Abstract
Objective
Lung cancer is a common malignancy in rheumatoid arthritis (RA). Since smoking is a risk factor for both (seropositive) RA and lung cancer, it remains unclear whether RA, in itself, increases lung cancer risk.
Methods
We performed a population-based cohort study of patients with RA and individually matched general population reference individuals identified in Swedish registers and from the Epidemiological Investigation of RA early RA study, prospectively followed for lung cancer occurrence 1995–2018. We calculated incidence rates and performed Cox regression to estimate HRs including 95% CIs of lung cancer, taking smoking and RA serostatus into account.
Results
Overall, we included 44 101 patients with RA (590 incident lung cancers, 56 per 100 000), and 216 495 matched general population individuals (1691 incident lung cancers, 33 per 100 000), corresponding to a crude HR (95% CI) of 1.76 (1.60 to 1.93). In subset analyses, this increased risk remained after adjustment for smoking (HR 1.77, 95% CI 1.06 to 2.97). Compared with general population subjects who were never smokers, patients with RA who were ever smokers had almost seven times higher risk of lung cancer. In RA, seropositivity was a significant lung cancer risk factor, even when adjusted for smoking, increasing the incidence 2–6 times. At 20 years, the risk in patients with RA was almost 3%, overall and over 4% for patients who were ever smokers and had at least one RA autoantibody.
Conclusions
Seropositive RA is a risk factor for lung cancer over and above what can be explained by smoking, although residual confounding by smoking or other airway exposures cannot be formally excluded. There is a need for increased awareness and potentially for regular lung cancer screening, at least in a subset of patients with RA.
Keywords: rheumatoid arthritis, autoantibodies, smoking
WHAT IS ALREADY KNOWN ON THIS TOPIC
Lung cancer is one of the most common malignancies in patients with rheumatoid arthritis (RA). Several risk determinants for lung cancer in RA have been proposed, including male sex, smoking and interstitial lung disease, but the aetiological questions of whether and to what extent the increased risk of lung cancer in RA is simply explained by the increased prevalence of smoking, and whether this risk differs between seropositive and seronegative RA, taking smoking into account, remain unresolved.
WHAT THIS STUDY ADDS
Our study showed that RA seropositivity, in particular anticitrullinated peptide antibody (ACPA) positivity and double seropositivity (rheumatoid factor and ACPA), is a strong and at least seemingly independent risk factor for lung cancer in RA. The magnitude of increased risk is 2–6 times, while the absolute risk at 20 years after diagnosis is almost 3%, overall, and over 4% for patients who were ever smokers and had at least one autoantibody.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results raise the question of whether regular CT lung screening of ever smokers who have autoantibody-positive RA, should be considered.
Introduction
During recent years, there has been an increasing interest in the role of the lungs in rheumatoid arthritis (RA), not only as a common extra-articular manifestation of the disease itself but also as a possible site of triggering of a pivotal immune response: autoimmune features such as the production of rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPAs) may appear years before the onset of joint symptoms and in the absence of systemic inflammation, and raise the hypothesis that the initial triggering of autoimmunity in RA may take place outside the joints.1 2
Smoking has been shown to be a strong environmental risk factor for development of ACPA positive RA, particular in the presence of one of the major genetic determinants of RA, the human leucocyte antigen class II shared epitope.3 Smoking, and potentially also other airway exposures, induce local inflammation and promote citrullination of proteins in the lungs.4 5 Signs of bronchial mucosal inflammation with germinal centre formation and local production of antibodies have been described in patients with early untreated RA.6 Together, these observations suggest that the lungs might be one site of initiation of RA. Smoking is also one of the strongest known risk factors for lung cancer. The link between chronic immune activation and tumorigenesis is well established.7 Tobacco smoke induces inflammatory and mutagenic effects in the lungs, which promote a procancer immune response. Indeed, the initial pathological hallmark of smoking is a widespread inflammatory infiltrate throughout the lung parenchyma.8
Lung cancer is one of the most common malignancies in patients with RA.9 In a meta-analysis of studies on cancer risks in RA versus the general population, the risk of lung cancer was significantly (an average 60%) increased in RA.10 Several risk determinants for lung cancer in RA have been proposed, including male sex, smoking and interstitial lung disease (ILD),11–13 but the aetiological questions of whether and to what extent the increased risk of lung cancer in RA is simply explained by the increased prevalence of smoking, and whether this risk differs between seropositive and seronegative RA, taking smoking into account, remain unresolved. Similarly, from a clinical point of view, assessments of absolute risk in patients with RA would be important to gauge the potential for routine screening for lung cancer.
Therefore, the aims of this study were to examine whether and to what extent the increased risk of lung cancer in RA may (or may not) be attributable to smoking, and to examine this association in terms of absolute and relative risks specifically in relation to RA serostatus.
Patients and methods
Study design
We performed a population-based matched cohort study in which patients with a clinical diagnosis of RA identified from different data sources, and individually matched reference individuals from the general population, were prospectively followed for lung cancer occurrence from 1 January 1995 to 31 December 2018. Patients in Swedish Rheumatology Quality (SRQ) and in Epidemiological Investigation of RA (EIRA) were diagnosed with RA and entered into the SRQ (and invited into EIRA) based on the clinical RA diagnosis by their treating rheumatologist, in turn based on the ACR 1987 or EULAR/ACR 2010 criteria. To identify patients with RA from the National Patient Register (NPR), we used previously validated algorithms based on ICD-codes.14 General population comparator subjects were matched for age, sex, calendar period and area of residence and received the same date of entry as their matched index-case with RA.
Setting, data sources, study population
This study was based on linkages between the SRQ Register and its adjunct biobank (SRQ biobank), the Swedish EIRA case–control study and the NPR, the Swedish Cancer Register, the Prescribed Drug Register, the Swedish Cause of Death Register and the Swedish Population Register (online supplemental figure 1). These data sources have been described elsewhere15 and are summarised in online supplemental file.
rmdopen-2022-002465supp001.pdf (240.2KB, pdf)
We defined our study cohort of incident RA from the following three overlapping data sources: SRQ, EIRA and NPR. For each unique patient with RA, we identified five matched general population comparator subjects, alive the year of entry of their index individual. Study population definition is described in detail in online supplemental file. In EIRA, a cap at 75 years of age at RA diagnosis was used.
Exposures
In a first analysis, the incidence of lung cancer (= outcome) in individuals in the overall RA cohort and in the EIRA subcohort (exposure=RA) was compared with that of the general population comparison cohorts.
In a second analysis, restricted to patients with RA from the EIRA cohort, exposure was defined based on RA autoantibody status (RF and ACPA). We collected information on seropositivity from three overlapping sources, in a hierarchical manner (online supplemental figure 2A and B): the SRQ biobank, EIRA and the SRQ. In SRQ biobank, information on RF and ACPA is defined based on central testing of RF IgM and ACPA using a fluoroenzyme-immunoassay (Elia assay, Phadia, Freiburg, Germany). In SRQ, information on seropositivity was based on the ICD-code (International Classification of Diseases code) for RA entered by the treating rheumatologist (online supplemental figure 2A and B). Seropositivity in EIRA was defined according to the clinical diagnosis (local immunology laboratory). On the basis of these sources of information, each RA patient’s exposure status (seropositivity) was categorised according to binary but not mutually exclusive definitions: (1) RF and/or ACPA positive vs all others; (2) RF positive vs all others; (3) ACPA-positive versus all others and (4) RF and ACPA double positive (vs all others). For those exposure definitions that were determined via information from SRQ biobank (n=174, 8% of all), which could have pertained to a time-point after the entry into the RA cohort (median time difference between entry into the RA cohort and entry into SRQ biobank was 21 months (IQR 4–60)), start of follow-up was set to the time of entry into SRQ biobank in order to avoid immortal time bias.
Follow-up
Follow-up started at the cohort (-specific) date of entry, and at the same date for their matched population control subjects. Follow-up ended at the first of: first ever occurrence of lung cancer, end of the study period (31 December 2018), death or emigration from Sweden. In each (RA and comparator) cohort, we assessed the total number of person-years at risk and incident lung cancers and calculated incidence rates counting from start of follow-up.
Outcome
We defined the primary outcome as a first ever lung cancer diagnosis (ICD code C34.9) registered in the Swedish Cancer register. Individuals with a history of lung cancer at start of follow-up were excluded. In separate analyses, we changed the outcome (from incident lung cancer) to death from lung cancer, using data from the Cause of Death register.
Covariates
We identified the following covariates through register linkages: age, sex, birth year, country of birth, county of residency, hospital resource utilisation and selected comorbidities up to 5 years before start of follow-up: number of hospitalisations, hospitalisations or specialty care outpatients visits listing ICD codes for respiratory diseases, chronic obstructive pulmonary disease (COPD), heart failure, ischaemic heart disease and renal failure.
Smoking status was collected from the EIRA questionnaire (incident cases with RA and their population controls), and was structurally missing for patients exclusively identified in the Patient Register. Analyses taking smoking into account were therefore restricted to individuals taking part in the EIRA study. We defined individuals who (by the time their smoking status was assessed) had never smoked as non-smokers, and those who were current or ex-smokers as ever smokers, irrespective of the reported number of cigarettes per day. We further calculated pack-years by multiplying the reported number of cigarettes smoked per day by the number of years the person had smoked.
Statistical methods
In a first set of analyses, we assessed the risk of lung cancer in the entire RA population (patients identified with RA in the NPR, SRQ or EIRA, total n=44 101) compared with their 216 495 matched general population comparator individuals. We performed Cox regression to estimate HRs and their corresponding 95% CIs, using time since start of follow-up as time-scale. Besides crude models conditioned on matching set, we also fitted two models: (1) model A, adjusted for age, sex, year of cohort entry, county of residency and (2) model B: adjusted for all the above, plus individual comorbidities. We also performed adjusted Cox regression analysis (same as model B above) with death from lung cancer as the outcome.
In a second set of analyses, we performed the above analyses in the subcohort of patients with RA participating in the EIRA study and their original matched population controls, for whom detailed smoking data up until RA diagnosis were available, and fitted a further adjusted model (C), similar to model B but additionally adjusted for smoking status (ever/never). Stratified analyses by smoking status (ever vs never, current vs past), and analyses with adjustment for pack-years of smoking (as a continuous instead of binary variable) were also performed.
In a third set of analyses, restricted to patients with RA from the EIRA cohort, we assessed the risk of lung cancer by smoking status, and by RF and anti-ACPA status, as available and using mutually non-exclusive categorisations: (1) RF and/or ACPA positive vs all others; (2) RF positive vs all others; (3) ACPA-positive versus all others, and (4) RF and ACPA double positive (vs all others)). Besides crude Cox regression, we fitted four models: model A, B and C as above, and model D, where instead of smoking as a dichotomous variable (ever vs never) we used pack-years for those subjects with available information (N=1794 (87%) of the 2060 patients with RA, and 2442 (88%% of the 2779 EIRA population control subjects).
All statistical analyses were performed using SPSS V.25.
Results
Table 1 summarises baseline characteristics for the whole study population and for the subcohort from EIRA. In the former, the mean age (SD) at RA diagnosis was 61 (16) years and the percentage of females was 69%. In the EIRA cohort, patients and controls were on average younger, which explains the lower prevalence of comorbid conditions (table 1).
Table 1.
Characteristics of the study population of Swedish patients with incident RA 1995–2018 and matched population referents
| All patients with RA | Subcohort of patients with RA included in EIRA | |||
| Incident RA (n=44 101) |
General population (n=216 495) |
Incident RA (n=2060) |
General population (n=2779) |
|
| Age (years), mean±SD | 61±16 | 61±16 | 53±13 | 54±13 |
| Sex (% male) | 31% | 31% | 29% | 29% |
| Education | ||||
| ≤9 years | 31% | 28% | 23% | 17% |
| 10–12 years | 44% | 42% | 47% | 42% |
| >12 years | 25% | 30% | 30% | 41% |
| Smoking at start of follow-up | ||||
| Never | 35% | 45% | ||
| Ever | 65% (N=1924) | 55% (N=2620) | ||
| Comorbid conditions at RA-diagnosis (start of follow-up)* | ||||
| Chronic obstructive pulmonary disease | 1.7% | 0.8% | 0.3% | 0.6% |
| All respiratory tract diseases | 9.9% | 5.5% | 5.7% | 4.9% |
| Interstitial lung disease | 3.6% | 1.6% | 1.0% | 1.4% |
| Heart failure | 1.3% | 0.7% | 0.3% | 0.3% |
| Ischaemic heart disease | 3.2% | 2.5% | 1.2% | 1.3% |
| Renal failure | 0.7% | 0.4% | 0.3% | 0.1% |
*Information about comorbiditied is presented in online supplemental table 2.
EIRA, Epidemiological Investigation of RA; RA, rheumatoid arthritis.
Occurrence, cumulative incidence and relative risk of lung cancer in RA versus population comparator subjects: results from all three RA cohorts combined
During a mean follow-up of 7.3 years for the overall RA cohort (n=44 101) and 7.6 years for the general population comparator (n=216 495), we identified 590 incident lung cancers (56 per 100 000) among the patients with RA and 1691 cases (33 per 100 000) in the general population comparator. Sixty-five per cent of the lung cancer cases in the RA group and 61% in the general population group were diagnosed during the first 5 years after diagnosis. During the same time frame, the cumulative incidence of lung cancer was 0.9% in the RA cohort and 0.5% in the general population comparison cohort. During the entire follow-up, the crude HR (95% CI) was 1.76 (1.60 to 1.93), which remained statistically significantly elevated even after adjustment for age, sex, index year, county of residency and comorbidities(HR (95% CI) = 1.70 (1.54 to 1.87)) (table 2).
Table 2.
Number of events, person-years of follow-up, number of events per 100 000 person-years and relative risk (HRs) of lung cancer in patients with RA versus general population, in all individuals and stratified by smoking status
| All patients | No of events (person years of follow-up; no of events/100 000 person-years) | Crude HR (95% CI) | Model A HR* (95% CI) | Model B HR** (95% CI) |
Model C HR** (95% CI) |
Model D HR** (95% CI) |
|
| Patients with RA | General population controls | ||||||
| All (NPR, SRQ and EIRA) | 590 (1 058 424; 55.7) | 1691 (5 195 880; 32.5) | 1.76 (1.60 to 1.93) | 1.74 (1.58 to 1.91) | 1.70 (1.54 to 1.87) | ||
| Patients with RA | EIRA population controls | ||||||
| EIRA cohort, all | 36 (49 440; 72.8) | 26 (66 696; 38.9) | 1.72 (1.04 to 2.86)* | 1.92 (1.15 to 3.20)* | 1.94 (1.16 to 3.24)* | 1.77 (1.06 to 2.97)* | 1.99 (1.16 to3.41)* |
| EIRA, ever smokers | 34 (49 440; 68.8) | 21 (66 696; 31.5) | 1.72 (1.01 to 2.96)† | 1.83 (1.06 to 3.18)† | 1.82 (1.06 to 3.17)† | N/A | N/A |
| EIRA, never smokers | 2 (49 440; 4.0) | 4 (66 696; 6.0) | 0.84 (0.15 to 4.58)‡ | 1.23 (0.22 to 6.76)‡ | 1.40 (0.25 to 7.77)‡ | N/A | N/A |
Five HRs are presented: (1) crude; (2) adjusted for age, sex, index year, county of residency (model A); (3) age, sex, index year, county of residency and comorbidities (renal failure, heart failure, ischaemic heart disease, COPD, respiratory infections, hospitalisation) (model B) (4) as model B plus smoking (ever/never) (model C) and (5) as model C with pack-years instead of smoking ever vs never (model D).
*RA versus Gen population.
†Ever smokers with RA versus ever smokers in general population.
‡Never smokers with RA versus never smokers in general population.
COPD, chronic obstructive pulmonary disease; EIRA, Epidemiological Investigation of RA; N/A, not available; NPR, National Patient Register; NPR, National Patient Register; RA, rheumatoid arthritis; SRQ, Swedish Rheumatology Quality.
Occurrence, cumulative incidence and relative risk of lung cancer in RA versus population comparator subjects: results from the EIRA study
During a mean follow-up of 9 years (same for patients with RA, n=2060 and their controls, n=2779), 36 incident lung cancers (73 per 100 000) occurred among the RA cases, compared with 26 (39 per 100 000) among their controls (table 2). Overall, the crude HR (1.72, 95% CI 1.04 to 2.86) remained largely unchanged following adjustment for comorbidities and smoking (table 2).
Analyses stratified by smoking status (never vs ever) at RA diagnosis (=inclusion into EIRA) revealed that 34 (94%) of the 36 incident lung cancer cases in the patients with RA and 21 (84%) out of the 25 lung cancer cases among their population control occurred among ever smokers. The HR for lung cancer in RA versus general population when adjusted for comorbidities was 1.82 (1.06–3.17) in the ever smoker subset and 1.40 (0.25–7.77) in the subset of never-smokers (table 2). When instead stratified by current versus past smoking, the HRs for lung cancer in RA versus general population in the subgroup of current smokers was 2.73 (95% CI 1.21 to 6.16) and 0.94 (95% CI 0.38 to 2.30) among past smokers, when adjusted for age, sex, calendar-year and pack-years of smoking.
Among the EIRA population comparator subjects without RA, ever (vs never) smoking was associated with a four-fold risk of lung cancer, HR=3.95 (95% CI) 1.36 to 11.52). Among patients with RA, ever (vs never) smoking was associated with almost ninefold increase in lung cancer risk, HR=8.68 (95% CI) 2.09 to 36.14). The combination of RA and ever smoking (vs never smokers without RA) was associated with almost seven times higher risk (HR (95% CI) = 6.80 (2.41 to 19.21)) than never-smokers without RA.
Absolute and relative risks of lung cancer in relation to RA serostatus, comparing between patients with RA: results from patients with RA in EIRA
When stratified by RA autoantibody status, positive autoantibody status was associated with an at least doubled risk of lung cancer in ACPA positive patients (vs ACPA negative patients) and in double seropositive (vs double seronegative) patients (table 3). The significant association between autoantibody positivity and risk for lung cancer remained even in the adjusted models B, C and D (table 3).
Table 3.
Number of events, person-years of follow-up, number of events per 100 000 person-years and relative risk of lung cancer according to autoantibody status in the EIRA subcohort
| No of events (person years of follow-up; no of events per 100 000 person years) | Crude HR (95% CI) | Model A HR (95% CI) | Model B HR (95% CI) |
Model C HR (95% CI) |
Model D with smoking as pack-years instead of ever/never | ||
| Positive | Negative | ||||||
| RF (n=2060) | 30 (49 440; 60.7) | 6 (49 440; 12.1) | 2.78 (1.16 to 6.69) | 3.01 (1.25 to 7.26) | 2.82 (1.17 to 6.82) | 2.44 (1.01 to 5.89) | 2.16 (0.88–5.28) |
| ACPA (n=2060) | 30 (49 440; 60.7) | 6 (49 440; 12.1) | 3.13 (1.30 to 7.51) | 3.43 (1.42 to 8.25) | 3.22 (1.33 to 7.77) | 2.88 (1.19 to 6.95) | 3.29 (1.26–8.58) |
| RF and/or ACPA (n=2060) | 34 (49 440; 68.8) | 2 (49 440; 4.0) | 6.38 (1.53 to 26.56) | 7.62 (1.83 to 31.83) | 7.20 (1.72 to 30.11) | 6.29 (1.51 to 26.30) | 5.76 (1.37–24.21) |
| RF and ACPA (positive vs double negative) (n=1608) | 26 (38 592; 67.4) | 2 (38 592; 5.2) | 6.67 (1.58 to 28.08) | 7.92 (1.87 to 33.50) | 7.08 (1.67 to 29.98) | 6.21 (1.47 to 26.33) | 5.86 (1.37–25.01) |
Five HRs are presented: (1) crude; (2) adjusted for age, sex, index year, county of residency (model A); (3) age, sex, index year, county of residency and comorbidities (renal failure, heart failure, ischaemic heart disease, COPD, respiratory infections, hospitalisation) (model B) (4) all the above plus smoking (model C) and (5) as model C with pack-years instead of smoking ever versus never.
ACPA, anticitrullinated peptide antibody; COPD, chronic obstructive pulmonary disease; EIRA, Epidemiological Investigation of RA; RF, rheumatoid factor.
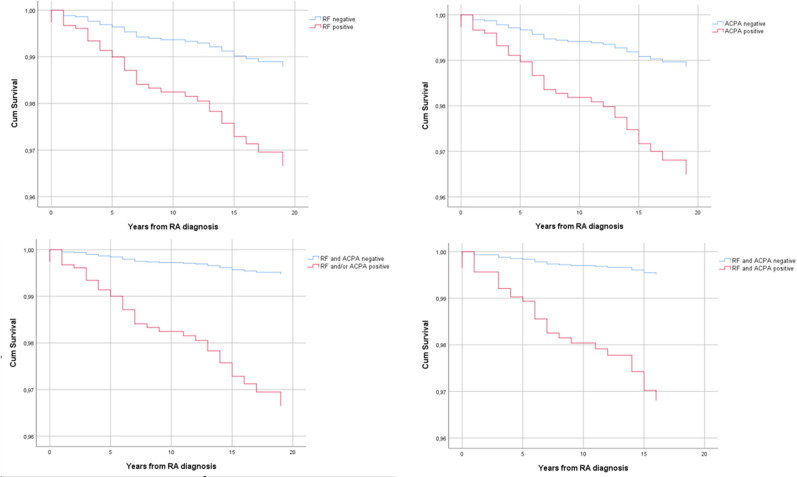
The cumulative incidence of lung cancer at 5 years was 0.8% in the RA cohort and 0.4% in their matched general population controls (figure 1A). At 20 years the risk in patients with RA was almost 3%, overall, and over 4% for patients who were ever smokers and had at least one autoantibody (figure 1C, D).
Figure 1.
Kaplan-Meier analysis for the risk of lung cancer in patients with RA versus population referents from the EIRA study: (A) for the whole group of patients; (B) for ever smokers patients with RA versus never smoker controls; (C) for patients with RA who are ACPA positive versus ACPA negative and (D) for patients with RA who were ever smokers (current and past) and were both RF and ACPA positive. ACPA, anticitrullinated peptide antibody; EIRA, Epidemiological Investigation of RA; RA, rheumatoid arthritis; RF, rheumatoid factor.
Analyses of mortality from lung cancer in the overall cohort
Changing the outcome from incident lung cancer to death from lung cancer, we noted 473 and 1359 deaths with lung cancer as cause of death in patients with RA and general population controls, respectively, during a mean of 7.3 years of follow-up from index date. The absolute risk for death from lung cancer in RA versus controls corresponded to 1% and 0.6%, respectively. The adjusted HR for death from lung cancer was 1.73, 95% CI 1.56 to 1.92.
Discussion
In this cohort study, we not only confirm the 50%–100% increase in risk of lung cancer in patients with RA, but further demonstrate that this risk increase differs by RA subtype and does not seem to be readily explained by smoking. During the first 5 years after RA diagnosis, 1% of patients with RA were diagnosed with lung cancer, a risk that increased to an overall 3% during the first 20 years.
We confirm previous results showing that RA is associated with approximately 70% increased risk of lung cancer as compared the general population.9 10 16 Most of these studies have assessed RA as one entity rather than factored in seropositivity, and have not factored in smoking. Our results demonstrated (as expected) a strong association between smoking and lung cancer, but also that adjustment for smoking did not readily explain the association between RA itself and risk for lung cancer. Interestingly, the association between RA, especially seropositive RA, and lung cancer remained also in models adjusted for pack-years.
ILD is a common extra-articular manifestation of RA, associated with increased risk for lung cancer.17 18 A previous study also suggested a possible link between RA-ILD and lung cancer mortality.19 Interstitial lung abnormalities have also been shown to be associated with lung cancer in the general population.20 Although we adjusted for comorbidities including respiratory diseases such as ILD, residual confounding connected to subclinical interstitial lung changes cannot be excluded. Since smoking may not be unique among airway exposures in its ability to induce an immune response, RA and lung cancer, we cannot formally exclude that some of the increased risks are linked to other factors, such as silica exposure. As shown in table 2, our results did not demonstrate any clear increase in the risk of lung cancer among never smokers with (vs without) RA, although precision was limited due to the limited number of lung cancer cases both among patients with RA and general population controls.
The earlier the diagnosis of lung cancer, the better the prognosis.21 Studies have evaluated different ways of detecting the disease earlier in order to reduce mortality.22 A recent population-based, randomised, controlled trial evaluated the effectiveness of screening with volume-based, low-dose CT in individuals at high risk for lung cancer23 showing a lower lung cancer related mortality at 10 years for those who underwent screening. In this study, high-risk individuals were defined as current or former smokers. In our study, we demonstrate an additional risk determinant, apart from smoking, for lung cancer, which suggests that two clinically easily ascertained pieces of information (RA serostatus and ever smoking status) can readily identify a group of individuals at particularly elevated risk of lung cancer. This observation calls for further research to evaluate the yield and optimal operation of targeted lung cancer screening in RA. In this regard, it is important to consider that lung cancer is a leading cause of death from cancer,24 mainly due to delayed diagnosis. It is interesting here to note that in the USA, annual screening for lung cancer with low-dose CT in adults aged 50–80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years, is recommended.
Our study has limitations. The paucity of individual-level data on smoking in the overall general population cohort forced us to restrict analyses of smoking to a subset (participants in the EIRA study). Nevertheless, in the combined overall RA cohort, we were able to adjust the Cox proportional hazards model for presence of COPD (strongly associated with smoking) and found that the increased risk for lung cancer in RA remained elevated. In analyses restricted to EIRA, in which the overall relative risk of lung cancer was on par with that in the overall RA cohort, RA was significantly associated with a higher risk for lung cancer even after adjustment for smoking (ever/never as well as intensity). Since smoking (intensity) is a risk factor for the development of RF and ACPA, it is, however, nearly impossible to formally rule out that the association between RA serostatus and lung cancer (even among smokers) includes an element of residual confounding by smoking intensity or pattern. Another limitation is the capture of smoking habits up until the time of RA diagnosis. Changes in smoking habits during follow-up could therefore not been accomodated. However, considering how even past smoking can increase the risk for lung cancer, we considered in the main analysis current and past smokers in the same group (even if a separate analysis with past vs current was also performed). So even if a significant number of patients had discontinued smoking, they would still be captured in the ever-smoking group (and the proportions of patients who starts to smoke once diagnosed with RA is likely to be very low).
Further, there is a possibility for detection bias due to pulmonary X-ray screening of patients with RA at the time of diagnosis and before starting antirheumatic treatment. However, the percentage of the total lung cancer cases detected during the first year after index date was similar in patients with RA (28%) and general population comparators (25%), arguing against a strong impact of detection bias, and the risk for detection bias should not explain the differential risks for lung cancer in autoantibody positive versus negative patients with RA. Further, the fact that the HR for incident lung cancer was similar to the HR for death from lung cancer argues against strong impact of detection (here: lead time) bias. Lastly, the comorbid conditions are sourced by ICD codes, which is a limitation. The low percentage of ILD most likely stem from the fact that only clinically diagnosed ILD is included, but not subclinical disease.
Our study has a number of strengths. It is a large, population-based cohort study, with high external validity. It is the largest study so far to our knowledge examining the complex relationship between RA, smoking and lung cancer, and could use of detailed data on smoking (intensity), comorbidities and other factors, for both patients with RA (by RA serostatus) and population controls. Follow-up and lung cancer were ascertained through linkage to external data-sources of high completeness.
In conclusion, RA seropositivity is a strong and at least seemingly independent risk factor for lung cancer in RA. Even though a definitive case for a direct causal biological relationship between seropositive RA and lung cancer cannot be made, due to the risk of residual confounding by smoking, the observation remains clinical significant and the results raise the question of whether regular CT lung screening of ever smokers who have autoantibody-positive RA should be considered.
rmdopen-2022-002465supp002.pdf (53.1KB, pdf)
Acknowledgments
This study has been previously presented at a conference and published as a conference abstract (K Chatzidionysiou, D Di Giuseppe, J Söderling, A Catrina, J Askling. POS0061 The risk of lung cancer in rheumatoid arthritis and in relation to autoantibody positivity and smoking. EULAR 2022.
Footnotes
Deceased: AC is deceased
Contributors: KC, JS, AC and JA contributed to study design. DdG and JS performed the data extraction and management. KC performed the analysis of raw data. All authors contributed to the interpretation of the results and in the preparation of the manuscript. KC and JA are responsible for the overall content as the guarantors.
Funding: The Swedish Research Council, the Swedish Heart Lung Foundation, The Swedish Cancer Society, Nordforsk, Vinnova.
Competing interests: KC: consultancy fees from Eli Lilly, AbbVie and Pfizer. JS: none. JA: Karolinska Institutet has entered into agreements between Karolinska Institutet (JA as principal investigator) with AbbVie, BMS, MSD, Eli Lilly, Galapagos, Pfizer, Roche, Samsung Bioepis, Sanofi and UCB, mainly regarding safety monitoring of anti-rheumatic therapies. DdG: none.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Swedish Ethical Review Authority 2015/1844-31/2.
References
- 1.Nielen MMJ, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthrit Rheumat 2004;50:380–6. 10.1002/art.20018 [DOI] [PubMed] [Google Scholar]
- 2.Rantapää-Dahlqvist S, de Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. 10.1002/art.11223 [DOI] [PubMed] [Google Scholar]
- 3.Characterisation of lung inflammation and identification of shared citrullinated targets in the lungs and joints of early rheumatoid arthritis. Ann Rheum Dis 2015;74:1772–7. [DOI] [PubMed] [Google Scholar]
- 4.Vossenaar ER, Smeets TJM, Kraan MC, et al. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum 2004;50:3485–94. 10.1002/art.20584 [DOI] [PubMed] [Google Scholar]
- 5.Makrygiannakis D, Hermansson M, Ulfgren A-K, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis 2008;67:1488–92. 10.1136/ard.2007.075192 [DOI] [PubMed] [Google Scholar]
- 6.Reynisdottir G, Olsen H, Joshua V, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis 2016;75:1722–7. 10.1136/annrheumdis-2015-208216 [DOI] [PubMed] [Google Scholar]
- 7.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer 2001;85:473–83. 10.1054/bjoc.2001.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bracke KR, D'hulst AI, Maes T, et al. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol 2006;177:4350–9. 10.4049/jimmunol.177.7.4350 [DOI] [PubMed] [Google Scholar]
- 9.Simon TA, Thompson A, Gandhi KK. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther 2008;10:R45. 10.1186/ar2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smitten AL, Simon TA, Hochberg MC, et al. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther 2008;10:R45. 10.1186/ar2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Q, Wang L, Li L, et al. Risk factors for progression and prognosis of rheumatoid arthritis-associated interstitial lung disease: single center study with a large sample of Chinese population. Clin Rheumatol 2019;38:1109–16. 10.1007/s10067-018-4382-x [DOI] [PubMed] [Google Scholar]
- 12.Nakajima A, Inoue E, Tanaka E, et al. Mortality and cause of death in Japanese patients with rheumatoid arthritis based on a large observational cohort, IORRA. Scand J Rheumatol 2010;39:360–7. 10.3109/03009741003604542 [DOI] [PubMed] [Google Scholar]
- 13.Mattey DL, Thomson W, Ollier WER, et al. Association of DRB1 shared epitope genotypes with early mortality in rheumatoid arthritis: results of eighteen years of followup from the early rheumatoid arthritis study. Arthritis Rheum 2007;56:1408–16. 10.1002/art.22527 [DOI] [PubMed] [Google Scholar]
- 14.Waldenlind K, Eriksson JK, Grewin B, et al. Validation of the rheumatoid arthritis diagnosis in the Swedish National Patient Register: a cohort study from Stockholm County. BMC Musculoskelet Disord 2014;15:432. 10.1186/1471-2474-15-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Askling J, Fored CM, Geborek P, et al. Swedish registers to examine drug safety and clinical issues in RA. Ann Rheum Dis 2006;65:707–12. 10.1136/ard.2005.045872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana R, Wolf R, Berney S. Risk of development of lung cancer is increased in patients with rheumatoid arthritis: a large case control study in US veterans. J Rheumatol 2008;35:1704–8. [PubMed] [Google Scholar]
- 17.Huang S, Kronzer VL, Dellaripa PF, et al. Rheumatoid arthritis–associated interstitial lung disease: current update on prevalence, risk factors, and pharmacologic treatment. Curr Treatm Opt Rheumatol 2020;6:337–53. 10.1007/s40674-020-00160-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung HI, Park JS, Lee M-Y, et al. Prevalence of lung cancer in patients with interstitial lung disease is higher than in those with chronic obstructive pulmonary disease. Medicine 2018;97:e0071. 10.1097/MD.0000000000010071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparks JA, Jin Y, Cho S-K, et al. Prevalence, incidence and cause-specific mortality of rheumatoid arthritis-associated interstitial lung disease among older rheumatoid arthritis patients. Rheumatology 2021;60:3689–98. 10.1093/rheumatology/keaa836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Axelsson GT, Putman RK, Aspelund T, et al. The associations of interstitial lung abnormalities with cancer diagnoses and mortality. Eur Respir J 2020;56:1902154. 10.1183/13993003.02154-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henschke CI, Yankelevitz DF, International Early Lung Cancer Action Program Investigators . Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med Overseas Ed 2006;355:1763–71. 10.1056/NEJMoa060476 [DOI] [PubMed] [Google Scholar]
- 22.Koning HJ, Aalst CM, Jong PA, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2020;382:503–13.31995683 [Google Scholar]
- 23.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–13. 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 24.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002465supp001.pdf (240.2KB, pdf)
rmdopen-2022-002465supp002.pdf (53.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.