Google Scholar]
- 11.Gladson CL, Hancock S, Arnold MM, et al. Stage-specific expression of integrin alphaVbeta3 in neuroblastic tumors. The American journal of pathology 1996;148(5):1423–34. [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparini G, Brooks PC, Biganzoli E, et al. Vascular integrin alpha(v)beta3: a new prognostic indicator in breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 1998;4(11):2625–34. [PubMed] [Google Scholar]
- 13.Brooks PC, Stromblad S, Klemke R, et al. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. The Journal of clinical investigation 1995;96(4):1815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai MS, Hornby AE, Lakins J, et al. Expression and function of CYR61, an angiogenic factor, in breast cancer cell lines and tumor biopsies. Cancer research 2000;60(20):5603–7. [PubMed] [Google Scholar]
- 15.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci 2008;33(10):461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang GP, Lau LF. Cyr61, product of a growth factor-inducible immediate early gene, is associated with the extracellular matrix and the cell surface. Cell Growth Differ 1991;2(7):351–7. [PubMed] [Google Scholar]
- 17.Tsai MS, Bogart DF, Li P, et al. Expression and regulation of Cyr61 in human breast cancer cell lines. Oncogene 2002;21(6):964–73. [DOI] [PubMed] [Google Scholar]
- 18.Tsai MS, Bogart DF, Castaneda JM, et al. Cyr61 promotes breast tumorigenesis and cancer progression. Oncogene 2002;21(53):8178–85. [DOI] [PubMed] [Google Scholar]
- 19.Babic AM, Kireeva ML, Kolesnikova TV, et al. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A 1998;95(11):6355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem 1998;273(5):3090–6. [DOI] [PubMed] [Google Scholar]
- 21.Menendez JA, Vellon L, Mehmi I, et al. A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene 2005;24(5):761–79. [DOI] [PubMed] [Google Scholar]
- 22.Sun ZJ, Wang Y, Cai Z, et al. Involvement of Cyr61 in growth, migration, and metastasis of prostate cancer cells. Br J Cancer 2008;99(10):1656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fromigue O, Hamidouche Z, Vaudin P, et al. CYR61 downregulation reduces osteosarcoma cell invasion, migration, and metastasis. J Bone Miner Res 2011;26(7):1533–42. [DOI] [PubMed] [Google Scholar]
- 24.Kireeva ML, Mo FE, Yang GP, et al. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Molecular and cellular biology 1996;16(4):1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leu SJ, Lam SC, Lau LF. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. The Journal of biological chemistry 2002;277(48):46248–55. [DOI] [PubMed] [Google Scholar]


