Abstract
Objectives
Ochronotic spondyloarthropathy represents one of the main clinical manifestations of alkaptonuria (AKU); however, prospective data and description of the effect of nitisinone treatment are lacking.
Methods
Patients with AKU aged 25 years or older were randomly assigned to receive either oral nitisinone 10 mg/day (N=69) or no treatment (N=69). Spine radiographs were recorded yearly at baseline, 12, 24, 36 and 48 months, and the images were scored for the presence of intervertebral space narrowing, soft tissue calcifications, vacuum phenomena, osteophytes/hyperostosis and spinal fusion in the cervical, thoracic and lumbosacral segment at each of the time points.
Results
At baseline, narrowing of the intervertebral spaces, the presence of osteophytes/hyperostosis and calcifications were the three most frequent radiographic features in AKU. The rate of progression of the five main features during the 4 years, ranked from the highest to lowest was as follows: intervertebral spaces narrowing, calcifications, vacuum phenomena, osteophytes/hyperostosis and fusions. The rate of progression did not differ between the treated and untreated groups in any of the five radiographic parameters except for a slower rate of progression (sum of all five features) in the treatment group compared with the control group (0.45 (1.11) nitisinone vs 0.74 (1.11) controls, p=0.049) in the thoracic segment.
Conclusion
The present study shows a relatively slow but significant worsening of radiographic features in patients with AKU over 4 years. Our results demonstrate a modest beneficial effect of 10 mg/day of nitisinone on the slowly progressing spondylosis in AKU during the relatively limited follow-up time.
Trial registration number
Keywords: Treatment, Osteoarthritis, Arthritis
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is a substantial lack of information about the pattern, localisation and the frequency of spine involvement in patients with alkaptonuria (AKU) as well as effects of nitisinone on the radiographic progression of the spondylosis.
WHAT THIS STUDY ADDS
Intervertebral disc-space narrowing, the presence of osteophytes/hyperostosis and calcification were the three most frequent radiological features in AKU.
The most affected spine region being lumbosacral, followed by the thoracic and cervical segments. Narrowing of the intervertebral spaces, calcifications and vacuum phenomena had the highest rate of radiographic progression, and nitisinone had a modest beneficial effect on the slowly progressing AKU spondylosis features in the thoracic spine as well as on the pain in the cervical segment.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results demonstrate a modest beneficial effect of nitisinone 10 mg/day on the slowly progressing AKU spondylosis over this time period.
Introduction
Alkaptonuria (AKU) is an autosomal recessive inherited condition (OMIM#203500) characterised by a lack of homogentisate 1,2 dioxygenase (HGD) activity (EC:1.13.11.5), due to mutations at the HGD gene loci, leading to accumulation of homogentisic acid (HGA).1–3 HGA undergoes oxidation via a benzoquinone acetate intermediary, resulting in deposition of melanin-like HGA pigment in joint and spine cartilage, tendons and ligaments, in a process known as ochronosis.4 The ochronotic spine becomes rigid and liable to further changes resulting in severe disability. The prevalence of AKU worldwide is estimated at 1:250 000 to 1:500 000, however the frequency as high as 1:19 000 has been observed in some countries such as Slovakia.5
Although spondylosis represents a clinical hallmark of AKU, little is known about the pattern, localisation and the frequency of spine involvement in untreated AKU. Current data, consisting mostly of case reports and cross-sectional studies, often provide inconclusive results.6 7 There is no information on potential factors that could affect AKU spondylosis such as age, gender, body weight and body mass index (BMI). There is incomplete data from objective assessments as to what age spondylosis takes hold and starts evolving over time. Spinal disease in AKU is said to begin at about age 30 years both in terms of symptoms as well as radiological change.6 Some of the features reported are intervertebral disc-space narrowing, intervertebral disc prolapse, intervertebral disc calcification, vacuum phenomenon, osteophyte formation, osteopenia, marginal sclerosis of adjoining vertebral bodies, fibrous and bony intervertebral bridging with eventual fusion and formation of block vertebrae with obliteration of disc spaces, calcification of spinal ligaments, spinal cord compression and vertebral fracture.6 7 As far as we are aware, there has never been a report on the spinal features’ progression using prospective data except for one study using scintigraphy rather than plain radiographs.7
A new therapy was approved by the European Medicines Agency in 2020; nitisinone, an inhibitor of 4-hydroxyphenylpyruvaye dioxygenase, has been shown to slow the progression of AKU disease overall by substantially decreasing HGA formation.8–10 Whether a nitisinone-mediated decrease in HGA could decrease spondylosis and stabilise spinal damage in AKU is not established. A statistically significant effect of nitisinone on the number of segments with spinal pain was, however, observed in a large cohort of patients with AKU who participated in the randomised control trial (SONIA 2; Suitability of Nitisinone in Alkaptonuria 2).8 Recent observations in human AKU suggests that ochronosis, visible externally in the ear and eyes, may reverse significantly post-nitisinone, bringing hope to those with advanced AKU disease.8 The current data analysis from the SONIA 2 study offers an opportunity to better understand how spine features in AKU evolve and to test the effect of nitisinone on spinal disease over a 4-year follow-up.
Materials and methods
The SONIA 2 study was a 4-year, open-label, evaluator-blinded, multicentre, randomised, no treatment controlled, parallel-group study to investigate the efficacy and safety of nitisinone 10 mg/day for patients with AKU, registered at ClinicalTrials.gov. The study was performed at three investigational sites between May 2014 and February 2019. The participating sites in this multinational study were: Liverpool University Hospitals NHS Foundation Trusts, Liverpool, UK; National Institute of Rheumatic Diseases, Piešťany, Slovakia; and Hôpital Necker-Enfants Malades, Paris, France. All patients provided written informed consent prior to inclusion in the study. Independent ethics committees at each centre approved the study. All patients have been previously reported.8 This prior article dealt with efficacy and safety of one time per day nitisinone for patients with AKU whereas in this manuscript we report radiographic features progression and effects of nitisinone on the spinal disease. A detailed description of the study participants and its design has been previously published.8 Briefly, 138 patients with a confirmed diagnosis of AKU, and any clinical manifestation in addition to increased HGA, were randomised so that 69 patients received nitisinone and 69 received no treatment. Use of placebo was not possible in the study because one of the signs of the disease is that the urine darkens due to oxidation of excreted HGA. Patients can therefore easily notice if they are receiving active drug or not. Instead, the study was evaluator blinded as far as possible. Assessments that did not require direct contact between the evaluator and the patient were masked during the entire study. In total, 108 patients completed the study. The main reason for discontinuation in the control group was withdrawal of consent (10 patients), whereas there were nine adverse events leading to withdrawal in the nitisinone group. SONIA 2 inclusion and exclusion criteria are described in the online supplemental tables S1 and S2.
rmdopen-2022-002422supp003.pdf (37.1KB, pdf)
rmdopen-2022-002422supp004.pdf (31KB, pdf)
Oral nitisinone (Orfadin, Swedish Orphan Biovitrum, Stockholm, Sweden) 10 mg/day was administered as a single capsule in the treated group. There were no restrictions regarding concomitant medications. Patients in both groups could freely use analgesics, anti-inflammatory drugs, and other drugs as needed to treat symptoms of AKU.
The assessments done at each visit are described in online supplemental table S3, including HGA measurements, medical history and physical examination, and a wide range of clinical outcome measures expressed as the clinical Alkaptonuria Severity Score Index (cAKUSSI).8 Standardised photographs of eyes and ears were taken at each site at baseline and then annually, that is, after 12, 24, 36 and 48 months. These digital photographs, from all centres, were scored with respect to pigmentation by a single trained analyst blinded for patient’s treatment. The presence or absence of pain in the cervical, thoracic, lumbar and sacral spine segments was recorded at baseline and after 12, 24, 36 and 48 months.
rmdopen-2022-002422supp005.pdf (40.7KB, pdf)
Spine radiographs, originally for the measurement of Cobb angles (scoliosis and kyphosis), consisting of images of cervical, thoracic and lumbosacral segments in the anteroposterior and lateral projections were recorded at baseline and yearly thereafter using GE Optima XR646 in (General Electric Company, Boston, Massachusetts, USA) or Kodak DirectView DR7500 (Carestream Health, Rochester New York, USA). The digital images of the spine segments were automatically merged using Auto Image Paste function of GE Optima service software. At the end of the study, two experienced radiologists (JS and MU), blinded for patients’ treatment, performed the image analysis for all centres. Differences between the two evaluators were subsequently reconciled and the agreed value was use for the statistical analysis. Five radiographic features, not included in the original analysis of study data, were assessed in the cervical, thoracic and lumbosacral segments and scored. These features and their respective scoring were following: presence of narrowing of the intervertebral space (0—none, 1—moderate, 2—extensive), soft tissue calcifications in intervertebral discs and spinal ligaments (0—none, 1—slightly visible, 2—extensive, clearly visible, vacuum phenomena, that is, presence of air space in a degenerated intervertebral disc (0—none, 1—one per segment, 2—two or more per segment), osteophytes and/or hyperostosis (0—none, 1—non-overbridging, 2—overbridging) and spinal fusion (0—none, 1—one per segment, 2—two or more per segment) (figure 1). The sum of scores was defined as score×number of patients at each of the scoring levels, that is, score=0, score=1 and score=2, and also calculated for all scores≥1 (each feature separately or combined features). Total scores were defined as sum of scores (cervical)+sum of scores (thoracic)+sum of scores (lumbosacral) at each of the scoring levels, and also calculated for all scores≥1 (each feature separately or combined features). The rate of progression was defined as the difference between sum of scores in all three spine regions between Month 48 and baseline. After the scoring of radiographic features in duplicates, that is, by two radiologists, the coefficient of variation (CV) was calculated using formula CV=(SD/mean)×100. For the CV calculation all score values were increased by 1 to avoid 0 value entering the formula. The CV values were following: narrowing of the intervertebral space 2.83%, soft tissue calcifications 3.78%, vacuum phenomena 1.57%, osteophytes and/or hyperostosis 3.77% and spinal fusion 0.94%.
Figure 1.

Examples of radiographic features in the cervical (A), thoracic (B) and lumbosacral (C) regions of the spine in woman in her 60s (A), man in his 60s (B) and man in his 50s (C) with alkaptonuria: narrowing of the intervertebral space (black full arrow), soft tissue calcifications (black dashed arrow), vacuum phenomena (white full arrow), osteophytes and/or hyperostosis (white dashed arrow) and spinal fusion (black dash dot arrow).
Statistical analyses were performed using IBM SPSS Statistics V.19 (SPSS, Chicago, Illinois, USA). All statistics carried out in the current analyses were post hoc. Group differences in mean values were analysed by the Student’s t-test or the Mann-Whitney U test, depending on the normality of the data distributions. Basic demographic and clinical data were correlated with X-ray analysis outcomes using the Pearson and partial correlation tests. The stepwise univariable linear regression analysis for each of the five radiographic features scores, as well as the sum of all five radiographic scorings for all three spine segments, were used as dependent variables; and age, body height, body weight, BMI, serum HGA (sHGA), 24-h urinary HGA excretion (uHGA24), eye and ear ochronosis as independent variables. The χ2 test was used to compare frequencies of scores 0, 1 and 2 between men and women at baseline. Fisher’s exact test was used to compare proportion of patients with improvement of spinal pain between the nitisinone and control groups. All values are expressed as mean SD unless stated otherwise. A p value of <0.05 was considered statistically significant.
Results
Baseline demographic data and clinical characteristics of patients with AKU included in the study are shown in table 1.
Table 1.
Baseline demographic, clinical characteristics and radiographic features of 69 (N=69) nitisinone, 69 (N=69) control as well as in all 138 patients with alkaptonuria enrolled in the Suitability of Nitisinone in Alkaptonuria 2 study. Homogentisic acid (HGA), clinical Alkaptonuria Severity Score Index (cAKUSSI). Spine pain, osteoarticular disease in spine, kyphosis, scoliosis, eye ochronosis and ear ochronosis values are presented as individual cAKUSSI subscores
| Nitisinone | Control | Total | |||||||
| All | Male | Female | All | Male | Female | All | Male | Female | |
| Patient number | N=69 | N=45 | N=24 | N=69 | N=40 | N=29 | N=138 | N=85 | N=53 |
| Age (years) | 49 (11.3) | 47.4 (11.9) | 51.9 (9.6) | 47.6 (10.1) | 48.1 (9.9) | 46.9 (10.5) | 48.3 (10.7) | 47.7 (10.9) | 49.2 (10.3) |
| Height (cm) | 166.3 (9.2) | 170.3 (7.1)*** | 158.6 (7.9) | 167 (9.5) | 172.5 (7.6)*** | 160.1 (6.8) | 166.8 (9.4) | 171.3 (7.4)*** | 159.4 (7.3) |
| Weight (kg) | 74.8 (14.8) | 79.2 (12.6)*** | 66.3 (15.1) | 74.1 (15.6) | 80.4 (13.3)*** | 65.6 (14.6) | 74.4 (15.1) | 79.8 (12.9)*** | 65.9 (14.7) |
| Body mass index (kg/m2) | 27 (4.4) | 27.3 (4.2) | 26.2 (4.8) | 26.4 (4.6) | 27.0 (4.1) | 25.5 (5.1) | 26.7 (4.5) | 27.2 (4.2) | 25.8 (4.9) |
| Serum HGA (mmol/L) | 30.3 (11) | 31.7 (11.2) | 27.9 (10.4) | 28.3 (8.7) | 29.1 (7.7) | 27.1 (9.8) | 29.3 (9.9) | 30.4 (9.7) | 27.5 (10.0) |
| Urinary HGA24 (mmol) | 3.5 (1.3) | 3.7 (1.3) | 3.1 (1.3) | 3.5 (1.4) | 3.9 (1.2)* | 3.1 (1.5) | 3.5 (1.3) | 3.8 (1.2)** | 3.1 (1.4) |
| cAKUSSI | 87 (34.2) | 90.9 (35.1) | 79.8 (31.9) | 80.5 (33.4) | 87.1 (31.2) | 71.3 (34.7) | 83.8 (33.8) | 89.1 (33.2)* | 75.2 (33.4) |
| mAKUSSI | 56.7 (26.7) | 60.2 (28.8) | 50.3 (21.3) | 54.1 (25) | 58.3 (25.1) | 48.5 (24.0) | 55.4 (25.8) | 59.2 (27.0)* | 49.3 (22.6) |
| Spinal stenosis | 3.8 (2.1) | 3.8 (2.1) | 3.8 (2.2) | 3.5 (2.3) | 3.7 (2.2) | 3.1 (2.4) | 3.6 (2.2) | 3.7 (2.1) | 3.4 (2.3) |
| Calcifications | 2.9 (1.8) | 3.0 (1.9) | 2.7 (1.8) | 2.6 (1.8) | 2.8 (1.7) | 2.4 (1.9) | 2.8 (1.8) | 2.9 (1.8) | 2.5 (1.8) |
| Vacuum phenomena | 2.3 (1.9) | 2.4 (2.0) | 2.3 (1.6) | 1.9 (1.6) | 2.0 (1.5) | 1.8 (1.7) | 2.1 (1.7) | 2.2 (1.8) | 2.0 (1.7) |
| Osteophytes/hyperostosis | 3.1 (2.1) | 3.1 (2.1) | 3.1 (2.1) | 2.8 (2.1) | 3.1 (2.1) | 2.5 (2.2) | 3.0 (2.1) | 3.1 (2.1) | 2.8 (2.2) |
| Fusions | 0.6 (1.1) | 0.5 (0.9) | 0.8 (1.4) | 0.5 (1.1) | 0.4 (0.8) | 0.7 (1.3) | 0.5 (1.1) | 0.4 (0.9) | 0.7 (1.3) |
| Spine pain | 4.6 (2.7) | 4.4 (2.7) | 5.0 (2.8) | 4.7 (2.4) | 4.3 (2.3) | 5.2 (2.5) | 4.7 (2.5) | 4.4 (2.5) | 5.1 (2.6) |
| Osteoarticular disease spine | 13.6 (8.5) | 13.7 (8.6) | 13.5 (8.6) | 12.1 (8.5) | 12.1 (8.1) | 12.1 (9.2) | 12.9 (8.5) | 12.9 (8.4) | 12.8 (8.9) |
| Kyphosis | 0.7 (1.4) | 0.5 (1.3) | 1.0 (1.4) | 0.5 (1.2) | 0.3 (0.9) | 0.7 (1.5) | 0.6 (1.3) | 0.4 (1.1) | 0.9 (1.5) |
| Scoliosis | 2.0 (0.7) | 1.9 (0.5) | 2.2 (1.0) | 2.0 (0.6) | 2.1 (0.7) | 2.0 (0.5) | 2.0 (0.7) | 2.0 (0.6) | 2.1 (0.8) |
| Eye ochronosis | 17.3 (9.2)# | 17.3 (8.9) | 17.2 (9.8)# | 14.1 (9.6) | 15.8 (9.7) | 11.7 (9.1) | 15.7 (9.5) | 16.6 (9.3) | 14.2 (9.7) |
| Ear ochronosis | 4.1 (3.0) | 4.2 (2.9) | 4.1 (3.1) | 3.9 (2.9) | 4.2 (2.7) | 3.5 (3.1) | 4.0 (2.9) | 4.2 (2.8) | 3.8 (3.1) |
Male versus female comparison *p<0.05, **p<0.01, ***p<0.001. Modified Alkaptonuria Severity Score Index (mAKUSSI).
mAKUSSI, Modified Alkaptonuria Severity Score Index.
Overall, there were more men (N=85, 66.6%) than women (N=53, 33.3%) entering the study. At baseline, male patients did not differ in age, BMI and serum HGA, but had higher body height (p<0.001), body weight (p<0.001), urinary HGA (p<0.01) and cAKUSSI (p<0.05) compared to women.
At baseline, patients in the treatment and non-treatment groups had comparable demographic and clinical characteristics except higher (p<0.05) eye ochronosis score in the nitisinone group compared with controls. When the demographic and clinical features at baseline were analysed in men and women separately, there were no significant differences between treatment and non-treatment groups in all studied parameters except higher (p<0.05) eye ochronosis in nitisinone-treated women compared with women in the control group.
At baseline, narrowing of the intervertebral spaces, the presence of osteophytes/hyperostosis and calcifications were the three most frequent radiographic pathologies found in patients with AKU with total scores in the spine of 500, 409 and 381 points in the three segments, respectively (table 2, online supplemental figure S2).
Table 2.
Patient numbers, frequencies and scores of five radiographic features, that is, narrowing of the intervertebral space, soft tissue calcifications, vacuum phenomena, osteophytes and/or hyperostosis and spinal fusion in the cervical, thoracic and lumbosacral regions of spine in 138 patients with alkaptonuria (N=138) at the baseline. Sum of scores=scoring×number of patients. Total score=sum of scores (cervical)+sum of scores (thoracic)+sum of scores (lumbosacral)
| Cervical | Thoracic | Lumbosacral | |||||
| Scoring | Number of patients (%) | Sum of scores | Number of patients (%) | Sum of scores | Number of patients (%) | Sum of scores | Cumulative score |
| Narrowing of the intervertebral space | |||||||
| 0 | 52 (37.7) | 0 | 40 (29.0) | 0 | 25 (18.1) | 0 | 0 |
| 1 | 38 (27.5) | 38 | 40 (29.0) | 40 | 16 (11.6) | 16 | 94 |
| 2 | 48 (34.8) | 96 | 58 (42.0) | 116 | 97 (70.3) | 194 | 406 |
| ≥1 | 86 (62.3) | 134 | 98 (71.0) | 156 | 113 (81.9) | 210 | 500 |
| Calcifications | |||||||
| 0 | 104 (75.4) | 0 | 36 (26.1) | 0 | 32 (23.2) | 0 | 0 |
| 1 | 32 (23.2) | 32 | 42 (30.4) | 42 | 29 (21.0) | 29 | 103 |
| 2 | 2 (1.4) | 4 | 60 (43.5) | 120 | 77 (55.8) | 154 | 278 |
| ≥1 | 34 (24.6) | 36 | 102 (73.9) | 162 | 106 (76.8) | 183 | 381 |
| Vacuum phenomena | |||||||
| 0 | 95 (68.8) | 0 | 96 (69.6) | 0 | 51 (37) | 0 | 0 |
| 1 | 16 (11.6) | 16 | 15 (10.9) | 15 | 23 (16.7) | 23 | 54 |
| 2 | 27 (19.6) | 54 | 27 (19.6) | 54 | 64 (46.4) | 128 | 236 |
| ≥1 | 43 (31.2) | 70 | 42 (30.5) | 69 | 87 (63.0) | 151 | 290 |
| Osteophytes/hyperostosis | |||||||
| 0 | 60 (43.5) | 0 | 46 (33.3) | 0 | 35 (25.4) | 0 | 0 |
| 1 | 60 (43.5) | 60 | 42 (30.4) | 42 | 35 (25.4) | 35 | 137 |
| 2 | 18 (13.0) | 36 | 50 (36.2) | 100 | 68 (49.3) | 136 | 272 |
| ≥1 | 78 (56.5) | 96 | 92 (66.7) | 142 | 103 (74.7) | 171 | 409 |
| Spinal fusions | |||||||
| 0 | 133 (96.4) | 0 | 119 (86.2) | 0 | 111 (80.4) | 0 | 0 |
| 1 | 3 (2.2) | 3 | 13 (9.4) | 13 | 11 (8.0) | 11 | 27 |
| 2 | 2 (1.4) | 4 | 6 (4.3) | 12 | 16 (11.6) | 32 | 48 |
| ≥1 | 5 (3.6) | 7 | 19 (13.8) | 25 | 27 (19.6) | 43 | 75 |
rmdopen-2022-002422supp002.pdf (133.9KB, pdf)
When analysed for the presence (score≥1) or absence (score=0) of any of the five distinct X-ray features studied, the most affected spine region was the lumbosacral, followed by the thoracic and the least affected cervical segments.
In general, the lower severity (score=1) was more common for calcifications and the presence of osteophytes/hyperostosis in the cervical spine, while more patients had narrowing of the intervertebral spaces and vacuum phenomena with high severity (score=2) in this spine segment. In the thoracic and lumbosacral regions, the high severity score (score=2) was more frequent than the low severity score (score=1) for all five pathologies except spinal fusions in the thoracic segment. Male and female patients with AKU did not differ in frequencies of score 0, 1 or 2 across the spine segments or in the five pathologies studied at baseline (table 1).
As expected, all the five studied spine pathologies correlated positively with age (p<0.001) (figure 2, online supplemental table S4). When adjusted for age; there were positive correlations among the radiographic features and cAKUSSI components related to the spine as well as non-spine osteoarticular disease (online supplemental table S4).
Figure 2.
Correlations between cumulative scores (cervical+thoracic+lumbosacral) for each of the five radiographic features, that is, narrowing of the intervertebral space (top left), soft tissue calcifications (bottom left), vacuum phenomena (top centre), osteophytes and/or hyperostosis (bottom centre) and spinal fusion (top right) and age in the spine of patients with alkaptonuria as well as between age and the sum of scoring in all five radiographic features (bottom right). The squared Pearson correlation coefficient (r2) and respective p value are showed under each of the panels.
rmdopen-2022-002422supp006.pdf (43.6KB, pdf)
Effects of age, body height, body weight, BMI, sHGA, uHGA24, cAKUSSI and eye and ear ochronosis on the X-ray features was analysed using the stepwise linear regression models (table 3).
Table 3.
The stepwise univariable linear regression analysis of each of the five radiographic features scorings for all three spine segments, as well as their sum, as dependent variables. Age, body height, body weight, body mass index, sHGA, uHGA24, eye and ear ochronosis of patients with alkaptonuria were entered into the regression as independent variables
| Variable | Model | R2 | Significance F change | B (SE) | Beta | Significance | Tolerance |
| Narrowing of the intervertebral space | Age | 0.60 | <0.0001 | Constant: −4.14 (0.56) | <0.0001 | ||
| Age: 0.16 (0.01) | 0.78 | <0.0001 | 1.00 | ||||
| Age Ear ochronosis |
0.63 | <0.0001 | Constant: −3.96 (0.56) | <0.0001 | |||
| Age: 0.15 (0.01) | 0.70 | <0.0001 | 0.84 | ||||
| Ear ochronosis: 0.15 (0.04) | 0.20 | <0.0001 | 0.84 | ||||
| Age Ear ochronosis sHGA |
0.65 | <0.0001 | Constant: −4.45 (0.58) | <0.0001 | |||
| Age: 0.14 (0.01) | 0.66 | <0.0001 | 0.80 | ||||
| Ear ochronosis: 0.15 (0.04) | 0.20 | 0.001 | 0.84 | ||||
| sHGA: 0.03 (0.01) | 0.13 | 0.015 | 0.95 | ||||
| Calcifications | Age | 0.55 | <0.0001 | Constant: −3.4 (0.50) | <0.0001 | ||
| Age: 0.13 (0.01) | 0.74 | <0.0001 | 1.00 | ||||
| Age Ear ochronosis |
0.60 | <0.0001 | Constant: −3.05 (0.50) | ||||
| Age: 0.11 (0.01) | 0.64 | <0.0001 | 0.84 | ||||
| Ear ochronosis: 0.16 (0.04) | 0.25 | <0.0001 | 0.84 | ||||
| Vacuum phenomena | Age | 0.21 | <0.0001 | Constant: −1.50 (0.61) | 0.019 | ||
| Age: 0.07 (0.01) | 0.46 | <0.0001 | 1.00 | ||||
| Age sHGA | 0.23 | 0.020 | Constant: −2.01 (0.65) | 0.002 | |||
| Age: 0.07 (0.01) | 0.42 | <0.0001 | 0.95 | ||||
| sHGA: 0.03 (0.01) | 0.18 | 0.020 | 0.95 | ||||
| Osteophytes or hyperostosis | Age | 0.53 | <0.0001 | Constant: −3.99 (0.57) | 0.02 | ||
| Age: 0.13 (0.01) | 0.66 | <0.0001 | 1.00 | ||||
| Age Ear ochronosis |
0.55 | <0.0001 | Constant: −3.76 (0.57) | <0.0001 | |||
| Age: 0.13 (0.01) | 0.65 | <0.0001 | 0.84 | ||||
| Ear ochronosis: 0.13 (0.05) | 0.17 | 0.005 | 0.84 | ||||
| Fusions | Age | 0.21 | <0.0001 | Constant: −1.65 (0.38) | <0.0001 | ||
| Age: 0.04 (0.01) | 0.46 | <0.0001 | 1.00 | ||||
| Age Height |
0.26 | 0.003 | Constant: 3.50 (1.73) | 0.044 | |||
| Age: 0.04 (0.01) | 0.36 | <0.0001 | 0.84 | ||||
| Height: −0.03 (0.01) | −0.25 | 0.003 | 0.84 | ||||
| Age Height sHGA |
0.29 | 0.015 | Constant: 3.06 (1.70) | 0.077 | |||
| Age: 0.03 (0.01) | 0.32 | <0.0001 | 0.81 | ||||
| Height: −0.03 (0.01) | −0.24 | 0.003 | 0.84 | ||||
| sHGA: 0.02 (0.01) | 0.18 | 0.015 | 0.95 | ||||
| Sum of all X-ray features | Age | 0.61 | <0.0001 | Constant: −14.62 (1.85) | <0.0001 | ||
| Age: 0.55 (0.04) | 0.78 | <0.0001 | 1.00 | ||||
| Age Ear ochronosis |
0.64 | <0.0001 | Constant: −13.99 (1.79) | <0.0001 | |||
| Age: 0.50 (0.04) | 0.71 | <0.0001 | 084 | ||||
| Ear ochronosis: 0.51 (0.15) | 0.20 | 0.001 | 0.84 | ||||
| Age Ear ochronosis sHGA |
0.66 | <0.0001 | Constant:−15.90 (1.90) | ||||
| Age: 0.48 (0.04) | 0.68 | <0.0001 | 0.80 | ||||
| Ear ochronosis: 0.50 (0.14) | 0.19 | 0.001 | 0.84 | ||||
| sHGA: 0.10 (0.04) | 0.13 | 0.013 | 0.95 |
Serum homogentisic acid (sHGA), 24-h urinary homogentisic acid excretion (uHGA24).
uHGA24, Serum homogentisic acid (sHGA), 24-h urinary homogentisic acid excretion.
An assessment of multicollinearity showed that models that included cAKUSSI as independent variable were highly correlated with age (tolerance <0.75), therefore cAKUSSI was excluded from the further linear regression analysis (data not shown). Correlations between baseline sHGA and age (r=0.23) as well as between baseline uHGA24 and age (r=−0.21) were relatively weaker compared with cAKUSSI versus age, therefore the biochemical parameters were entered to the regression as independent variables. The regression models consisting of age alone or in combination with ear ochronosis were the best indicators of the studied radiographic features (table 3).
The 4-year follow-up of the SONIA 2 clinical trial allowed us to analyse radiographic progression of the spondylosis in the spine of patients with AKU (figure 3). The rate of progression during the 4-year observation period in patients with untreated AKU who completed the study (N=55) was the following (mean (SD) difference of scores between Year 4 and baseline): narrowing of the intervertebral spaces 0.46 (0.82), calcifications 0.46 (0.77), vacuum phenomena 0.44 (0.88), osteophytes/hyperostosis 0.30 (0.50) and fusions 0.07 (0.33). The rate of progression during the 4-year observation period in nitisinone-treated patients who completed the study at visit 6 (N=57) was the following: narrowing of the intervertebral spaces 0.46 (0.71), calcifications 0.36 (0.67), osteophytes/hyperostosis 0.30 (0.54), vacuum phenomena 0.29 (0.73) and fusions 0.11 (0.37).
Figure 3.
Radiographic scores (mean, SE), that is, narrowing of the intervertebral space (top left), soft tissue calcifications (top right), vacuum phenomena (mid left), osteophytes and/or hyperostosis (mid right), spinal fusion (bottom left) and sum of all features in all spine segments (bottom right) in 69 (N=69) patients with alkaptonuria on nitisinone 10 mg/day (black circles, full line) and in 69 (N=69) untreated patients during 48 months.
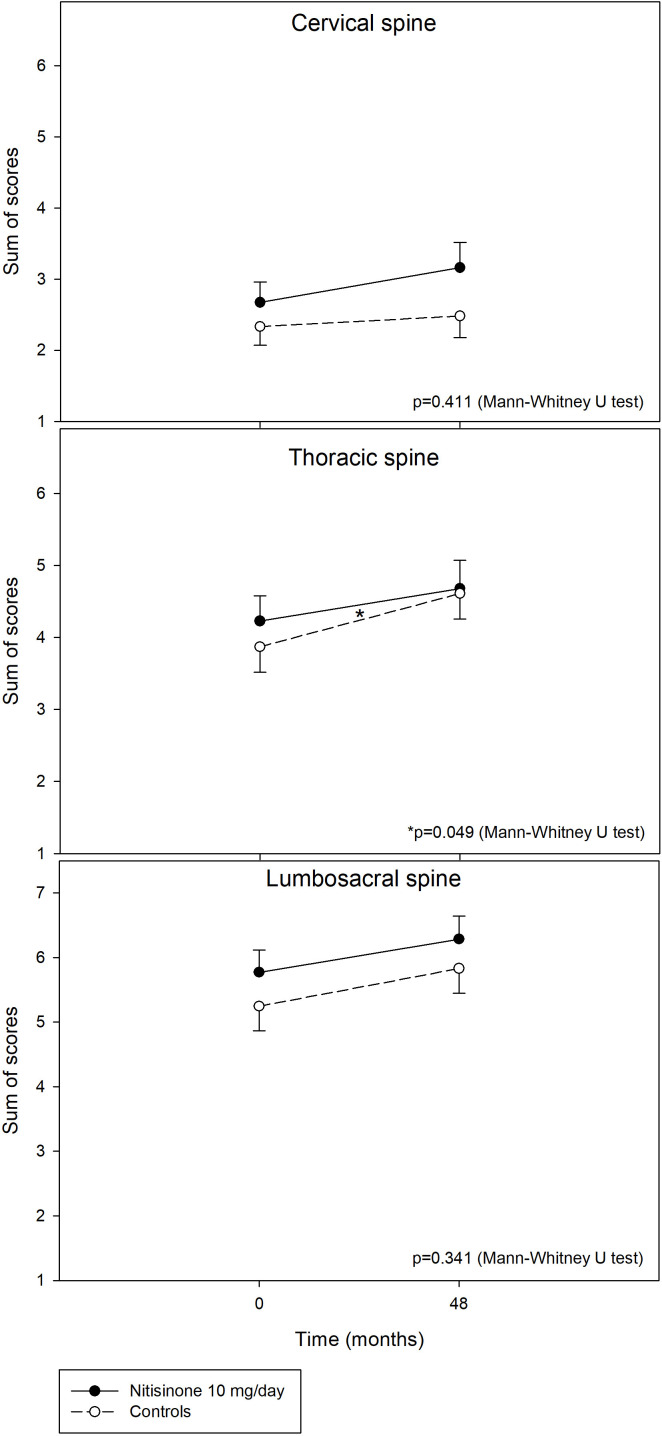
The rate of progression during the 4-year period did not differ between the treated and untreated groups in any of the five major X-ray features. When the rate of progression was analysed for each of the three spinal segments separately, the patients on nitisinone and controls had comparable increment of scores in each of the five radiographic features. When the rate of progression was analysed for the separate spine segments as a sum of all scores for the five features, the rate of progression was significantly slower (0.45 (1.11) in the in nitisinone group compared with 0.74 (1.11) in the control group, p=0.049) in the thoracic segment (figure 4). The general linear model for repeated measures (with or without age as a covariate) did not find any significant time versus treatment group interaction indicating no effect of nitisinone on radiographic feature progression during the 4-year observation interval.
Figure 4.
Change in sum of scores (mean, SE) for all five radiographic features (narrowing of the intervertebral space+soft tissue calcifications+vacuum phenomena+osteophytes and/or hyperostosis+spinal fusion) in the cervical (top), thoracic (centre) and lumbosacral (bottom) regions of the spine in 69 (N=69) patients with alkaptonuria on nitisinone 10 mg/day (black circles, full line) and in 69 untreated patients from the baseline (Month 0) to Month 48. P value (Mann-Whitney U test) denotes treatment versus non-treatment difference in increments (Month 48 minus Month 0).
The radiographic progression of thoracic narrowing of the intervertebral spaces and calcifications, in the pooled patient population irrespective of treatment group, was significantly faster in the women than in the men (narrowing: 0.11 (0.32) in men vs 0.27 (0.50) in women, p=0.05; calcifications: 0.11 (0.36) in men vs 0.32 (0.57) in women, p=0.02). The rate of progression of osteophytes/hyperostosis in all spine segments combined was also faster in the women than in the men (0.23 (0.49) in men vs 0.41 (0.55), p=0.033).
The proportion of patients reporting an improvement in cervical spine pain was significantly higher in the nitisinone group compared with the control group (20 out of 55 in the nitisinone vs 8 out of 54 in the control group, p=0.015) at Month 48. The proportion of patients reporting the pain improvement in the thoracic, lumbar and sacral segments were comparable between nitisinone and control groups at Month 48.
Discussion
The present study shows a significant worsening of radiographic features, that is, narrowing of intervertebral spaces, calcifications, vacuum phenomena, osteophytes and/or hyperostosis and spinal fusions, in patients with untreated AKU during the 4-year period, offering direct evidence for progression of AKU spondylosis in this prospective cohort. The present results are in line with previous cross-sectional data and case reports confirming that AKU spondyloarthropathy represents a hallmark feature of the disease’s natural history.6 11 12 Regarding a specific anatomical location, our findings of the lumbosacral region being the most affected suggest that AKU spondylitis to a great extend mirrors degeneration of intervertebral discs leading to spondyloarthropathy in the general population.13
The age-related distribution of the radiographic features at baseline in our pooled (nitisinone and control group) data set shows that except for the spinal fusions, all the studied radiographic features can be detected before age 40 years, and in two of our patients they were present even before age 30. This agrees with Phornphutkul and colleagues who showed low composite radiographic score for the severity of spinal and joint disease until age 30, after which it rose in a roughly linear fashion with age in their cross-sectional analysis.6 More recent data have confirmed the presence of spondyloarthropathy in about 9% of patients with AKU younger than 25 years with 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) uptake, suggesting even earlier development of bone and cartilage structural changes in AKU.7
The second aim of the present study was to investigate effects of the 4-year treatment with 10 mg/day nitisinone on the radiographic progression of AKU spondylosis. The results demonstrate only a minor effect of the treatment on the slowly progressing spine pathology limited to the thoracic region. The relatively weak effect of nitisinone on delaying radiographic progression can be attributed to the slow progressing nature of the disease, spanning several decades, in relation to the relatively short duration of our study. In her pioneering nitisinone trial in AKU, Introne and colleagues did not find an effect on the Schober distance after 3 years of nitisinone treatment.14 Unlike the spine pathology, an arrest and even regression of eye and ear ochronosis have been observed in patients with AKU on nitisinone.8 9 Given the slow progressive nature of the intervertebral disc ageing and degeneration, a longer observational period will be necessary to confirm whether there are positive effects of nitisinone on spondyloarthropathy in AKU. The modest effect of nitisinone in the thoracic but not in cervical and lumbar regions found in our study may reflect the fact mechanical stresses on the regions of the spine can vary. The cervical spine is more mobile compared with the thoracic region and this may explain some of these results. The more mobile cervical region could potentially show more advanced and more irreversible changes and less response to nitisinone unlike the relatively less mobile thoracic spine. It is also possible that nitisinone therapy earlier in life, as opposed to therapy at an advanced age of 49 (11.3) years, the average age in the SONIA 2 study, could prevent these spinal features from appearing or progressing.
As expected, our results confirm age as the most important factor involved in AKU spondylosis. This is in line with other studies showing a positive relationship between age and spine pathologies in AKU.6 11 12 15 16 In addition to age, we analysed the relative contribution of other demographic parameters in our cohort on the radiographic presentation of the AKU spondylosis. The less steep regression slope of spinal fusion scores versus age when compared with the slopes of the other four radiological features versus age found in the present study are consistent with the well-accepted clinical notion that fusion occurs later during the disease progression, mostly after 50 years of age.6
The circulating concentration of HGA in AKU is determined by at least three major factors; dietary intake of tyrosine and phenylalanine, residual HGD activity and renal excretion of HGA.17–20 In the present study, we attempted to provide evidence linking HGA and spine pathology in AKU; however, a single ‘snapshot’ measurement of sHGA and uHGA24 has limited value compared with the cumulative HGA load over the lifetime of a patient. In line with the latter, age but not sHGA or uHGA24 was a strong indicator of the studied radiographic features in the regression analysis. In addition to age and HGA, our study shows that ear ochronosis, a marker of lifetime exposure to HGA, is a relatively good indicator of the intervertebral narrowing, calcifications and appositions. Collectively, our results suggest that the ochronotic process in visible cartilages such as ears may indeed reflect the status of ochronotic spondyloarthropathy in AKU. Interestingly, body height but not body weight was identified as an independent factor of spinal fusion scores among other radiographic features in the regression analysis. This may reflect the higher load stresses on a longer spine resulting in greater damage although this finding may also simply reflect relative decrease in body height due to intervertebral space narrowing progressing into development of the spinal fusions.
One of the limitations of the present study is the relatively short duration of the SONIA 2 trial, which does not allow full evaluation of the effect of nitisinone on AKU spondyloarthropathy as well as some specific aspects of long-term radiographic progression. Since intervertebral disc ageing and degeneration with age are common in the general population, an analysis of spondylosis as a part of the AKU natural history, would require additional age-matched and gender-matched, healthy control group data, for comparison. Reporting of pain by patients could have been biased due to SONIA 2 being a non-blinded clinical trial. Due to relatively long asymptomatic phase of AKU with only dark urine and a gradual onset of joint and spine pain approximately in the third decade of life, disease duration was not assessed in the present study.
In conclusion, the present study shows a relatively slow but significant worsening of radiographic features, that is, narrowing of the intervertebral spaces, calcifications, vacuum phenomena, osteophytes and/or hyperostosis and spinal fusions in patients with untreated AKU during the 4-year period. Our results also demonstrate a modest beneficial effect of nitisinone 10 mg/day on the slowly progressing AKU spondylosis over this time period.
rmdopen-2022-002422supp001.pdf (71.3KB, pdf)
rmdopen-2022-002422supp007.pdf (296.7KB, pdf)
Acknowledgments
We thank the European Commission for the Seventh Framework Programme grant (DevelopAKUre, project number 304985) that was crucial to conduct the SONIA 2 study. We also thank Výskumná agentúra (Research Agency) for the BIOFORD grant (ITMS 313011AFG5) co-financed by the European Regional Development Fund. We thank all patients in SONIA 2 for their participation, and the patient societies for supporting the patients in the study and for their efforts in successfully recruiting many patients.
Footnotes
Contributors: RI performed radiological data analyses, wrote the manuscript and assisted with the conduct of the study in Piešťany, Slovakia and is the guarantor of the present study. JS and MU did the radiograph analyses and scorings. MG and RJ assisted with the statistical analysis. BO and MR contributed to the study design and contributed to the manuscript writing. AZ did the biomarker and genetic analyses. J-BA assisted with the conduct of the study in Paris, France. OL, VM, RS, EV, JR and EZ assisted with the conduct in Piešťany. EL, HB and MK assisted with the conduct of the study in Liverpool, UK. LRR and JG pioneered the idea for SONIA 2, secured funding and managed the study.
Funding: The study was supported by the Seventh Framework Programme grant (DevelopAKUre, project number 304985) from the European Commission and by BIOFORD grant (ITMS 313011AFG5) co-financed by the European Regional Development Fund.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data access will be granted in response to qualified research requests. All de-identified individual participant data, for patients with separate consent signed for this purpose, can be made available to researchers. Data will be shared on the basis of the scientific merit of the proposal (ie, the proposal should be scientifically sound, ethical and have the potential to contribute to the advancement of public health) and the feasibility of the research proposal (ie, the requesting research team must be scientifically qualified and have the resources to conduct the proposed project). The data files would exclude data dictionaries that require user licenses. Data could be made available following finalised regulatory authority review and at the end of any data exclusivity periods, and ending 36 months after the regulatory authority review decision has been received or until the corresponding author is able to fulfil this obligation, whichever is earlier. Furthermore, the study protocol and statistical analysis plan can be made available. Proposals should be directed to j.a.gallagher@liverpool.ac.uk. Data requestors will need to sign a data access agreement.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by EC Liverpool (NRES Committee North-West – Liverpool Central) Reference number: 13/NW/0567; EC Piešťany (NURCH Ethica Committee, National Institute of Rheumatic Diseases, Ivana Krasku 4,92101 Piešťany, Slovak Republic) Reference number: 04196/0029/001/001; EC Paris (EC Ile De France II, hospital Necker 149 Rue de Sevres 75 743 Paris Cedex 15, Porte N2, 1er etage, France) Reference number: 2013-08-08. Participants gave informed consent to participate in the study before taking part.
References
- 1.Granadino B, Beltrán-Valero de Bernabé D, Fernández-Cañón JM, et al. The human homogentisate 1,2-dioxygenase (HGO) gene. Genomics 1997;43:115–22. 10.1006/geno.1997.4805 [DOI] [PubMed] [Google Scholar]
- 2.Nemethova M, Radvanszky J, Kadasi L, et al. Twelve novel HGD gene variants identified in 99 alkaptonuria patients: focus on 'black bone disease' in Italy. Eur J Hum Genet 2016;24:66–72. 10.1038/ejhg.2015.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascher DB, Spiga O, Sekelska M, et al. Homogentisate 1,2-dioxygenase (HGD) gene variants, their analysis and genotype-phenotype correlations in the largest cohort of patients with AKU. Eur J Hum Genet 2019;27:888–902. 10.1038/s41431-019-0354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranganath LR, Norman BP, Gallagher JA. Ochronotic pigmentation is caused by homogentisic acid and is the key event in alkaptonuria leading to the destructive consequences of the disease-A review. J Inherit Metab Dis 2019;42:776–92. 10.1002/jimd.12152 [DOI] [PubMed] [Google Scholar]
- 5.Zatková A, de Bernabé DB, Poláková H, et al. High frequency of alkaptonuria in Slovakia: evidence for the appearance of multiple mutations in HGO involving different mutational hot spots. Am J Hum Genet 2000;67:1333–9. 10.1016/S0002-9297(07)62964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med 2002;347:2111–21. 10.1056/NEJMoa021736 [DOI] [PubMed] [Google Scholar]
- 7.Ranganath LR, Khedr M, Vinjamuri S, et al. Characterizing the alkaptonuria joint and spine phenotype and assessing the effect of homogentisic acid lowering therapy in a large cohort of 87 patients. J Inherit Metab Dis 2021;44:666–76. 10.1002/jimd.12363 [DOI] [PubMed] [Google Scholar]
- 8.Ranganath LR, Psarelli EE, Arnoux J-B, et al. Efficacy and safety of once-daily nitisinone for patients with alkaptonuria (SONIA 2): an international, multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2020;8:762–72. 10.1016/S2213-8587(20)30228-X [DOI] [PubMed] [Google Scholar]
- 9.Ranganath LR, Khedr M, Milan AM, et al. Nitisinone arrests ochronosis and decreases rate of progression of alkaptonuria: evaluation of the effect of nitisinone in the United Kingdom national alkaptonuria centre. Mol Genet Metab 2018;125:127–34. 10.1016/j.ymgme.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 10.Ranganath LR, Milan AM, Hughes AT, et al. Suitability of Nitisinone in alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis 2016;75:362–7. 10.1136/annrheumdis-2014-206033 [DOI] [PubMed] [Google Scholar]
- 11.Palazzi C, D'Angelo S, Leccese P, et al. Ochronotic arthropathy of the spine limited to the thoracic section. Rheumatology 2013;52:799. 10.1093/rheumatology/kes396 [DOI] [PubMed] [Google Scholar]
- 12.Perry MB, Suwannarat P, Furst GP, et al. Musculoskeletal findings and disability in alkaptonuria. J Rheumatol 2006;33:2280–5. [PubMed] [Google Scholar]
- 13.Adams MA, Dolan P. Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat 2012;221:497–506. 10.1111/j.1469-7580.2012.01551.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab 2011;103:307–14. 10.1016/j.ymgme.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalevski SK, Haritonov DG, Peev NA. Alcaptonuria with lumbar disc prolapse: case study and review of the literature. Spine J 2007;7:495–8. 10.1016/j.spinee.2006.06.399 [DOI] [PubMed] [Google Scholar]
- 16.Reddy DR, Prasad VS. Alkaptonuria presenting as lumbar disc prolapse: case report and review of literature. Spinal Cord 1998;36:523–4. 10.1038/sj.sc.3100562 [DOI] [PubMed] [Google Scholar]
- 17.Judd S, Khedr M, Milan AM, et al. The nutritional status of people with alkaptonuria: an exploratory analysis suggests a protein/energy dilemma. JIMD Rep 2020;53:45–60. 10.1002/jmd2.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranganath LR, Milan AM, Hughes AT, et al. Homogentisic acid is not only eliminated by glomerular filtration and tubular secretion but also produced in the kidney in alkaptonuria. J Inherit Metab Dis 2020;43:737–47. 10.1002/jimd.12181 [DOI] [PubMed] [Google Scholar]
- 19.Hughes JH, Wilson PJM, Sutherland H, et al. Dietary restriction of tyrosine and phenylalanine lowers tyrosinemia associated with nitisinone therapy of alkaptonuria. J Inherit Metab Dis 2020;43:259–68. 10.1002/jimd.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez JM, Timm DE, Titus GP, et al. Structural and functional analysis of mutations in alkaptonuria. Hum Mol Genet 2000;9:2341–50. 10.1093/oxfordjournals.hmg.a018927 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002422supp003.pdf (37.1KB, pdf)
rmdopen-2022-002422supp004.pdf (31KB, pdf)
rmdopen-2022-002422supp005.pdf (40.7KB, pdf)
rmdopen-2022-002422supp002.pdf (133.9KB, pdf)
rmdopen-2022-002422supp006.pdf (43.6KB, pdf)
rmdopen-2022-002422supp001.pdf (71.3KB, pdf)
rmdopen-2022-002422supp007.pdf (296.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data access will be granted in response to qualified research requests. All de-identified individual participant data, for patients with separate consent signed for this purpose, can be made available to researchers. Data will be shared on the basis of the scientific merit of the proposal (ie, the proposal should be scientifically sound, ethical and have the potential to contribute to the advancement of public health) and the feasibility of the research proposal (ie, the requesting research team must be scientifically qualified and have the resources to conduct the proposed project). The data files would exclude data dictionaries that require user licenses. Data could be made available following finalised regulatory authority review and at the end of any data exclusivity periods, and ending 36 months after the regulatory authority review decision has been received or until the corresponding author is able to fulfil this obligation, whichever is earlier. Furthermore, the study protocol and statistical analysis plan can be made available. Proposals should be directed to j.a.gallagher@liverpool.ac.uk. Data requestors will need to sign a data access agreement.