Abstract
Macrophages are the immune cells that accumulate the most in the majority of established tumors and this accumulation is associated with a poor prognosis. Tumor-associated macrophages (TAMs) produce inflammatory cytokines and growth factors that promote tumor expansion and metastasis. TAMs have recently emerged as targets of choice to restore an efficient antitumor response and to limit tumor growth. Many molecules targeting TAMs are actually evaluated in clinical trials, alone or in combination. While these molecules induce tumor regression and stimulate cytotoxic responses in mouse models of tumor development, results from early clinical trials are less impressive. In this review, we list the biological differences between human and mouse macrophages that help explain the different efficacy of antitumor strategies targeting TAMs between human and animal studies. Differences in the impact of survival and polarization factors and in the cytokines produced and markers expressed as well as the limitations of extrapolations based on in vitro models of TAM-like generation should be considered in order to improve the design and efficacy of antitumor drugs targeting TAMs.
Keywords: Macrophages; Immunity, Innate; Tumor Microenvironment; Immunotherapy
Introduction
Macrophages, which are the most abundant infiltrating immune cells in the majority of solid tumors, facilitate tumor growth and metastasis. Indeed, tumor-associated macrophages (TAMs) participate in most of the mechanisms involved in tumor growth, namely cell proliferation, angiogenesis, metastasis, immune suppression and smoldering inflammation. TAMs have thus emerged as targets and different strategies, such as functional reprogramming or inhibition of their recruitment, gave encouraging results in several preclinical models of tumor development. Nevertheless, the results in humans may be less impressive.1 2 Considering the biological differences between human and mouse macrophages and the limits of the models and markers used to assess their functions should improve the design and effectiveness of antitumor strategies based on targeting/manipulating TAMs. In this review, we highlight the differences between human and murine TAMs (summarized in table 1) that need to be considered in the design of clinical trials.
Table 1.
Differences between mouse and human macrophages that may impact the efficacy of antitumor strategies targeting TAM
| Parameters | Murine Mφ | Human Mφ | Tumor microenvironment and human TAMs | Therapeutic implications |
| Roles of GM-CSF | Induces DCs and Mφ generation | Induces Mφ generation | Present in inflammatory and tumor sites Modulates the phenotype of human TAMs |
M-CSF neutralization may boost the impact of GM-CSF and thereby favor DC generation in mice but not in human |
| Type II IFN expression | High expression | Low expression by both in vitro generated human Mφ and TAMs | Type II IFN production by murine TAMs may favor cytotoxic T lymphocyte responses and their reprogramming into antitumor Mφ | |
| M1/M1-type versus M2/M2-type polarizing factors | M1: IFNγ+LPS | M1-type: GM-CSF, GM-CSF+IFNγ M-CSF+IFNγ |
Cancers are mostly infiltrated by Th1, Th17 and Treg cells rather than Th2 cells | The M1/M2 model is not relevant for human TAM characterization |
| M2: M-CSF+IL-4 | M2-type: M-CSF+IL-4 (or IL-1 or IL-10) |
The M1/M2 classification (based on the Th1/Th2 dichotomy) and IL-4-induced M2-like cells as a model of TAMs are poorly relevant in human cancer | ||
| M1/M1-type and M2/M2-type markers | M1: iNOS, CD80, MHC-II, IFNγ | M1-type: inflammatory cytokines (IL-1β, IL-6 and TNFα), IL-12 | Human TAMs exhibit both M1 and M2 characteristics (concomitant expression of inflammatory and regulatory cytokines and of growth factors) | Prototypic mouse M1 and M2 markers cannot be used to characterize human TAMs |
| M2: Arg1, Ym1, VEGF … | M2-type: growth factors (TGF-β, VEGF, EGF, PDGF), IL-10 | |||
| Polarization versus activation | Resting (M2) and LPS-stimulated (M1) cells are usually compared | Human Mφ and TAMs require to be stimulated (such as via CD40 or TLRs) to reveal their phenotypes | Human Mφ polarization and activation are two independent processes | |
Arg1, arginase 1; DCs, dendritic cells; EGF, epithelial growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; M-CSF, macrophage colony-stimulating factor; MHC-II, major histocompatibility complex class II; Mφ, macrophage; PDGF, platelet-derived growth factor; PRR, pattern recognition receptors; TAMs, tumor-associated macrophages; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Macrophages, a heterogeneous and multifunctional cell population
Macrophages are myeloid cells of the innate immune system, present in almost all tissues. They perform diverse and essential functions. In addition to their antimicrobial activity, they ensure the maintenance of tissue homeostasis and control all phases of healing and tissue repair. They are also involved in the initiation and resolution of the inflammation and can present antigens to memory (but not naive) T cells.3
Resident macrophages exhibit highly specialized functions (eg, Kupffer cells in the liver or microglia in the central nervous system). Monocytes can also be recruited to inflamed or injured tissues, where they undergo local differentiation into macrophages. This process is a critical step in their functional polarization, as the phenotype they acquire is driven by the nature of the differentiation signals present in their environment. Moreover, macrophages are plastic, meaning that they continuously adapt their phenotype to the demands of the tissue, as they permanently screen their environment.4 5 A multitude of factors/signals, such as microbes, cytokines, changes in nutrients or metabolite concentrations, extracellular pH, oxygen tension and pressure alteration, induces metabolic and epigenetic modifications that are associated with their functional adaptation. Depending on the factors they encounter, macrophages can thus adopt a multiplicity of phenotypes, which makes it difficult to classify them according to their phenotype.6 However, and although incomplete and simplifying, the classification of proinflammatory M1 versus reparative M2 macrophages remains widely used.
As detailed later in this review, the M1/M2 classification was originally defined by in vitro generated murine macrophage subsets and is based on well characterized phenotypic signatures. However, such signatures are rarely, if ever, observed in human macrophages. In order to circumvent this limitation, and despite being simplistic, a classification of human macrophages into M1-like and M2-like subtypes has been proposed. In this review, we will use these terms as a functional classification (antitumor M1-like vs protumor M2-like cells).
TAMs: a complex diversity
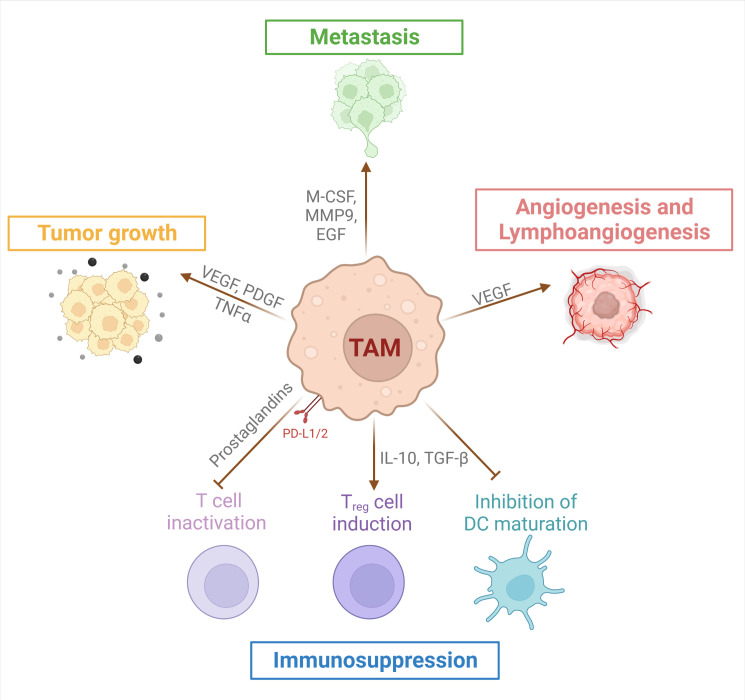
In the majority of established tumors, macrophages accumulate in larger numbers than the other immune cells7–10 and their accumulation is associated, in most cancers, with a poor prognosis.7 11 12 TAMs favor tumor growth and metastasis, angiogenesis and lymphangiogenesis and tumor-associated inflammation, and decrease effector CD8+ T-cell recruitment and function13–16 (figure 1).
Figure 1.
Protumor roles of tumor-associated macrophages (TAMs). TAMs secrete factors into the tumor microenvironment and express membrane molecules that promote tumorigenesis by favoring tumor cell proliferation, angiogenesis/lymphoangiogenesis, metastasis and immunosuppression. TAMs mediate immunosuppression through induction of regulatory T cells (Treg), inactivation of effector T cell function and inhibition of dendritic cell (DC) maturation. EGF, epithelial growth factor; IL, interleukin; M-CSF, macrophage colony-stimulating factor; MMP, matrix metalloprotease; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
TAMs derive from both resident tissue macrophages and newly recruited monocytes.17 18 In a mouse model of lung cancer, lineage tracing experiments revealed that TAMs are initially composed of resident macrophages that acquire protumor functions and proliferate, and that, in later phases of tumor development, TAMs are predominantly composed of recruited monocytes that have differentiated locally.18 While the proliferation of murine macrophages has been widely reported,19–21 that of human macrophages remains poorly documented.22–24 In humans, the importance of monocyte recruitment in maintaining pools of TAMs was recently demonstrated in patients with cancer after bone marrow transplantation.25 Moreover, it appears obvious that the density of TAMs in a fast-growing tissue may probably result from the recruitment of monocytes rather than a massive expansion of resident macrophages whose proliferative capacity remains questionable in humans.
Many molecules targeting TAMs are currently in clinical trials, either alone or in combination with chemotherapy, radiotherapy or immunotherapy (especially immune checkpoint inhibitors (ICI)).26–29 TAM-targeting approaches, described in recent reviews, are of two types: those preventing TAM accumulation and/or survival (eg, by inhibiting the macrophage colony-stimulating factor (M-CSF)/CD115 or C-C motif chemokine ligand 2 (CCL2)/CCR2 axis)30 31 and those making use of the phagocytic properties of TAMs (eg, blocking the SIRP1α/CD47 interaction) or of their cytotoxic activity.30–33 These strategies decrease tumor growth and stimulate cytotoxic CD8+ T-cell responses in combination with chemotherapy, radiotherapy, or ICI in different mouse models of tumor development. Based on these results, clinical trials aiming to evaluate the impact of strategies targeting TAMs are currently underway. Here, we provide an overview of some of the key observations that need to be considered when extrapolating results obtained in mouse models to humans.
Differences in the processes of generation and functional polarization of mouse and human macrophages
Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the generation of dendritic cells in mice and macrophages in humans
As discussed earlier, the accumulation of TAMs in established tumors results principally from monocyte recruitment induced by attracting molecules, including mainly M-CSF (CSF-1) and CCL2 (also known as MCP-1). This process can be also induced by other chemokines, such as C-X-C chemokine receptor type 4 (CXCR4) and CXCR5 ligands.13–15 A number of clinical trials are assessing neutralization of CD115 (or c-fms), the receptor for M-CSF and interleukin (IL)-34, as a means of preventing TAM accumulation. In mouse models, neutralization of the M-CSF–IL-34/CD115 axis acts in synergy with chemotherapy and ICI to reduce tumor growth and to favor CD8+ T-cell responses.26 34 However, clinical trials in humans have given disappointing results1 35 and some TAM subsets seem resistant to this approach.22 It also seems likely that in vivo neutralization of the M-CSF–IL-34/CD115 axis amplifies the impact of GM-CSF (also known as CSF-2) on myeloid cells.36
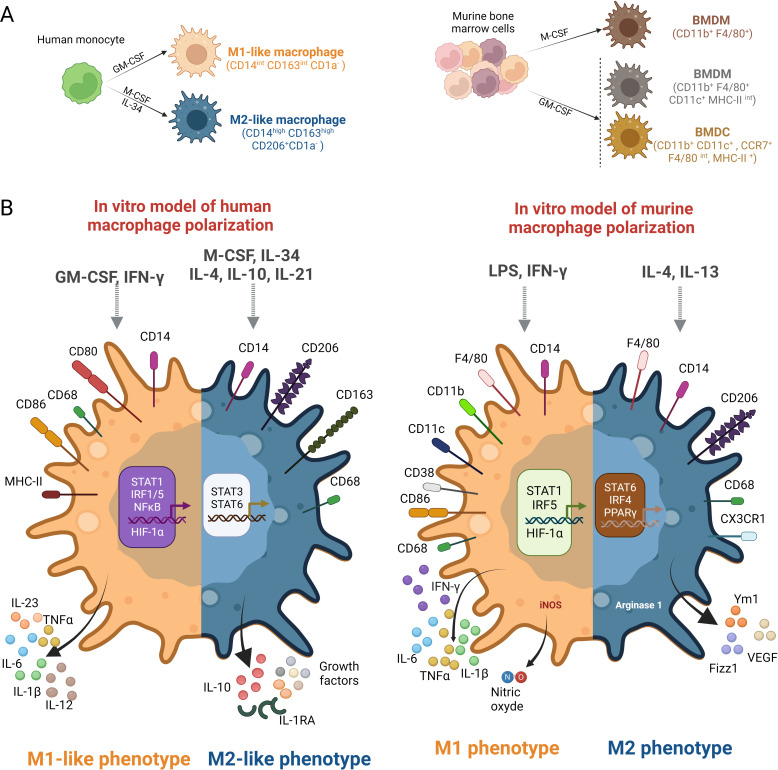
Importantly, GM-CSF has different effects on myeloid cell differentiation in human and mice.37 M‐CSF is constitutively expressed in many tissues, whereas GM-CSF is present principally at sites of inflammation, including tumor sites, as it is produced by activated immune and non-immune cells and by some tumors.37–39 GM-CSF triggers the differentiation of murine myeloid precursors into mature dendritic cells,40 41 and favors antitumor responses by facilitating dendritic cell recruitment and activation.37 42 For example, in a murine melanoma model, vaccination with irradiated melanoma cells engineered to secrete GM-CSF stimulates robust and long-lasting antitumor immunity.43 44 Conversely, GM-CSF neutralization affects murine dendritic cell recruitment in vivo.45 Based on these observations, GM-CSF has been used in dendritic cell-based tumor vaccine trials as an inducer of dendritic cell maturation, and is currently included in antitumor immunotherapies.42 However, in humans, both M-CSF and GM-CSF promote monocyte survival and differentiation into macrophages (figure 2A, left panel). M-CSF triggers in vitro the generation of regulatory macrophages that maintain tissue homeostasis, whereas GM-CSF induces the generation of macrophages that produce inflammatory cytokines (including IL-6, IL-1β and tumor necrosis factor α) on stimulation (figure 2B), the production of these mediators being upregulated by interferon gamma (IFNγ).37 38 46 47 Moreover, high concentrations of GM-CSF promote the recruitment of immunosuppressive murine myeloid cells within tumors.39 48 This, and the opposite effects of GM-CSF on the differentiation of human and murine myeloid precursors into macrophages and dendritic cells, respectively, may provide some explanation for the poor efficacy of GM-CSF as an adjuvant for antitumor vaccines in humans.42
Figure 2.
Differences in the generation and polarization of human and murine macrophages. (A) Left panel, human macrophages are usually generated in vitro from peripheral blood monocytes cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) (in the presence or absence of IFNγ) or macrophage colony-stimulating factor (M-CSF)/interleukin (IL)-34 (in the presence or absence of IL-4) to generate M1 and M2 cells, respectively. To reveal their phenotype, human macrophages must be activated. Right panel: Murine macrophages are primarily generated from bone marrow myeloid cell progenitors maintained in M-CSF. The use of GM-CSF leads to the generation of both dendritic cells and macrophages.87 (B) Left panel, human markers of macrophage polarization are shown. Right panel: murine markers of macrophage polarization. BMDC, bone-marrow derived dendritic cells; BMDM, bone-marrow derived macrophages; HIF-1α, hypoxia inducible factor; IFNg, Interferon-gamma; IRF, Interferon regulatory factor; LPS, lipopolysaccharide; MHC-II, major histocompatibility complex class II; STAT, Signal transducer and activator of transcription.
Importantly, a recent study reported, in a mouse model of breast-to-brain metastasis, that the inhibition of CD115 induced a compensatory increase of GM-CSF synthesis by pericytes that induced the acquisition by TAM of an inflammatory and tissue repair profile.49 One can thus suspect that, in vivo, the neutralization of the M-CSF–IL-34/CD115 axis may exacerbate the role of GM-CSF, which would then promote the generation, in humans, of inflammatory macrophages favoring tumor recurrence.
Moreover, we have recently shown that the simultaneous presence of GM-CSF and lactic acid, a metabolite that accumulates in glycolytic tumors, induces the generation of macrophages producing inflammatory cytokines and tumor-promoting factors that display similarities to the ovarian cancer TAMs.50 Another study also reported that lactic acid increases the expression of VEGF by murine macrophages.51 These results suggest that some features of TAMs are induced independently of M-CSF, a prototypic M2 inducer, contributing to explain the lack of sensitivity to CD115 blockade of some TAM subsets, such as angiogenic TAMs.52
It could be argued that, in the presence of IL-4 and/or IL-13, GM-CSF induces the differentiation of human monocytes into dendritic cells.53 While lactic acid is frequently found in cancers, the expression of IL-4 or IL-13 has only been reported in a restricted number of cancers, and mouse, but not human myeloid cells, express IL-4 and IL-13, excluding an autocrine role in the generation of human TAMs. Collectively, these results suggest that the role of IL-4 and IL-13 in the functional polarization of human TAM should be limited.54
It should be noted that other strategies aiming to reduce macrophage accumulation by neutralizing the CCL2/CCR2, CXCL12 (or SDF1)/CXCR4 or IL-1/IL-1 receptor axes, together with strategies reducing the recruitment of neutrophil precursors, which exhibit suppressive properties (eg, by targeting the C5a receptor, by jointly neutralizing CCR2 and CCR5, or by using a CD11b agonist), are also currently evaluated in clinical trials.55 56
Given the difference in factors controlling dendritic cell and macrophage generation between humans and mice, the impact of these strategies on the recruitment and differentiation of precursors and/or activation of human macrophages (as well as of dendritic cells) should systematically be evaluated.
Polarization and activation of human macrophages should be distinguished as two independent processes
Macrophages express different phenotypes, depending on the signals present in the environment. In vitro experiments with human monocytes have shown that this process, referred to as polarization, is time dependent.57 Moreover, human macrophages require stimulation (via CD40 or using TLR agonists) to reveal their cytokine profile (except those constitutively produced, such as CCL2).46 50 For murine M1 and M2 cells, the terms polarization and activation are not dissociated, and resting and activated cells are compared in studies using murine cells.
Myeloid cell activators, including TLR agonists, are currently evaluated in clinical trials as inducers of TAM reprogramming into antitumor cells.26 30 Importantly, TLR9 is expressed by murine but not human myeloid cells, a fundamental difference that may profoundly impact the consequences of TLR9 agonists on macrophage polarization.
TLRs and CD40 agonists induce dendritic cell maturation and facilitate the generation of a protective antitumor response. In contrast, the activation of TAM is likely to enhance the production of immunosuppressive cytokines and growth factors, thus strengthening their protumor phenotype. Thus, acting on both polarization and activation may be required to reprogram human TAMs into antitumor cells. Repolarization could be achieved by modulating the metabolic and/or cytokine microenvironment. Consistent with the view of tumors as a wound that never heals,58 we and others have shown that factors classically associated with tissue lesions, such as lactic acid, contribute to the phenotype of TAMs.50 51 Various molecules affecting tumor cell metabolism (by inhibiting glycolysis or other metabolic pathways) are currently in clinical trials59 60; the impact of these molecules, used alone or, preferentially, in combination with other TAM-targeting molecules, should also be studied on human TAMs. Combination treatments that repolarize TAMs and activate TAMs and dendritic cells should be beneficial.
Phenotypic differences between human and mouse macrophages/TAMs
Mouse and human macrophages differ in their capacity to produce IFNγ
IFNγ, which has an important role in immune surveillance, facilitates the generation and activation of Th1 lymphocytes and the initiation of cytotoxic T lymphocyte responses. IFNγ is also a potent activator of myeloid cells: it polarizes dendritic cells into IL-12-producing cells (known as DC1), thereby facilitating the generation of Th1 lymphocytes. Previously known as macrophage activating factor, IFNγ induces the generation of inflammatory macrophages that are potentially cytotoxic to tumor cells. IFNγ may also have protumor effects through a feedback effect. For example, IFNγ helps to maintain peripheral tolerance by increasing PD-L1 expression by tumor cells.
Due to its potent antitumor activity, IFNγ has been used in the treatment of certain cancers and is currently being tested, in association with other antitumor molecules, in clinical trials.61 It is currently thought that the dose and duration of IFNγ treatment need to be established, to optimize its beneficial effect when combined with antitumor therapies.61 Nevertheless, while murine macrophages are IFNγ-producing cells (figure 2B, right panel), human myeloid cells produce IFNγ at a very low level compared with lymphoid cells62 63 and, importantly, the combination IL-12 plus IL-18 appears as the most effective in inducing IFNγ production by human macrophages. Of note, IL-12, a prototypic antitumor cytokine,64 is poorly expressed in most tumors and IL-18 exhibits protumor activity (favoring cancer progression, metastasis, angiogenesis and immune suppression).65 This will make unlikely an in situ reprogramming of human TAM into antitumor cells in the absence of IFNγ-producing lymphoid cells. Moreover, the activation/reprogramming by IFNγ may lower the efficacy of anti-PD-1/-PD-L1 antibody treatment (consecutive to an upregulation of PD-L1 expression by tumor cells), in contrast to the beneficial impact of combining TAM activation (that induces IFNγ production) and PD1 targeting in murine models of tumor development.66
The M1/M2 model does not reflect the phenotypic diversity of TAMs
Murine TAMs are commonly characterized by transcriptome analysis, thanks to the specific expression of genes defined as ‘M2/protumor’ and ‘M1/antitumor’ cells, according to the M1/M2 classification which distinguishes macrophages differentiated in the presence of M-CSF with either IL-4 (M2) or IFNγ plus LPS (M1) (figure 2A, right panel).67
Although IL-4 and IL-13 expressions have been detected in some cancers,68–70 the use of macrophages generated in the presence of IL-4 as a model for protumor macrophages is of little physiological relevance, as discussed earlier. The M1/M2 classification was based on the Th1/Th2 dichotomy, itself derived from an analysis of the physiological mechanisms associated with autoimmune diseases in mice of different genetic backgrounds. The pathophysiology of autoimmune diseases is now characterized by a Th1–Th17/Treg imbalance rather than a Th1/Th2 imbalance. Similarly, cancer development is associated with a sustained inflammation and profound immune suppression involving Th1, Th17 and Treg cells, rather than Th2 accumulation. Moreover, although the expression of IL-4 and IL-13 has been detected in some cancers, M2 cells generated in the presence of IL-4 are considered as prototypic tissue repair macrophages rather than bona fide protumor macrophages.
Macrophages play an essential role in controlling all stages of the tissue repair process,3 but the expression of IL-4 or IL-13 is not a characteristic of healing areas, and other factors present locally in the injured areas, such as IL-21, IL-25 and IL-33,71 may also confer a wound healing phenotype to macrophages.72–74 Thus, the study of genes associated with M2 cells generated in the presence of IL-4 are of more relevance in mouse models of allergic reactions or helminth infections than in cancers or wound-healing process.
Most of the prototypic murine M1 and M2 markers are not suitable for the characterization of human macrophages
The phenotypes of macrophage subsets drastically differ between human and mouse (figure 2B). Human M1-like macrophages are characterized by an elevated expression of membrane CD80 and CD86 and by the secretion of inflammatory cytokines and IL-12. In contrast, murine M1 macrophages express CD11c, CD11b and CD38 and produce nitric oxide in addition to proinflammatory cytokines. Human M2-like macrophages are characterized by an elevated expression of CD163 and CD206 and by the production of IL-10 and of several growth factors when murine M2 macrophages are defined as arginase 1 (Arg1)-expressing and VEGF-expressing cells. Moreover, the genes overexpressed in the presence of IL-4 or IFNγ greatly differ between human and mouse macrophages.67 73 75 Either there are no human homologs of particular genes (eg, Ym1 and Fizz1) or their expression is differentially regulated. The most widely used signature is the expression of inducible nitric oxide synthase by murine M1 cells and of Arg1 by murine M2 cells, two molecules not expressed by human macrophages.67 76 More generally, there is little correspondence between the genes upregulated by IL-4 in human and mouse macrophages.61 In addition, most of the genes used to characterize murine M1 and M2 cells have no known function, making it difficult to extrapolate their protumor versus antitumor functions. Reinforcing this result, Zilionis et al nicely demonstrated that, in lung tumors, TAMs exhibit species-specific profiles,77 emphasizing the importance of characterizing human macrophages instead of extrapolating from mouse phenotypes. Finally, the transcription factors involved in the polarization of M1-like and M2-like macrophages differ between human and mouse (figure 2B).
The most informative and reliable way to characterize the function of human macrophages is based on the profiling of soluble mediators, including growth factors, inflammatory cytokines and immunosuppressive molecules. This approach is all the more relevant as cumulative single-cell analysis studies in different tumors revealed/confirmed that human TAM exhibit mixed M1-like and M2-like signatures.50 78–82 Surface markers on human macrophages display lower levels of modulation than cytokine production, and, when modulated (eg, CD163, which reflects M-CSF consumption; CD206, which reflects IL-4 consumption), their expression is not predictive of their protumor or antitumor functions.
Concluding remarks
In conclusion, the different impacts of GM-CSF on human versus murine myeloid cell differentiation, the absence of IFNγ and TLR9 expression by human macrophages and the limits of models using IL-4 as an inducer of repair macrophages are all features that distinguish human macrophages from mouse macrophages and that must be considered when translating from the preclinical to the clinical therapeutic application.
All these information have to be taken account to optimize strategies that directly target TAM, especially with the emergence of promising new antitumor therapies that promote the phagocytic activity of macrophages by targeting the CD47/SIRPα axis, as recently reported for B-cell lymphoma and some solid tumors.83–86
Footnotes
MM and LP contributed equally.
Correction notice: This article has been corrected. The affiliations of Alberto Mantovani have been updated.
Contributors: PJ conceived and supervised this study. MM and LP drafted the manuscript and the figures. AM and YD provided critical revision of the manuscript. All authors read and approved the submitted version.
Funding: This study did not receive specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work was realized in the context of (i) the LabEx ImmunoGraftOnco program (National Research Agency; ANR-11-LABX-0016-01) and (ii) the University Hospital of Angers - University of Angers joint program (project 3I-Impact).
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Gomez-Roca CA, Italiano A, Le Tourneau C, et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol 2019;30:1381–92. 10.1093/annonc/mdz163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noel M, O'Reilly EM, Wolpin BM, et al. Phase 1B study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest New Drugs 2020;38:800–11. 10.1007/s10637-019-00830-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010;184:3964–77. 10.4049/jimmunol.0903356 [DOI] [PubMed] [Google Scholar]
- 4.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol 2016;17:9–17. 10.1038/ni.3320 [DOI] [PubMed] [Google Scholar]
- 5.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol 2020;15:123–47. 10.1146/annurev-pathmechdis-012418-012718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014;40:274–88. 10.1016/j.immuni.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938–45. 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787–95. 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39:782–95. 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity 2019;51:411–2. 10.1016/j.immuni.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 11.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 2018;17:887–904. 10.1038/nrd.2018.169 [DOI] [PubMed] [Google Scholar]
- 12.Sinha VC, Rinkenbaugh AL, Xu M, et al. Single-cell evaluation reveals shifts in the tumor-immune niches that shape and maintain aggressive lesions in the breast. Nat Commun 2021;12:5024. 10.1038/s41467-021-25240-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399–416. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allavena P, Sica A, Garlanda C, et al. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 2008;222:155–61. 10.1111/j.1600-065X.2008.00607.x [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–55. 10.1016/s1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 16.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41:49–61. 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loyher P-L, Hamon P, Laviron M, et al. Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med 2018;215:2536–53. 10.1084/jem.20180534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casanova-Acebes M, Dalla E, Leader AM, et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 2021;595:578–84. 10.1038/s41586-021-03651-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013;38:792–804. 10.1016/j.immuni.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein I, Cornejo JC, Polakos NK, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood 2007;110:4077–85. 10.1182/blood-2007-02-073841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chorro L, Sarde A, Li M, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med 2009;206:3089–100. 10.1084/jem.20091586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Li Z, Skrzypczynska KM, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 2020;181:e29:442–59. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Wang Y, Chu Y, et al. Tumor-derived adenosine promotes macrophage proliferation in human hepatocellular carcinoma. J Hepatol 2021;74:627–37. 10.1016/j.jhep.2020.10.021 [DOI] [PubMed] [Google Scholar]
- 24.Yang N, Isbel NM, Nikolic-Paterson DJ, et al. Local macrophage proliferation in human glomerulonephritis. Kidney Int 1998;54:143–51. 10.1046/j.1523-1755.1998.00978.x [DOI] [PubMed] [Google Scholar]
- 25.Kurashige M, Kohara M, Ohshima K, et al. Origin of cancer-associated fibroblasts and tumor-associated macrophages in humans after sex-mismatched bone marrow transplantation. Commun Biol 2018;1:131. 10.1038/s42003-018-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anfray C, Ummarino A, Andón FT, et al. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells 2019;9. 10.3390/cells9010046. [Epub ahead of print: 23 12 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 2019;12:76. 10.1186/s13045-019-0760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowal J, Kornete M, Joyce JA. Re-education of macrophages as a therapeutic strategy in cancer. Immunotherapy 2019;11:677–89. 10.2217/imt-2018-0156 [DOI] [PubMed] [Google Scholar]
- 29.Pittet MJ, Michielin O, Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol 2022;19:402–21. 10.1038/s41571-022-00620-6 [DOI] [PubMed] [Google Scholar]
- 30.Piechutta M, Berghoff AS. New emerging targets in cancer immunotherapy: the role of cluster of differentiation 40 (CD40/TNFR5). ESMO Open 2019;4:e000510. 10.1136/esmoopen-2019-000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takimoto CH, Chao MP, Gibbs C, et al. The Macrophage 'Do not eat me' signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol 2019;30:486–9. 10.1093/annonc/mdz006 [DOI] [PubMed] [Google Scholar]
- 32.Jalil AR, Andrechak JC, Discher DE. Macrophage checkpoint blockade: results from initial clinical trials, binding analyses, and CD47-SIRPα structure-function. Antib Ther 2020;3:80–94. 10.1093/abt/tbaa006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Huang Q, Xiao W, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front Immunol 2020;11:18. 10.3389/fimmu.2020.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peranzoni E, Lemoine J, Vimeux L, et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci U S A 2018;115:E4041–50. 10.1073/pnas.1720948115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesolowski R, Sharma N, Reebel L, et al. Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors. Ther Adv Med Oncol 2019;11:1758835919854238. 10.1177/1758835919854238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pradel LP, Ooi C-H, Romagnoli S, et al. Macrophage susceptibility to Emactuzumab (RG7155) treatment. Mol Cancer Ther 2016;15:3077–86. 10.1158/1535-7163.MCT-16-0157 [DOI] [PubMed] [Google Scholar]
- 37.Jeannin P, Paolini L, Adam C, et al. The roles of CSFs on the functional polarization of tumor-associated macrophages. Febs J 2018;285:680–99. 10.1111/febs.14343 [DOI] [PubMed] [Google Scholar]
- 38.Hamilton JA, Cook AD, Tak PP. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat Rev Drug Discov 2016;16:53–70. 10.1038/nrd.2016.231 [DOI] [PubMed] [Google Scholar]
- 39.Bronte V, Chappell DB, Apolloni E, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 40.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992;176:1693–702. 10.1084/jem.176.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helft J, Böttcher J, Chakravarty P, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity 2015;42:1197–211. 10.1016/j.immuni.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 42.Yan W-L, Shen K-Y, Tien C-Y, et al. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017;9:347–60. 10.2217/imt-2016-0141 [DOI] [PubMed] [Google Scholar]
- 43.Borrello I, Pardoll D. GM-CSF-based cellular vaccines: a review of the clinical experience. Cytokine Growth Factor Rev 2002;13:185–93. 10.1016/s1359-6101(01)00034-x [DOI] [PubMed] [Google Scholar]
- 44.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 1993;90:3539–43. 10.1073/pnas.90.8.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louis C, Cook AD, Lacey D, et al. Specific contributions of CSF-1 and GM-CSF to the dynamics of the mononuclear phagocyte system. J Immunol 2015;195:134–44. 10.4049/jimmunol.1500369 [DOI] [PubMed] [Google Scholar]
- 46.Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007;110:4319–30. 10.1182/blood-2007-02-072587 [DOI] [PubMed] [Google Scholar]
- 47.Lacey DC, Achuthan A, Fleetwood AJ, et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol 2012;188:5752–65. 10.4049/jimmunol.1103426 [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharya P, Budnick I, Singh M, et al. Dual role of GM-CSF as a pro-inflammatory and a regulatory cytokine: implications for immune therapy. J Interferon Cytokine Res 2015;35:585–99. 10.1089/jir.2014.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klemm F, Möckl A, Salamero-Boix A, et al. Compensatory CSF2-driven macrophage activation promotes adaptive resistance to CSF1R inhibition in breast-to-brain metastasis. Nat Cancer 2021;2:1086–101. 10.1038/s43018-021-00254-0 [DOI] [PubMed] [Google Scholar]
- 50.Paolini L, Adam C, Beauvillain C, et al. Lactic acidosis together with GM-CSF and M-CSF induces human macrophages toward an inflammatory protumor phenotype. Cancer Immunol Res 2020;8:383–95. 10.1158/2326-6066.CIR-18-0749 [DOI] [PubMed] [Google Scholar]
- 51.Colegio OR, Chu N-Q, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014;513:559–63. 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Li Z, Skrzypczynska KM, et al. Single-cell analyses inform mechanisms of Myeloid-Targeted therapies in colon cancer. Cell 2020;181:e29:442–59. 10.1016/j.cell.2020.03.048 [DOI] [PubMed] [Google Scholar]
- 53.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994;179:1109–18. 10.1084/jem.179.4.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Junttila IS. Tuning the cytokine responses: an update on Interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol 2018;9:888. 10.3389/fimmu.2018.00888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou W, Guo S, Liu M, et al. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr Med Chem 2019;26:3026–41. 10.2174/0929867324666170830111531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panni RZ, Herndon JM, Zuo C, et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med 2019;11. 10.1126/scitranslmed.aau9240. [Epub ahead of print: 03 07 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preisser L, Miot C, Le Guillou-Guillemette H, et al. Il-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology 2014;60:1879–90. 10.1002/hep.27328 [DOI] [PubMed] [Google Scholar]
- 58.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res 2015;3:1–11. 10.1158/2326-6066.CIR-14-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Counihan JL, Grossman EA, Nomura DK. Cancer metabolism: current understanding and therapies. Chem Rev 2018;118:6893–923. 10.1021/acs.chemrev.7b00775 [DOI] [PubMed] [Google Scholar]
- 60.Luengo A, Gui DY, Vander Heiden MG. Targeting metabolism for cancer therapy. Cell Chem Biol 2017;24:1161–80. 10.1016/j.chembiol.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaidi MR. The interferon-gamma paradox in cancer. J Interferon Cytokine Res 2019;39:30–8. 10.1089/jir.2018.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pietilä TE, Veckman V, Kyllönen P, et al. Activation, cytokine production, and intracellular survival of bacteria in Salmonella-infected human monocyte-derived macrophages and dendritic cells. J Leukoc Biol 2005;78:909–20. 10.1189/jlb.1204721 [DOI] [PubMed] [Google Scholar]
- 63.Darwich L, Coma G, Peña R, et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology 2009;126:386–93. 10.1111/j.1365-2567.2008.02905.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb Perspect Biol 2018;10:a028530. 10.1101/cshperspect.a028530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park S, Cheon S, Cho D. The dual effects of interleukin-18 in tumor progression. Cell Mol Immunol 2007;4:329–35. [PubMed] [Google Scholar]
- 66.Ma HS, Poudel B, Torres ER, et al. A CD40 agonist and PD-1 antagonist antibody reprogram the microenvironment of Nonimmunogenic tumors to allow T-cell-mediated anticancer activity. Cancer Immunol Res 2019;7:428–42. 10.1158/2326-6066.CIR-18-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez FO, Helming L, Milde R, et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 2013;121:e57–69. 10.1182/blood-2012-06-436212 [DOI] [PubMed] [Google Scholar]
- 68.Gaggianesi M, Turdo A, Chinnici A, et al. IL4 primes the dynamics of breast cancer progression via DUSP4 inhibition. Cancer Res 2017;77:3268–79. 10.1158/0008-5472.CAN-16-3126 [DOI] [PubMed] [Google Scholar]
- 69.Srabovici N, Mujagic Z, Mujanovic-Mustedanagic J, et al. Interleukin 13 expression in the primary breast cancer tumour tissue. Biochem Med 2011;21:131–8. 10.11613/bm.2011.021 [DOI] [PubMed] [Google Scholar]
- 70.Suzuki A, Leland P, Joshi BH, et al. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine 2015;75:79–88. 10.1016/j.cyto.2015.05.026 [DOI] [PubMed] [Google Scholar]
- 71.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44:450–62. 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serezani APM, Bozdogan G, Sehra S, et al. IL-4 impairs wound healing potential in the skin by repressing fibronectin expression. J Allergy Clin Immunol 2017;139:142–51. 10.1016/j.jaci.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daley JM, Brancato SK, Thomay AA, et al. The phenotype of murine wound macrophages. J Leukoc Biol 2010;87:59–67. 10.1189/jlb.0409236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ingersoll MA, Spanbroek R, Lottaz C, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010;115:e10–19. 10.1182/blood-2009-07-235028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raes G, Van den Bergh R, De Baetselier P, et al. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol 2005;174:6561–2. 10.4049/jimmunol.174.11.6561 [DOI] [PubMed] [Google Scholar]
- 77.Zilionis R, Engblom C, Pfirschke C, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 2019;50:e10:1317–34. 10.1016/j.immuni.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moganti K, Li F, Schmuttermaier C, et al. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology 2017;222:952–9. 10.1016/j.imbio.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 79.Chevrier S, Levine JH, Zanotelli VRT, et al. An immune atlas of clear cell renal cell carcinoma. Cell 2017;169:e18:736–49. 10.1016/j.cell.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Azizi E, Carr AJ, Plitas G, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 2018;174:e36:1293–308. 10.1016/j.cell.2018.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Müller S, Kohanbash G, Liu SJ, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol 2017;18:234. 10.1186/s13059-017-1362-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitsi E, Kamng’ona R, Rylance J, et al. Human alveolar macrophages predominately express combined classical M1 and M2 surface markers in steady state. Respir Res 2018;19:66. 10.1186/s12931-018-0777-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y-C, Shi W, Shi J-J, et al. Progress of CD47 immune checkpoint blockade agents in anticancer therapy: a hematotoxic perspective. J Cancer Res Clin Oncol 2022;148:1–14. 10.1007/s00432-021-03815-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi R, Chai Y, Duan X, et al. The identification of a CD47-blocking "hotspot" and design of a CD47/PD-L1 dual-specific antibody with limited hemagglutination. Signal Transduct Target Ther 2020;5:16. 10.1038/s41392-020-0121-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dheilly E, Moine V, Broyer L, et al. Selective blockade of the ubiquitous checkpoint receptor CD47 is enabled by dual-targeting bispecific antibodies. Mol Ther 2017;25:523–33. 10.1016/j.ymthe.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu B, Guo H, Xu J, et al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs 2018;10:315–24. 10.1080/19420862.2017.1409319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Helft J, Böttcher J, Chakravarty P, et al. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity 2015;42:1197–211. 10.1016/j.immuni.2015.05.018 [DOI] [PubMed] [Google Scholar]