Abstract
We examined the effect of a human immunodeficiency virus (HIV)-specific immune-based therapy in Thailand, where access to antiviral drug therapy is limited. A 40-week trial was conducted with 297 asymptomatic, HIV-infected Thai subjects with CD4-cell counts greater than 300 μl/mm3. Subjects were randomized to receive either HIV type 1 (HIV-1) immunogen (Remune; inactivated HIV-1 from which gp120 is depleted in incomplete Freund's adjuvant or adjuvant control at 0, 12, 24, and 36 weeks at five different clinical sites in Thailand. Neither group received antiviral drug therapy. The a priori primary endpoint for the trial was changes in CD4-cell counts with secondary parameters of percent changes in CD8-cell counts (percent CD4, CD8, and CD4/CD8) and body weight. Subsets of subjects were also examined for changes in plasma HIV-1 RNA levels, Western blot immunoreactivity, and HIV-1 delayed-type hypersensitivity (DTH) skin test reactivity. There was a significant difference in changes in CD4-cell counts that favored the HIV-1 immunogen-treated group compared to those for the adjuvant-treated control group (P < 0.05). On average, for HIV-1 immunogen-treated subjects CD4-cell counts increased by 84 cells by week 40, whereas the increase for the control group was 38 cells by week 40. This increase in CD4-cell count was associated with increased HIV-specific immunogenicity, as shown by Western blotting and enhanced HIV-1 DTH skin reactivity. No significant differences in adverse events were observed between the groups. The results of this trial suggest that HIV-1 immunogen is safe and significantly increases CD4-cell counts and HIV-specific immunity compared to those achieved with the adjuvant control in asymptomatic HIV-1-infected subjects not taking antiviral drugs.
Recent advances in human immunodeficiency virus (HIV) type 1 (HIV-1) antiviral drug therapy have had a significant impact on AIDS morbidity and mortality in industrialized countries (2, 25; R. A. Torres and M. Barr, Letter, N. Engl. J. Med. 336:1531–1532, 1997). In North America and Europe, antiviral drug “cocktails” (antiretroviral therapies [ARTs]) which include a protease inhibitor have become the standard of care, but their access is greatly limited in the countries where more than 90% of the individuals with HIV-1 infection live (4, 40).
In Thailand, despite aggressive public health measures and a possible slowing of the epidemic, an estimated 1 million deaths will occur from AIDS by the year 2014 (27, 37). Thus, cost-effective strategies including preventive and therapeutic approaches to further slow progression of the disease are urgently needed. Recently, there has been increased interest in the relationship between strong HIV-1-specific immune function, improved clinical outcomes, and the potential to use immune-based therapies to stimulate similar immune responses (12, 28, 31, 33). Previous studies with HIV-1 immunogen as a monotherapy (inactivated HIV-1 from which gp120 is depleted in incomplete Freund's adjuvant [IFA]; Remune) had shown activity in the slower increase in the number of proviral DNA copies and increased the percentage of CD4 cells, body weight, and HIV-1-specific immune function in asymptomatic subjects prior to the advent of potent antiviral drug therapy (38, 39). Furthermore, phase I trials of the HIV-1 immunogen as a monotherapy with 30 asymptomatic Thai subjects not taking ARTs confirmed the safety and immunogenicity of this approach (8, 20). We therefore hypothesized that use of this HIV-1-specific immune-based approach as monotherapy might improve clinical outcomes as measured by CD4-cell counts in Thai subjects who have little access to antiviral drug therapy. We conducted a phase II double-blind, adjuvant-controlled, multisite clinical trial to examine the effects of the HIV-1 immunogen in an asymptomatic Thai cohort not taking ARTs.
MATERIALS AND METHODS
Approval for the study described here was obtained from the Technical Subcommittee on AIDS Vaccines under the Ministry of Public Health, Bangkok, Thailand, and from the Ethical Review Committee for Research in Human Subjects of the National AIDS Committee. Informed consent was obtained from 297 HIV-infected, asymptomatic Thai subjects with CD4-cell counts >300 μl/mm3. HIV-1-infected individuals were positive by Western blotting and enzyme-linked immunosorbent assay (ELISA) and were randomized to receive either HIV-1 immunogen or IFA adjuvant, with the subjects and the investigators blinded to the treatment codes. Five different clinical sites in Thailand participated in this protocol, and 33 patients were enrolled at each site (allowing a 10% dropout rate). The subjects were randomized to be treated at a 2:1 ratio with an intramuscular injection into the triceps muscle of HIV-1 immunogen or IFA control on day 1 and at weeks 12, 24, and 36. HIV-1 immunogen consisted of inactivated HIV-1 from which gp120 was depleted and was used at a dose of 10 U of p24 antigen in IFA. HIV-1 from which gp120 is depleted is highly purified by ultrafiltration and ion-exchange chromatography (35) from filtered (pore size, 0.45 μm) extracellular supernatant of HIV-1 HZ321-infected HuT-78 cells (5). The a priori primary endpoint of the study was a change in CD4+-cell counts, and these were evaluated at day 1 and weeks 12, 24, 36, and 40. Secondary parameters were percent CD4, CD8 CD4/CD8 cells, body weight, plasma HIV-1 RNA levels, and HIV-1-specific immune function markers.
Lymphocyte phenotyping analysis for the counting of CD4+ and CD8+ cells was done at the BioService Unit, Mahidol University (BSU), and at the Armed Forces Research Institute of Medical Sciences with fresh cells by the same validated methodology (42) at both locations. Enumeration of the CD4+ and CD8+ cells in blood samples of HIV-infected patients was determined by three-color immunofluorescence (e.g., CD3/CD4/CD45 and CD3/CD8/CD45 [27a]) and the use of a TRUECOUNT Absolute Tube with known amounts of fluorescent dye beads to allow calculation of the absolute counts of the leukocytes using the equation recommended by the reagent supplier: number of events in the upper right quadrant containing cell population/number of events as obtained from absolute count bead region × total number of beads/blood volume (in microliters).
The absolute cell numbers were calculated by using the average value for the values from both reactions. The percentage of CD4 and CD8 cells was obtained directly from the percentages of the gated lymphocyte population. Monitoring of CD4 and CD8 levels from blood samples drawn from HIV-infected patients was performed upon receipt of HIV-1 immunogen and IFA (control) on day 1 and at weeks 12, 24, 36, and 40.
The two laboratories calculated absolute cell numbers by using the average value of the number of standard bead events.
Secondary analyses included an HIV-1 antigen delayed-type hypersensitivity (DTH) skin test performed with one cohort to assess functional HIV-specific cell-mediated immunity as described elsewhere (39). Western blot analysis was used to measure the antibody responses to the HIV-1 immunogen in seven cohorts (8). Plasma HIV RNA level determination was done by the NUCLISENS method with a subset of four cohorts (n = 124). Viral subtyping was performed by the Department of Microbiology, Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand, by a peptide ELISA (30) and heteroduplex mobility assay (10). The 14 amino acids specific for subtype E (env subtype E; T S I T I G P G Q V F Y R T) and subtype B (env subtype B; K S I H L G P G Q A W Y T T) were used with the ELISA for this study. The primers ED 3/14 and ED 5/12 were used in the heteroduplex mobility assay, and their PCR products were compared in 5% polyacrylamide gels. Blood chemistry studies for assessment of safety and toxicities were done with samples from all cohorts, as were complete physical examinations by the site physician.
Study data were recorded on case report forms and were entered into a database, which was verified by the Biostatistics Department, Faculty of Public Health, Mahidol University, prior to unblinding.
HIV-1 immunogen (Remune) is obtained by concentration and purification from the supernatant of HIV-1 HZ321-infected Hut-78 cells (13). HZ321 is a Zairian isolate classified as a clade envelope A and clade G gag (5). Antigen preparations were inactivated through a sequential application of β-propiolactone (21) and 60Co irradiation (16). HIV-1 immunogen was prepared by combining equal volumes of inactivated HIV-1 antigen containing 10 U of p24 in isotonic saline and IFA. IFA is formulated by adding one part of the surfactant Montanide 80 (mannide monoleate; Seppic, Paris, France) to nine parts of Drakeol 6 VR light mineral oil (Penreco, Karnes City, Pa.). Subjects were monitored at each visit during the trial for adverse events, and the body weight was also recorded. Safety laboratory investigations included liver function tests (aspartate aminotransferase, alanine aminotransferase) and renal function (blood urea nitrogen, creatinine). For the primary statistical analysis, the Wilcoxon rank, Fisher's exact, and chi-square tests were used. All P values are two tailed.
RESULTS
The baseline demographic characteristics of the HIV-1 immunogen-treated (n = 198) and the IFA-treated (n = 99) groups are shown in Table 1. Both groups are comparable in terms of baseline percent CD4 and CD8 cells, age and gender. The baseline mean CD4 count for the HIV-1 immunogen-treated group was 545.05 cells/mm3, whereas it was 552.21 cells/mm3 for the IFA-treated group, as shown in Table 2. Baseline mean body weights were also similar for the HIV-1 immunogen-treated and IFA-treated groups (56.21 compared to 53.70 kg, respectively). For the subset of subjects for whom plasma RNA levels were determined, the HIV-1 immunogen-treated group (n = 82) and the IFA-treated group (n = 42) likewise had similar levels at the baseline (3.53 and 3.66 log10 copies/ml, respectively). None of the subjects was on antiviral drug therapy at the baseline or during the trial. The predominant subtype of HIV-1 in this cohort was determined to be E, which occurred in 94% of the subjects, and the rest of the subjects (6%) were infected with subtype B. By week 40, data were available for 289 of the 297 subjects.
TABLE 1.
Baseline demographic characteristics
| Characteristic | Treatment group
|
|
|---|---|---|
| IFA | HIV-1 Immunogen | |
| Total sample size (no. of subjects) | 99 | 198 |
| Age (yr) | ||
| Minimum–maximum | 18–46 | 18–43 |
| Mean ± SD | 29.5 ± 7.1 | 28.4 ± 6.1 |
| Median | 29 | 27 |
| Sex | ||
| % Male | 31.3 | 28.1 |
| % Female | 68.7 | 71.9 |
| % With childbearing potential | 10.1 | 10.7 |
| % Without childbearing potential | 58.6 | 61.2 |
| % Homosexual | 2.3 | 3.4 |
| % IVDUa | 1.1 | 0.0 |
| % HIV-positive partner | 96.0 | 88.0 |
| % Hemophiliac | 0.0 | 0.6 |
| % Transfusion recipient | 0.0 | 1.1 |
| % Occupational exposure | None | None |
IVDU, intravenous drug user.
TABLE 2.
Mean baseline CD4 and CD8 counts, percent CD4/CD8 cells, viral load, and body weight
| Group | No. of cells/mm3
|
% Cells
|
Viral load (log10 no. of copies/ml) | Body wt (kg) | |||
|---|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | CD4/CD8 | |||
| IFA | 552.21 | 1,213.54 | 22.42 | 48.95 | 0.487 | 3.66 (n = 82) | 53.70 |
| HIV-1 immunogen | 545.05 | 1,201.26 | 22.26 | 48.72 | 0.493 | 3.53 (n = 42) | 56.21 |
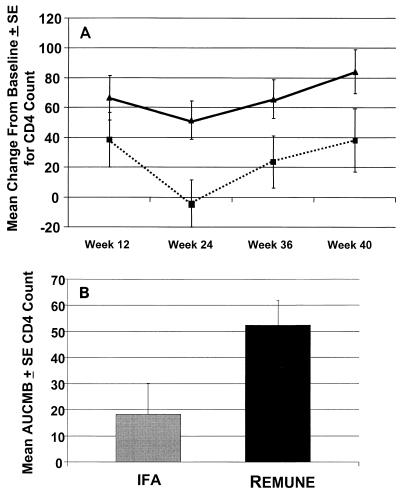
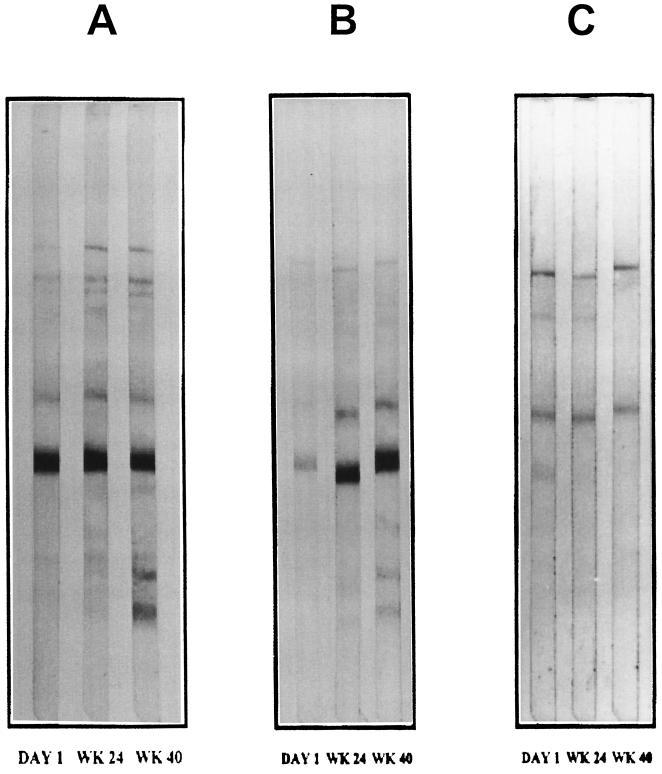
The mean change in the CD4+ cell count from the baseline is displayed in Fig. 1A. Over the course of the study there was a statistically significant increase in the CD4+-cell count (the primary endpoint) in the HIV-1 immunogen-treated group compared to that in the IFA-treated group by area-under-the-curve-minus-baseline analysis, as shown in Fig. 1B (P ≤ 0.05). By week 40, after four treatments, the counts in the subjects in the HIV-1 immunogen-treated group increased by 84 cells, on average, from the baseline count, and the counts in the IFA-treated group increased by 38 cells, on average, as shown in Table 3. Similarly, trends in the values for the other cytologic parameters favoring the HIV-1 immunogen-treated group, as shown in Table 3. Stable viral loads were found for subsets of patients in both treatment groups (Table 3). There was no reactivity to HIV-1 as measured by DTH skin test reactivity at the baseline. The reactivity to HIV-1 antigens in vivo as measured by DTH skin test reactivity in the HIV-1 immunogen-treated group compared to that in the IFA-treated control group increased by week 40 (Table 4). Furthermore, as shown in Table 5 and Fig. 2, there was increased HIV-1-specific antibody immunoreactivity on Western blots in terms of the increased generation of new bands and less of an attenuation of bands for the HIV-1 immunogen-treated group compared to that for the IFA-treated group over the study period. There were no significant differences in laboratory findings, results of physical examinations (data not shown), or adverse events between the two treatment groups, as depicted in Table 6. The most common adverse event was transient local injection site reaction, and this occurrence was similar between the HIV-1 immunogen- and IFA-treated groups (P > 0.05). These reactions subsided without medication.
FIG. 1.
(A) Mean ± standard error change in CD4 count from the baseline (P < 0.05). ■, IFA; ▴, HIV-1 immunogen. (B) Mean area under the curve minus the baseline (AUCMB) for CD4 count at weeks 12, 24, 36, and 40.
TABLE 3.
Mean change from baseline in CD4 count, percent CD4/CD8, viral load, and body weighta at week 40
| Group | No. of CD4 cells/mm3 | Percent
|
Viral load (log10 no. of copies/ml) | Body wt (kg) | |
|---|---|---|---|---|---|
| CD4 | CD4/CD8 | ||||
| IFA | 37.95 ± 21.2 | −0.74 ± 0.47 | −0.009 ± 0.01 | −0.021 ± 0.092 (n = 82) | 0.65 ± 0.3 |
| HIV-1 immunogen | 84.32 ± 14.86 | −0.16 ± 0.37 | 0.013 ± 0.01 | −0.029 ± 0.066 (n = 42) | 0.65 ± 0.2 |
Values are means ± standard errors.
TABLE 4.
Distribution of DTH skin test reactivities at Week 40a
| Group | No. of subjects | % (no.) of subjects with induration (mm) of:
|
|||
|---|---|---|---|---|---|
| 0 | 5–15 | 15–20 | 20 | ||
| IFA | 10 | 40.0 (4) | 40.4 (4) | 20.0 (2) | 0.0 (0) |
| HIV-1 immunogen | 18 | 17.0 (3) | 17.0 (3) | 33.0 (6) | 33.0 (6) |
P = 0.042 (Fisher exact test).
TABLE 5.
Distribution of Western blot results at week 40a
| Group | No. of subjects | % (no.) of subjects with the following change in Western blot immunoreactivity:
|
||
|---|---|---|---|---|
| Decrease | No change | Increase | ||
| IFA | 71 | 18.3 (13) | 38.0 (27) | 43.8 (31) |
| HIV-1 immunogen | 147 | 9.5 (14) | 21.1 (31) | 69.4 (102) |
P = 0.001 by the chi-square test.
FIG. 2.
Western blots for three subjects each at day 1 and weeks 24 and 40. Subjects A and B are representative responders in terms of increased breadth and intensity of bands with immunization. Subject C is a nonresponder.
TABLE 6.
Adverse events at week 40a
| Group | No. of subjects | % Subjects with adverse event | % Subjects with adverse event that was:
|
|
|---|---|---|---|---|
| Mild | Moderate | |||
| IFA | 95 | 20.6 | 20.6 | |
| HIV-1 immunogen | 194 | 16.6 | 14.2 | 2.4 |
P = 0.548 by the Fisher exact test.
DISCUSSION
This is the first report of a phase II double-blind, placebo-controlled trial of an HIV-specific immunotherapeutic agent in Thailand. In this study, asymptomatic HIV-infected Thai subjects not taking antiviral drug therapy were randomized to receive an HIV-1 immunogen or the adjuvant control (IFA). Subjects received four treatments of the HIV-1 immunogen, and their absolute CD4+ cell counts increased significantly without any associated significant toxicity. The changes in CD4 counts represented increases of approximately 15 and 7% relative to baseline for the HIV-1 Immunogen and IFA groups, respectively—a twofold difference favoring active immunization. Changes in CD4 counts, independent of changes in viral loads, have been examined extensively with respect to their ability to predict clinical outcomes for patients with HIV-1 infection. For example, in one study, an increase in CD4 count of approximately 100 cell/mm3 after 6 months of zidovudine therapy was associated with improved survival of 78% after adjusting for baseline covariates (15). More recently, CD4 counts obtained at the 3rd month of potent antiviral drug therapy have been demonstrated to be a reliable independent predictor of long-term clinical outcome (24). It should be noted, though, that both CD4-cell counts and viral load are imperfect in their ability to completely explain a clinical treatment effect (3, 6). Nevertheless, these two markers are clinically useful and are the only two accepted, validated clinical markers for HIV-1 infection (22). Interestingly, the results of a recent AIDS cohort study suggested that the CD4-cell count increases in response to potent antiviral drug therapy can be quite variable in a clinical setting (9). Factors such as drug resistance, toxicity, and noncompliance are the main factors associated with poor clinical responses to antiviral drug therapy (32). Such factors may be less important for an immune-based therapy, which is administered infrequently and which has low levels of toxicity, such as the HIV-1 immunogen, for which in this study an extremely low rate of discontinuation and loss to follow-up was demonstrated (<5%).
The results presented in this report confirmed the results of previous studies with the HIV-1 immunogen in terms of its ability to augment CD4-cell numbers and HIV-1-specific immune function in asymptomatic subjects not taking antiviral drug therapy (19, 38, 39). Furthermore, the augmentation of absolute CD4-cell numbers observed in this study is in contrast to the lack of beneficial effects observed with gp160 or gp120 therapeutic vaccines (11, 14, 34). It is tempting to speculate that immune responses (both cell-mediated and antibody responses) against core proteins but not the more variable envelope proteins may be more pivotal for the induction of a clinically relevant effect (23, 36).
This increase in absolute CD4+ cell numbers in the HIV-1 immunogen-treated group was also associated with enhanced HIV-specific immunogenicity, as determined by DTH skin test reactivities and Western blotting. These data suggest that this treatment increased both humoral and cell-mediated immune responses to core proteins of the virus. Clearly, the effects on virus-specific immune function also differentiate this approach from antiviral drug therapy, which potently suppresses viral replication but which has yet to demonstrate an effect on HIV-1-specific immunity, with the exception of when treatment is administered very early during primary infection (1, 18, 36). Thus, it is likely that the magnitude of increase in absolute CD4-cell numbers as observed in this study is related to the induction of HIV-specific memory T-helper clones, which represent a small subpopulation of total CD4 T cells.
Recently, persistent reservoirs of virus have been demonstrated in subjects on long-term, potent antiviral drug therapy (7, 43). This may in part explain some recent reports that have suggested the occurrence of virologic failure in approximately 20 to 40% of patients after 2 years of potent antiviral drug therapy (17, 41). Thus, additional treatment modalities are warranted to limit viral reservoirs in subjects on even the most potent antiviral drug therapies. Therefore, the effect of treatment with the HIV-1 immunogen in combination with potent antiviral drug therapy on virologic failure in various reservoirs is now being examined in other studies (29).
Interestingly, both absolute CD4-cell counts and HIV-1-specific immune function were enhanced from those at the baseline in the IFA treatment group in this study. This could be potentially explained by the improved care that subjects received by participating in a clinical trial. Alternatively, these findings could be explained by a direct immunostimulatory activity of IFA, as observed in infected subjects in other clinical trials who express HIV-1-specific memory CD4 T-cell clones at a low frequency in the presence of ongoing viral replication (38, 39). Nevertheless, this trial and others have noted the superiority of HIV-1 in IFA compared to IFA alone on CD4-cell counts and HIV-1 functional immunity. No effect on viral load was demonstrated in the subset of patients for whom this was assayed during the 40 weeks of this trial. Interestingly, the viral loads remained stable in both treatment groups for the duration of this study. This result is consistent with those of previous studies in which the effects on viral load were observed after only three treatments (38). Longer follow-up of the subjects treated with HIV-1 immunogen in an open-label extension has observed decreases or stabilization of the viral loads for approximately 90% of subjects at week 88 of the trial. Viral load decreases in the absence of antiviral drug therapy (an average of 1 log from the baseline) have been observed in 30% of these subjects (V. Churdboonchart, C. Sakondhavat, S. Kupradist, B. Isarangkura Na Ayudthya, V. Chandeying, S. Rugpao, C. Boonshuyar, W. Sukeepaisarncharoen, W. Sirawaraporn, and R. B. Moss, Proc. 2000 Palm Springs Symposium on HIV/AIDS, 2000), and no subjects have developed opportunistic infections. Thus, unlike the acute viral load effects observed with antiviral drugs, this immune-based therapy may require the induction of specific anti-HIV immune responses which may then require time to affect viral replication.
This trial has demonstrated that the HIV-1 immunogen can enhance CD4-cell counts and HIV-specific immunity in HIV-1-infected Thai subjects. One can speculate on the public health ramifications of the use of such a therapy on the basis of the epidemiology of the evolving AIDS epidemic. With such a therapy, good compliance would be anticipated on the basis of the excellent safety profile of this approach observed in this and other trials. Furthermore, by use of a killed whole-virus immunogen, which can stimulate immune responses to the genetically conserved core proteins of HIV-1, it is likely that such an approach can be used in different geographical areas where different clades of HIV-1 exist (26). In fact, the predominant HIV-1 subtype among the subjects in this trial and Thailand in general is E. This is in contrast to the predominant subtype in the United States and Europe, which is subtype B.
In summary, the results of this trial demonstrate that HIV-1 immunogen (Remune) is safe and significantly increased CD4-cell counts and HIV-specific immunity compared to those achieved with the adjuvant control in HIV-1 infected Thai subjects. While significant gains in the treatment of HIV-1 infection with more potent antiviral therapies have been made in industrialized countries, only prevention or cost-effective therapies may affect the global AIDS epidemic. This study further suggests that this HIV-1-specific immune-based therapy may be an important treatment alternative in countries where access to antiviral drugs is limited. Longer-term studies are under way to further optimize the use of this immune-based therapy and also to combine this intervention with cost-effective antiviral drugs or other biologic approaches in developing countries.
REFERENCES
- 1.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Baum S E, Morris J T, Gibbons R V, Cooper R. Reduction in human immunodeficiency virus patient hospitalizations and nontraumatic mortality after adoption of highly active antiretroviral therapy. Mil Med. 1999;164:609–612. [PubMed] [Google Scholar]
- 3.Boutitie F, Pocock S J. Predictive value of repeated measurements of CD4 lymphocyte counts on progression to AIDS. AIDS. 1994;8:35–41. doi: 10.1097/00002030-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society-USA Panel. JAMA. 1998;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 5.Choi D J, Dube S, Spicer T P, Slade H B, Jensen F C, Poiesz B J. Sequence note: HIV type 1 isolate Z321, the strain used to make a therapeutic HIV type 1 immunogen, is intersubtype recombinant. AIDS Res Hum Retroviruses. 1997;13:357–361. doi: 10.1089/aid.1997.13.357. [DOI] [PubMed] [Google Scholar]
- 6.Choi S, Lagakos S W, Schooley R T, Volberding P A. CD4+ lymphocytes are an incomplete surrogate marker for clinical progression in persons with asymptomatic HIV infection taking zidovudine. Ann Intern Med. 1993;118:674–680. doi: 10.7326/0003-4819-118-9-199305010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Chun T-W, Carruth L, Finzi D, Shen X, DiGiuseepe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo T-H, Brookmeye R, Zelger M A, Barditch-Crovo P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 8.Churdboonchart V, Moss R B, Sirawaraporn W, Smutharaks B, Sutthent R, Jensen F C, Vacharak P, Grimes J, Theofan G, Carlo D J. Effect of HIV-specific immune-based therapy in subjects infected with HIV-1 subtype E in Thailand. AIDS. 1998;12:1521–1527. doi: 10.1097/00002030-199812000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Deeks S G, Hecht F M, Swanson M, Elbeik T, Loftus R, Cohen P T, Grant R M. HIV RNA and CD4 cell count response to protease inhibitor therapy in an urban AIDS clinic: response to both initial and salvage therapy. AIDS. 1999;13:F35–F43. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- 10.Delwart E L, Shaper E G, Loawagie J, McCutchan F E, Grez M, Rubsamen-Waigmann H, Mullins J I. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993;262:1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- 11.Eron J J, Jr, Ashby M A, Giordano M F, Chernow M, Reiter W M, Deeks S G, Lavelle J P, Conant M A, Yangco B G, Pate P G, Torres R A, Mitsuyasu R T, Twaddell T. Randomised trial of MNrgp120 HIV-1 vaccine in symptomless HIV-1 infection. Lancet. 1996;348:1547–1551. doi: 10.1016/s0140-6736(96)05283-x. [DOI] [PubMed] [Google Scholar]
- 12.Garzino-Demo A, Moss R B, Margolick J B, Cleghorn F, Sill A, Blattner W A, Cocchi F, Carlo D J, DeVico A L, Gallo R C. Spontaneous and antigen-induced production of HIV-inhibitory β-chemokines are associated with AIDS-free status. Proc Natl Acad Sci USA. 1999;96:11986–11991. doi: 10.1073/pnas.96.21.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Getchell J P, Hicks D R, Srinivasan A, Heath J L, York D A, Malonga M, Forthal D N, Mann J M, McCormick J B. Human immunodeficiency virus isolated from a serum sample collected in 1976 in Central Africa. J Infect Dis. 1987;156:833–837. doi: 10.1093/infdis/156.5.833. [DOI] [PubMed] [Google Scholar]
- 14.Goebel F D, Mannhalter J W, Belshe R B, Eibl M M, Grob P J, de Gruttola V, Griffiths P D, Erfle V, Kunschak M, Engl W. Recombinant gp160 as a therapeutic vaccine for HIV-infection: results of a large randomized, controlled trial. European Multinational IMMUNO AIDS Vaccine Study Group. AIDS. 1999;13:1461–1468. doi: 10.1097/00002030-199908200-00004. [DOI] [PubMed] [Google Scholar]
- 15.Graham N M, Piantadosi S, Park L P, Phair J P, Rinaldo C R, Fahey J L. CD4+ lymphocyte response to zidovudine as a predictor of AIDS-free time and survival time. J Acquir Immune Defic Syndr. 1993;6:1258–1266. [PubMed] [Google Scholar]
- 16.Kitchen A D, Mann G F, Harrison J F, Zuckerman A J. Effect of gamma irradiation on the human immunodeficiency virus and human coagulation proteins. Vox Sang. 1989;56:223–229. doi: 10.1111/j.1423-0410.1989.tb02033.x. [DOI] [PubMed] [Google Scholar]
- 17.Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, Vernazza P, Sudre P, Flepp M, Furrer H, Francioli P, Weber R. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV cohort study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 18.Lederman M M, Connick E, Landay A, Kuritzkes D R, Spritzler J, St. Clair M, Kotzin B L, Fox L, Chiozzi M H, Leonard J M, Rousseau F, Wade M, Roe J D, Martinez A, Kessler H. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 19.Levine A M, Groshen S, Allen J, Munson K M, Carlo D J, Daigle A E, Ferre F, Jensen F C, Richieri S P, Trauger R J, Parker J W, Salk P L, Salk J. Initial studies on active immunization of HIV-infected subjects using a gp120-depleted HIV-1 immunogen: long-term follow-up. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:351–364. doi: 10.1097/00042560-199604010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Limsuwan A, Churdboonchart V, Moss R B, Sirawaraporn W, Sutthent R, Smutharaks B, Glidden D, Trauger R, Theofan G, Carlo D J. Safety and immunogenicity of REMUNETM in HIV-infected Thai subjects. Vaccine. 1998;16:142–149. doi: 10.1016/s0264-410x(97)88327-2. [DOI] [PubMed] [Google Scholar]
- 21.LoGrippo G A. Investigations of the use of beta-propiolactone in virus inactivation. Ann N Y Acad Sci. 1960;83:578–594. doi: 10.1111/j.1749-6632.1960.tb40931.x. [DOI] [PubMed] [Google Scholar]
- 22.Mildvan D, Landay A, De Gruttola V, Machado S G, Kagan J. An approach to the validation of markers for use in AIDS clinical trials. Clin Infect Dis. 1997;24:764–774. doi: 10.1093/clinids/24.5.764. [DOI] [PubMed] [Google Scholar]
- 23.Mofenson L M, Harris D R, Rich K, Meyer III W A, Read J S, Moye J, Jr, Nugent R P, Korelitz J, Bethel J, Pahwa S. Serum HIV-1 p24 antibody, HIV-1 RNA copy number and CD4 lymphocyte percentage are independently associated with risk of mortality in HIV-1-infected children. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. AIDS. 1999;13:31–39. doi: 10.1097/00002030-199901140-00005. [DOI] [PubMed] [Google Scholar]
- 24.Monforte A, Valeria T, Adorni F, Castelelnuovo B, Bini T, Testa L, Moscatelli G, Chiesa E, Rusconi S, Abeli C, Sollima S, Musicco M, Meroni L, Galli M, Moroni M. CD4 cell counts at the third month of HAART may predict clinical failure. AIDS. 1999;13:1669–1676. doi: 10.1097/00002030-199909100-00010. [DOI] [PubMed] [Google Scholar]
- 25.Moore R D, Chaisson R E. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13:1933–1942. doi: 10.1097/00002030-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 26.Moss R B, Giermakowska W, Lanza P, Turner J L, Wallace M R, Jensen F C, Theofan G, Richieri S P, Carlo D J. Cross-clade immune responses after immunization with a whole-killed gp120-depleted human immunodeficiency virus type-1 immunogen in incomplete Freund's adjuvant (HIV-1 immunogen, REMUNETM) in human immunodeficiency virus type-1 seropositive subjects. Viral Immunol. 1997;10:221–228. doi: 10.1089/vim.1997.10.221. [DOI] [PubMed] [Google Scholar]
- 27.Nelson K E, Celentano D D, Eiumtrakol S, Hoover D R, Beyrer C, Suprasert S, Kuntolbutra S, Khamboonruang C. Changes in sexual behavior and a decline in HIV infection among young men in Thailand. N Engl J Med. 1996;335:297–303. doi: 10.1056/NEJM199608013350501. [DOI] [PubMed] [Google Scholar]
- 27a.Nicholson J, Kidd P, Mandy I F, Livnat D, Kagan J. Three-color supplement to the NIAID guideline for flow cytometric immunophenotyping. Cytometry. 1996;26:227–230. doi: 10.1002/(SICI)1097-0320(19960915)26:3<227::AID-CYTO8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donahoe S M, Demoitie M A, Kakimoto W M, Ketas T, Clas B, Heymann J J, Zhang L, Cao Y, Hurley A, Moore J P, Ho D D, Markowitz M. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Invest. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson B K, Carlo D J, Kaplan M H, Marecki M, Pawha S, Moss R B. Cell-associated HIV-1 messenger RNA and DNA in T-helper cell and monocytes in asymptomatic HIV-1-infected subjects on HAART plus an inactivated HIV-1 immunogen. AIDS. 1999;13:1607–1611. doi: 10.1097/00002030-199909100-00002. [DOI] [PubMed] [Google Scholar]
- 30.Pau C-P, Lee-Thomas S, Auwanit W, George J R, Ou C Y, Parekh B S, Granade T C, Holloman D L, Phillips S, Schochetman G, et al. Highly specific V-3 peptide enzyme immunoassay for serotyping HIV-1 specimens from Thailand. AIDS. 1993;7:337–340. doi: 10.1097/00002030-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 32.Pomerantz R J. Primary HIV-1 resistance: a new phase in the epidemic? JAMA. 1999;282:1177–1179. doi: 10.1001/jama.282.12.1177. [DOI] [PubMed] [Google Scholar]
- 33.Pontesilli O, Carotenuto P, Kerkhof-Garde S R, Roos M T, Keet I P, Coutinho R A, Goudsmit J, Miedema F. Lymphoproliferative response to HIV type 1 p24 in long-term survivors of HIV type 1 infection is predictive of persistent AIDS-free infection. AIDS Res Hum Retrovir. 1999;15:973–981. doi: 10.1089/088922299310485. [DOI] [PubMed] [Google Scholar]
- 34.Pontesilli O, Guerra E C, Ammassari A, Tomino C, Carlesimo M, Antinori A, Tamburrini E, Prozzo A, Seeber A C, Vella S, Ortona L, Aiuti F. Phase II controlled trial of post-exposure immunization with recombinant gp160 versus antiretroviral therapy in asymptomatic HIV-1-infected adults. VaxSyn Protocol Team AIDS. 1998;12:473–480. doi: 10.1097/00002030-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Prior C P, Gore R, Harter J, Berbaum M, Duffy C, Ferre F, Hancock R, Lowry P, Musil R, Stiglitz M, Carlo D J, Richieri S P, Maigetter R Z. Inactivation and purification of human immunodeficiency virus-1 as antigen for the treatment of HIV-1 infection. BioPharm. 1996;9:22–34. [Google Scholar]
- 36.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 37.Surasiengsunk S, Kiranandana S, Wongboonsin K, Garnett G P, Anderson R M, van Griensven G J. Demographic impact of the HIV epidemic in Thailand. AIDS. 1998;12:775–784. doi: 10.1097/00002030-199807000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Trauger R J, Ferre F, Daigle A E, Jensen F C, Moss R B, Mueller S H, Richieri S P, Slade H B, Carlo D J. Effect of immunization with inactivated gp120-depleted human immunodeficiency virus type 1 (HIV-1) immunogen on HIV-1 immunity, viral DNA, and percentage of CD4 cells. J Infect Dis. 1994;169:1256–1264. doi: 10.1093/infdis/169.6.1256. [DOI] [PubMed] [Google Scholar]
- 39.Turner J L, Trauger R J, Daigle A E, Carlo D J. HIV-1 immunogen induction of HIV-1-specific delayed-type hypersensitivity: results of a double-blind, adjuvant-controlled, dose-ranging trial. AIDS. 1994;8:1429–1435. [PubMed] [Google Scholar]
- 40.UNAIDS and World Health Organization. Report on the global HIV/AIDS epidemic. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 41.Wit F W N M, van Leeuwen R, Weverling G J, Jurriaans S, Nauta K, Steingrover R, Schuijtemaker J, Eyssen X, Fortuin D, Weeda M, de Wolf F, Reiss P, Danner S A, Lange J M A. Outcome and predictors of failure of highly active antiretroviral therapy: one year follow-up of a cohort of human immunodeficiency virus type 1-infected persons. J Infect Dis. 1999;179:790–798. doi: 10.1086/314675. [DOI] [PubMed] [Google Scholar]
- 42.Young N L, Ponglertnapakorn P, Shaffer N, Srisak K, Chaowanachan T, On-Thern V, Kittinunvorakoon C, Bunwattanakul A, Suksaweang S, Pobkeeree V, Punnotok J, Mastro T D. Clinical field site evaluation of the FACSCount for absolute CD3+, CD3+ CD4+, and CD3+ CD8+ cell count determinations in Thailand. Clin Diagn Lab Immunol. 1997;4:783–786. doi: 10.1128/cdli.4.6.783-786.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D, Guo Y, Duran M, Hurley A, Tsay J, Huang Y C, Wang C C. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]