Abstract
Purpose
Exposures early in life, beginning in utero, have long-term impacts on mental and physical health. The ECHO prenatal and early childhood pathways to health consortium (ECHO-PATHWAYS) was established to examine the independent and combined impact of pregnancy and childhood chemical exposures and psychosocial stressors on child neurodevelopment and airway health, as well as the placental mechanisms underlying these associations.
Participants
The ECHO-PATHWAYS consortium harmonises extant data from 2684 mother–child dyads in three pregnancy cohort studies (CANDLE [Conditions Affecting Neurocognitive Development and Learning in Early Childhood], TIDES [The Infant Development and Environment Study] and GAPPS [Global Alliance to Prevent Prematurity and Stillbirth]) and collects prospective data under a unified protocol. Study participants are socioeconomically diverse and include a large proportion of Black families (38% Black and 51% White), often under-represented in research. Children are currently 5–15 years old. New data collection includes multimodal assessments of primary outcomes (airway health and neurodevelopment) and exposures (air pollution, phthalates and psychosocial stress) as well as rich covariate characterisation. ECHO-PATHWAYS is compiling extant and new biospecimens in a central biorepository and generating the largest placental transcriptomics data set to date (N=1083).
Findings to date
Early analyses demonstrate adverse associations of prenatal exposure to air pollution, phthalates and maternal stress with early childhood airway outcomes and neurodevelopment. Placental transcriptomics work suggests that phthalate exposure alters placental gene expression, pointing to mechanistic pathways for the developmental toxicity of phthalates. We also observe associations between prenatal maternal stress and placental corticotropin releasing hormone, a marker of hormonal activation during pregnancy relevant for child health. Other publications describe novel methods for examining exposure mixtures and the development of a national spatiotemporal model of ambient outdoor air pollution.
Future plans
The first wave of data from the unified protocol (child age 8–9) is nearly complete. Future work will leverage these data to examine the combined impact of early life social and chemical exposures on middle childhood health outcomes and underlying placental mechanisms.
Keywords: public health, fetal medicine, developmental neurology & neurodisability, paediatric thoracic medicine
Strengths and limitations of this study.
The ECHO prenatal and early childhood pathways to health consortium (ECHO-PATHWAYS) brings together three pregnancy cohort studies (N=2684) to investigate a wide range of chemical and psychosocial exposures during pregnancy and their association with child neurodevelopment and airway health in the early and middle childhood periods.
ECHO-PATHWAYS also includes extensive prenatal bioassay results as well as a biorepository with samples spanning the prenatal period through childhood.
ECHO-PATHWAYS is generating the largest transcriptomics data set to date (N=1083) with which to elucidate placental mechanisms that link maternal exposures during pregnancy to child outcomes.
ECHO-PATHWAYS has some sampling limitations: the component cohorts (1) were not designed to recruit representative samples from their respective sites, (2) do not contribute equally to the full sample and (3) had minimal overlap in the calendar years during which recruitment occurred.
Introduction
Childhood should be a time of healthy development, yet too many children in the USA experience a number of social and health-related challenges early in life that lead to poor physical and mental health outcomes across the life course. The Developmental Origins of Health and Disease (DOHaD)1 framework posits that experiences and exposures across the life course, beginning in utero, can have both long-term and latent impacts on both mental and physical health. Studies across multiple health outcomes highlight pregnancy as an important sensitive period of development. Women can experience numerous modifiable chemical and psychosocial stressors during pregnancy, which are transmitted to the fetus through multiple physiological mechanisms. Pregnant women in the USA are not only commonly exposed to air pollutants associated with roadway, industrial and agricultural emissions, but many are now exposed to extreme levels due to increasing length and severity of wildfire seasons.2 3 Exposure to phthalates, a commonly used synthetic chemical additive found in dust, diet and personal care products, is nearly ubiquitous. Many pregnant women also experience significant psychosocial stressors, with 13.5% of women of childbearing age4 living in poverty and 75% of women reporting at least one major stressful life event 12 months prior to giving birth.5 Further, people from racially and ethnically minoritised groups and low-income families are disproportionately exposed to both chemical exposures and psychosocial stressors, contributing to health disparities. While mounting evidence suggests that chemical and psychosocial exposures during pregnancy and early childhood have long-term impacts on child mental and physical health outcomes,6 7 prior epidemiological research investigating how these exposures combine and interact to influence the developing fetus and subsequent child outcomes is notably inconsistent, with limited understanding of underlying physiological and psychosocial mechanisms or attention to heterogenous effects of exposures within subgroups.8 9
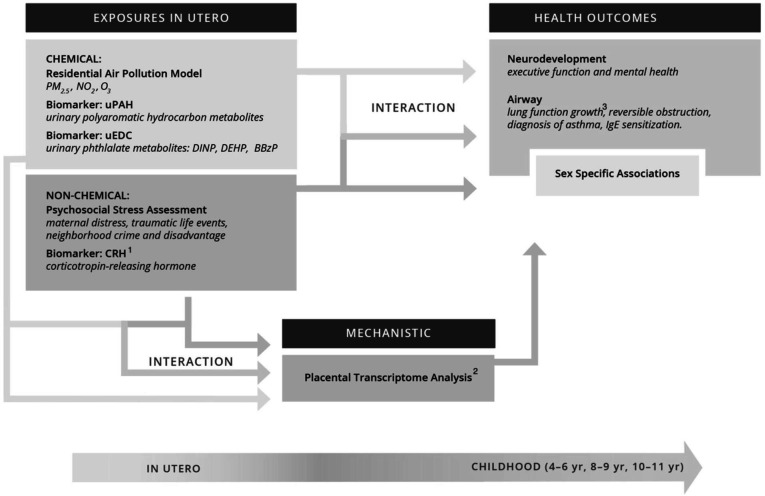
The ECHO prenatal and early childhood pathways to health consortium (ECHO-PATHWAYS) addresses these important gaps by bringing together an interdisciplinary team of investigators and three richly characterised pregnancy cohorts from diverse populations across the country. The core aims of the ECHO-PATHWAYS study are to examine the independent and combined impact of common chemical exposures during pregnancy (air pollutants and phthalates) and psychosocial stressors experienced by the mother during pregnancy and in her childhood (maternal childhood traumatic life events, and exposures to stressful events and neighbourhood crime during pregnancy) on child neurodevelopment and airway health (figure 1). Secondary aims of this study extend investigations to include a child’s own early life exposures to chemical and psychosocial stressors. Primary goals for exposure characterisation were to harmonise existing data, develop new data collection protocols to produce a databank of prenatal and early life environmental exposures, and use existing and newly collected maternal and child specimens for analyses. The substantial biorepository collected from the pregnancy period across the component cohorts, combined with ongoing biospecimen collection, is a major strength of ECHO-PATHWAYS and provides unique opportunities for the well-powered investigation of biomarkers related to psychosocial stress (ie, placental corticotropin releasing hormone (pCRH)) as well as chemical exposures (ie, urinary polycyclic aromatic hydrocarbon metabolites and urinary phthalate metabolites) during pregnancy. We also leverage existing placental samples to better understand the extent to which differences in placental biology (reflected in perturbations in the placental transcriptome) serve as mechanisms mediating the relation between pregnancy exposures and child health outcomes. This ongoing work represents the largest study to characterise placental mechanisms in relation to child health outcomes to date.
Figure 1.
Conceptual model of the ECHO-PATHWAYS study and specific aims. 1CRH is being measured in the CANDLE and GAPPS-PW cohorts only. 2Placental transcriptomes are being sequenced in the CANDLE and GAPPS-PW cohorts only. 3Lung function growth will be estimated in the CANDLE cohort; TIDES and GAPPS-PW will measure lung function at one time point (age 8-9 years). BBzP, benzyl butyl phthalate; CRH, corticotropin releasing hormone; DINP, diisononyl phthalate; DEHP, di(2-ethylhexyl) phthalate; NO2, nitrogen dioxide; O3, ozone; PM2.5, particulate matter of 2.5 microns in diameter or less; uEDC, urinary endocrine disrupting chemical.
For the airway and neurodevelopment outcome domains, ECHO-PATHWAYS developed a comprehensive, multimodal assessment battery that combines maternal report, child self-report and child task-based assessment, including a range of gold standard data elements less commonly collected in large prospective, pregnancy cohort studies. For the airways outcome domain, measures include spirometry to objectively measure lung functional development, as well as validated surveys to assess major chronic airway disorders including asthma, allergic rhinitis and related atopic conditions (eg, atopic dermatitis/eczema).10 Neurodevelopmental outcome assessments include task-based measures of executive function and child cognitive performance, as well as child self-report of depression and anxiety symptoms using gold-standard survey measures. These robust outcome assessments combined with rich extant and ongoing exposure assessment make ECHO-PATHWAYS well suited to advance our understanding of how early childhood environments contribute to neurodevelopment and airway health. The ECHO-PATHWAYS sample is racially and socioeconomically diverse and, for many analyses, is adequately powered to examine whether specific subgroups are more likely to experience negative outcomes in the context of harmful exposures. For example, there is mounting evidence that risk of poor airway and neurodevelopmental outcomes is associated with exposures across multiple domains in a sex-specific manner; however, many studies are underpowered to detect sex-specific effects and thus far, findings are often inconsistent.11–14 The ECHO-PATHWAYS consortium, with its large, well-powered sample, is poised to robustly evaluate sex-specific associations (ie, effect modification), which are central to all ECHO-PATHWAYS analyses.
Cohort description
ECHO-PATHWAYS brings together three pregnancy cohort studies: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study, The Infant Development and Environment Study (TIDES) and a subset of the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) study sample that has been enrolled in ECHO-PATHWAYS (GAPPS-PW). Each study collected extensive data during the pregnancy period and at birth, a wide range of biospecimens and implemented varying degrees of follow-up after the pregnancy period.
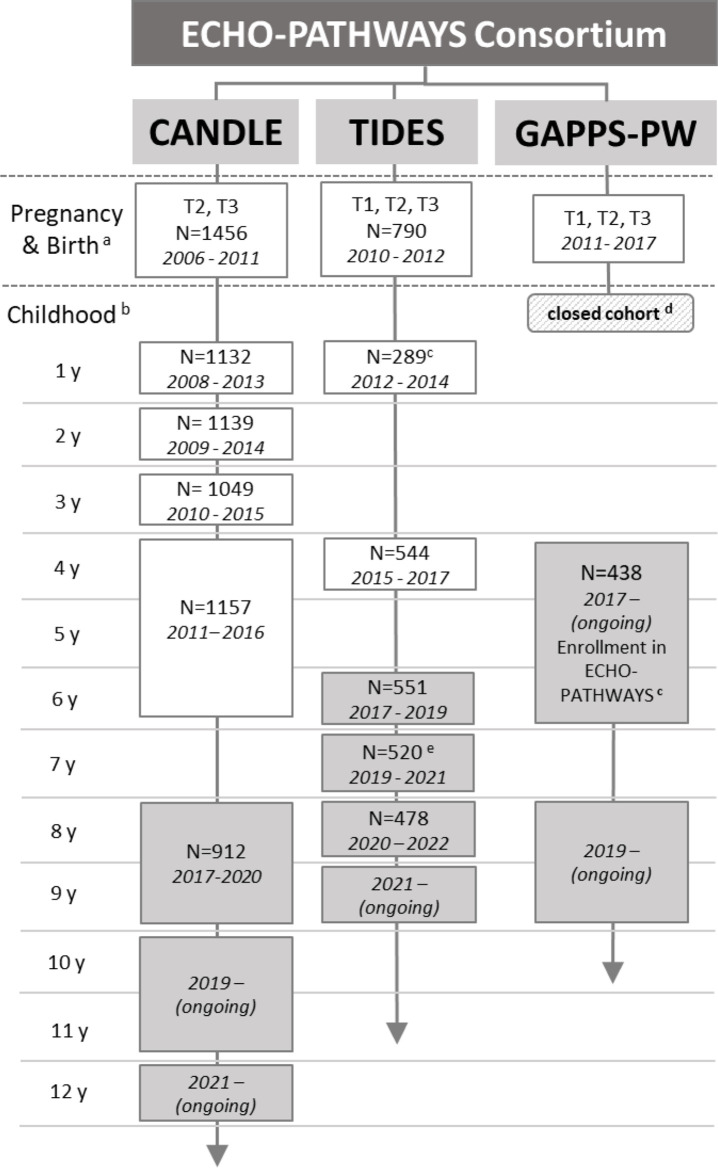
The CANDLE study at the University of Tennessee Health Science Center was designed to examine factors in the pregnancy and early childhood environment that influence child neurodevelopmental outcomes and was extended to include a broad range of child health outcomes (eg, cardiometabolic and airway health). Pregnant women were recruited from Shelby County/Memphis, Tennessee, from 2006 to 2011 during the second trimester of pregnancy. Primary recruitment sites included an urban hospital obstetric clinic and community obstetric practices. Inclusion criteria were: planning to deliver at one of the five hospitals in Shelby County; maternal age of 16–40 years; residence in Shelby County; having a low medical risk, singleton pregnancy; and being able to speak and understand English.15 A total of 1503 pregnant mothers were enrolled, and mother–child dyads were followed up at regular intervals with between 912 and 1157 participants attending each follow-up visit (figure 2).
Figure 2.
ECHO-PATHWAYS visit schedule across cohorts. White boxes indicate cohort-specific data collection prior to enrolment in ECHO-PATHWAYS; shaded grey boxes indicate ECHO-PATHWAYS data collection. aThe study population was defined as participants with birth outcome data (either birth weight or gestational age at birth) for CANDLE and TIDES. bCANDLE and TIDES collected data outside of these in-person clinic visits as well: CANDLE conducted home visits at ages 1 month and 24 months and phone surveys at 3, 6, 9, 15, 18, 21, 27, 30, 33, 42, 48 and 56 months, and TIDES conducted a mail survey at age 2–3 years old; cTIDES age 1 visit was only conducted for boys; dGAPPS was a closed cohort after birth, participants were recruited into GAPPS-PW at the age 4–6 years visit; eTIDES age 7 visit consisted of maternal surveys administered by email.
The multisite TIDES study was designed to examine prenatal phthalate exposure and psychosocial stress in relation to hormone-sensitive and sex-dependent child health outcomes, including reproductive and neurodevelopmental outcomes. Eligibility criteria for TIDES included: less than 13 weeks pregnant, singleton pregnancy, English-speaking, age 18 or over, no serious threat to the pregnancy and plans to deliver at a study hospital.16 Participants were recruited in their first trimester from four university-based prenatal clinics including the University of San Francisco, California (San Francisco, California), the University of Rochester Medical Center (Rochester, New York), the University of Minnesota (Minneapolis, Minnesota) and University of Washington/Seattle Children’s (Seattle, Washington).16 In total, TIDES enrolled 969 women between 2010 and 2012, and collected prenatal information on 790 mother–child dyads (figure 2).
The GAPPS study was designed to establish a large biorepository during the pregnancy and early postnatal period that could be used for research on reducing adverse birth outcomes.17 Since 2007, over 2500 women have been enrolled from the University of Washington in Seattle, Washington; Swedish Hospital in Seattle, Washington; Yakima Valley Memorial Hospital in Yakima, Washington; and Loma Linda University Medical Center in Loma Linda, California, at various time points in pregnancy. Pregnant women were eligible if they were older than 18 years of age, English-speaking and had plans to deliver at the hospital in which they enrolled. Unlike TIDES and CANDLE, GAPPS was a closed cohort (participant engagement ended at birth) at the time of ECHO-PATHWAYS combined cohort development; however, participants had previously been given the option to consent to be contacted for future research studies. Eligibility for ECHO-PATHWAYS included the following: recruitment at a Washington study site, consent to contact for future research, at least one pregnancy urine sample available, pregnancy questionnaire data collected and child age between 4–8 years old. Eligible women (N=1271) have been contacted by phone, email and/or mail and enrolment into GAPPS-PW is ongoing under the purview of Seattle Children’s Research Institute (figure 2). GAPPS-PW has two study sites, Seattle, Washington, and Yakima, Washington. The initial enrolment visit for GAPPS-PW is currently ongoing (figure 2).
Participants from the component cohorts were consented into ECHO-PATHWAYS at their first ECHO-PATHWAYS-specific in-person visit (GAPPS-PW age 4–8 years, TIDES age 6 years and CANDLE age 8–9 years; figure 2). The pooled ECHO-PATHWAYS cohort includes 2684 mother–child dyads from the seven study sites, which increases as more GAPPS-PW dyads are enrolled. Children actively engaged in current research range in age from 5 to 15 years. In the combined ECHO-PATHWAYS sample (table 1) 51% of mothers self-identify as white, 38% as black, with the remainder identifying as being of multiple racial groups, Asian, or other; 6% identify as Hispanic. At enrolment, 45% of women had a high school education or less; 50% of children are women. There is notable sociodemographic variation across the cohorts reflecting regional differences, variations in sampling strategies, and underlying source populations.
Table 1.
Characteristics of participants in the ECHO-PATHWAYS consortium
| Overall (n=2684) |
CANDLE* (n=1456) |
TIDES* (n=790) |
GAPPS-PW† (n=438) |
|||||
| Variable (categorical)‡ | N | % | N | % | N | % | N | % |
| Child sex | 2683 | 1456 | 790 | 437 | ||||
| Male | 50 | 50 | 51 | 49 | ||||
| Female | 50 | 50 | 49 | 51 | ||||
| Maternal race | 2645 | 1454 | 773 | 418 | ||||
| White | 51 | 31 | 70 | 82 | ||||
| Black/African-American | 38 | 62 | 13 | 2 | ||||
| Asian | 3 | 1 | 6 | 3 | ||||
| Native Hawaiian/Pacific Islander | 0 | 0 | 1 | 0 | ||||
| American Indian/Alaska Native | 0 | 0 | 1 | 1 | ||||
| Multiple | 5 | 5 | 4 | 9 | ||||
| Other | 2 | 0 | 6 | 3 | ||||
| Maternal ethnicity | 2664 | 1454 | 779 | 431 | ||||
| Hispanic | 6 | 2 | 9 | 14 | ||||
| Not Hispanic | 94 | 98 | 91 | 86 | ||||
| Maternal education§ | 2660 | 1454 | 781 | 425 | ||||
| <High school | 10 | 12 | 8 | 4 | ||||
| High school completion | 35 | 46 | 19 | 26 | ||||
| College degree | 32 | 30 | 31 | 44 | ||||
| Graduate/professional degree | 23 | 12 | 43 | 26 | ||||
| Prenatal smoking (any)¶ | 2655 | 7 | 1455 | 10 | 775 | 6 | 425 | 3 |
| Prenatal alcohol use | 2657 | 10 | 1455 | 8 | 785 | 13 | 417 | 9 |
| Preterm birth (<37 weeks) | 2681 | 11 | 1456 | 9 | 789 | 10 | 436 | 18 |
| Breast feeding (any) | 2161 | 78 | 1188 | 66 | 541 | 91 | 432 | 94 |
| Breastfeeding duration | 1669 | 770 | 494 | 405 | ||||
| <2 months | 14 | 19 | 7 | 11 | ||||
| 2–4 months | 17 | 23 | 10 | 12 | ||||
| 5–6 months | 13 | 17 | 8 | 11 | ||||
| >6 months | 57 | 41 | 74 | 66 | ||||
| Variable (continuous)‡ | N | Median IQR |
N | Median IQR |
N | Median IQR |
N | Median IQR |
| Gestational age at birth (days) | 2681 | 275 | 1456 | 274 | 789 | 277 | 436 | 274 |
| 269–281 | 269–280 | 270–284 | 264–281 | |||||
*The study population was defined as participants with birth outcome data (either birth weight or gestational age at birth) in CANDLE and TIDES.
†GAPPS was a closed cohort, which was reopened for ECHO-PATHWAYS and the study population in this summary includes those consented into GAPPS-PW as of 31 December 2020.
‡N is defined as the total N with non-missing data. Percent is shown for each category.
§College degree includes college or technical school degree. Graduate/professional defined as some graduate work or graduate/professional degree.
¶Maternal smoking during pregnancy was defined based on self-report.
**
††
CANDLE, Conditions Affecting Neurocognitive Development and Learning in Early Childhood; GAPPS, Global Alliance to Prevent Prematurity and Stillbirth; TIDES, The Infant Development and Environment Study.
Patient and public involvement
Prior to joining ECHO-PATHWAYS, each component cohort had their own strategies for engaging with participants and the public, which included returning appropriate study results and sharing study findings with local end user stakeholders. In addition to continuing with prior methods, ECHO-PATHWAYS also is creating lay summaries of published manuscripts to be used by cohorts for inclusion in participant communications.
Extant data collection
Prior to joining the ECHO-PATHWAYS consortium, each component cohort had completed varying degrees of pregnancy and postnatal follow-up (figure 2). Prior CANDLE cohort data collection was extensive and included two clinical visits during pregnancy and three clinical visits in early childhood, with additional home visits, questionnaires and medical record abstraction including at delivery. Early childhood clinical visits included: biospecimen collection, task-based assessments of neurodevelopment; extensive mother-reported surveys on measures of child behaviour and development, asthma and allergy, family environment, adverse experiences and child diet; observed and coded parent–child interactions and home environment; as well as task-based assessments of maternal cognitive performance and reading ability. In TIDES, study procedures included prenatal visits every trimester, a birth examination including a thorough genital examination with anogenital distance measurement, birth record review, a child exam at 12 months (boys only), a maternal survey at child age 2–3 years and a postnatal visits at 4 years of age. Study visits included anthropometric assessments, biospecimen collection, survey-based assessments (including sociodemographics, medical history, health behaviours, maternal stress and depression, as well as other factors) and task-based assessments of child neurodevelopment and maternal cognitive performance. GAPPS participants completed multiple surveys during prenatal visits (the number and timing varied by participant), collecting information on demographics, reproductive history, health history, pregnancy conditions, health behaviours, prenatal stress, nutrition and other factors, and had the opportunity to complete a postnatal survey when their child was a newborn. Medical record abstraction provided data about pregnancy, delivery and newborn health. Biospecimens were collected at prenatal visits, during delivery and after birth, contributing to a large biorepository that we have leveraged for ECHO-PATHWAYS research.
PATHWAYS study visits
PATHWAYS study visits (figure 2) were designed to: (1) collect data from the younger cohorts (TIDES and GAPPS-PW) on key exposures and outcomes that could be harmonised with extant data from the older CANDLE cohort; (2) implement the ECHO-PATHWAYS flagship assessment battery at the age 8–9 and 10–11 year visit; and (3) collect data on critical covariates (confounders, precision variables, effect modifiers). GAPPS-PW and TIDES each completed an ECHO-PATHWAYS visit between the ages of 4 and 6 years focused on data harmonisation with the CANDLE cohort. All ECHO-PATHWAYS cohorts will complete visits in the 8–9 year range; for CANDLE and GAPPS-PW this is a single visit, while TIDES will collect these data across two study visits conducted at ages 8 and 9 years. CANDLE participants will also complete a 10–11 year visit and 12+ year visit.
Key measurements
Prenatal chemical exposures
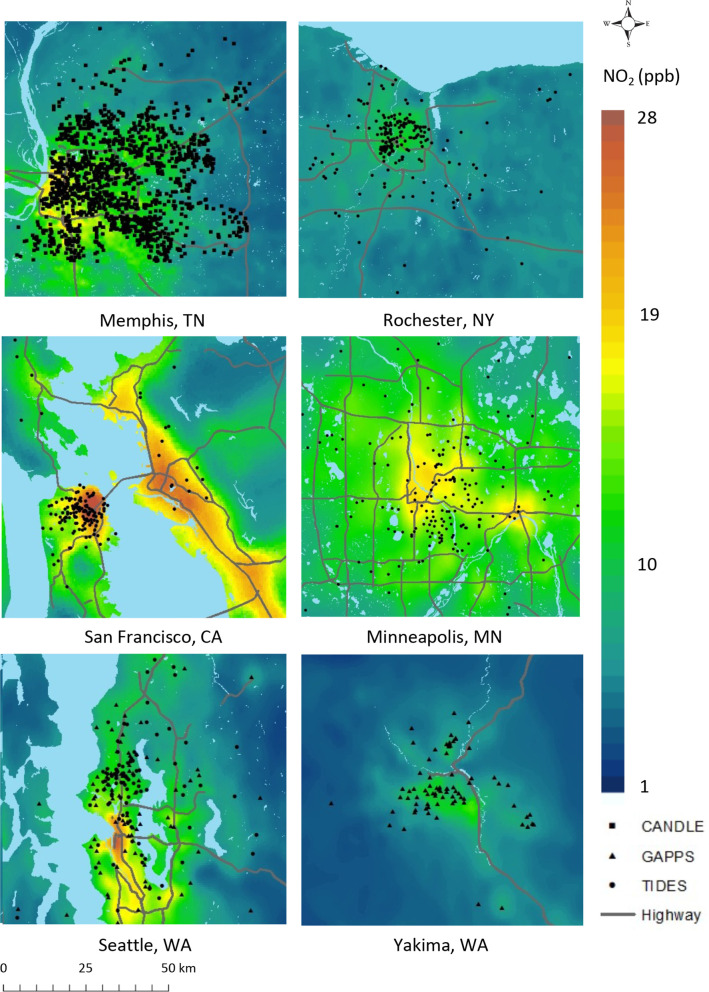
The ECHO-PATHWAYS study focuses on key chemical exposures that are ubiquitous but also show wide variation in levels of exposure across the USA and in association with sociodemographic characteristics. Chemical stressors of interest include air pollutants (ambient air particulate matter less than 10 in diameter (PM10), PM2.5, ozone and nitrogen dioxide (NO2)) as well as phthalates and polycyclic aromatic hydrocarbons (PAHs), assessed as urinary biomarkers. The team’s expertise in advanced statistical air pollution modelling was leveraged to produce national spatiotemporal models of outdoor PM2.5, ozone and NO2.18 These models provide temporal resolution at biweekly intervals, which enables investigation of critical periods of exposure during pregnancy,19 20 and point-based spatial resolution, which is key for the study of pollutants with a high degree of spatial variability, like NO2. This adds significant strengths to the current literature in terms of exposure characterisation.18 We observe notable variation in air pollution exposure both within and between ECHO-PATHWAYS study sites (see figure 3 for example of NO2). PAHs are a class of chemicals produced during incomplete combustion, as such they are encountered commonly in indoor and outdoor air from traffic and industrial combustion-related emissions as well as tobacco smoke. Exposure may occur through the diet when foods are contaminated during their growth such as crops encountering PAH in soil or water or during food preparation such as grilling meats. PAHs include compounds that are known carcinogens, mutagens and teratogens and in recent years have been implicated as endocrine disrupting chemicals. Epidemiological studies of prenatal PAH exposure and associations with common child health outcomes are relatively few. In ECHO-PATHWAYS we observe somewhat higher PAH exposure in CANDLE compared with the other cohorts, likely reflecting underlying differences in the sample population as well as sources of exposures, which is a focus on ongoing ECHO-PATHWAYS research (table 2). Phthalates are a well characterised class of endocrine disrupting chemicals that can impact hormone activity as well as other inflammatory ECHO-PATHWAYS. Prenatal phthalate exposure is associated with numerous adverse childhood health outcomes.21 22 Prenatal concentrations vary by calendar year of pregnancy, geographical location and cohort. For example, low molecular weight phthalates (monoethyl phthalate or monobutyl phthalate) are higher within the CANDLE cohort compared with TIDES (table 2); these low molecular weight phthalates are primarily found in personal care products,21 23 but also these concentrations have decreased over time and CANDLE children were born before TIDES children. Overall, concentrations are similar to those measured within the US-based National Health and Nutrition Examination Survey (NHANES)24 based on year of birth (table 2). Eight PAH biomarkers (N=1891) and 18 phthalate metabolites (N=2204) were determined in mid-pregnancy urine samples in ECHO-PATHWAYS mothers (table 2).
Figure 3.
Estimated outdoor NO2 exposures at ECHO-PATHWAYS sites. Participant locations are jittered to obscure true locations. NO2 predictions are based on the annual average for 2012, for all sites. Gray lines show major roadways. CA, California;
Table 2.
Primary prenatal exposure measures in the ECHO-PATHWAYS consortium
| Overall (n=2684) |
CANDLE* (n=1456) |
TIDES* (n=790) |
GAPPS-PW† (n=438) |
|||||
| Variable (continuous) | Median | IQR | Median | IQR | Median | IQR | Median | IQR |
| Prenatal period mean air pollution exposures‡ | N=2572 | N=1417 | N=762 | N=393 | ||||
| PM2.5 (µg/m3) | 9.6 | 7.9–10.9 | 10.8 | 10.0–11.3 | 8.2 | 7.0–8.8 | 5.7 | 4.9–7.1 |
| NO2 (ppb) | 8.4 | 6.4–10.8 | 8.5 | 6.7–10.4 | 9.0 | 67.0–11.6 | 6.8 | 5.0–10.5 |
| O3 (ppb) | 25.8 | 23.3–30.0 | 26.6 | 24.9–28.7 | 24.5 | 21.4–27.2 | 23.3 | 19.7–26.6 |
| Prenatal psychosocial stress | ||||||||
| Maternal SLEs during pregnancy§ | N=1878 | N=904 | N=540 | N=435 | ||||
| 1 | 0–2 | 1 | 0–3 | 1 | 0–2 | 1 | 0–2 | |
| Maternal childhood trauma § | N=2386 | N=1393 | N=540 | N=435 | ||||
| 0 | 0–1 | 0 | 0–1 | 0 | 0–1 | 0 | 0–1 | |
| Crime rate ¶ | N=2550 | N=1417 | N=744 | N=389 | ||||
| 7.0 | 2.8–17.1 | 15.5 | 5.7–21.9 | 4.1 | 2.1–9.2 | 2.8 | 12.0–4.6 | |
| Placental corticotropin releasing hormone (pCRH; pg/mL) | N=1338 | N=1338 | ||||||
| At time 1 | 38.5 | 21.6–72.7 | 38.5 | 21.6–72.7 | ||||
| At time 2 | 242.1 | 131.4–440.3 | 242.1 | 131.4–440.3 | ||||
| Rate of rise from time 1 to 2 | 187.2 | 99.1–357.6 | 187.2 | 99.1–357.6 | ||||
| Prenatal urinary hydroxyl PAH metabolite concentrations (ng/mL)** | N=1891 | N=990 | N=658 | N=243 | ||||
| 1-NAP | 0.58 | 0.20–1.58 | 0.98 | 0.46–2.50 | 0.19 | 0.03–0.62 | 0.38 | 0.17–0.83 |
| 2-NAP | 3.65 | 1.50–7.71 | 4.78 | 2.43–8.94 | 2.30 | 0.94–6.03 | 2.82 | 1.14–5.35 |
| 2-PHEN | 0.07 | 0.03–0.12 | 0.08 | 0.05–0.14 | 0.06 | 0.02–0.11 | 0.04 | 0.01–0.07 |
| 3-PHEN | 0.07 | 0.03–0.13 | 0.09 | 0.05–0.16 | 0.05 | 0.02–0.10 | 0.04 | 0.01–0.07 |
| 4-PHEN | 0.02 | 0.02–0.04 | 0.02 | 0.02–0.05 | 0.02 | 0.01–0.04 | 0.01 | 0.01–0.03 |
| 1/9-PHEN | 0.18 | 0.05–0.41 | 0.32 | 0.14–0.59 | 0.10 | 0.03–0.23 | 0.08 | 0.03–0.16 |
| 2/3/9-FLUO | 0.54 | 0.34–1.30 | 0.95 | 0.50–1.83 | 0.34 | 0.34–0.73 | 0.14 | 0.06–0.25 |
| 1-PYR | 0.08 | 0.01–0.23 | 0.14 | 0.07–0.27 | 0.01 | 0.01–0.20 | 0.03 | 0.01–0.08 |
| Prenatal urinary phthalate metabolite concentration (ng/mL)†† | N=2204 | N=1155 | N=663 | N=386 | ||||
| MMP | 2.1 | 0.10–8.1 | 5.5 | 0.099–13 | 0.019 | 0.019–2.9 | 1.9 | 0.51–4.2 |
| MEP | 66 | 21–192 | 115 | 45–274 | 28 | 10–91 | 29 | 12–85 |
| MCPP | 1.8 | 0.99–3.7 | 1.9 | 1.1–3.4 | 1.63 | 0.89–3.6 | 1.7 | 0.68–4.8 |
| MEHHP | 14.5 | 5.9–31 | 25 | 15–50 | 5.5 | 2.7–10 | 9.3 | 4.3–18 |
| MBZP | 12 | 4.4–26 | 19 | 9.1–39 | 4.6 | 1.8–12 | 8.2 | 3.0–18 |
| MHXP | 0.16 | 0.071–0.35 | 0.25 | 0.13–0.47 | 0.076 | 0.013–0.18 | 0.10 | 0.059–0.22 |
| MHPP | 0.73 | 0.32–1.5 | 1.1 | 0.54–2.0 | 0.40 | 0.14–0.91 | 0.56 | 0.20–1.2 |
| MCIOP | 7.8 | 2.8–22 | 12 | 5.8–28 | 1.7 | 0.77–5.0 | 18 | 5.9–69.7 |
| MCINP | 2.3 | 1.04–4.8 | 3.1 | 1.7–5.3 | 1.1 | 0.40–2.8 | 2.2 | 0.97–5.0 |
| MEHP | 3.8 | 1.2–9.2 | 7.1 | 3.3–14 | 1.5 | 0.35–3.8 | 1.9 | 0.66–4.5 |
| MBP | 16 | 6.7–36 | 30 | 16–51 | 6.6 | 3.4–13 | 10 | 4.0–23 |
| MIBP | 7.9 | 3.6–16 | 12 | 6.4–20 | 4.2 | 2.1–8.1 | 5.9 | 2.3–11 |
| MECPP | 12 | 6.2–24 | 16 | 9.7–32 | 7.1 | 3.6–13.2 | 11 | 5.0–23 |
| MCMHP | 12 | 5.3–25 | 16 | 8.8–30 | 6.2 | 3.2–15 | 8.0 | 3.7–21 |
| MCHPP | 0.12 | 0.079–0.43 | 0.18 | 0.034–0.42 | 0.079 | 0.079–0.32 | 0.35 | 0.11–1.0 |
| MEOHP | 7.7 | 3.6–16 | 12 | 7.1–23 | 3.58 | 1.9–6.9 | 5.2 | 2.5–9.6 |
| MINP | 0.43 | 0.043–1.9 | 0.36 | 0.035–0.93 | 2.12 | 0.013–5.9 | 0.11 | 0.043–0.73 |
| ƩDEHP | 0.13 | 0.061–0.28 | 0.21 | 0.12–0.41 | 0.0603 | 0.032–0.12 | 0.097 | 0.044–0.18 |
*The study population was defined as participants with birth outcome data (either birth weight or gestational age at birth) in CANDLE and TIDES.
†GAPPS was a closed cohort which was reopened for ECHO-PATHWAYS. The study population in this summary includes participants consented into GAPPS-PW as of 31 December 2020.
‡NO2, nitrogen dioxide; O3, ozone; PM2.5, particulate matter less than 2.5.
§Prenatal stressful life events (SLEs, 14 questions) and maternal childhood trauma (3 questions) scored as count of affirmative responses.
¶Violent crime rate per 1000 residents in 2012 at the census block group level, averaged across the address history during pregnancy.
**Only metabolites detected in >50% of samples in the combined cohort were included; four PAHs were not included due low detection in this sample (1-BAA: 1-hydroxybenz[a]anthracene, 3-BCP: 3-hydroxybenzo[c]phenanthrene, 1-CHRY: 1-hydroxychrysene, and 6-CHRY: 6-hydroxychrysene). All PAH values are shown for raw values (not adjusted for specific gravity), using LOD/sqrt(2) for those below the limit of detection. 1-NAP, 1-hydroxynaphthalene; 2-NAP, 2-hydroxynaphthalene; 2-PHEN, 2-hydroxyphenanthrene; 3-PHEN, 3-hydroxyphenanthrene; 4-PHEN, 4-hydroxyphenanthrene; 1/9-PHEN, the sum of 1-hydroxyphenanthrene and 9-hydroxyphenanthrene; 2/3/9-FLUO, the sum of 2-hydroxyfluorene, 3-hydroxyfluorene and 9-hydroxyfluorene; and 1-PYR, 1-hydroxypyrene.
††Only metabolites detected in >50% of samples in the combined cohort were included. CANDLE phthalates were obtained from the enrolment visit during the second trimester. TIDES phthalates were obtained from the second trimester visit. GAPPS-PW phthalates were obtained from the second trimester if available; if not, measures from the first or third trimesters were used instead. All phthalate values are shown for raw values (not adjusted for specific gravity), using LOD/sqrt(2) for those below the limit of detection. MMP, mono-methyl phthalate; MEP, mono-ethyl phthalate; MCPP, mono-(3-carboxypropyl) phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MBzP, mono-benzyl phthalate; MHXP, mono-n-hexyl phthalate; MHPP, mono-n-heptyl phthalate; MCIOP, mono-(carboxyisooctyl) phthalate; MCINP, mono-(carboxynonyl) phthalate; MEHP, mono-2-ethylhexyl phthalate; MBP, mono-n-butyl phthalate; MiBP, mono-isobutyl phthalate; MECPP, mono-(2-ethyl-5-carboxypentyl) phthalate; MCMHP, mono-(2-carboxymethyl-hexyl) phthalate; MCHPP, mono-carboxy-n-heptyl phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; MINP, mono-isononyl phthalate; ƩDEHP, sum of metabolites of di-2-ethylhexyl phthalate parent compound in µmol/L calculated as (MEHP*(1/278.34)) + (MEHHP*(1/294.34)) + (MEOHP*(1/292.33)) + (MECPP*(1/308.33)).
CANDLE, Conditions Affecting Neurocognitive Development and Learning in Early Childhood; GAPPS, Global Alliance to Prevent Prematurity and Stillbirth; PAHs, polycyclic aromatic hydrocarbons; TIDES, The Infant Development and Environment Study.
Prenatal psychosocial stressors
The ECHO-PATHWAYS study focuses on psychosocial stress exposures that are fairly common in the USA, but with some variation in levels of exposure by sociodemographic characteristics. Building on life course health development and DOHaD frameworks, stressors from two key maternal life course exposure periods (childhood and pregnancy) are examined as potential contributors to offspring development in utero. Maternal exposure to stressful life events during pregnancy was ascertained using retrospective maternal report of 14 events adapted from the Centers for Disease Control and Prevention Pregnancy Risk Assessment Monitoring System,25 which included illness, death, relationship problems, housing difficulties, legal issues and financial problems during pregnancy (table 2). In addition, maternal residential address across pregnancy (duration-weighted for women who moved) was geospatially linked to multisource crime reports to create a comprehensive measure of potential exposure to violent crime at the neighbourhood level.26 ECHO-PATHWAYS has also assessed maternal histories of exposure to traumatic events in childhood, including physical and sexual abuse, violence in the home, loss of a close family member or friend, family upheaval (eg, divorce) and illness/injury before age 18. There is notable variation in exposure to these stress exposures in ECHO-PATHWAYS as well as some differences between cohorts (table 2). Together, these measures allow for the investigation of intergenerational transmission of stress effects on health and the consideration of multiple domains and periods of stress exposures in relation to child well-being.
Neurodevelopment and mental health
Extant and planned data collection from the 4–6 year visit in the CANDLE, TIDES and GAPPS-PW cohorts included a comprehensive IQ assessment (using the Stanford Binet, Wechsler Intelligence Scale for Children-V, and Wechsler Preschool and Primary Scale of Intelligence-IV, respectively)27–29 as well as parent report on the Child Behaviour Checklist (CBCL),30–32 which is a widely used measure of multiple dimensions of child behaviour and includes both continuous and clinically relevant cut-off scores for symptoms related to internalising and externalising behaviour, as well as specific symptoms associated with depression, anxiety and attention deficit/hyperactivity disorder (ADHD) (table 3).
Table 3.
Primary child health outcomes in the ECHO-PATHWAYS consortium at age 4–6
| Age 4–6 year visit* | Overall (n=2179) |
CANDLE (n=1149) |
TIDES (n=593) |
GAPPS-PW (n=437) |
||||
| Variable (categorical) | N | % | N | % | N | % | N | % |
| Asthma and allergy measures† | 2172 | 1145 | 591 | 436 | ||||
| Current wheeze | 16 | 19 | 10 | 13 | ||||
| Current asthma | 12 | 16 | 5 | 9 | ||||
| Ever asthma | 12 | 15 | 7 | 8 | ||||
| Strict asthma | 9 | 12 | 6 | 6 | ||||
| Ever Eczema | 33 | 32 | 35 | 33 | ||||
| Ever Rhinitis | 25 | 27 | 21 | 27 | ||||
| Neurodevelopment measures | ||||||||
| Child Behaviour Checklist‡ | 1997 | 1025 | 537 | 435 | ||||
| Externalising (T-score >63) | 5 | 4 | 9 | 4 | ||||
| Internalising (T-score >63) | 7 | 5 | 10 | 8 | ||||
| Affective scale (T-score >69) | 3 | 4 | 4 | 2 | ||||
| Anxiety scale (T-score >69) | 2 | 2 | 2 | 4 | ||||
| ADHD scale (T-score >69) | 2 | 2 | 3 | 3 | ||||
| Full scale IQ§ | 1859 | 1050 | 427 | 382 | ||||
| Very high/extremely high | 12 | 8 | 20 | 17 | ||||
| High average | 23 | 21 | 24 | 29 | ||||
| Average | 45 | 48 | 39 | 44 | ||||
| Low average | 12 | 15 | 9 | 6 | ||||
| Very low/extremely low | 7 | 9 | 8 | 3 | ||||
| Variable (continuous) | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Child Behaviour Checklist T-scores | N=1997 | N=1025 | N=537 | N=435 | ||||
| Externalising score | 46 | 10 | 45 | 10 | 49 | 10 | 44 | 10 |
| Internalising score | 46 | 11 | 45 | 11 | 49 | 10 | 46 | 11 |
| Affective scale | 53 | 5 | 53 | 5 | 54 | 6 | 53 | 5 |
| Anxiety scale | 53 | 6 | 52 | 5 | 54 | 6 | 53 | 6 |
| ADHD scale | 53 | 5 | 53 | 5 | 54 | 6 | 52 | 5 |
| Full scale IQ§ | N=1859 | N=1050 | N=427 | N=382 | ||||
| Composite score | 103 | 15 | 100 | 15 | 106 | 16 | 107 | 13 |
*Total count for the age 4–6 year visit defined as those participants having at least one neurodevelopment or airway outcome.
†Airway and allergy measures were assessed via the International Study of Asthma and Allergies in Childhood survey. Current wheeze was defined as an affirmative response to two questions: ‘Has your child ever had wheezing or whistling in the chest at any time in the past?’ and ‘Has your child ever had wheezing or whistling in the chest in the last 12 months?’ Ever asthma was defined as an affirmative response to the question: ‘Has your child ever had asthma?’ Current asthma was defined as at least two of current wheeze, ever asthma and asthma medication use (‘In the past 12 months has the child used any medications for asthma or wheeze’). Strict asthma was defined as ever asthma AND at least one of current wheeze and current asthma medication use. Eczema was defined as an affirmative response to the question ‘Has your child ever had eczema?’ Rhinitis was defined as an affirmative response to the question ‘Has your child ever had a problem with sneezing, or a runny, or blocked nose when he/she did not have a cold or the flu?’.
‡T-scores were used to identify children with clinically significant behaviour problems on the Child Behaviour Checklist using published norms; ADHD, attention deficit/hyperactivity disorder.
§Full scale IQ was categorised as follows: 120 and higher as very high/extremely high, 110–119 as high average, 90–109 as average, 80–89 as low average and 79 and less as very low/extremely low.
CANDLE, Conditions Affecting Neurocognitive Development and Learning in Early Childhood; GAPPS, Global Alliance to Prevent Prematurity and Stillbirth; TIDES, The Infant Development and Environment Study.
The age 8–9 year ECHO-PATHWAYS neurodevelopmental and mental health battery provides both dimensional and clinically relevant insights into potential child behaviour and mental health problems, with a focus on symptoms and diagnostically relevant cut-off scores for ADHD, depression and anxiety, the three most common mental health problems in children within this age range. Neurodevelopmental measures at this visit are primarily child self-report and task-based assessment, in addition to parent report on the CBCL. ECHO-PATHWAYS includes a gold standard, child self-report survey measure of depression symptoms at this age, the Children’s Depression Inventory,33 34 which provides continuous scores and cutoffs related to clinical significance as well as a similarly robust measure of child anxiety, the Screen for Child Anxiety Related Disorders (table 4).35 The battery also includes task-based assessments of executive function, which refers to a number of core cognitive skills related to self-regulation, attention and planning that are positively associated with multiple social and educational outcomes, and negatively associated with psychopathology.36 37 Three executive functioning tasks are administered at all in person visits and capture cognitive flexibility (Hearts and Flowers),38 39 attention and inhibitory control (Flanker)40 and working memory (Digit Span) (table 4).28
Table 4.
Primary child health outcomes in the ECHO-PATHWAYS consortium at age 8–9
| Age 8–9 year visit* | N=912 | |||||
| Variable (categorical) | N | % | Variable (continuous) | N | Mean | SD |
| Asthma and allergy | ||||||
| ISAAC survey† | 912 | |||||
| Current wheeze | 13 | |||||
| Current asthma | 14 | |||||
| Ever asthma | 19 | |||||
| Strict asthma | 12 | |||||
| Eczema | 35 | |||||
| Rhinitis | 31 | |||||
| Spirometry‡ | 739 | Spirometry‡ | 739 | |||
| FEV1<LLN | 3 | FEV1 Z-score | 0.28 | 1.00 | ||
| FVC<LLN | 2 | FVC Z-score | 0.39 | 0.99 | ||
| FEV1/FVC<LLN | 8 | FEV1/FVC Z-score | −0.17 | 1.10 | ||
| FEF 25–75<LLN | 7 | FEF 25–75 Z-score | −0.16 | 0.95 | ||
| IgE§ | 545 | |||||
| Food Phadiotop (≥0.35) | 29 | |||||
| Food Phadiotop (≥0.10) | 58 | |||||
| Aeroallergen Phadiotop (≥0.35) | 50 | |||||
| Aeroallergen Phadiotop (≥0.10) | 63 | |||||
| Any allergy (≥0.35) | 56 | |||||
| Any allergy (≥0.10) | 76 | |||||
| Neurodevelopment and mental health | ||||||
| Child Behaviour Checklist¶ | 912 | Child Behaviour Checklist ¶ | 912 | |||
| Externalising score (T-score >63) | 6 | Externalising score | 48 | 10 | ||
| Internalising score (T-score >63) | 8 | Internalising score | 48 | 10 | ||
| Affective scale (T-score >69) | 3 | Affective scale | 54 | 6 | ||
| Anxiety scale (T-score >69) | 3 | Anxiety scale | 54 | 5 | ||
| ADHD scale (T-score >69) | 3 | ADHD scale | 54 | 6 | ||
| Depression | 820 | Depression** | 820 | |||
| Elevated CDI-2 (≥65) | 17 | CDI-2 t-score | 55 | 11 | ||
| Very elevated CDI-2 (≥70) | 10 | |||||
| Anxiety | 820 | Anxiety †† | ||||
| SCARED score ≥25 | 55 | SCARED score | 822 | 28 | 14 | |
| Digit Span scaled score | 748 | 9.5 | 3.0 | |||
| Hearts and Flowers | ||||||
| Overall accuracy | 744 | 0.84 | 0.12 | |||
| Flanker | ||||||
| Age-corrected standard score | 720 | 95.6 | 13.8 | |||
*Total count for the age 8–9 year visit in this table is defined as those participants having at least one neurodevelopment or airway outcome in the CANDLE cohort. Age 8–9 year visits are currently ongoing in the TIDES and GAPPS-PW cohorts, and thus data are not included here.
†ISAAC, International Study of Asthma and Allergies in Childhood. Current wheeze was defined as an affirmative response to two questions: ‘Has your child ever had wheezing or whistling in the chest at any time in the past?’ and ‘Has your child ever had wheezing or whistling in the chest in the last 12 months?’ Ever asthma was defined as an affirmative response to the question: ‘Has your child ever had asthma?’ Current asthma was defined as at least two of current wheeze, ever asthma and asthma medication use (‘In the past 12 months has the child used any medications for asthma or wheeze’). Strict asthma was defined as ever asthma AND at least one of current wheeze and current asthma medication use. Eczema was defined as an affirmative response to the question ‘Has your child ever had eczema?’ Rhinitis was defined as an affirmative response to the question ‘Has your child ever had a problem with sneezing, or a runny, or blocked nose when he/she did not have a cold or the flu?’.
‡FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25−75, forced expiratory flow at 25–75% of FVC; LLN, lower limit of normal, defined as a Z-score of −1.645.
§Serum IgE screen was measured from child participants’ blood samples collected at the age 8–9 year visit. The food allergen mix included chicken egg, cow’s milk, peanut, soybean, codfish and wheat. Values less than the limit of detection (<0.1) were set to 0.1/√2.
¶Continuous scales are T-scores that were calculated using standard Child Behaviour Checklist norms; T-scores were dichotomised using clinical cut-points; ADHD, attention deficit/hyperactivity disorder.
**CDI-2, Children’s Depression Inventory, Second Edition. Continuous measures are T-scores using age and gender specific norms.
††SCARED, Screen for Child Anxiety Related Disorders. Continuous measures are raw scores; possible range is 0–82.
Possible Digit Span scaled score range is 1–19.
CANDLE, Conditions Affecting Neurocognitive Development and Learning in Early Childhood; GAPPS, Global Alliance to Prevent Prematurity and Stillbirth; TIDES, The Infant Development and Environment Study.
Airway health
Respiratory health measurement focuses on the globally recognised International Study of Asthma and Allergies in Childhood Survey based assessment10 of asthma and atopic diseases as well as spirometry to characterise child lung function, including repeat assessment in some participants, allowing characterisation of lung functional growth over middle childhood.41 Spirometry is conducted using American Thoracic Society guidelines with assessment of key parameters including forced vital capacity and forced expiratory volume in 1 s, including pre and post bronchodilator to ascertain reversibility of airway obstruction, a hallmark of asthma.42 Asthma, allergic rhinitis and eczema are conditions associated with an atopic predisposition in children,43 with assessment of current symptoms and associated medication and healthcare use. Blood seroatopy is assessed using a multiallergen screen for aeroallergens (Phadiatop, mix proprietary) in combination with the food allergen mix (fx5, chicken egg, cow’s milk, peanut, soybean, codfish and wheat).44 Application of these screens has been more effective than individual allergen-specific IgE measurements in characterising the atopic status of children (table 4).
Biological mechanisms
The placental transcriptome provides insight into the developmental programming of perinatal and child outcomes. Gene expression is assessed using whole-genome RNA sequencing and allows for identification of mechanistic links between chemical and non-chemical prenatal exposures and perinatal and childhood health outcomes.45 46 Transcriptomics data reflects gene expression and thus can reveal underlying functional differences related to exposures or outcomes at a molecular level. The placental epigenome, transcriptome and proteome change across gestation47 48 and respond to cues from the maternal environment.49 Using specimens banked at the time of delivery, we have generated placental transcriptomics data on 289 participants in the GAPPS-PW and 794 participants in the CANDLE cohorts, which combined is the largest collection of placental transcriptomics data available to date. The ability to examine these genetic transcriptomic data in a well-characterised cohort with robustly assessed outcomes and exposures, provides an unprecedented opportunity to understand the role of the placental transcriptome as a mediator between prenatal exposures, fetal development and childhood health.
In addition, as a potential biomarker that is sensitive to social stress exposures and chemical exposures,50 pCRH was measured via repeated blood samples during the second and third trimesters of pregnancy. CRH plays a key role in regulating the activity of the hypothalamic–pituitary–adrenal axis, the body’s major neuroendocrine stress response system and is synthesised and secreted by the placenta during pregnancy. Variation in pCRH levels and rise across pregnancy is critical for fetal development51 and birth timing processes, but excessive levels are related to adverse birth and developmental outcomes. Thus, pCRH during pregnancy may provide mechanistic insight into the biological embedding of both maternal psychosocial stressors and chemical exposures. In ECHO-PATHWAYS, pCRH has been ascertained in 1338 women in the CANDLE cohort and is planned for GAPPS-PW cohort as well (table 2).
Additional exposure and covariate characterisation
An additional strength of the ECHO-PATHWAYS cohort is rich characterisation of potential covariates and confounders. For example, multiple studies examining associations between phthalates and both airway and neurodevelopmental outcomes have only adjusted for maternal education to account for confounding by socioeconomic position.52 ECHO-PATHWAYS also includes robust income assessments (adjusting for household size, inflation and regional differences in purchasing power) as well as neighbourhood deprivation, which includes indicators related to poverty, unemployment, educational attainment and home ownership derived from the US Census.53 ECHO-PATHWAYS also includes directly assessed maternal IQ, a potential confounder for many studies examining child neurodevelopmental outcomes and rarely included in large, pregnancy cohort studies.52 54 Additional covariates in the ECHO-PATHWAYS study include birth weight, gestational age, maternal mental health, maternal smoking and alcohol use during pregnancy, secondhand tobacco smoke exposure during pregnancy and childhood, breast feeding, as well as a range of postnatal environmental factors such as parenting and child diet. Ongoing work also includes the characterisation of primary exposure domains (chemical and psychosocial stress) and potential protective factors (eg, neighbourhood opportunity, positive parenting, sleep quality) throughout childhood, which will be examined in future analyses.
Findings to date
Each cohort was developed to address specific study aims or goals and, thus, has their own cohort-specific foci and resulting publications before they joined the ECHO-PATHWAYS consortium. ECHO-PATHWAYS has leveraged all three of its cohorts to examine important scientific questions regarding each of the three primary ECHO-PATHWAYS exposures (phthalates, air pollution, psychosocial stress) in relation to each health outcome domain (neurodevelopment/mental health, airway health) with some early analyses focusing on single cohorts and more recent work in pooled samples harmonising data across all three cohorts.
As CANDLE is the oldest cohort in the ECHO-PATHWAYS consortium with data available through age 6 at study initiation, early ECHO-PATHWAYS analyses focused on analyses within this cohort and, in addition to chemical and psychosocial exposure data, leveraged detailed nutrition characterisation during pregnancy (plasma vitamin D and folate, dietary patterns, etc). These early ECHO-PATHWAYS products have examined maternal pregnancy exposures to vitamin D,55 dietary patterns,56 air pollutants,12 57 phthalates52 and maternal stress58 in relation to child neurodevelopment59 and other health outcomes.19 60 For example, prenatal exposure to PM10 in pregnancy was associated with reduced IQ in children and the magnitude of effect increased with reduced folate levels, suggesting that prenatal folate may protect against the neurotoxic effects of air pollution.12 Similar results were observed in associations with child behaviour problems.57 ECHO-PATHWAYS analyses in the CANDLE study population also suggest a positive association between maternal vitamin D and childhood IQ,55 links between eating fast food in the second trimester of pregnancy and childhood obesity56 and that maternal stressors during pregnancy are associated with her child’s early life socioemotional development61 and executive functioning,62 with some evidence that high quality parenting may buffer these associations.62 In one of the largest studies of pCRH and child behaviour outcomes to date, we reported that maternal exposure to traumatic events during her childhood resulted in higher pCRH levels and rise during pregnancy.58 We also observed that prenatal phthalate mixtures were associated with alterations in pCRH, and that women with pregnancy complications (gestational diabetes and pre-eclampsia) may be most strongly impacted.63 The first study examining the CANDLE placental transcriptome in relation to prenatal phthalate exposures found that maternal urinary concentrations of several phthalate metabolites were associated with changes in the expression of 38 genes within the placenta.64 We also noted additional relations between prenatal phthalate exposure and placental gene expression that were only significant in male or female infants. In ECHO-PATHWAYS analyses focused on the TIDES cohort, we found that prenatal phthalate exposure was associated with child behaviours as well as social responsiveness at age 4–6 in a sex-specific manner, indicating that phthalates may have sex-specific impacts on child behaviour and development.65 Additional work in TIDES demonstrated that maternal stressful life events and distress during pregnancy were associated with more child behaviour problems and lower levels of adaptive functioning.66 Since GAPPS-PW had been a closed cohort, no child health data was available until ECHO-PATHWAYS began and therefore no child health analyses using extant data could be leveraged within this cohort. Currently, GAPPS-PW has collected data on over 600 ECHO-PATHWAYS participants, and consortium analyses including these data are ongoing.
The first multicohort analysis within the ECHO-PATHWAYS consortium combined the TIDES and CANDLE cohorts and found that prenatal phthalate exposure mixtures were associated with increased risk of asthma and wheeze in boys but not girls.67 A second paper combining these two cohorts leveraged the high temporal resolution of modelled ECHO-PATHWAYS air pollution estimates to examine novel hypotheses related to the timing of exposure in relation to phases of fetal lung development. We observed associations between air pollution exposure during the saccular phase (24–36 weeks) of prenatal lung development and childhood risk of asthma.20 The first ECHO-PATHWAYS studies using all three cohorts have now been published. One study found that prenatal air pollution exposure (PM2.5 and NO2) was positively associated with child behaviour problems and negatively associated with cognitive functioning, with some evidence suggesting stronger associations among girls.68 Another three-cohort study suggests that the duration of exclusive breast feeding is associated with reduced risk of asthma.69 Another found that maternal exposure to trauma during childhood and stressful life events during pregnancy was associated with greater child mental health problems at age 4–6 (Bush et al, under review).
Strengths and limitations
The ECHO-PATHWAYS consortium has developed a rich data resource that includes prenatal bioassay results (urinary PAHs, phthalates and cotinine; plasma pCRH; and placental gene expression) as well as a biorepository with samples spanning the prenatal period through childhood. Data collected from ECHO-PATHWAYS participants include extensive survey data on both chemical and psychosocial exposures during pregnancy (maternal report only) and childhood (child and maternal report); robust neurodevelopmental and airway assessments in the early and middle childhood periods; and extensive data on potential covariates, allowing for rigorous adjustment for potential confounders and precision variables across analyses. Notably, with a gender-balanced, socioeconomically and racially diverse sample, ECHO-PATHWAYS is also well-powered to investigate effect modification by these characteristics, as well as investigate inequities in prenatal experiences and exposures that may presage notable health disparities in neurodevelopmental and airway outcomes. Moreover, ECHO-PATHWAYS has the capacity to identify prenatal and postnatal protective factors (eg, maternal nutrition during pregnancy, parenting, neighbourhood opportunity) that promote resilience and health—a key component of evidence used to inform prevention and intervention strategies.70 While outside the scope of the current paper, ECHO-PATHWAYS data have also provided an opportunity for additional research in areas of major public health concern that are beyond the primary ECHO-PATHWAYS outcome areas, including adverse pregnancy and birth outcomes as well as childhood obesity and hypertension.
One limitation of the ECHO-PATHWAYS consortium is that the component cohorts were not designed to recruit a representative sample from their respective sites, which vary in their representativeness of the underlying target population with respect to sociodemographic characteristics. Related, the cohorts also vary with respect to their demographic characteristics and relative contribution to the final sample, which we address in analyses with robust adjustment for sociodemographic characteristics, study site and site by covariate interactions when conceptually indicated. We also examine the robustness of associations to site and cohort differences by conducting leave-one-site and leave-one-cohort out sensitivity analyses. While the ongoing CANDLE, TIDES and GAPPS-PW cohorts have experienced some differential loss to follow-up, participant characteristics have remained largely similar over time with respect to key demographic characteristics including maternal and paternal education, family income and marital status.52 Finally, as with many pooled cohort studies, ECHO-PATHWAYS faces various challenges with respect to data harmonisation.71 One challenge salient to paediatric health research is harmonisation of household income, a confounder of many associations between early life exposure to stressors and child health outcomes. The ECHO-PATHWAYS team has developed a novel approach to standardising reported household income across regions with vastly different costs of living (eg, San Francisco and Memphis) so that the measure represents approximately equivalent purchasing power (Masterson et al, under review). Other pooled, multisite studies will be able to use this approach to harmonise a key predictor of many exposures and outcome, mitigating the potential for residual confounding.
Supplementary Material
Acknowledgments
We would like to thank the participating families and study team members across the The Infant Development and Environment Study (TIDES), Global Alliance to Prevent Prematurity and Stillbirth in ECHO-PATHWAYS (GAPPS-PW) and Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) cohorts. This manuscript has been reviewed by ECHO-PATHWAYS for scientific content and consistency of data interpretation with previous ECHO-PATHWAYS publications. This research was conducted using specimens and data collected and stored on behalf of the GAPPS Repository. Dr Kannan analysed phthalates, polycyclic aromatic hydrocarbons and cotinine in TIDES with support from the NYU ECHO Cohort Center (UG3/UH3OD023305) (principal investigator: Leonardo Trasande).
Footnotes
Twitter: @emilysbarrettphd
Collaborators: We would like to acknowledge the contributions of the following staff members across our multiple sites and cohorts. The following individuals contributed to data collection as part of the CANDLE study: Maureen Sorrells, April Huggins, Regina Werkhoven, Anum Abbas, Ryan Carr, L. Ashley Robinson, and Morgan Bromley. The following individuals contributed to data collection as part of the TIDES study: Stephanie Grover, Simar Singh, Beomyun Han, Zainab Ghadialy, Abigail Gelineau, Lauren Berg, Pamela Carr-Manthe, Alexandra Gowdy-Jaehnig, Andrea Hart, Annabel Victor-Halliday, and Thomson Kelly. The following individuals contributed to data collection as part of the GAPPS-PW study: Maria Hernandez, Daisy Hernandez, Madison Grady, Ifeoma Okafor, Kendall Dunlop Korsness, Janine Mifsud, Diane Bowman and Mehar Maju. Logan Dearborn contributed to data management. All non-author contributors gave permission to be included.
Contributors: All authors contributed to the design of this study. KZL, CL and SS developed the data analytical plan. CL and ES supervised data analyses and MH conducted data analyses and developed tables and figures. KZL interpreted the data and drafted the manuscript. CL, SS, CJK, KC, RN, EB, SHS, AAS, AP, PM, LY, AS, TC, NB, LST, SM, SW, AC, AMat, JP, TJ, AN-Z, AMas and NRB edited the manuscript contributing critical content expertise and/or study design knowledge. AS, TC, NB, LST, SM, SW, AC, AMat, JP and TJ also conducted study visits. Authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approve the final manuscript. KZL is responsible for the overall content as guarantor.
Funding: Research reported in this publication was supported by the ECHO-PATHWAYS consortium (NIH grants: UG300023271 and UH3OD023271), NIH grant P30ES007033 and NCATS/NIH grants: UL1 TR002319, KL2 TR002317 and TL1 TR002318. The CANDLE study is also funded by the Urban Child Institute, NIH grant 1R01HL109977, R01HL132338 and CIHR award number MWG-146331. The TIDES study is also supported by the NIH Office of Director (UG3OD023305 and UH3OD023305), NIEHS intramural funding ZIA10331 and NIH grants: R01ES016863 and R01ES25169. Air pollution data were developed under STAR research assistance agreements, RD831697 (MESA Air), RD-83830001 (MESA Air Next Stage), RD83479601 (UW Center for Clean Air Research) and R83374101 (MESA Coarse), awarded by the US Environmental Protection Agency (EPA). The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. This publication has not been formally reviewed by the EPA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This manuscript has been reviewed by ECHO-PATHWAYS for scientific content and consistency of data interpretation with previous ECHO-PATHWAYS publications.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographical or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
The data utilised for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement. Contact the corresponding author for more information. Most ECHO-PATHWAYS data has been shared with the ECHO consortium and can be used for approved ECHO analysis proposals that leverage the US NIH ECHO-wide Cohort data platform. Policies describing the use of ECHO data are available through the ECHO Coordinating Center, echocc@duke.edu.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by University of Washington Institutional Review Board: STUDY00000638. Participants gave informed consent to participate in the study before taking part.
References
- 1.Barker DJP. The origins of the developmental origins theory. J Intern Med 2007;261:412–7. 10.1111/j.1365-2796.2007.01809.x [DOI] [PubMed] [Google Scholar]
- 2.Brumberg HL, Karr CJ, COUNCIL ON ENVIRONMENTAL HEALTH . Ambient air pollution: health hazards to children. Pediatrics 2021;147:e2021051484. 10.1542/peds.2021-051484 [DOI] [PubMed] [Google Scholar]
- 3.Holm SM, Miller MD, Balmes JR. Health effects of wildfire smoke in children and public health tools: a narrative review. J Expo Sci Environ Epidemiol 2021;31:1–20. 10.1038/s41370-020-00267-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flood S, King M, Rodgers R. Data from: integrated public use microdata series, current population survey: version 9.0. IPUMS-CPS 2021.
- 5.Burns ER, Farr SL, Howards PP, et al. Stressful life events experienced by women in the year before their infants' births--United States, 2000-2010. MMWR Morb Mortal Wkly Rep 2015;64:247–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Bergh BRH, van den Heuvel MI, Lahti M, et al. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev 2020;117:26–64. 10.1016/j.neubiorev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award paper. Psychoneuroendocrinology 2015;62:366–75. 10.1016/j.psyneuen.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padula AM, Rivera-Núñez Z, Barrett ES. Combined impacts of prenatal environmental exposures and psychosocial stress on offspring health: air pollution and metals. Curr Environ Health Rep 2020;7:89–100. 10.1007/s40572-020-00273-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett ES, Padula AM. Joint impact of synthetic chemical and non-chemical stressors on children's health. Curr Environ Health Rep 2019;6:225–35. 10.1007/s40572-019-00252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J 1995;8:483–91. 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 11.Chiu Y-HM, Hsu H-HL, Coull BA, et al. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int 2016;87:56–65. 10.1016/j.envint.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loftus CT, Hazlehurst MF, Szpiro AA, et al. Prenatal air pollution and childhood IQ: preliminary evidence of effect modification by folate. Environ Res 2019;176:108505. 10.1016/j.envres.2019.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radke EG, Braun JM, Nachman RM, et al. Phthalate exposure and neurodevelopment: a systematic review and meta-analysis of human epidemiological evidence. Environ Int 2020;137:105408. 10.1016/j.envint.2019.105408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bale TL. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues Clin Neurosci 2016;18:459–64. 10.31887/DCNS.2016.18.4/tbale [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sontag-Padilla L, Burns R, Shih R. The urban child Institute candle study: methodological overview and baseline sample description. Santa Monica, CA: RAND Corporation; 2015. https://www.rand.org/content/dam/rand/pubs/research_reports/RR1300/RR1336/RAND_RR1336.pdf [Google Scholar]
- 16.Barrett ES, Sathyanarayana S, Janssen S, et al. Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur J Obstet Gynecol Reprod Biol 2014;176:119–25. 10.1016/j.ejogrb.2014.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global alliance to prevent prematurity and stillbirth. Available: https://www.gapps.org [Accessed 8 Dec 2021].
- 18.Kirwa K, Szpiro AA, Sheppard L, et al. Fine-Scale air pollution models for epidemiologic research: insights from approaches developed in the multi-ethnic study of atherosclerosis and air pollution (MESA Air). Curr Environ Health Rep 2021;8:113–26. 10.1007/s40572-021-00310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni Y, Szpiro AA, Young MT, et al. Associations of pre- and postnatal air pollution exposures with child blood pressure and modification by maternal nutrition: a prospective study in the CANDLE cohort. Environ Health Perspect 2021;129:47004. 10.1289/EHP7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazlehurst MF, Carroll KN, Loftus CT, et al. Maternal exposure to PM2.5 during pregnancy and asthma risk in early childhood: consideration of phases of fetal lung development. Environ Epidemiol 2021;5. 10.1097/ee9.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sathyanarayana S. Phthalates and children's health. Curr Probl Pediatr Adolesc Health Care 2008;38:34–49. 10.1016/j.cppeds.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 22.Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children's health. Curr Opin Pediatr 2013;25:247–54. 10.1097/MOP.0b013e32835e1eb6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan M, Mita C, Bellavia A, et al. Racial/Ethnic disparities in pregnancy and prenatal exposure to endocrine-disrupting chemicals commonly used in personal care products. Curr Environ Health Rep 2021;8:98–112. 10.1007/s40572-021-00317-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect 2011;119:878–85. 10.1289/ehp.1002727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitehead NS, Brogan DJ, Blackmore-Prince C, et al. Correlates of experiencing life events just before or during pregnancy. J Psychosom Obstet Gynaecol 2003;24:77–86. 10.3109/01674820309042805 [DOI] [PubMed] [Google Scholar]
- 26.Goldman-Mellor S, Margerison-Zilko C, Allen K, et al. Perceived and objectively-measured neighborhood violence and adolescent psychological distress. J Urban Health 2016;93:758–69. 10.1007/s11524-016-0079-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roid G, Barram R. Essentials of Stanford-Binet intelligence scales (SB5) assessment. Hoboken, NJ: John Wiley & Sons, Inc, 2004. [Google Scholar]
- 28.Wechsler D. WISC-V: technical and interpretive manual. Bloomington, MN: Pearson, 2014. [Google Scholar]
- 29.Wechsler D. WPPSI-IV: technical and interpretive manual. Bloomington, MN: Pearson, 2012. [Google Scholar]
- 30.Achenbach TM. The Achenbach system of empirically based Assessemnt (ASEBA): development, findings, theory, and applications. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families, 2009. [Google Scholar]
- 31.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families, 2000. [Google Scholar]
- 32.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families, 2001. [Google Scholar]
- 33.Smucker MR, Craighead WE, Craighead LW, et al. Normative and reliability data for the children's depression inventory. J Abnorm Child Psychol 1986;14:25–39. 10.1007/BF00917219 [DOI] [PubMed] [Google Scholar]
- 34.Sun S, Wang S. The children’s depression inventory in worldwide child development research: a reliability generalization study. J Child Fam Stud 2015;24:2352–63. 10.1007/s10826-014-0038-x [DOI] [Google Scholar]
- 35.Birmaher B, Brent DA, Chiappetta L, et al. Psychometric properties of the screen for child anxiety related emotional disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry 1999;38:1230–6. 10.1097/00004583-199910000-00011 [DOI] [PubMed] [Google Scholar]
- 36.Zelazo PD, Carlson SM. The neurodevelopment of executive function skills: implications for academic achievement gaps. Psychol Neurosci 2020;13:273–98. 10.1037/pne0000208 [DOI] [Google Scholar]
- 37.Romer AL, Pizzagalli DA. Is executive dysfunction a risk marker or consequence of psychopathology? A test of executive function as a prospective predictor and outcome of general psychopathology in the adolescent brain cognitive development study®. Dev Cogn Neurosci 2021;51:100994. 10.1016/j.dcn.2021.100994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson MC, Amso D, Anderson LC, et al. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 2006;44:2037–78. 10.1016/j.neuropsychologia.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diamond A, Barnett WS, Thomas J, et al. Preschool program improves cognitive control. Science 2007;318:1387–8. 10.1126/science.1151148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gershon RC, Wagster MV, Hendrie HC, et al. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013;80:S2–6. 10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 42.Saglani S, Menzie-Gow AN. Approaches to asthma diagnosis in children and adults. Front Pediatr 2019;7:148. 10.3389/fped.2019.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno MA. Atopic diseases in children. JAMA Pediatr 2016;170:96. 10.1001/jamapediatrics.2015.3886 [DOI] [PubMed] [Google Scholar]
- 44.Szefler SJ, Wenzel S, Brown R, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol 2012;129:S9–23. 10.1016/j.jaci.2011.12.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konwar C, Del Gobbo G, Yuan V, et al. Considerations when processing and interpreting genomics data of the placenta. Placenta 2019;84:57–62. 10.1016/j.placenta.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deyssenroth MA, Rosa MJ, Eliot MN, et al. Placental gene networks at the interface between maternal PM2.5 exposure early in gestation and reduced infant birthweight. Environ Res 2021;199:111342. 10.1016/j.envres.2021.111342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim YC, Li J, Ni Y, et al. A complex association between DNA methylation and gene expression in human placenta at first and third trimesters. PLoS One 2017;12:e0181155. 10.1371/journal.pone.0181155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sitras V, Fenton C, Paulssen R, et al. Differences in gene expression between first and third trimester human placenta: a microarray study. PLoS One 2012;7:e33294. 10.1371/journal.pone.0033294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cox B, Leavey K, Nosi U, et al. Placental transcriptome in development and pathology: expression, function, and methods of analysis. Am J Obstet Gynecol 2015;213:S138–51. 10.1016/j.ajog.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 50.Wang XK, Agarwal M, Parobchak N, et al. Mono-(2-Ethylhexyl) phthalate promotes pro-labor gene expression in the human placenta. PLoS One 2016;11:e0147013. 10.1371/journal.pone.0147013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perkins AV, Linton EA, Eben F, et al. Corticotrophin-Releasing hormone and corticotrophin-releasing hormone binding protein in normal and pre-eclamptic human pregnancies. Br J Obstet Gynaecol 1995;102:118–22. 10.1111/j.1471-0528.1995.tb09063.x [DOI] [PubMed] [Google Scholar]
- 52.Loftus CT, Bush NR, Day DB, et al. Exposure to prenatal phthalate mixtures and neurodevelopment in the conditions affecting neurocognitive development and learning in early childhood (CANDLE) study. Environ Int 2021;150:106409. 10.1016/j.envint.2021.106409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health 2006;83:1041–62. 10.1007/s11524-006-9094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeWinn KZ, Bush NR, Batra A, et al. Identification of modifiable social and behavioral factors associated with childhood cognitive performance. JAMA Pediatr 2020;174:1063–72. 10.1001/jamapediatrics.2020.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melough MM, Murphy LE, Graff JC, et al. Maternal plasma 25-hydroxyvitamin D during gestation is positively associated with neurocognitive development in offspring at age 4-6 years. J Nutr 2021;151:132–9. 10.1093/jn/nxaa309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu Z, Tylavsky FA, Kocak M, et al. Effects of maternal dietary patterns during pregnancy on early childhood growth trajectories and obesity risk: the candle study. Nutrients 2020;12:465. 10.3390/nu12020465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loftus CT, Ni Y, Szpiro AA. Exposure to ambient air pollution and early childhood behavior: a longitudinal cohort study 2020;183:109075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steine IM, LeWinn KZ, Lisha N, et al. Maternal exposure to childhood traumatic events, but not multi-domain psychosocial stressors, predict placental corticotrophin releasing hormone across pregnancy. Soc Sci Med 2020;266:113461. 10.1016/j.socscimed.2020.113461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shih EW, Ahmad SI, Bush NR, et al. A path model examination: maternal anxiety and parenting mediate the association between maternal adverse childhood experiences and children's internalizing behaviors. Psychol Med 2021:1–11. 10.1017/S0033291721001203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ni Y, Szpiro A, Loftus C, et al. Associations between maternal nutrition in pregnancy and child blood pressure at 4-6 years: a prospective study in a community-based pregnancy cohort. J Nutr 2021;151:949–61. 10.1093/jn/nxaa395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad SI, Rudd KL, LeWinn KZ, et al. Maternal childhood trauma and prenatal stressors are associated with child behavioral health. J Dev Orig Health Dis 2022;13:1–11. 10.1017/S2040174421000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmad SI, Shih EW, LeWinn KZ, et al. Intergenerational transmission of effects of women's stressors during pregnancy: child psychopathology and the protective role of parenting. Front Psychiatry 2022;13:838535. 10.3389/fpsyt.2022.838535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barrett ES, Corsetti M, Day D, et al. Prenatal phthalate exposure in relation to placental corticotropin releasing hormone (pCRH) in the CANDLE cohort. Environ Int 2022;160:107078. 10.1016/j.envint.2022.107078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paquette AG, MacDonald J, Lapehn S, et al. A comprehensive assessment of associations between prenatal phthalate exposure and the placental transcriptomic landscape. Environ Health Perspect 2021;129:97003. 10.1289/EHP8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Day DB, Collett BR, Barrett ES, et al. Phthalate mixtures in pregnancy, autistic traits, and adverse childhood behavioral outcomes. Environ Int 2021;147:106330. 10.1016/j.envint.2020.106330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudd KL, Cheng SS, Cordeiro A, et al. Associations between maternal stressful life events and perceived distress during pregnancy and child mental health at age 4. Res Child Adolesc Psychopathol 2022;50:977-986. 10.1007/s10802-022-00911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adgent MA, Carroll KN, Hazlehurst MF, et al. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ Int 2020;143:105970. 10.1016/j.envint.2020.105970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ni Y, Loftus CT, Szpiro AA, et al. Associations of pre- and postnatal air pollution exposures with child behavioral problems and cognitive performance: a U.S. multi-cohort study. Environ Health Perspect 2022;130:67008. 10.1289/EHP10248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson K, Gebretsadik T, Adgent MA, et al. The association between duration of breastfeeding and childhood asthma outcomes. Ann Allergy Asthma Immunol 2022;129:205–11. 10.1016/j.anai.2022.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huizink AC, de Rooij SR. Prenatal stress and models explaining risk for psychopathology revisited: generic vulnerability and divergent pathways. Dev Psychopathol 2018;30:1041–62. 10.1017/S0954579418000354 [DOI] [PubMed] [Google Scholar]
- 71.Lesko CR, Jacobson LP, Althoff KN, et al. Collaborative, pooled and harmonized study designs for epidemiologic research: challenges and opportunities. Int J Epidemiol 2018;47:654–68. 10.1093/ije/dyx283 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilised for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement. Contact the corresponding author for more information. Most ECHO-PATHWAYS data has been shared with the ECHO consortium and can be used for approved ECHO analysis proposals that leverage the US NIH ECHO-wide Cohort data platform. Policies describing the use of ECHO data are available through the ECHO Coordinating Center, echocc@duke.edu.