Abstract
Objective
There is limited experience regarding the use of biological disease-modifying antirheumatic drug (bDMARD) and JAK inhibitor (JAKi) for the management of immune checkpoint inhibitors (ICI)-induced inflammatory arthritis. We aimed to assess their efficacy and safety in this setting.
Methods
Using the Club Rhumatismes and Inflammation French network, we conducted a multicentre, retrospective, observational study of patients with cancer diagnosed with inflammatory arthritis under ICI(s) and treated with bDMARD or JAKi. Clinical data were collected using a standardised case report form.
Results
Twenty patients (60% men, median age 69.5 years) were included, with rheumatoid arthritis (RA)-like (n=16), polymyalgia rheumatica-like (n=2) or psoriatic arthritis-like (n=2) clinical presentation. Two patients had pre-existing RA. 90% were treated with glucocorticoids as first-line therapy and 60% received methotrexate prior to bDMARD or JAKi. Anti-interleukin-6 receptor (IL-6R) therapy was used in 13/20 patients (65%), leading to clinical improvement in 11/13 patients (85%), but one patient experienced intestinal perforation and cancer progression was noticed in 6/13 patients (46%). Tumour necrosis factor inhibitors were used in 5/20 patients (25%), with improvement in 4/5 patients (80%) and cancer progression was observed in 3/5 patients (60%). Two infections (diverticulitis and pneumonitis) were reported. Anakinra, baricitinib and ustekinumab were each used in one patient. Median duration of the bDMARD or JAKi was 17 weeks.
Conclusion
Anti-IL-6R therapy is currently the most common strategy in patients with ICI-induced inflammatory arthritis and insufficient response to glucocorticoids and methotrexate, leading to improvement in >80%. Overall, cancer progression occurred in about half of patients and whether the bDMARD/JAKi impacted the tumour response remains to be determined.
Keywords: arthritis, biological therapy, immune system diseases, therapeutics
WHAT IS ALREADY KNOWN ON THIS TOPIC
Rheumatic immune-related adverse events (irAEs) mimic classical rheumatic musculoskeletal diseases but there is limited experience regarding use of biological disease-modifying antirheumatic drugs (bDMARDs) and JAK inhibitor (JAKi) in this setting.
WHAT THIS STUDY ADDS
The use of anti-interleukin-6 receptor (IL-6R) is currently the most common strategy in patients with severe immune checkpoint inhibitors (ICI)-induced inflammatory arthritis, with improvement in >80% and no specific safety signal.
ICI is usually held and rarely administrated concurrently with anti-IL-6R.
The use of JAKi and bDMARDs other than anti-IL-6R remains scarce in patients with ICI-induced inflammatory arthritis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
This study supports clinical trials evaluating the use anti-IL-6R therapy and its optimal timing in the management of rheumatic irAEs.
Introduction
Checkpoint inhibitors targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or programmed cell death-(ligand) 1 (PD-1/PD-L1) coinhibitory receptors are increasingly used to treat various types of advanced cancers. By releasing the brakes on T-cell activation, they can be responsible for inflammatory side effects, the so-called immune-related adverse events (irAEs), that can affect every organ system.1 While described rather rarely in clinical trials, rheumatic and musculoskeletal symptoms are observed in 5%–10% of patients with cancer treated with immune checkpoint inhibitors (ICI).2 Rheumatoid arthritis (RA)-like and polymyalgia rheumatica (PMR)-like clinical presentations are frequently reported, which sometimes overlap between the two entities.3 4 First-line therapy of ICI-induced inflammatory arthritis is based on glucocorticoids, as recommended by several oncological and EULAR guidelines.5 6 Although most patients respond well to low-to-moderate dose of prednisone allowing ICI continuation, few patients require treatment escalation with conventional and/or biological disease-modifying antirheumatic drugs (DMARDs) owing to severe irAE or as steroid-sparing strategy. So far, few data are available regarding the use of bDMARDs and JAK inhibitor (JAKi) in this setting, therefore we aimed to assess their effectiveness and tolerance in patients with ICI-induced inflammatory arthritis.
Patients and methods
We conducted a multicentre, retrospective, observational study using the Club Rhumatismes and Inflammation (CRI) French network. A national call for observations was launched from February 2021 to February 2022 in order to identify patients with cancer treated with ICI who experienced ICI-induced inflammatory arthritis requiring the use of bDMARD or JAKi. French rheumatology and internal medicine practitioners were contacted by electronic newsletters and through a dedicated website to collect cases. After patient consent, characteristics, treatments and outcomes of both rheumatic irAE and cancer were collected by the treating physician through a standardised case report form. Notably, data assessed were the cancer type and its outcomes, the type of ICI(s) used, its starting date, whether ICI regimen was discontinued or not following the rheumatic irAE, the type of rheumatic irAE, the presence of a pre-existing autoimmune disease, the bDMARD or targeted synthetic DMARD (tsDMARD) used with its start and end date, the initial and lower dosage of glucocorticoids, the use of conventional synthetic DMARD(s) (csDMARD(s)) prior or concomitant to bDMARD/JAKi and the side effects potentially related to bDMARD/JAKi use. Tumour response was assessed with Response Evaluation Criteria in Solid Tumours criteria and irAE response was based on clinical judgement of the treating physician and classified as no improvement, partial improvement or resolution.
Results
Patient characteristics
Twenty patients have been identified including 60% of men, with a median age of 69.5 years, and a diagnosis of lung cancer (n=11), melanoma (n=7), renal carcinoma (n=1) or lymphoma (n=1) (table 1). All patients were receiving an anti-PD-1 (nivolumab or pembrolizumab), in combination with an anti-CTLA-4 (ipilimumab) for two patients. The median time between ICI initiation and the rheumatic irAE onset was 22 weeks, ranging from 1 to 156 weeks. The type of inflammatory arthritis was RA-like (n=16), PMR-like (n=2) or psoriatic arthritis-like (n=2). Of note, two patients had pre-existing RA and experienced flare. Only two patients with de novo inflammatory arthritis (RA-like pattern) tested positive for rheumatoid factor and one had anti-cyclic citrullinated peptide(CCP) antibodies. Most patients (95%) were treated with glucocorticoids as first-line therapy and 12/20 patients (60%) received a csDMARD (methotrexate) before the bDMARD use, the latter stopped in seven patients at bDMARD initiation.
Table 1.
Patient characteristics, n (%)
| Age, median (range), year | 69.5 (48–77) |
| Gender | |
| Male | 12 (60) |
| Female | 8 (40) |
| Tumour type | |
| Lung | 11 (55) |
| Melanoma | 7 (35) |
| Renal | 1 (5) |
| Lymphoma | 1 (5) |
| Cancer therapy | |
| Anti-PD-1 | 18 (90) |
| Combination anti-CTLA-4/anti-PD-1 | 2 (10) |
| Pre-existing autoimmune disease | |
| No | 18 (90) |
| Yes (RA) | 2 (10) |
| Type of rheumatic irAE | |
| RA-like | 16 (80) |
| PMR-like | 2 (10) |
| PsA-like | 2 (10) |
| Type of bDMARD/tsDMARD used | |
| Anti-IL-6R | 13 (65) |
| Tocilizumab | 12 (57) |
| Sarilumab | 1 (5) |
| TNF inhibitors | 5 (25) |
| Etanercept | 2 (9.5) |
| Adalimumab | 2 (9.5) |
| Infliximab | 1 (5) |
| Others | 3 (15) |
| Anakinra | 1 (5) |
| Baricitinib | 1 (5) |
| Ustekinumab | 1 (5) |
| ICI discontinuation | |
| No | 3 (15) |
| Temporarily | 5 (25) |
| Permanently* | 12 (60) |
*For other irAE (n=2: colitis and pneumonitis).
bDMARD, biologic disease-modifying antirheumatic drug; CTLA-4, cytotoxic T-lymphocyte antigen 4; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed cell death protein 1; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; RA, rheumatoid arthritis; TNF, tumour necrosis factor; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
bDMARD/JAKi used
Anti-interleukin-6 receptor (IL-6R) therapy was prescribed for 13/20 patients (65%), mostly tocilizumab (n=12, 60%), all but one with subcutaneous administration, and tumour necrosis factor (TNF) inhibitors were used for 5/20 patients (25%) (adalimumab n=2, etanercept n=2 and infliximab n=1). Anakinra, baricitinib and ustekinumab were each prescribed for one patient. Median duration of bDMARD/JAKi was 17 weeks, still ongoing for 10 patients. Overall, the use of bDMARD or JAKi led to complete (7/20; 35%) or partial (10/20; 50%) resolution of the rheumatic irAE and glucocorticoids were stopped (6/20, 30%) or reduced (7/20; 35%) following bDMARD initiation. Four patients (20%), all with RA-like phenotype, did not have improvement while receiving tocilizumab (n=2), etanercept (n=1) or anti-IL-1 (n=1), with only one patient receiving a second targeted DMARD (baricitinib after tocilizumab failure). At the last follow-up, 8/20 patients (40%) were still receiving the bDMARD or JAKi with ongoing tumour response (table 2).
Table 2.
Characteristics, management and outcomes of rheumatic irAEs
| Patient | Rheumatic irAE | Time to onset (weeks) | bDMARD/ tsDMARD | Previous treatment for irAE | bDMARD/ tsDMARD duration (weeks) | irAE outcome | Cancer outcome | GC tapering (mg/day) |
| 1 | RA-like | 131 | Etanercept | GC, MTX | 86 (ongoing) | Resolution | Remission | 0 |
| 2 | RA-like | 22 | Infliximab | GC, MTX | 8 | Resolution | Progression | 0 |
| 3 | RA-like | 14 | Tocilizumab | GC, MTX | 8 | Partial improvement | Progression | 20 |
| 4 | RA-like | 16 | Tocilizumab | GC | 34 | Partial improvement | Progression* | 10 |
| 5 | PMR-like | 3 | Tocilizumab | GC | 53 (ongoing) | Partial improvement | Stable | 2 |
| 6 | RA-like | 24 | Tocilizumab | GC, MTX | 69 (ongoing) | Resolution | Remission | 0 |
| 7 | RA-like | 2 | Tocilizumab | GC, MTX | 8 | Resolution | Progression* | 10 |
| 8 | PMR-like | 48 | Tocilizumab | GC, MTX | 48 (ongoing) | Resolution | Remission | 0 |
| 9 | PsA-like | 8 | Tocilizumab | NSAIDs, GC, MTX | 43 (ongoing) | Partial improvement | Progression | 2.5 |
| 10 | RA-like | 33 | 1/Tocilizumab 2/Baricitinib | GC, MTX | 1/6 2/78 (ongoing) |
1/No improvement 2/Resolution | Progression | 0 |
| 11 | PsA-like | 34 | Ustekinumab | GC | 60 (ongoing) | Partial improvement | Stable | 5 |
| 12 | RA-like | 20 | Adalimumab | GC, MTX | 8 (ongoing) | Resolution | Remission | 0 |
| 13 | RA-like | 26 | Adalimumab | GC | 34 | Partial improvement | Progression | 5 |
| 14 | RA-like | 16 | Tocilizumab | GC, MTX | 8 | No improvement | Remission | 5 |
| 15 | RA-like | 64 | Tocilizumab | GC, MTX | 17 | Partial improvement | Progression | 5 |
| 16 | RA-like | 156 | Tocilizumab | GC | 8 (ongoing) | Partial improvement | Stable | 5 |
| 17 | RA-like | 22 | Sarilumab | NSAIDs, GC, MTX | 12 (ongoing) | Partial improvement | Partial response | 7.5 |
| 18 | RA flare | 0 | Tocilizumab | GC | 22 | Partial improvement | Progression* | 15 |
| 19 | RA-like | 69 | Anakinra | Colchicine | 1 | No improvement | Stable | 0 |
| 20 | RA flare | 1 | Etanercept | GC | 8 | No improvement | Progression* | 80 |
*Death.
bDMARD, biologic disease-modifying antirheumatic drug; GC, glucocorticoids; irAE, immune-related adverse event; MTX, methotrexate; NSAIDs, non-steroidal anti-inflammatory drugs; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; RA, rheumatoid arthritis; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
The bDMARD was discontinued in 7 patients after improvement or resolution of the irAE, with a median duration of bDMARD of 17 weeks, and with only 1 patient experiencing a flare (patient 1). Indeed, this patient discontinued ICI for RA-like irAE and received almost 1 year later etanercept during 4 months with resolution of irAE then restarted 1 month later due to relapse.
Oncological outcomes
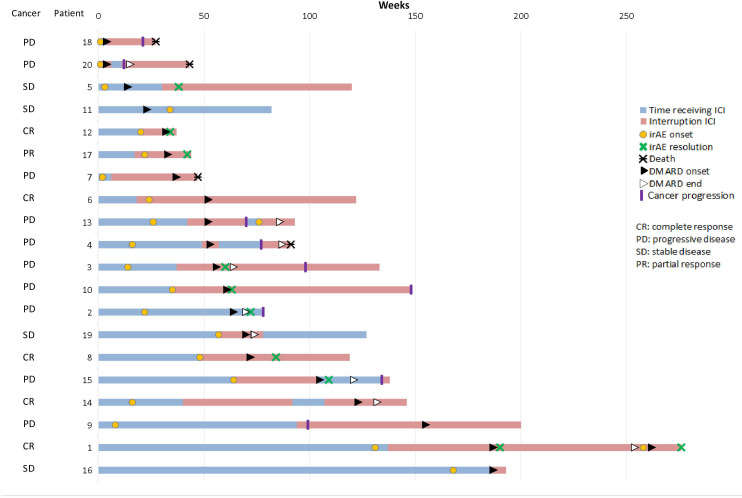
Regarding tumour response, 6 of 20 patients (30%) had an objective response, either complete (n=5) or partial (n=1), and 4 patients (20%) had stable disease. Cancer progression was observed in 10 patients (50%), which occurred prior to bDMARD initiation in 6 patients, and led to death in 4 patients treated with tocilizumab (n=3) or etanercept (n=1) (figure 1).
Figure 1.
Swimmer plot representing time receiving immune checkpoint inhibitor (ICI), onset and resolution of rheumatic immune-related adverse event (irAE) and use of disease-modifying antirheumatic drug (DMARD). Cancer response is mentioned on the left side.
Three patients continued ICI treatment in combination with bDMARD, with one receiving the combination anti-PD-1+anti-CTLA-4 relayed to anti-PD-1 monotherapy. ICI was discontinued permanently in 12/20 patients (60%) due to the rheumatic irAE (n=10) or another toxicity (n=2), and temporarily in 5/20 patients (25%). Among these five patients rechallenged with ICI, in combination with bDMARD, only one had to permanently discontinue ICI owing to rheumatic irAE relapse despite ongoing adalimumab treatment.
Outcomes according to the type of targeted DMARD
Description of irAEs and patient’s outcomes according to the type of bDMARD or JAKi used is summarised in table 3. Anti-IL-6R partially or completely resolves rheumatologic irAE in 11/13 patients (85%) with cancer progression reported in almost half of patients (6/13; 46%), stable disease in 3/13 patients (23%) and partial or complete cancer response in 4/13 patients (31%). TNF inhibitors (TNFi) partially or completely resolve 4/5 patients (80%) of rheumatologic irAE when prescribed in this setting. There was cancer progression in 3/5 patients (60%) and remission in 2/5 patients (40%). Anakinra was used daily during 1 week in a patient with ICI-induced RA-like presentation, with no efficacy on irAE and no impact on tumour outcomes. JAKi (baricitinib) was used as second-line therapy after tocilizumab failure (patient 10) allowing a complete resolution of RA-like symptoms but the cancer progressed. Ustekinumab was used in a patient with pre-existing psoriasis who experienced de novo arthritis under pembrolizumab after worsening of psoriasis lesions. Effectiveness was noticed on psoriasis but less on arthritis, requiring addition of methotrexate with stable oncological outcomes.
Table 3.
Patient and irAE outcomes according to bDMARD/tsDMARD type
| bDMARD/ tsDMARD | Type of cancer | Type of irAE | DMARD duration (median, weeks) | Association with ICI | Safety | Efficacy | Cancer outcomes |
| Anti-IL-6R | Lung (n=6) Melanoma (n=6) Renal (n=1) |
RA-like (n=10) | 17 (6 ongoing) | Yes (n=3) | 0 | Partial improvement (n=3) | Progression (n=2) Stable (n=1) |
| PMR-like (n=2) | No (10) | Small intestine perforation (n=1) Septic shock (n=1) (ongoing chemotherapy) |
Resolution (n=3) Partial improvement (n=5) No efficacy (n=2) |
Progression (n=4) Stable (n=2) Partial response (n=1) Remission (n=3) |
|||
| PsA-like (n=1) | |||||||
| TNF inhibitors | Lung (n=4) Lymphoma (n=1) |
RA-like (n=5) | 8 (2 ongoing) | Yes (n=3) | Diverticulitis (n=1) Pneumonitis (n=1) |
Resolution (n=1) Partial improvement (n=1) No efficacy (n=1) |
Progression (n=3) |
| No (n=2) | 0 | Resolution (n=2) | Remission (n=2) | ||||
| Anakinra | Melanoma | RA-like (n=1) | 1 | Yes | 0 | No efficacy | Stable |
| Baricitinib | Lung | RA-like (n=1) | 78 (ongoing) | No | 0 | Resolution | Progression |
| Ustekinumab | Lung | PsA-like (n=1) | 60 (ongoing) | Yes | 0 | Partial improvement | Stable |
bDMARD, biologic disease-modifying antirheumatic drug; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; RA, rheumatoid arthritis; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
Considering patients treated with TNFi and anti-IL-6R who experienced cancer progression, 4/9 (44%) had already objective tumour progression before bDMARD onset (3 patients receiving anti-IL-6R and 1 receiving TNFi). Methotrexate use was reported in 5/9 patients (56%) with cancer progression and in 6/8 patients (75%) without cancer progression. Glucocorticoids were stopped in 4/8 (50%) who had good oncological outcomes and in 1/9 patients (11%) with cancer progression. Proportion of patients according to tumour type was similar between patients experiencing cancer progression and those who did not. The median duration of TNFi or anti-IL-6R use was 30 weeks in patients with good oncological outcomes and 17 weeks in patients with cancer progression.
Safety
There were few side effects related to bDMARD/JAKi use, with two infections (diverticulitis and pneumonitis) reported under TNFi and one intestinal perforation revealing an unknown small intestine metastasis after two injections of tocilizumab, leading to definitive discontinuation of the bDMARD. One patient experienced septic shock while receiving tocilizumab and chemotherapy, 6 months after ICI discontinuation. No death was related to DMARD use.
Discussion
This national observational study revealed that the use of bDMARD and JAKi in the setting of rheumatic irAEs remains scarce, but most often improved or resolved irAEs after glucocorticoids and/or methotrexate failure. Anti-IL-6R therapy was mostly used, followed by TNFi, while other bDMARDs (anti-IL-1, anti-IL-12/IL-23) and JAK inhibitors were each used in one patient. For eight patients, the bDMARD was used in combination with ICI, immediately or when ICI was resumed, and there were no safety signals. However, caution is advised with the combination of bDMARD and chemotherapy since one patient had septic shock.
Our study is in accordance with the nationwide Canadian Research Group of Rheumatology in Immuno-Oncology cohort, reporting the need of bDMARD in only 3 of 117 patients with rheumatic irAEs (2 TNFi and 1 rituximab).7 Furthermore, the use of targeted therapy concurrently to ICI has been safely reported in seven patients with rheumatic irAE or pre-existing RA, with an average duration of 4.5 months.8 In this series, TNFi (adalimumab or infliximab) and tocilizumab were also mostly used and one patient received tofacitinib, all after systemic glucocorticoids failure and methotrexate for three patients. Cancer progression was more frequently reported under TNFi than under IL-6R inhibitors, but the small number of patients and heterogeneity in cancer type does not allow to draw firm conclusions regarding the impact of bDMARD type on tumour response.
Concurrent therapy with TNFi and ICIs has been successfully and safely reported by gastroenterologists in patients with ICI-related colitis.9 Overall, there are reassuring retrospective data about a temporary use of TNFi to treat irAEs. However, some studies raise concerns about its impact on tumour response as slightly better outcomes were reported under vedolizumab compared with infliximab.10 11 The use of tocilizumab has also been successfully reported in various irAEs, including few patients with rheumatic irAEs.12–14 Nevertheless, rheumatic irAE may persist several weeks or months even after ICI discontinuation and there is limited experienced regarding long-term use of TNF and IL-6R inhibitors for irAE management.15 Our study brings data on the use of other targeted DMARDs (anti-IL-1, anti-IL-12/IL-23) but single cases do not allow any conclusion regarding effectiveness, safety and tumour outcomes. We did not identify patients with rheumatic irAE treated with anti-IL-17 or anti-IL-23 therapy, but their use has been reported in this setting.16 The use of JAKi has also been reported in few patients with ICI-induced colitis, arthritis and myocarditis.17 However, the current European Medicines Agency and Food and Drug Administration warning on JAKi in patients with cancer as well as some concerns regarding tumour response when blocking T helper (Th)1 immunity prompt caution in using JAKi for irAEs. The importance of JAK signalling in anti-PD-1 response is also discussed with the identification of clonal selection for JAK1 or JAK2 inactivating mutations in few patients with melanoma experiencing acquired resistance to anti-PD-1 therapy.18
Combination of bDMARD and ICI is being actively studied by oncologists, that will inform rheumatologists about the safety of such combination and its impact on tumour response. Encouraging preclinical data showing a synergistic effect of TNFi with ICIs led to the phase Ib TNF-Inhibitor as Immune Checkpoint Inhibitor for Advanced MELanoma(TICIMEL) trial combining either infliximab or certolizumab to anti-CTLA-4 and anti-PD-1 in patients with metastatic melanoma.19 20 Both triple combinations were safe and resulted in an increased Th1 immune response which likely promote antitumour response, particularly noticed in the certolizumab cohort with all patients considered as responders. Similarly, the use of IL-6R inhibitors in an experimental model of ICI-treated tumour showed a decoupling effect by increasing antitumour response while mitigating toxicity.21 First data of an ongoing phase II clinical trial assessing the combination of tocilizumab with nivolumab and ipilimumab (NCT03999749) are promising with a reduction of severe irAEs and a similar or possibly increased tumour response compared with patients without tocilizumab.22 Combination of anti-PD-1 and rituximab has been safely used in patients with relapsed follicular lymphoma but recent insights into the role of B cells in the response to ICI would deserve further data on such combination.23 24
Glucocorticoids are recommended as first-line therapy in all guidelines for management of irAEs, including rheumatic irAEs. Despite reassuring data on their use for managing irAEs, there are concerns about their potential impact on tumour response, notably with dosage >10 mg/day of prednisone equivalent.25 26 The use of csDMARDs has been reported in ICI-induced inflammatory arthritis as glucocorticoids-sparing strategy, mostly methotrexate, with a median follow-up of more than a year and no specific signal on tumour outcomes.15 27 Hydroxychloroquine and sulfasalazine use was also reported in this setting.28 29 Further research is needed to assess csDMARDs impact on tumour response and one should also consider a longer delay in the onset of response compared with bDMARD/JAKi. Our study confirms that the use of bDMARD/JAKi allowed glucocorticoids sparing, therefore may support the paradigm shift currently discussed.30 Indeed, owing to encouraging preclinical data and ongoing oncological trials, one may discuss the use of targeted therapy, mainly bDMARD, earlier in the management of rheumatic irAE to avoid long-term use of glucocorticoids. This approach is also discussed in patients with pre-existing autoimmune disease by preferring targeted therapies to non-selective immunosuppressive therapies at ICI initiation.31 Prospective clinical trials will likely assess such strategy in the future.
Our study acknowledges some limitations, beyond its retrospective design, with reporting and investigator biases. First, patients were identified through a national network and reported on a voluntary basis, which is not exhaustive. Second, the treating physician decided on the therapeutic strategy, notably the type of DMARD (routine care, no funding). While discussion with oncologist was reported for all patients, reasons regarding the choice of a specific DMARD are unknown. There were no comorbidities reported but some patients experienced concomitant irAE, which probably influenced the choice of bDMARD. Of note, such patients (concomitant colitis, concomitant pneumonitis) were treated with anti-IL-6. Furthermore, there was neither specific classification criteria nor standardised assessment of disease activity, which was based on clinical judgement of the treating physician. Finally, our series included a high proportion of patients with lung cancer, who tend to have worse prognosis, and this may be independent of any DMARD received.
To conclude, collaborative efforts are definitively needed to share experience and develop clinical trials of bDMARD/tsDMARD use in ICI-induced inflammatory arthritis, notably with anti-IL-6R, being currently the most common strategy.
Acknowledgments
We thank the Club Rhumatismes and Inflammation (CRI) French network for his support in conducting this national observational study.
Footnotes
Twitter: @crichez33, @mariekostine
Contributors: All the authors contributed to the manuscript: conception and design (MK, CR, MET, TS), collection of data (all authors) and analyses (FDLF, MK). FDLF and MK drafted the manuscript, and all the authors critically reviewed and approved the final version of the manuscript. MK is the guarantor of this study, accepting full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Research Ethics Committee of the University Hospital of Bordeaux (CER-BDX-2022-23).
References
- 1. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020;6:38. 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018;77:393–8. 10.1136/annrheumdis-2017-212257 [DOI] [PubMed] [Google Scholar]
- 3. Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol 2018;14:569–79. 10.1038/s41584-018-0074-9 [DOI] [PubMed] [Google Scholar]
- 4. Calabrese C, Cappelli LC, Kostine M, et al. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD Open 2019;5:e000906. 10.1136/rmdopen-2019-000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kostine M, Finckh A, Bingham CO, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis 2021;80:36–48. 10.1136/annrheumdis-2020-217139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts J, Ennis D, Hudson M, et al. Rheumatic immune-related adverse events associated with cancer immunotherapy: a nationwide multi-center cohort. Autoimmun Rev 2020;19:102595. 10.1016/j.autrev.2020.102595 [DOI] [PubMed] [Google Scholar]
- 8. Calabrese C, Esfahani K, Calabrese L. Concomitant Targeted Therapy with Ongoing Immune Checkpoint Inhibitor (ICI) Therapy for Severe Immune Related Adverse Events (irAEs): Clinical Experience from Two Centers [abstract]. Arthritis Rheumatol 2021;73 https://acrabstracts.org/abstract/concomitant-targeted-therapy-with-ongoing-immune-checkpoint-inhibitor-ici-therapy-for-severe-immune-related-adverse-events-iraes-clinical-experience-from-two-centers/ [Google Scholar]
- 9. Badran YR, Cohen JV, Brastianos PK, et al. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer 2019;7:226. 10.1186/s40425-019-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lesage C, Longvert C, Prey S, et al. Incidence and clinical impact of Anti-TNFα treatment of severe immune checkpoint inhibitor-induced colitis in advanced melanoma: the Mecolit survey. J Immunother 2019;42:175–9. 10.1097/CJI.0000000000000268 [DOI] [PubMed] [Google Scholar]
- 11. Zou F, Faleck D, Thomas A, et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer 2021;9:e003277. 10.1136/jitc-2021-003277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2019;25:551–7. 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
- 13. Kim ST, Tayar J, Trinh VA, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017;76:2061–4. 10.1136/annrheumdis-2017-211560 [DOI] [PubMed] [Google Scholar]
- 14. Dimitriou F, Hogan S, Menzies AM, et al. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer 2021;157:214–24. 10.1016/j.ejca.2021.08.031 [DOI] [PubMed] [Google Scholar]
- 15. Braaten TJ, Brahmer JR, Forde PM, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis 2020;79:332–8. 10.1136/annrheumdis-2019-216109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma VT, Lao CD, Fecher LA, et al. Successful use of secukinumab in two melanoma patients with immune checkpoint inhibitor-induced inflammatory arthropathy. Immunotherapy 2022;14:593–8. 10.2217/imt-2021-0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson Berg M-H, Del Rincón SV, Miller WH. Potential therapies for immune-related adverse events associated with immune checkpoint inhibition: from monoclonal antibodies to kinase inhibition. J Immunother Cancer 2022;10:e003551. 10.1136/jitc-2021-003551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016;375:819–29. 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bertrand F, Montfort A, Marcheteau E, et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun 2017;8:2256. 10.1038/s41467-017-02358-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montfort A, Filleron T, Virazels M, et al. Combining nivolumab and ipilimumab with infliximab or Certolizumab in patients with advanced melanoma: first results of a phase Ib clinical trial. Clin Cancer Res 2021;27:1037–47. 10.1158/1078-0432.CCR-20-3449 [DOI] [PubMed] [Google Scholar]
- 21. Hailemichael Y, Johnson DH, Abdel-Wahab N, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022;40:509–23. 10.1016/j.ccell.2022.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber JS, Muramatsu T, Hamid O, et al. 1040O phase II trial of ipilimumab, nivolumab and tocilizumab for unresectable metastatic melanoma. Ann Oncol 2021;32:S869–905. 10.1016/j.annonc.2021.08.1425 [DOI] [Google Scholar]
- 23. Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 2014;15:69–77. 10.1016/S1470-2045(13)70551-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020;577:549–55. 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Draghi A, Borch TH, Radic HD, et al. Differential effects of corticosteroids and anti-TNF on tumor-specific immune responses: implications for the management of irAEs. Int J Cancer 2019;145:1408–13. 10.1002/ijc.32080 [DOI] [PubMed] [Google Scholar]
- 26. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 27. Leipe J, Christ LA, Arnoldi AP, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open 2018;4:e000714. 10.1136/rmdopen-2018-000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts J, Smylie M, Walker J, et al. Hydroxychloroquine is a safe and effective steroid-sparing agent for immune checkpoint inhibitor-induced inflammatory arthritis. Clin Rheumatol 2019;38:1513–9. 10.1007/s10067-019-04451-2 [DOI] [PubMed] [Google Scholar]
- 29. Ford M, Sahbudin I, Filer A, et al. High proportion of drug hypersensitivity reactions to sulfasalazine following its use in anti-PD-1-associated inflammatory arthritis. Rheumatology 2018;57:2244–6. 10.1093/rheumatology/key234 [DOI] [PubMed] [Google Scholar]
- 30. Chatzidionysiou K, Liapi M, Tsakonas G, et al. Treatment of rheumatic immune-related adverse events due to cancer immunotherapy with immune checkpoint inhibitors-is it time for a paradigm shift? Clin Rheumatol 2021;40:1687–95. 10.1007/s10067-020-05420-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haanen J, Ernstoff MS, Wang Y, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol 2020;31:724–44. 10.1016/j.annonc.2020.03.285 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.