Abstract
Background
The IONESCO (IFCT-1601) trial assessed the feasibility of neoadjuvant durvalumab, for early-stage resectable non-small-cell lung cancer (NSCLC).
Methods
In a multicenter, single-arm, phase II trial, patients with IB (≥4 cm)-IIIA, non-N2, resectable NSCLC received three doses of durvalumab (750 mg every 2 weeks) and underwent surgery between 2 and 14 days after the last infusion. The primary endpoint was the complete surgical resection rate. Secondary endpoints included tumor response rate, major histopathological response (MPR: ≤10% remaining viable tumor cells), disease-free survival (DFS), overall survival (OS), durvalumab-related safety, and 90-day postoperative mortality (NCT03030131).
Results
Forty-six patients were eligible (median age 60.9 years); 67% were male, 98% were smokers, and 41% had squamous cell carcinoma. Regarding tumor response, 9% had a partial response, 78% had stable disease, and 13% had progressive disease. Among the operated patients (n=43), 41 achieved complete resection (89%, 95% CI 80.1% to 98.1%)), and eight achieved MPR (19%). The 12-month median OS and DFS rates were 89% (95% CI 75.8% to 95.3%) and 78% (95% CI 63.4% to 87.7%), respectively (n=46). The median follow-up was 28.4 months (12.8–41.1). All patients in whom MPR was achieved were disease-free at 12 months compared to only 11% of those with >10% residual tumor cells (p=0.04). No durvalumab-related serious or grade 3–5 events were reported. The unexpected 90-day postoperative mortality of four patients led to premature study termination. None of these four deaths was considered secondary to direct durvalumab-related toxicity.
Conclusions
Neoadjuvant durvalumab given as monotherapy was associated with an 89% complete resection rate and an MPR of 19%. Despite an unexpectedly high rate of postoperative deaths, which prevented us from completing the trial, we were able to show a significant association between MPR and DFS.
Keywords: Immunotherapy; Lung Neoplasms; Biomarkers, Tumor; Programmed Cell Death 1 Receptor
WHAT IS ALREADY KNOWN ON THIS TOPIC
The role of immune checkpoint inhibitors (ICIs) in early-stage resectable non-small-cell lung cancer (NSCLC) is unclear. Phase III studies with neoadjuvant ICIs in combination with chemotherapy or as adjuvant monotherapy after chemotherapy are positive. This phase II study tested durvalumab as neoadjuvant in patients with localized NSCLC.
WHAT THIS STUDY ADDS
There was a significant association between major pathological response (MPR) and disease-free survival (DFS), despite a small number of patients due to an early termination of the study because of a high 90-day postoperative mortality rate. This mortality was related to postoperative complications in a population with cardiovascular and respiratory comorbidities.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The association between MPR and DFS shown in this study is an additional argument for using MPR as a possible surrogate marker for neoadjuvant treatment with immunotherapy as a single agent.
The high rate of death due to postoperative complications suggests the need to better select patients with few comorbidities and operative risk factors for these immunotherapy-based neoadjuvant strategies.
Background
The role of immunotherapy in patients with early-stage resectable non-small-cell lung cancer (NSCLC) is unclear. A statistically significant improvement in disease-free survival (DFS) with atezolizumab, an anti-PD-L1 therapy, following surgery and chemotherapy has been shown in the interim analysis of the phase III IMPower010 study for patients with resectable stage II-IIIA NSCLC with positive PD-L1 expression.1 More recently, a second phase III trial, the PEARLS/KEYNOTE-091 study, showed significant improvement in DFS with pembrolizumab, an anti-PD-1 therapy, in adjuvant setting in patients with resectable stage II-IIIA NSCLC regardless of PD-L1 expression.2 With regard to the neoadjuvant setting, nivolumab plus chemotherapy was found to increase the pathological complete response (pCR) rate compared with chemotherapy alone in the phase III CheckMate-816 trial.3 More recently, significant improvement in event-free survival (EFS) was reported with a 37% reduction in the risk of progression, recurrence or death.3 Compared with adjuvant therapy, the superior efficacy of neoadjuvant therapy has been suggested in animal model studies.4 Moreover, histopathological responses such as major pathological response (MPR) may be used as surrogate markers of effectiveness of neoadjuvant treatments, since overall survival (OS) has been associated with MPR following neoadjuvant cisplatin-based chemotherapy,5 although this association has not been demonstrated for immunotherapy as a single agent. Multiple phase II studies using neoadjuvant immunotherapy (anti-PD-L1, anti-PD-1 or anti-CTLA4 antibody) have shown encouraging signals.6–10
With the IONESCO trial, we aimed to assess the feasibility of neoadjuvant treatment with single-therapy durvalumab, a human monoclonal anti-PD-L1 antibody, for early-stage resectable NSCLC.
Methods
Study design
We performed a multicenter, prospective, single-arm, phase II trial of durvalumab as neoadjuvant treatment in patients with early-stage, resectable NSCLC. Patient enrollment lasted 32 months, and the follow-up period was 1 year. The study protocol was approved by the Comité de Protection and the French Health Authority (ANSM).
Study population
The main inclusion criteria were histologically confirmed diagnosis of NSCLC, classified as stage IB (only ≥4 cm), IIA, IIB, or IIIA non-N2. There was no patient selection based on PD-L1 expression. Patients with an Eastern Cooperative Oncology Group performance status of 0–1 were eligible. Neoadjuvant platinum-based or other chemotherapy and preoperative radiation therapy were not allowed. Anti-cancer therapy after surgery was at the discretion of the investigator. Adjuvant platinum-based chemotherapy and adjuvant radiation were allowed, according the current guidelines. Full inclusion and exclusion criteria are described in the study protocol.
Drug administration
Patients received durvalumab (750 mg) via 60 min intravenous infusion on days 1, 15, and 29. Then, they underwent surgical resection between 2 and 14 days after the last infusion. No premedication was needed.
Clinical assessments
Primary endpoint
The primary endpoint was complete surgical resection, defined as the complete removal of the tumor, with no microscopic evidence of cancerous cells at any of the resected margins (R0). Complete resection was evaluated via histopathological assessment of paraffin-embedded tissue.
Secondary endpoints
Secondary endpoints included the time between the first durvalumab infusion and surgery; the tumor response rate according to Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1; MPR, defined as ≤10% remaining viable tumor cells in the primary tumor (MPR includes complete pathological response, which was defined as tumors without any viable tumor cells in the resected lung cancer specimen and all sampled regional lymph nodes); DFS, defined as the time from inclusion to tumor recurrence or death; OS, defined as the time from inclusion to death of any cause; safety and tolerance to durvalumab; postoperative adverse events (AEs, occurring up to 4 weeks after surgery); and 90-day postoperative mortality.
Tumor response was assessed by radiological review, mainly based on a contrast-enhanced CT scan. FDG-PET was also performed after durvalumab administration and prior to surgery. Responses were evaluated locally by each investigator. MPR was assessed with surgical tissue specimens (tumor and lymph nodes) by two thoracic expert pathologists using a semiquantitative method described by Cottrell and colleagues.11 All surgically removed lymph nodes were analyzed. Safety and tolerance to durvalumab were monitored for 100 days after the last treatment. AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events V.4.0.
Data analysis
The sample size was calculated based on α, β, and the expected effect size using a test for single binomial proportions with a two-stage design and O’Brien-Fleming stopping rules, which allow early termination for futility. East V.6.0 software was used. A complete resection rate (primary endpoint) of ≤85% (P0) was considered unacceptable, while a 95% complete resection rate was considered good (P1). Therefore, the computation was based on the following assumptions: P0=85%; P1=95%; statistical power of 0.90; and a type I error rate (one-sided) of 0.05. The null hypothesis (the rate of complete resection is P0=85%) was tested against a one-sided alternative and was rejected if ≥71 complete resections were observed in 77 eligible patients (≥92%). First, 39 eligible patients were to be recruited, and if 34 or fewer complete resections were achieved in these 39 patients, the study would be terminated for futility. Considering a 5% patient exclusion rate, 81 patients were planned to be included.
We censored follow-up on October 1, 2020. Median follow-up was calculated with the reverse Kaplan-Meier method. The probability of survival was estimated using the Kaplan-Meier method.
Descriptive statistics were performed on the intention-to-treat (ITT) population (all included patients), the efficacy population (eligible patients without any major deviations from the inclusion/exclusion criteria), and the safety population (all patients who had received at least one dose of durvalumab). Data analyses were performed by using SAS® V.9.4 software.
Results
Patients
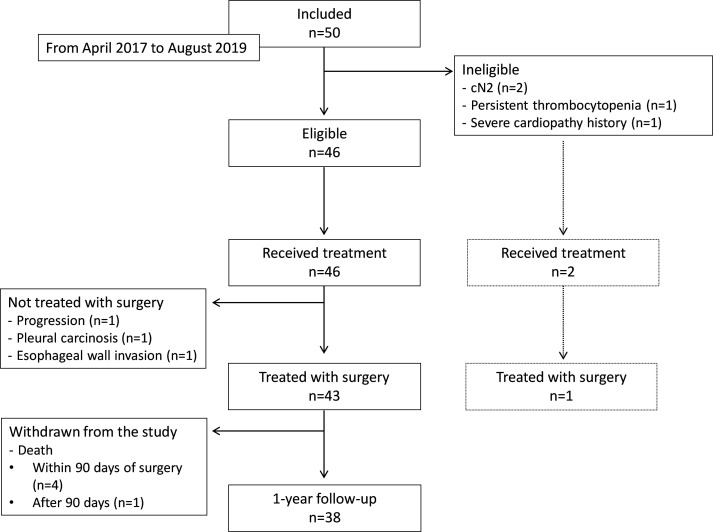
Fifty patients in the ITT population were recruited from April 2017 to August 2019, of whom 46 met the eligibility criteria (figure 1). Interim analysis was performed, and the independent committee decided that enrollment would be terminated in the case of a new death (any cause) occurring within 90 days of the date of surgery starting from the 46th patient enrolled. Enrolment was stopped on August 28, 2019, at the request of the independent committee due to excessive 90-day postoperative mortality, with four unexpected deaths (9% of the 46 eligible patients). Patient characteristics are presented in table 1. Among the 46 patients who were eligible for treatment, all were treated, and 43 underwent surgery. The remaining 3 patients (7%) did not undergo surgery. After surgery, 27 patients received adjuvant therapy (chemotherapy, n=22; and chemotherapy and radiotherapy, n=5).
Figure 1.
CONSORT flowchart of the IONESCO trial
Table 1.
Patient characteristics
| Efficacy population, n=46 No of patients (%) |
|
| Sex | |
| Female | 15 (32.6) |
| Male | 31 (67.4) |
| Age (median (range) years) | 60.9 (46.7–80.5) |
| Smoking status | |
| Yes | 45 (97.8) |
| No | 1 (2.2) |
| Packs-years (median (range)) | 40.0 (2.0–100.0) |
| ECOG performance status | |
| 0 | 38 (82.6) |
| 1 | 8 (17.4) |
| Histology | |
| Adenocarcinoma | 23 (50.0) |
| Squamous cell carcinoma | 19 (41.3) |
| Other | 4 (8.7) |
| Stage | |
| IB | 5 (10.9) |
| IIA | 13 (28.3) |
| IIB | 27 (58.7) |
| IIIA | 1 (2.2) |
| Preoperative respiratory function | |
| VEMS % (median (range)) | 87.5 (49.0–122.0) |
| DLCO % (median (range)) | 79.5 (42.0–129.0) |
| SaO2 % (median (range)) | 97.0 (93.0–100.0) |
| Patients receiving 3 doses of durvalumab | 43 (93.5) |
| Surgical procedure posttreatment* | |
| Bilobectomy | 3 (7.0) |
| Upper right | 1 |
| Lower right | 2 |
| Lobectomy | 30 (69.8) |
| Upper right | 12 |
| Lower right | 6 |
| Upper right | 11 |
| Lower right | 1 |
| Pneumonectomy | 10 (23.3) |
| Right | 5 |
| Left | 5 |
| Surgical approaches* | |
| Thoracotomy | 34 (79.1) |
| Video-assisted thoracic surgery (VATS) | 9 (20.9) |
*Three of the 46 patients did not undergo resection surgery due to disease progression (n=1) and pleural/esophageal invasion (n=2).
ECOG, Eastern Cooperative Oncology Group; SaO2, arterial oxygen saturation.
Efficacy
Complete resection (primary endpoint)
Of the 46 patients who were eligible for inclusion and received treatment (efficacy population), 41 (89%, 95% CI 80.1% to 98.1%) achieved complete resection (R0), and 2 (4%, 95% CI 0.0% to 10.2%) had microscopically incomplete resection (R1: presence of cancerous cells on bronchial margin section).
Time between the first/last durvalumab infusion and surgery
In 43 patients who underwent resection surgery, the time (median (range)) between the first durvalumab infusion and surgery was 37 days (29–46), while the time between the last infusion and surgery was 11 days (3–33).
Tumor response
RECIST tumor response was evaluated by investigators in the 46 eligible patients who received treatment prior to surgery: 4 (9%) patients achieved partial response, 36 (78%) had stable disease, and 6 (13%) had progressive disease.
Major pathological response
Among 43 patients who underwent resection surgery, 8 (19%) achieved MPR, of whom 3 (7%) achieved pCR (no viable tumor cells). There was a significant association between the radiographic and pathological response (n=43, p=0.03), and 3/4 patients achieving a partial response had MPR (online supplemental table 1).
jitc-2022-005636supp001.pdf (118.2KB, pdf)
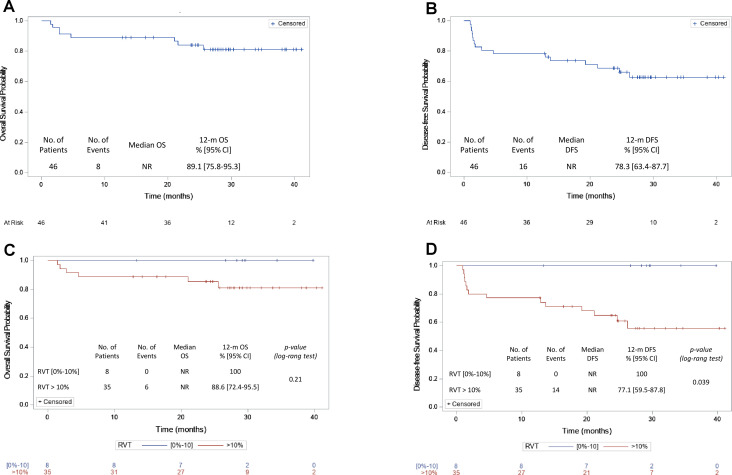
There was a significant association between MPR and DFS (n=43, log-rank test p=0.04): all 8 patients with MPR were disease-free (12-month DFS: 100%) vs 27/35 patients with >10% residual tumor cells (12-month DFS: 77%, 95% CI 59.5% to 87.8%). A positive trend between MPR and OS was also observed (n=43, log-rank test p=0.21): all 8 patients with MPR were alive (12-month OS: 100%) vs 31/35 patients with >10% residual tumor cells (12-month OS: 89%, 95% CI 72.4% to 95.6%) (figure 2C, D).
Figure 2.
Survival probability depends on achieving a major histopathological response. OS and DFS are shown for 46 durvalumab-treated patients (A, B, respectively). The median follow-up was 28.4 months. (C, D) shows the association between MPR and OS (C) and between MPR and DFS (D). Regarding RVT, the category (0%–10%) corresponds to patients who achieved MPR; the category >10% corresponds to patients who did not achieve MPR. DFS, disease-free survival; MPR, major pathological response; NR, not reported; OS, overall survival; RVT, residual viable tumor cells (%).
Survival
The median follow-up of the 46 eligible and treated patients was 28.4 months (12.8;41.1). Median survival was not reached at the data cut-off, and the 12-month OS rate was 89% (95% CI 75.8% to 95.3%). Median DFS was also not reached, and the 12-month DFS rate was 78% (95% CI 63.4% to 87.7%) (figure 2A, B).
Safety
Adverse events
In the safety population (n=48 patients who received treatment, see figure 1), durvalumab treatment was generally well tolerated, with no serious AEs and no grade 3–5 AEs related to durvalumab. A total of 16 patients (33%) experienced mild or moderate durvalumab-related AEs within 100 days after the end of treatment (table 2). The most common related AEs were asthenia (n=9), diarrhea (n=3), nausea (n=3), and pruritus (n=3). All-cause AEs are shown in online supplemental table 2.
Table 2.
Durvalumab-related adverse events
| Safety population, n=48 No of patients (%) |
||||||
| Any Grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Any AE | 16 (33.3) | 11 (22.9) | 5 (10.4) | 0 (0) | 0 (0) | 0 (0) |
| Serious AE | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| General disorders or administrative site conditions | 9 (18.8) | 7 (14.6) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) |
| Asthenia | 9 (18.8) | 7 (14.6) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal disorders | 7 (14.6) | 6 (12.5) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 3 (6.3) | 3 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 3 (6.3) | 3 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Abdominal pain | 1 (2.1) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 1 (2.1) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 1 (2.1) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Skin and subcutaneous tissue disorders | 4 (8.3) | 2 (4.2) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) |
| Pruritus | 3 (6.3) | 1 (2.1) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) |
| Photosensitivity reaction | 1 (2.1) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rash | 1 (2.1) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ear and labyrinth disorders | 2 (4.2) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vertigo | 2 (4.2) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Investigations | 2 (4.2) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Decreased serum thyroid stimulating hormone | 2 (4.2) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Metabolism and nutrition disorders | 2 (4.2) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Decreased appetite | 2 (4.2) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Musculoskeletal and connective tissue disorders | 2 (4.2) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Myalgia | 1 (2.1) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Polyarthritis | 1 (2.1) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Infections and infestations | 1 (2.1) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Oral fungal infection | 1 (2.1) | 1 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
AE, adverse event.
Ninety-day postoperative mortality
Mortality at 90 days after surgery was unexpectedly high, with four deaths reported (9% of the 46 eligible patients who were treated (table 3). Three of them suffered from cardiovascular comorbidities or other comorbidities (severe chronic obstructive pulmonary disease and diabetes in one patient each).
Table 3.
Ninety-day postoperative mortality
| Age | Sex | Smoking status | Stage | Histology | Comorbidities | Surgical procedure | Surgical resection | RVT | Cause of death | Days from surgery |
| 76 | M | Yes | IIA | Squamous cell carcinoma | Arterial hypertension, paroxysmal atrial fibrillation, diabetes | Right pneumonectomy | R0 | >10% | Surgical complication: early tracheal fistula with revision surgery | 8 |
| 78 | M | Yes | IIB | Squamous cell carcinoma | Arterial hypertension, ischemic heart disease, peripheral arterial disease, hypercholesterolemia | Right lower lobectomy extended to the middle lobe | R0 | >10% | Infectious respiratory distress | 21 |
| 64 | F | Yes | IIB | Adenocarcinoma | Arterial hypertension, COPD | Right lower lobectomy | R0 | >10% | Sudden death at home | 40 |
| 49 | F | Yes | IIB | Squamous cell carcinoma | None | Right upper lobectomy with parietectomy 2,3,4 ribs | R0 | >10% | Surgical complication: hemoptysis due to an arterial lesion; respiratory distress | 45 |
COPD, chronic obstructive pulmonary disease; F, female; M, male; RVT, residual viable tumor cells (%).
Postoperative complications
The complications occurring up to 4 weeks postoperatively in the safety population are shown in online supplemental table 3. A total of 19 events were reported in 14 patients, 16 of which were serious.
Discussion
Neoadjuvant durvalumab treatment produced a complete surgical resection rate of 89% in patients with early-stage resectable NSCLC who were enrolled in the IFCT-1601 IONESCO trial. The sample size was small due to early study termination, and the observed rate was not ≥92%, which prevented the trial from reaching its primary endpoint.
The rates of radiographic partial response (9%) and MPR (19%) are in line with those observed in the largest trial to date of neoadjuvant anti-PD-L1 monotherapy (the LCMC3 study).8 Higher MPR rates have been reported in smaller trials, specifically, 45% after two doses of nivolumab (n=20)6 and 41% after two doses of sintilimab (n=37).9 In the PRINCEPS trial (n=30), a lower rate of 14% was observed after a single injection of atezolizumab.10
Efficacy may be enhanced by using a combination of immune checkpoint inhibitors (ICIs), as suggested by the NEOSTAR trial, in which the MPR rates were 50% (8/16) with nivolumab plus ipilimumab vs 24% (5/21) with nivolumab alone.7 However, combining ICIs may raise safety issues, as shown by a dual checkpoint blockade trial in which the study arm combining nivolumab and ipilimumab was terminated early due to toxicity.12
Higher MPR rates have also been reported in studies exploring the combination of neoadjuvant chemotherapy and immunotherapy for resectable NSCLC, ranging from 57%13 to 83%.14 Finally, the CheckMate 816 phase III trial recently confirmed the benefit of using a combined approach: significantly higher rates of pCR were reported with three cycles of neoadjuvant nivolumab plus platinum-doublet chemotherapy versus chemotherapy alone (24% vs 2%). MPR rates were also higher with the combination compared with chemotherapy alone (37% vs 9%).3 A 37% reduction in EFS was reached, with a median of 31.6 months vs 20.8 months (p=0.0052).3 Although the FDA has approved (March 2022) this combination for patients with resectable NSCLC in the neoadjuvant setting.
A key finding of the IONESCO trial is the significant association between MPR and DFS. A strong relationship between MPR and OS had already been shown for neoadjuvant chemotherapy5 and chemoimmunotherapy,14 but this is the first study, to the best of our knowledge, to support such a link between MPR and DFS in patients receiving immunotherapy alone. Nonetheless, MPR should not be used as a standalone marker of efficacy. One should also consider the percentage of operated patients. In the IONESCO trial, 7% (3/46) of eligible treated patients did not undergo surgery. This is comparable to other studies on single-agent immunotherapies, in which up to 12% of eligible patients did not undergo surgery.6–9 This rate can reach 24% in studies on ICI combinations7 and ranges from 11% to 18% in studies on chemotherapy combinations.3 7 13–15
We did not find a correlation between pathological response and pretreatment PD-L1 expression in pre-surgical biopsies. However, it is difficult to draw conclusions from such a small number of samples and data on this relationship are generally inconclusive.6 7 9 12–14 16 17 These discrepancies may partly be explained by a potential underestimation of the PD-L1 status from the biopsy specimens compared with the whole tissue sample.18
The study was terminated due to excessive 90-day postoperative mortality (four deaths). A fifth death related to infection and an undocumented cardiac complication (figure 1) occurred 94 days after surgery and was therefore not included in the 90-day postoperative mortality calculations. This patient was a male in his mid-60s with cardiovascular comorbidities who underwent left pneumonectomy with microscopically incomplete resection (R1) for a squamous cell carcinoma stage IIB. The acceptable level of surgical complexity after a short period of ICI administration remains an open question, suggesting the need to record surgical difficulty scores for mediastinal lymph node dissection after immunotherapy. The 90-day postoperative mortality rate is difficult to compare with previous studies using ICIs in monotherapy since the mortality rate is not always explicitly provided3 6 or measured over a shorter timeframe.9 However, the longer time window used in this study is unlikely to explain the 9% mortality rate, which may instead be due to inadequate patient selection based on cardiovascular or respiratory history.7 Indeed, characteristics of the study population and the type of surgeries that were performed (in particular, the rate of pneumonectomy) may have contributed to the high mortality rate. In the IONESCO trial, 41% of patients had squamous cell carcinomas, 98% were previous/current smokers and the rate of pneumonectomy was relatively high (23%). Squamous cell carcinomas are more proximal tumors, potentially making surgical resection and hilar dissection more difficult. Male sex and smoking history are also negative prognostic factors of survival. The prevalence of squamous cell carcinomas in the IONESCO trial was higher than that in the pilot study by Forde and colleagues (29%),6 comparable to that in the LCMC3 trial (38%)16 and the NEOSTAR trial (39%).7 Almost all patients in the IONESCO trial were previous/current smokers compared with 80%–90% in previous studies.7–9 11 Interestingly, the lowest mortality rate at 90 days was reported in the LCMC3 trial (1%), which also had the lowest rate of pneumonectomy (9%) and a relatively low proportion of male patients (49%), and squamous cell carcinomas (38% of patients).8 In the Chinese study by Gao et al, in which EGFR mutations were excluded, 33% of patients underwent pneumonectomy, and 83% of patients had squamous cell carcinoma. Although the mortality rate at 90 days was not provided in this study, the rate at 30 days was already quite high (5%).9
In the CheckMate-816 trial, the combination of nivolumab and chemotherapy did not impact the surgical procedure compared with chemotherapy alone. There were two deaths related to surgery in the experimental arm. Other phase III studies combining chemotherapy and immunotherapy are ongoing. It will then be necessary to understand the impact of chemotherapy combined with immunotherapy vs immunotherapy alone on the surgical procedure.
Finally, the involvement of 20 active centers is likely to have introduced heterogeneity within the IONESCO trial. Despite high initial hopes, real-life multicenter experience has shown that there are certain complications arising from this approach, possibly suggesting that such a multimodal strategy should be limited to highly experienced surgical centers or to the fittest and least comorbid patients.
Conclusions
The IONESCO phase II trial exploring neoadjuvant durvalumab as a single agent in patients with early-stage resectable NSCLC did not reach its primary endpoint because of an excessive rate of postoperative deaths. However, such a strategy was still able to lead to R0 complete resection in 89% of the patients, which is acceptable. As a secondary endpoint, MPR was of 19% and was significantly correlated with DFS. The high rate of death due to postoperative complications suggests the need to better select patients with few comorbidities and operative risk factors for these immunotherapy-based neoadjuvant strategies.
Acknowledgments
This study was funded by the Intergroupe Francophone de Cancérologie Thoracique (IFCT) and by AstraZeneca and was sponsored by the IFCT. We thank the participating patients and their families as well as the study teams involved in the trial, the clinical research assistants, the study coordinators, the IFCT neoadjuvant working group and the IFCT operations staff. We also thank Dr Martine Antoine and Prof Diane Damotte for the central pathology review of the study and all the participating investigators: Prof Jacques Cadranel (Paris - Tenon), Prof Julien Mazieres (Toulouse - Centre Hospitalier Universitaire), Prof Gérard Zalcman (Paris - Bichat), Dr Olivier Carre (Amiens - Clinique de l’Europe), Dr Catherine Dubos-Arvis (Caen - Centre Régional de Lutte Contre le Cancer), Dr Thomas Egenod (Limoges - Centre Hospitalier Universitaire), Dr Philippe Girard (Paris - Institut Mutualiste Montsouris), Dr Gaelle Jeannin (Clermont - Ferrand-Centre Hospitalier Universitaire), Prof Denis Moro-Sibilot (Grenoble - Centre Hospitalier Universitaire), Dr Olivier Molinier (Le Mans - Centre Hospitalier), Dr Marie-Ange Massiani (Saint-Cloud - Centre René Huguenin), Dr Charles Dayen (Saint-Quentin - Centre Hospitalier), Dr Sophie Schneider (Bayon - Centre Hospitalier), Dr Patrick Dumont (Chauny-Centre Hospitalier Universitaire), Dr Jean-Bernard Auliac (Mantes La Jolie - Centre Hospitalier), Prof Fabrice Barlesi (Marseille - Assistance Publique Hôpitaux de Marseille), Dr Judith Raimbourg (Nantes - Centre Régional de Lutte Contre le Cancer), Prof Jaafar Bennouna (Nantes - Hôpital Laennec), Prof Marie Wislez (Paris - Hôpital Cochin), and Dr Bertrand Mennecier (Strasbourg - Nouvel Hôpital Civil). Medical writing support was provided by Dr Leonarda Di Candia and Gaele Ducher (Scinopsis).
Footnotes
Twitter: @IFCTlung
Presented at: This work was presented in 2020 at the ESMO virtual congress and in 2021 at the ESMO congress (Paris, France) and at the 25th and at the 26th CPLF (Congrès de Pneumologie de Langue Française).
Contributors: Marie Wislez: conceptualization, writing–original draft, writing–review and editing; Julien Mazières: investigation, writing-review and editing; Armelle Lavole: investigation, writing-review and editing; Gérard Zalcman: investigation, writing-review and editing; Olivier Carre: investigation, writing—review and editing; Thomas Egenod: investigation, writing-review and editing; Raffaele Caliandro: investigation, writing-review and editing; Catherine Dubos-Arvis: investigation, writing-review and editing; Gaelle Jeannin: investigation, writing-review and editing; Olivier Molinier: investigation, writing-review and editing; Marie-Ange Massiani: investigation, writing-review and editing; Françoise Le Pimpec Barthes: investigation, writing-review and editing; Laurent Brouchet: investigation, writing-review and editing; Jalal Assouad: investigation, writing-review and editing; Bernard Milleron: investigation, writing-review and editing; Diane Damotte: investigation, writing-review and editing; Martine Antoine: investigation, writing-review and editing; Alexandra Langlais: methodology, formal analysis, writing-review and editing; Franck Morin: project administration, writing-review and editing; Virginie Westeel: guarantor, supervision.
Funding: This study was funded by the Intergroupe Francophone de Cancérologie Thoracique (IFCT-1601) and by AstraZeneca (ESR-15-11017).
Competing interests: Marie Wislez, reports grants from Astra-Zeneca, honoraria for speaker’s bureau from Roche, Bristol Myers Squibb and Boehringer Ingelheim, participation on a Data Safety Monitoring Board or Advisory Board from Astra-Zeneca, Merck Sharp and Dohme and Novartis, non-financial interests from Astra-Zeneca, Bristol Myers Squibb, Merck Sharp and Dohme, Novartis, Lilly Merck KgA, Merus, GlaxoSmithKline and Amgen, outside the submitted work. Julien Mazières reports honoraria for lectures from Bristol Myers Squibb, Roche Genentech, Astra-Zeneca and Merck Sharp and Dohme, outside the submitted work. Gérard Zalcman reports grants from Roche and Bristol Myers Squibb, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astra-Zeneca and Bristol Myers Squibb, support for attending meetings and/or travel from Astra-Zeneca, Abbvie, Bristol Myers Squibb and Pfizer, participation on a Data Safety Monitoring Board or Advisory Board from Astra-Zeneca, Inventiva, Da Volterra and Bristol Myers Squibb, outside the submitted work. Olivier Molinier reports participation on a Data Safety Monitoring Board or Advisory Board from Astra-Zeneca, outside the submitted work. Virginie Westeel reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astra-Zeneca, Bristol Myers Squibb, Merck Sharp and Dohme and Roche, Support for attending meetings and/or travel from Astra-Zeneca, Bristol Myers Squibb, Merck Sharp and Dohme, Pfizer and Roche, Participation on a Data Safety Monitoring Board or Advisory Board from Astra-Zeneca, Bristol Myers Squibb, Merck Sharp and Dohme, Takeda and Roche, outside the submitted work. All other authors have nothing to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The trial protocol did not include a data sharing plan; therefore, data from the trial will not be shared publicly, as data sharing was not included when ethical approval was requested.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Comité de Protection des Personnes Ile De France X (reference number: 24-2016). Participants gave informed consent to participate in the study before taking part.
References
- 1.Wakelee HA, Altorki NK, Zhou C, et al. IMpower010: primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). JCO 2021;39:8500. 10.1200/JCO.2021.39.15_suppl.8500 [DOI] [Google Scholar]
- 2.Paz-Ares L, O'Brien MER, Mauer M, et al. VP3-2022: pembrolizumab (pembro) versus placebo for early-stage non-small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (chemo) when indicated: randomized, triple-blind, phase III EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 study. Annals of Oncology 2022;33:451–3. 10.1016/j.annonc.2022.02.224 [DOI] [Google Scholar]
- 3.Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–85. 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382–99. 10.1158/2159-8290.CD-16-0577 [DOI] [PubMed] [Google Scholar]
- 5.Hellmann MD, Chaft JE, William WN, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42–50. 10.1016/S1470-2045(13)70334-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–86. 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascone T, William WN, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504–14. 10.1038/s41591-020-01224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Chaft J, Nicholas A, et al. PS01.05 surgical and clinical outcomes with neoadjuvant Atezolizumab in resectable stage IB–IIIB NSCLC: LCMC3 trial primary analysis. J Thorac Oncol 2021;16:S59–61. 10.1016/j.jtho.2021.01.320 [DOI] [Google Scholar]
- 9.Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816–26. 10.1016/j.jtho.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 10.Besse B, Adam J, Cozic N, et al. 1215O - SC Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): results from the phase II princeps trial. Annals of Oncology 2020;31:S794–5. 10.1016/j.annonc.2020.08.1417 [DOI] [Google Scholar]
- 11.Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853–60. 10.1093/annonc/mdy218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuss JE, Anagnostou V, Cottrell TR, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer 2020;8:e001282. 10.1136/jitc-2020-001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786–95. 10.1016/S1470-2045(20)30140-6 [DOI] [PubMed] [Google Scholar]
- 14.Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413–22. 10.1016/S1470-2045(20)30453-8 [DOI] [PubMed] [Google Scholar]
- 15.Rothschild SI, Zippelius A, Eboulet EI, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non–small-cell lung cancer—a multicenter single-arm phase II trial. JCO 2021;39:2872–80. 10.1200/JCO.21.00276 [DOI] [PubMed] [Google Scholar]
- 16.Carbone D, Lee J, Kris M, et al. OA06.06 clinical/biomarker data for neoadjuvant Atezolizumab in resectable stage IB-IIIB NSCLC: primary analysis in the LCMC3 study. Journal of Thoracic Oncology 2021;16:S115–6. 10.1016/j.jtho.2021.01.294 [DOI] [Google Scholar]
- 17.Bar J, Urban D, Ofek E, et al. Neoadjuvant pembrolizumab (pembro) for early stage non-small cell lung cancer (NSCLC): updated report of a phase I study, MK3475-223. JCO 2019;37:8534. 10.1200/JCO.2019.37.15_suppl.8534 [DOI] [Google Scholar]
- 18.Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016;27:147–53. 10.1093/annonc/mdv489 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005636supp001.pdf (118.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The trial protocol did not include a data sharing plan; therefore, data from the trial will not be shared publicly, as data sharing was not included when ethical approval was requested.