Abstract
The discovery of multiple subtypes of human immunodeficiency virus type 1 (HIV-1) worldwide has created new challenges for the development of both therapeutic and preventive AIDS vaccines. We examined T-helper proliferative responses to HIV-1 clade A, B, C, G, and E whole-killed virus and to HIV-1 clade G and B core (p24) antigens in HIV-1-infected subjects taking potent antiviral drugs who received HIV immunogen (Remune) therapeutic vaccination. Subjects who were immunized mounted strong proliferative responses to both whole virus and core antigens of the different clades. These results suggest that a whole-killed immunogen may have broad applications as a therapeutic as well as a preventive vaccine in the current multiclade HIV-1 pandemic.
The development of variations of envelope with the resulting subtypes of human immunodeficiency virus type 1 (HIV-1) has created new challenges for the development of both therapeutic and preventive AIDS vaccines worldwide (27). For example, HIV-1 clade C, which is endemic in Africa and parts of Asia, may now account for one-half of the infections with HIV-1 worldwide (6, 12, 19, 26). Subtype-specific HIV preventive vaccines are being tested, but an alternative, more global, approach might utilize a whole-killed vaccine, which might be capable of inducing cross-clade CD4 and CD8 antiviral immune responses.
The lack of CD4 T-helper cell activity in response to HIV-1 antigens is characteristic of very early HIV infection and is a defect not restored in chronic HIV infection with antiviral drug treatment (1, 2, 8, 10, 15, 21, 22, 24). The rare exceptions to this are individuals with nonprogressive HIV-1 disease, and they may represent the best model of control of HIV-1 by the immune system. HIV-1-seropositive individuals with nonprogressive disease typically have low but measurable HIV-1 viral loads but do not progress clinically or develop profound CD4 depletion for at least 10 years (20, 28). One explanation of such sustained control of viral replication is that cell-mediated immunity is able to suppress viral replication below a threshold that results in clinical disease. A low level of viral replication may be an important source of antigenic stimulation necessary for the immune system to maintain host immunosurveillance.
We hypothesized that an inactivated, gp120-depleted HIV immunogen (clade A/G) (Remune) might be capable of inducing T-helper immune responses to multiple HIV-1 clades. Previously we had characterized and quantitated viral antigens within this immunogen (22, 23). We therefore examined the lymphocyte proliferative response to different HIV-1 whole-killed virus preparations as well as p24 protein antigens of different clades in 11 subjects with chronic HIV-1 infection. The subjects were treated with a gp120-depleted, whole-killed therapeutic vaccine (HIV-1 immunogen) (Remune) while concurrently receiving antiviral drug therapy. Previously we reported that some cross-clade CD4 T-helper cell responses could be elicited to whole virus types B and E in HIV-1 immunogen-treated patients (13). Here we extend those observations and show that subjects were able to mount cross-clade lymphocyte proliferative immune responses to different whole and p24 antigens, including HIV-1 type C. These results suggest that a gp120-depleted, whole-killed virus is capable of inducing T-helper immune responses and may have important implications for HIV-1 therapy and prevention.
MATERIALS AND METHODS
The HIV-1 Immunogen (Remune) is composed of an HIV-1 isolate (HZ321) from serum collected from a patient in Zaire in 1976. This virus has been sequenced and classified as clade A envelope and clade G Gag and grown in the Hut 78 T-cell line (3). Eleven HIV-1-seropositive subjects were enrolled in an open-label research study as part of an expanded access program. Institutional review boards approved all research. Subjects received one intramuscular injection of the HIV-1 immunogen on day 1 and every 12 weeks, which consisted of 10 U of p24 (100 μg of total protein inactivated) gp120-depleted HIV-1 (HZ321) in incomplete Freund's adjuvant. Lymphocyte proliferative assays were performed on 11 HIV-1-seropositive subjects, using strain HZ321 (clade A), strain BaL (clade B), strain TH022E (clade E), strain 96BW01 (clade C), and native p24 (np24) (clade G), recombinant p24 (rp24) (clade B), and IIIB p24 (clade B) antigens for comparison.
The HIV-1 HZ321 immunogen is obtained by concentration and purification from the supernatant fluid of HZ321-infected Hut-78 T cells (7). In the preparation of the immunogen, envelope gp120 is depleted during freezing and thawing during the purification process. HIV-1 BaL antigen (Advanced Biotechnologies Incorporated, Columbia, Md.) was propagated in primary human macrophages. HZ321 virus was collected from a 72-h harvest obtained from a 14-day-infected culture exhibiting extensive syncytium formation, maximum p24 antigen production, and reverse transcriptase activity. The supernatant was clarified at 250 × g for 15 min, aliquoted, and frozen at −70°C. The suspending buffer consisted of Dulbecco modified Eagle medium (high glucose), 20% fetal bovine serum, and 50 mg of gentamicin per ml. HIV-1 clade E (strain TH022E; Advanced Biotechnologies Incorporated) and clade C (18) (kindly provided by the Harvard AIDS Institute) were propagated in primary adult human phytohemagglutinin-stimulated peripheral blood mononuclear cells (PBMCs). Infectious fluids were clarified by differential pelleting, and virus was purified through a 20% sucrose cushion. The suspending buffer consisted of 10 mM Tris, 150 mM NaCl, and 1 mM EDTA (pH 7.5). Clade E, clade C, and HZ321 antigen preparations were inactivated through a sequential application of beta-propiolactone (BPL) (11) and 60Co irradiation (9). Whole IIIB (clade B) and p24 IIIB (clade B) were obtained from Advanced Biotechnologies Incorporated. rp24 (clade B) was obtained from Protein Science (Meriden, Conn.).
Native p24 was preferentially lysed from purified inactivated HIV-1 (HZ321) with 2% Triton X-100 and then purified using Pharmacia Sepharose Fast Flow S resin. Chromatography was carried out at pH 5.0, and p24 was eluted using a linear salt gradient. The purity of the final product was estimated by both sodium dodecyl sulfate-polyacrylamide gel electrophoresis and reverse-phase high-pressure liquid chromatography to be >99%.
For the lymphocyte proliferation assays, fresh PBMCs from HIV-1-seropositive subjects were cultured in RPMI medium with 10% human AB serum at a concentration of 2 × 105 cells per well with medium alone or with inactivated HIV antigens, including whole gp120-depleted HZ321 (clade A) (5 μg/ml), native p24 (clade G) (5 μg/ml), whole BaL (clade B) (1 μg/ml), whole clade E (5 μg/ml), clade C (5 μg/ml), IIIB (5 μg/ml), IIIB p24 (5 μg/ml), and rp24 (5 μg/ml).
PBMCs were seeded in a round-bottom 96-well plate (Falcon) at 2 × 105 cells/well in RPMI (Gibco) containing 10% heat-inactivated (56°C for 30 min) human AB serum (Gemini) and 1% antibiotics (100 U of penicillin per ml and 100 μg of streptomycin per ml) (Gibco). All assays were done in triplicate. After 6 days of incubation, supernatants were harvested from each well (100 μl), and the cells were labeled with 1 μCi of [3H]thymidine in complete RPMI. On day 7 before the harvest, 20 μl of BPL (1:400 final concentration) was added to each well to neutralize any virus produced during the incubation period. Cells were harvested after a 2-h incubation in BPL at 37°C, and incorporated label was determined by scintillation counting in a beta counter. Geometric mean counts per minute were calculated from the triplicate wells with and without antigen. Results were calculated as a lymphocyte stimulation index (LSI), which is the geometric mean counts per minute for the cells incubated with antigen divided by the geometric mean counts per minute for the cells without antigen (cells incubated in medium alone). Spearman rank correlation was performed to examine relationships between lymphocyte proliferative responses to different antigens. The Mann-Whitney nonparametric U test was utilized to compare lymphocyte proliferation responses before and after immunization. All P values are two tailed.
RESULTS
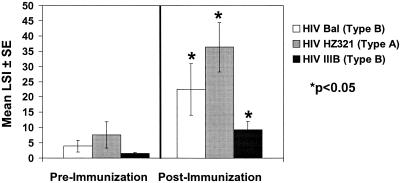
The baseline characteristics of the HIV-1-infected subjects on potent antiviral drug therapy who also received the HIV-1 immunogen and of unimmunized controls are listed in Table 1. As reported previously, subjects treated with the HIV-1 immunogen responded with strong lymphocyte proliferative responses to whole HIV antigens, including the gp120-depleted immunizing antigen (HZ321) (clade A) (P = 0.0008) and whole virus BaL (clade B) (P = 0.003), as shown in Fig. 1. We also demonstrated that these same subjects developed strong lymphocyte proliferative immune responses to HIV-1 IIIB (clade B) (P = 0.002). Overall, unimmunized controls showed weaker proliferative responses to HZ321 (n = 4; mean LSI ± standard error [SE] = 9.1 ± 2.0), BaL (n = 4; mean LSI ± SE = 3.1 ± 1.8), and IIIB (n = 4; mean LSI ± SE = 5.0 ± 3.1) whole HIV-1 antigens.
TABLE 1.
Baseline demographics for immunized subjects
| Subject | CD4 cells/mm3 (absolute no.) on Day 1 | RNA copies/ml on day 1 | HIV medication (mo receiving prior to baseline) |
|---|---|---|---|
| 1 | 426 | <400 | Zidovudine (40) |
| Lamivudine (35) | |||
| Indinavir (19) | |||
| 2 | 607 | <400 | Lamivudine (32) |
| Stavudine (31) | |||
| Nelfinavir (16) | |||
| 3 | 1,969 | <400 | Lamivudine (21) |
| Stavudine (21) | |||
| Nelfinavir (21) | |||
| 4 | 1,270 | <400 | Zidovudine (30) |
| Lamivudine (30) | |||
| Indinavir (30) | |||
| 5 | 657 | <400 | Zidovudine (88) |
| Zalcitabine (49) | |||
| Indinavir (60) | |||
| 6 | 954 | <400 | Efavirenz (3) |
| Stavudine (15) | |||
| Lamivudine (15) | |||
| 7 | 597 | <400 | Zidovudine (29) |
| Lamivudine (29) | |||
| Efavirenz (2) | |||
| 8 | 834 | <400 | Zidovudine (37) |
| Lamivudine (37) | |||
| Indinavir (32) | |||
| 9 | 568 | <400 | Zidovudine (17) |
| Lamivudine (17) | |||
| Nelfinavir (17) | |||
| 10 | 630 | <400 | Stavudine (10) |
| Lamivudine (10) | |||
| Nevirapine (10) | |||
| 11 | 520 | <400 | Stavudine (38) |
| Lamivudine (43) | |||
| Sustiva (7) | |||
| Mean | 821 | <400 |
FIG. 1.
Lymphocyte proliferative responses to whole antigens of different clades before and 1 month after three immunizations (n = 11).
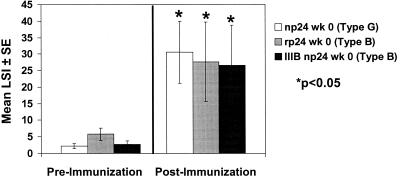
We also examined the response to core proteins of different clades. As shown in Fig. 2, subjects responded to np24 (clade G) (P = 0.0001), rp24 (clade B) (P = 0.01), and IIIB p24 (clade B) (P = 0.001). These core protein immune responses correlated with whole-protein responses (e.g., np24 correlated with HIV-1 [r = 0.88; P < 0.0001]). Overall, unimmunized subjects (n = 4) displayed weaker proliferative responses to np24 (mean LSI ± SE = 4.2 ± 1.1), rp24 (mean LSI ± SE = 15.4 ± 6.8), and IIIB p24 (mean LSI ± SE = 8.3 ± 2.9).
FIG. 2.
Lymphocyte proliferative responses to core protein antigens of different clades before and 1 month after three immunizations (n = 11).
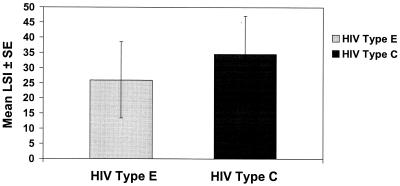
Finally, we examined responses to both clade E and clade C whole virus in these subjects. Strong lymphocyte proliferative responses to both HIV-1 type E (mean LSI ± SE = 26.0 ± 12.6) and HIV-1 type C (mean LSI ± SE = 34.5 ± 12.7) were observed, as shown in Fig. 3. HIV-1 type C T-helper immune responses correlated with type E (r = 0.87; P = 0.0009), BaL (r = 0.8; P = 0.005), IIIB (r = 0.8; P = 0.006), np24 IIIB (r = 0.9; P = 0.002), and rp24 (r = 0.9; P = 0.0007) compared to other whole-virus antigens tested. Unimmunized subjects (n = 4) displayed weaker proliferative responses to HIV-1 clade E (mean LSI ± SE = 0.8 ± 0.1) and HIV-1 clade C (mean LSI ± SE = 3.4 ± 1.8).
FIG. 3.
Lymphocyte proliferative responses to clade E and C 3 months after the third immunization (n = 11).
DISCUSSION
In this study we tested T-helper immune responses to a number of HIV-1 whole and core antigens from different clades of HIV-1. Subjects were on potent antiviral drug therapy and concomitantly received therapeutic HIV-1 immunogen. In unimmunized subjects and at baseline prior to immunization, subjects expressed low proliferative responses to HIV-1 antigens. This is consistent with work by others suggesting that the partial immune reconstitution with potent antiviral drug therapy does not include the full repertoire of HIV-specific clones (4, 14). Furthermore, recent work suggests that the frequency of both CD4 and CD8 HIV-specific T cells may decrease in subjects on potent antiviral drug therapy (R. Koup, M. Betts, J. Casazza, D. Douek, L. Picker, Abstr. 2000 Palm Springs Symposium on HIV/AIDS, p. 30, 2000). In this study we utilized HIV-1 protein antigens which most likely stimulate the class II major histocompatibility complex pathway to activate CD4 T-helper cells (17). Studies using HIV-1 peptides which may activate the class I major histocompatibility complex pathway in order to better examine the CD8 T-cell response to this immunogen are ongoing.
This study further suggests that proliferative responses to clade B, C, and E whole-virus antigens can be stimulated in HIV-1-infected subjects on antiviral drug therapy who receive the HIV-1 immunogen. This observation expands our previous findings and suggests that treatment with an envelope-depleted clade A envelope and clade G Gag can stimulate T-helper responses to a number of clades of HIV-1. While the exact mechanism is unknown, this is probably due to the cellular response to the more conserved proteins of the virus. The response demonstrated here is most likely not due solely to alloantigen stimulation or nonspecific stimulation, as these immune responses to whole-virus antigens correlated with the highly purified core proteins.
Recently, strong core protein T-helper immune responses have been observed both in subjects with primary HIV-1 infection on potent antiviral drug therapy and in subjects with nonprogressive HIV disease receiving no therapy (5, 25). Studies to determine whether the inducement of such responses with this immunogen can delay viral load rebound in patients on potent antiviral drug therapy or during structured treatment interruption are ongoing. Additionally, such an approach may offer a logical prototype for a preventive vaccine, particularly if it can elicit antiviral immune responses against different clades in seronegative subjects.
In summary, HIV-1-infected subjects on potent antiviral drug therapy were able to mount strong proliferative responses to different whole-killed HIV-1 and core proteins from different clades after treatment with HIV-1 immunogen (Remune). Such an immunogen may have broad applications as a therapeutic vaccine as well as a preventive vaccine in the current multiclade HIV-1 epidemic (16).
REFERENCES
- 1.Angel J, Kumar A, Parato K, Filion L, Diaz-Mitoma F, Daftarian P, Pham B, Sun E, Leonard J, Cameron D. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J Infect Dis. 1998;177:898–904. doi: 10.1086/515244. [DOI] [PubMed] [Google Scholar]
- 2.Autran B, Carcelain G, Li T, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostatis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 3.Choi D, Dube S, Spicer T P, Slade H B, Jensen F C, Poiesz B J. Sequence note: HIV type 1 isolate Z321, the strain used to make a therapeutic HIV type 1 immunogen, is intersubtype recombinant. AIDS Res Hum Retroviruses. 1997;13:357–361. doi: 10.1089/aid.1997.13.357. [DOI] [PubMed] [Google Scholar]
- 4.Connick E, Lederman M, Kotzin B, Spritzler J, Kuritzkes D, St. Clar M, Sevin A, Fox L, Chiozzi M, Leonard J, Fousseau F, Roe J A, Martinez A, Kessler H, Landay A. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J Infect Dis. 2000;181:358–363. doi: 10.1086/315171. [DOI] [PubMed] [Google Scholar]
- 5.deQuiros J, Shupert W, McNeil A, Gea-Banacloche J, Flanigan M, Savage A, Martino L, Weiskopf E, Imamichi H, Zhang Y, Adelsburger J, Stevens R, Murphy P, Zimmerman P, Hallahan C, Davey R J, Connors M. Resistance to replication of human immunodeficiency virus challenge in SCID-Hu mice engrafted with peripheral blood mononuclear cells of nonprogressors is mediated by CD8+ T cells and associated with a proliferative response to p24 antigen. J Virol. 2000;74:2023–2028. doi: 10.1128/jvi.74.4.2023-2028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essex M. Human immunodeficiency viruses in the developing world. Adv Virus Res. 1999;53:71–88. doi: 10.1016/s0065-3527(08)60343-7. [DOI] [PubMed] [Google Scholar]
- 7.Getchell J, Hicks D, Srinivasan A, Heath J, York D, Malonga M, Forthal D, Mann J, McCormick J. Human immunodeficiency virus isolated from a serum sample collected in 1976 in Central Africa. J Infect Dis. 1987;156:833–837. doi: 10.1093/infdis/156.5.833. [DOI] [PubMed] [Google Scholar]
- 8.Kelleher A, Carr A, Zaunders J, Cooper D. Alterations in the immune response of human immunodeficiency virus (HIV)-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J Infect Dis. 1996;173:321–329. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 9.Kitchen A, Mann G, Harrison J, Zuckerman A. Effect of gamma irradiation on the human immunodeficiency virus and human coagulation proteins. Vox Sang. 1989;56:223–229. doi: 10.1111/j.1423-0410.1989.tb02033.x. [DOI] [PubMed] [Google Scholar]
- 10.Lederman M, Connick E, Landay A, Kuritzkes D, Spritzler J, St. Clair M, Kotzin B, Fox L, Chiozzi M, Leonard J, Rousseau F, Wade M, Roe J, Martinez A, Kessler H. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 11.LoGrippo G. Investigations of the use of beta-propiolactone in virus inactivation. Ann NY Acad Sci. 1960;83:578–594. doi: 10.1111/j.1749-6632.1960.tb40931.x. [DOI] [PubMed] [Google Scholar]
- 12.Lole K, Bollinger R, Paranjape R, Gadkari D, Kulkarni S, Ng N, Ingersoll R, Sheppard H, Ray S. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss R, Giermakowska W, Lanza P, Turner J, Wallace M, Jensen F, Theofan G, Richieri S, Carlo D. Cross-clade immune responses after immunization with a whole-killed gp120-depleted human immunodeficiency virus type-1 immunogen in incomplete Freund's adjuvant (HIV-1 Immunogen, REMUNE) in human immunodeficiency virus type-1 seropositive subjects. Viral Immunol. 1997;10:221–228. doi: 10.1089/vim.1997.10.221. [DOI] [PubMed] [Google Scholar]
- 14.Moss R, Jensen F, Carlo D. Insights into HIV-specific immune function: implications for therapy and prevention in the new millennium. Clin Immunol. 2000;95:79–85. doi: 10.1006/clim.2000.4856. [DOI] [PubMed] [Google Scholar]
- 15.Moss R, Richieri S, Ferre F, Daigle A, Trauger R, Theofan G, Giermakowska W, Lanza P, Brostoff S, Carlo D, Jensen F. HIV-1-specific functional immune measurements as markers of disease progression. J Biomed Sci. 1997;4:127–131. doi: 10.1007/BF02255640. [DOI] [PubMed] [Google Scholar]
- 16.Moss R B, Diveley J, Jensen F, Carlo D J. In vitro immune function after vaccination with an inactivated, gp120-depleted HIV-1 antigen with immunostimulatory oligodeoxynucleotides. Vaccine. 2000;18:1081–1087. doi: 10.1016/s0264-410x(99)00368-0. [DOI] [PubMed] [Google Scholar]
- 17.Moss R B, Wallace M R, Giermakowska W K, Webb E, Savary J, Chamberlin-Brandt C, Theofan G, Musil R, Richieri S P, Jensen F C, Carlo D J. Phenotypic analysis of human immunodeficiency virus (HIV) type 1 cell-mediated immune responses after treatment with an HIV-1 immunogen. J Infect Dis. 1999;180:641–648. doi: 10.1086/314924. [DOI] [PubMed] [Google Scholar]
- 18.Novitsky V, Montano M, McLane M, Renjifo B, Vannberg F, Foley B, Ndung'u T, Rahman M, Makhema M, Marlink R, Essex M. Molecular cloning and phylogenetic analysis of human immunodeficiency virus type 1 subtype C: a set of 23 full-length clones from Botswana. J Virol. 1999;73:4427–4432. doi: 10.1128/jvi.73.5.4427-4432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oelrichs R, Shrestha I, Anderson D, Deacon N. The explosive human immunodeficiency virus type 1 epidemic among injecting drug users of Kathmandu, Nepal, is caused by a subtype C virus of restricted genetic diversity. J Virol. 2000;74:1149–1157. doi: 10.1128/jvi.74.3.1149-1157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L K, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 21.Pitcher C, Quittner C, Peterson D, Connors M, Koup R, Maino V, Picker L. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 22.Prior C, Bay P, Ebert B, Gore R, Holt J, Irish T, Jensen F, Leone C, Mitschelen J, Stiglitz M, Tarr C, Trauger R J, Weber D, Hrinda M. Process development for the manufacture of inactivated HIV-1. BioPharmacology. 1995;8:25–35. [Google Scholar]
- 23.Richieri S P, Bartholomew R, Aloia R C, Savary J, Gore R, Holt J, Ferre F, Musil R, Tian H R, Trauger R, Lowry P, Jensen F, Carlo D J, Maigetter R Z, Prior C P. Characterization of highly purified, inactivated HIV-1 particles isolated by anion exchange chromatography. Vaccine. 1998;16:119–129. doi: 10.1016/s0264-410x(97)00196-5. [DOI] [PubMed] [Google Scholar]
- 24.Rinaldo J C R, Liebman J, Huang X-L, Fan Z, Al-Shboul Q, McMahon D, Day R, Riddler S, Mellors J. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J Infect Dis. 1999;172:329–336. doi: 10.1086/314599. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 26.Tien P, Chiu T, Latif A, Ray S, Batra M, Contag C, Zejena L, Mbizvo M, Delwart E, Mullins J, Katzenstein D. Primary subtype C HIV-1 infection in Harare, Zimbabwe. J Acquir Immune Defic Syndr Hum Retroviral. 1999;20:147–153. doi: 10.1097/00042560-199902010-00006. [DOI] [PubMed] [Google Scholar]
- 27.van der Groen G, Nyambi P, Beirnaert E, Davis D, Fransen K, Heyndrickx L, Ondoa P, Van der Auwera G, Janssens W. Genetic variation of HIV type 1: relevance of interclade variation to vaccine development. AIDS Res Hum Retroviruses. 1998;14(Suppl. 3):S211–S221. [PubMed] [Google Scholar]
- 28.Vesanen M, Stevens C, Taylor P, Rubinstein P, Saksela K. Stability in controlling viral replication identifies long-term nonprogressors as a distinct subgroup among human immunodeficiency virus type 1-infected persons. J Virol. 1996;70:9035–9040. doi: 10.1128/jvi.70.12.9035-9040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]