Abstract
Chlamydia pneumoniae and Mycoplasma pneumoniae immunoglobulin G (IgG) and IgA antibody seroprevalence rates and antibody levels related to age and gender were studied. The samples (n = 742) were collected during a nonepidemic period and analyzed by quantitative enzyme immunoassays (EIAs). Seroprevalence to C. pneumoniae was found to increase sharply in young children, and in the 15- to 19-year-old group it reached levels as high as 70 and 60% for IgG and IgA, respectively. After adolescence, seroprevalence showed a transient decrease and then continued to increase, although less dramatically than in early childhood. In the elderly the seroprevalence of IgG antibodies reached 75 and 100% in women and men, respectively. The corresponding rates of IgA antibodies were 73 and 100%. When a randomly selected subgroup of samples (n = 66) was analyzed in parallel by a microimmunofluorescence test and an EIA for C. pneumoniae IgA antibodies, similar seroprevalence rates were obtained (36 versus 35%). Seroprevalence to M. pneumoniae was already found to increase very sharply in 2- to 4-year-old children, reaching 16% for IgG and 8% for IgA. Seroprevalence to M. pneumoniae also continued to increase in adolescence, but in contrast to that to C. pneumoniae, the increase leveled off at about 40 to 50% in adulthood. In subjects aged over 65 years, prevalence did not exceed 60% for IgG or 35% for IgA. The seroprevalence patterns as well as the medians and variations of levels of C. pneumoniae and M. pneumoniae IgG antibodies were similar to those of corresponding IgA antibodies. Compared to IgG antibodies, IgA antibodies do not seem to be of additional value in the diagnosis of infections caused by these pathogens when single serum specimens are studied.
Chlamydia pneumoniae and Mycoplasma pneumoniae are common human pathogens causing asymptomatic, mild, or, rarely, severe upper and lower respiratory tract infections. The infections are usually not identified in general health care, because the etiology of respiratory infections is investigated in only a small proportion of patients, in cases of prolonged nonresponsiveness to conventional antimicrobial therapy or pneumonias requiring hospitalization.
Despite the acknowledged importance of these pathogens, only a few reports have been published on the seroprevalence of antibodies against them (2–4). The most extensive study of C. pneumoniae immunoglobulin G (IgG) antibodies, involving sample size, geographic distribution, and long-term surveillance, has been carried out in Finland (3). All studies published so far (2–4) have been carried out using an in-house microimmunofluorescence assay (MIFA) which, compared to the enzyme immunoassay (EIA) is subjective, utilizes a noncontinuous scale, and is highly variable in performance (7). None of the studies has provided data on the prevalence of C. pneumoniae IgA antibodies, which have recently received much attention as a possible marker of chronic C. pneumoniae infection (9, 10). M. pneumoniae infection has been found to account for 15 to 20% of all cases of pneumonia (1). However, to our knowledge, no population-based study of the seroprevalence of M. pneumoniae IgG and IgA antibodies has been carried out.
The aims of this study were (i) to provide epidemiological data during a nonepidemic period on the distribution of C. pneumoniae and M. pneumoniae IgG and IgA antibodies in relation to age and gender using quantitative and comparable EIA techniques, (ii) to compare the sensitivity of the EIA to C. pneumoniae IgA antibodies with that of the respective MIFA, and (iii) to detect differences in the epidemiologies of C. pneumoniae and M. pneumoniae infections.
MATERIALS AND METHODS
Samples.
The sample donors were selected randomly. They represented different age groups of a predominantly urban Finnish population. The samples were donated voluntarily, and the donors reported no heart or respiratory diseases and were otherwise apparently healthy. The National Public Health Institute of Finland did not report C. pneumoniae or M. pneumoniae epidemics during the sample collection periods (1996 to 1997 and 1999). Table 1 provides details of the sample groups. The sera were grouped according to the ages of the donors without knowledge of their personal identities.
TABLE 1.
Distribution of the ages, genders, and geographical locations of subjects giving serum specimens and the year of sampling
| Sample group | No. of females | No. of males | Location | Source and collection date |
|---|---|---|---|---|
| 7 mo–1 y | 12 | 9 | Turku | Department of Virology, University of Turku; collected prospectively from children born in 1989 |
| 2–4 yr | 25 | 22 | Turku | Department of Virology, University of Turku; collected prospectively from children born in 1989 |
| 7 | 9 | Rovaniemi and Turku archipelago | Department of Medical Microbiology, University of Turku; collected in 1996 | |
| 5–9 yr | 29 | 30 | Turku | Department of Virology, University of Turku; collected prospectively from children born in 1989 |
| 19 | 17 | Rovaniemi and Turku archipelago | Department of Medical Microbiology, University of Turku; collected in 1996 | |
| 10–14 yr | 39 | 31 | Rovaniemi and Turku archipelago | Department of Medical Microbiology, University of Turku; collected in 1996 |
| 15–19 yr | 36 | 29 | Rovaniemi and Turku archipelago | Department of Medical Microbiology, University of Turku; collected in 1996 |
| 20–29 yr | 55 | 11 | Helsinki | Finnish Red Cross; collected in 1996–1997 |
| 30–39 yr | 49 | 26 | Helsinki | Finnish Red Cross; collected in 1996–1997 |
| 40–49 yr | 23 | 49 | Helsinki | Finnish Red Cross; collected in 1996–1997 |
| 50–59 yr | 26 | 57 | Helsinki | Finnish Red Cross; collected in 1996–1997 |
| 60–65 yr | 10 | 16 | Helsinki | Finnish Red Cross; collected in 1996–1997 |
| 9 | 12 | Helsinki | Laakso City Hospital, Helsinki outpatient department; collected in 1999 | |
| Over 65 yr | 51 | 34 | Helsinki | Laakso City Hospital, Helsinki outpatient department; collected in 1999 |
The samples from children below 9 years of age consisted of both consecutive samples from the same subjects and cross-sectional samples of different age groups. The consecutive samples (n = 127) had been collected from children aged between 7 months and 9 years, born in 1989, and belonging to the population of the Special Turku Coronary Risk Factor Intervention Project (STRIP baby project) (11). These sera were obtained from the Department of Virology, University of Turku, Turku, Finland. The cross-sectional samples (n = 187) from children aged from 2 to 19 years were obtained from the Department of Medical Microbiology, University of Turku. These sera were collected in 1996 from healthy children living in Rovaniemi and the Turku archipelago for studies of the seroprevalence of antibodies to Borrelia burgdorferi.
Sera (n = 322) from subjects aged from 20 to 65 years were obtained from the Finnish Red Cross. These subjects were healthy blood donors living in the metropolitan Helsinki area in 1996 and 1997. The sera (n = 106) from persons above 60 years of age were from patients visiting the Laakso City Hospital (Helsinki) outpatient department. All these sera were from individuals without a history of acute respiratory illness during the previous 3 months.
EIA and MIFA.
C. pneumoniae IgG and IgA antibodies were measured in a blind fashion using C. pneumoniae IgG and IgA EIAs (Labsystems, Helsinki, Finland). The C. pneumoniae EIAs utilize stabilized C. pneumoniae elementary body as an antigen. The assays measure only antibodies to surface-exposed proteins and not antibodies targeted to the genus-specific lipopolysaccharide of C. pneumoniae. The assays have been proven more sensitive than the in-house MIFAs in defining acute C. pneumoniae infection (7a). The EIAs gave borderline positive results with sera with very high concentrations of C. trachomatis antibodies, whereas they consistently gave negative results with sera with lower C. trachomatis antibody concentrations.
IgA antibodies were also measured in a blind fashion using a C. pneumoniae IgA MIFA (Labsystems) with samples from a randomly selected subgroup of 20- to 29-year-old blood donors. The cut-off for positivity in the MIFA was a dilution of 1:8. The sera were analyzed at a dilution of 1:8 and evaluated as definitely positive, borderline positive, or definitely negative. The IgA antibody prevalence rates were assessed using the MIFA in two ways: either taking into consideration only the definitely positive sera or taking into consideration both the definitely and borderline positive sera.
An in-house EIA was developed for the analysis of IgG and IgA antibodies to M. pneumoniae. Enhanced binding plates (Labsystems) were sensitized with 150 μl of enriched P1 antigen of M. pneumoniae (Aalto Bioreagents Ltd., Dublin, Ireland) in a concentration of 0.75 μg/well in 0.1 M phosphate-buffered saline, pH 7.4, containing 0.5% (vol/vol) Tween 20. The serum samples were diluted 1:200 in 0.1 M phosphate-buffered saline, pH 7.5, containing 0.5% bovine serum albumin, 0.02% Tween 20, and proprietary proteins, and were incubated at 37°C for 1 h. After the plates were washed five times with a citrate buffer containing 1% Tween 20, horseradish peroxidase-labeled anti-human IgG or IgA antibodies were incubated in the wells as described above. The plates were washed as described above and then incubated with 100 μl of tetramethyl benzidene substrate for 30 min. The reaction was stopped with 100 μl of 0.5 M sulfuric acid, and optical densities were read at 450 nm (Multiskan Ascent; Labsystems). The same calibrators, containing 100 arbitrary enzyme immunoassay units (EIU), and the same positive controls and cut-off controls were used throughout the study.
For comparison, all values were determined in EIUs as the net absorbance of the sample divided by the net absorbance of the respective calibrator multiplied by 100. The positivity thresholds (cut-offs) were adjusted to those of other well-established serologic methods. Thus, the cut-offs in C. pneumoniae IgG and IgA EIAs were comparable to the respective cut-offs in C. pneumoniae IgG and IgA MIFAs. The cut-off for the M. pneumoniae IgG EIA was adjusted to the same level as that of the M. pneumoniae IgG EIA of Sanofi Pasteur (Marnes la Coquette, France), and that of the Mycoplasma IgA EIA was adjusted to the same level as that of the Sero MP IgA EIA from Savyon (Ashdod, Israel).
Statistical analysis.
One-tailed variance analysis and Student's t test for unpaired samples were applied to the sample groups of subjects over 29 years old to investigate the statistical significance of the differences in antibody levels between males and females.
RESULTS
Comparison of C. pneumoniae IgA prevalence rates assessed by the MIFA and EIA.
The randomly selected subgroup of samples (n = 66) was analyzed by both the MIFA and EIA for C. pneumoniae IgA antibodies. When the MIFA data were analyzed considering both definite positives and borderline positives as positives, the seroprevalence of IgA was 53% (35 of 66). On the other hand, when only definite positives were taken into consideration, the seroprevalence was 36% (24 of 66), which was quite comparable to the respective prevalence of 35% (23 of 66) obtained by the EIA.
Distribution of seroprevalence rates and antibody levels by age and gender.
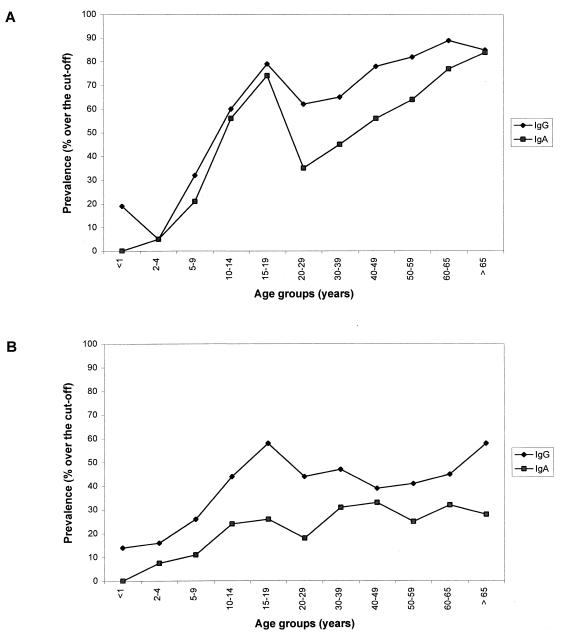
Figure 1 shows the comparative distribution of C. pneumoniae and M. pneumoniae IgG and IgA antibodies in relation to age. The seroprevalence of IgG and IgA antibodies to C. pneumoniae increased sharply in preschool children, reaching 71% for IgG and 57% for IgA in teenagers; the prevalence then slightly decreased, to increase again but not so dramatically as in childhood. In the elderly, prevalence rates reached 84% for IgG and 85% for IgA.
FIG. 1.
Age distribution of the seroprevalence of IgG and IgA antibodies to C. pneumoniae (A) and M. pneumoniae (B) in a healthy Finnish population.
The seroprevalence of M. pneumoniae IgG antibodies in young children (2- to 4 years old) was higher than that of C. pneumoniae IgG antibodies (13 to 19% versus 4 to 6%). As with the seroprevalence of C. pneumoniae, the seroprevalence of M. pneumoniae increased with age, but it did not, even in the elderly, exceed 58% for IgG and 28% for IgA. The seroprevalence rates of both M. pneumoniae and C. pneumoniae showed a tendency to be higher in males than in females, but the differences did not reach statistical significance at a P value of 0.05 (data not shown). The median levels of IgA antibodies to both microbes were close to those of the corresponding IgG antibodies (Table 2). When the data are presented as 2.5 and 97.5 percentiles, the variations in IgG and IgA antibody levels are large in all age groups, and the increases of the 97.5 percentile values resemble those of the respective prevalence curves (compare Fig. 1 and Table 2).
TABLE 2.
Medians and 2.5 and 97.5 percentiles of the levels of IgG and IgA antibodies to C. pneumoniae and M. pneumoniae in a healthy Finnish population in relation to age
| Age group (yr) |
C. pneumoniae antibodies (EIU)
|
M. pneumoniae antibodies (EIU)
|
||
|---|---|---|---|---|
| IgG median (2.5–97.5 percentile) | IgA median (2.5–97.5 percentile) | IgG median (2.5–97.5 percentile) | IgA median (2.5–97.5 percentile) | |
| <1 | 8 (3–36) | 0 (0–13) | 10 (5–47) | 4 (2–25) |
| 2–4 | 8 (5–22) | 3 (0–57) | 12 (6–138) | 8 (4–197) |
| 5–9 | 12 (7–172) | 3 (3–110) | 14 (8–220) | 13 (8–113) |
| 10–14 | 61 (10–304) | 30 (10–233) | 22 (7–238) | 19 (2–164) |
| 15–19 | 82 (35–222) | 47 (23–260) | 56 (22–168) | 29 (16–128) |
| 20–29 | 37 (12–123) | 20 (10–120) | 37 (22–147) | 27 (16–86) |
| 30–39 | 38 (15–145) | 23 (10–127) | 44 (27–135) | 30 (21–122) |
| 40–49 | 51 (26–147) | 30 (13–147) | 37 (15–140) | 31 (18–84) |
| 50–59 | 60 (30–206) | 40 (17–197) | 35 (20–145) | 29 (19–85) |
| 60–65 | 95 (51–255) | 57 (27–200) | 40 (15–90) | 33 (20–71) |
| >65 | 62 (44–229) | 67 (27–183) | 42 (18–180) | 32 (23–164) |
DISCUSSION
Our results show that the seroprevalence of IgG and IgA antibodies to C. pneumoniae among healthy Finnish persons above 14 years of age is high, increasing until old age. The prevalence of the corresponding M. pneumoniae antibodies also increases rapidly in young people but levels off at the age of 20. Because the samples analyzed in this study were collected during a nonepidemic period, the results reflect the baseline seroprevalence in a given population. This high seroprevalence emphasizes the necessity of analyzing paired samples in identifying acute infection if only IgG and IgA antibodies are measured. It also emphasizes the importance of caution in the interpretation of results concerning the association between antibodies to C. pneumoniae and noninfectious-disease manifestations.
Seroprevalence figures are dependent on the chosen cut-off levels. In our study, the cut-offs used for diagnostic purposes were selected for the determination of seroprevalence rates. However, the cut-off levels selected are not very important for the study of relative trends of antibody prevalences in relation to age.
As for the pattern of C. pneumoniae IgG antibody seroprevalence related to age and gender, our results are generally in agreement with earlier data (2, 4). We also found that prevalence rates sharply increase in healthy Finnish children until the age of 20 years, continuing to increase in older age groups, although less dramatically. The seroprevalence rates of C. pneumoniae in some groups of young children in our study were higher than those reported earlier from Seattle and Denmark (2, 4). The reason for the difference between Finland and Seattle may be the higher degree of institutionalization of day care in Finland. When sampling is focused on a few kindergartens, unrecognized C. pneumoniae infections, manifesting as local outbreaks of flulike illness, may cause biased results. Our results concerning seroprevalence in Finnish children differ slightly from those published earlier by Saikku (10). In his study, the seroprevalence of C. pneumoniae IgG antibodies was only 0 to 10% among children aged 6 to 10 years. Differences between the sensitivity of the in-house MIFA used by Saikku and that of our EIA may be a reason for the discrepancy. In agreement with the results of earlier studies (2–4), our data show the tendency towards higher seroprevalence rates of IgG and IgA antibodies in males than in females for both pathogens studied, but the difference was not statistically significant. It has been suggested that the higher seroprevalence in males indicates that the transmission of the infection mainly occurs outside the home (2). This speculation may, however, not be valid in the Finnish social setting.
Our study shows a slight decrease in the seroprevalence rates of both IgG and IgA antibodies to C. pneumoniae in people aged 20 to 30 years. This decrease may be an artifact attributable to the limited size of each age group in our study. Another reason for the bias may be that subjects below 20 years of age lived in areas different from the areas where the older subjects lived. The younger subjects were from the southern archipelago, a rural area, and from the town of Rovaniemi in northern Finland, whereas the older subjects were mainly from the metropolitan Helsinki area. On the other hand, the decrease could be real, caused by a complex interplay between immunity and changes in the rate of exposure to C. pneumoniae in early adulthood. Whether the transient decrease occurs or not, it does not negate the fact of a steady increase in seroprevalence towards almost complete saturation in senescence. The increase implies that relatively high antibody levels persist in the elderly. The high seroprevalence rates may be due to repeated reinfections, persistent antigenic stimulus, or long-lasting immunological memory. Long-term follow-up studies on the persistence of IgG and IgA antibodies to C. pneumoniae are needed to clarify the role of the persistence.
The steady increase in antibody seroprevalence has been attributed to multiple exposures to C. pneumoniae during the subjects' lifetimes (2, 4). However, Chlamydia-like organisms, e.g., Simkania negevensis and Parachlamydia acanthamoebae, and the four recently discovered organisms with Chlamydia-like sequences may have some impact on the steady increase in antibodies with age because of their possible antigenic cross-reactivities (5, 6).
Earlier, it was suggested that IgA antibodies to C. pneumoniae, by virtue of their short half-life, can be markers of chronic C. pneumoniae infection (9). However, recent studies of the association between IgA antibodies to C. pneumoniae and ischemic heart disease have provided conflicting results (12; L. von Hertzen, R. Isoaho, S.-L. Kivelä, and P. Saikku, Letter, BMJ 318:1575–1576, 1999). Our results show that the seroprevalence patterns and levels of IgA antibodies to C. pneumoniae were rather similar to those of IgG in all age groups. Further studies are needed to determine how the persistence of antibodies in general and IgA antibodies in particular is related to chronic C. pneumoniae infection.
All earlier seroprevalence data on IgG and IgA antibodies to C. pneumoniae were obtained using in-house MIF methods. We have shown that our IgG EIA gives results compatible with those obtained using MIF methods (2, 4). On the other hand, no comparative data were available for the comparison of IgA MIF methods and IgA EIA. Therefore, these tests were compared in a subgroup of specimens randomly selected from the present material. When only definite positives by the MIFA (Labsystems) were considered, similar seroprevalence rates were obtained with the MIFA and the EIA (36 versus 35%). Thus, we conclude that, with the cut-off selected in the present study, the EIA does not overestimate the seroprevalence of IgA antibodies to C. pneumoniae.
IgG and IgA antibodies to M. pneumoniae were measured by in-house EIAs using the enriched P1 protein of Mycoplasma as an antigen. This 168-kDa protein is an adhesin reported to be a highly immunogenic antigen eliciting a strong antibody response in infected hosts while eliciting a low or negligible response in asymptomatic carriers (8). Our results show that seroprevalence to M. pneumoniae increases more sharply in early childhood than that to C. pneumoniae, but then it levels off above the age of 20 years. This confirms the earlier assumption that M. pneumoniae infection is more prevalent than C. pneumoniae infection among children and adolescents, affecting fewer middle-aged and elderly people.
We used samples left over from other studies. The samples, therefore, did not represent the whole country, nor were they collected simultaneously. Further, the gender and age distributions were not uniform in our groups. Despite these weaknesses, we believe that our data reliably reflect true baseline seroprevalence rates and age-related trends. Our results show that high levels of both IgG and IgA antibodies to C. pneumoniae are relatively common, particularly in old age. Efficient treatment trials and long-term follow-up studies are needed to find out whether these antibody levels are maintained by a persistent C. pneumoniae infection, repeated exposures to the organism itself, or some other microbes cross-reacting antigenically with C. pneumoniae. Our data also confirm that C. pneumoniae and M. pneumoniae infections have different epidemiological patterns.
ACKNOWLEDGMENTS
We thank Jorma Ilonen from the Department of Virology and Jaakko Uksila from the Department of Medical Microbiology, University of Turku, for providing us with the specimens.
REFERENCES
- 1.Foy H M. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin Infect Dis. 1993;17(Suppl. 1):37–47. doi: 10.1093/clinids/17.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 2.Grayston T J, Campbell L A, Kuo C-C, Mordhorst C, Saikku P, Thom D H, Wang S-P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;161:618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- 3.Karvonen M, Tuomilehto J, Pitkäniemi J, Naukkarinen A, Saikku P. Chlamydia pneumoniae IgG antibody prevalence in South-Western and Eastern Finland in 1982 and 1987. Int J Epidemiol. 1994;23:176–184. doi: 10.1093/ije/23.1.176. [DOI] [PubMed] [Google Scholar]
- 4.Kuo C-C, Jackson L A, Campbell L A, Grayston T J. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman D, Kahane S, Lieberman D, Friedman M G. Pneumonia with serological evidence of acute infection with the Chlamydia-like microorganism “Z.”. Am J Respir Crit Care Med. 1997;156:578–582. doi: 10.1164/ajrccm.156.2.9608081. [DOI] [PubMed] [Google Scholar]
- 6.Ossewaarde J M, Meijer A. Molecular evidence for the existence of additional members of the other Chlamydiales. Microbiology. 1999;145:411–417. doi: 10.1099/13500872-145-2-411. [DOI] [PubMed] [Google Scholar]
- 7.Peeling, R. W., S. P. Wang, J. T. Grayston, F. Blasi, J. Boman, A. Clad, et al. Chlamydia serology: inter-laboratory variation in microimmunofluorescence results, p. 159–162. In R. S. Stephens, G. I. Byrne, et al. (ed.), Chlamydial infections. Proceedings of the 9th International Symposium on Human Chlamydial Infections.
- 7a.Persson K, Boman J. Comparison of five serologic tests for diagnosis of acute Chlamydia pneumoniae infections. Clin Diagn Lab Immunol. 2000;7:739–744. doi: 10.1128/cdli.7.5.739-744.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastawicki W, Räty R, Kleemola M. Detection of antibodies to Mycoplasma pneumoniae adhesin P1 in serum specimens from infected and non-infected subjects by immunoblotting. Diagn Microbiol Infect Dis. 1996;26:141–143. doi: 10.1016/s0732-8893(96)00216-7. [DOI] [PubMed] [Google Scholar]
- 9.Saikku P, Leinonen M, Mattila K, Ekman M-R, Nieminen M S, Mäkelä P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia TWAR with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 10.Saikku P. Diagnosis of acute and chronic Chlamydia pneumoniae infections. In: Orfila J, et al., editors. Chlamydia infection. Proceedings of the 8th International Symposium on Human Chlamydial Infections. Bologna, Italy: Societa Editrice Esculapio; 1994. pp. 163–172. [Google Scholar]
- 11.Simell O, Niinikoski H, Viikari J, Rask Nissila L, Tammi A, Ronnemaa T. Cardiovascular disease risk factors in young children in the STRIP baby project. Special Turku Coronary Risk Factor Intervention Project for Children. Ann Med. 1993;31(Suppl. 1):55–61. [PubMed] [Google Scholar]
- 12.Strachan D P, Carrington D, Mendall M A, Ballam L, Morris J, Butland B K, Sweetnam P M, Elwood P C. Relation of Chlamydia pneumoniae serology to mortality and incidence of ischaemic heart disease over 13 years in the Caerphilly prospective heart disease study. BMJ. 1999;318:1035–1040. doi: 10.1136/bmj.318.7190.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]