Abstract
Introduction
Survival amongst posterior fossa tumour (PFT) patients is improving. Clinical endpoints such as overall survival fail to depict QoL. There is yet to be a review of current QoL instruments used for adult PFTs. Aim of this review is to outline the QoL reporting in the management of PFTs and measure participation level.
Methods
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis. A search strategy to identify adult patients with PFTs who took part in QoL metrics was conducted. Observational and experimental studies published from 1990 to date were included. Studies with a sample size less than 10 and performance measures such as Karnofsky Performance Status were not considered.
Results
A total of 116 studies were included in the final analysis. Vestibular schwannomas were the most common tumour pathology (n = 23,886, 92.6%) followed by pilocytic astrocytomas (n = 657, 2.5%) and meningiomas (n = 437, 1.7%) Twenty-five different QoL measures were used in the study pool. SF-36 was the most common (n = 55, 17 47.4%) QoL metric in the whole study pool, followed by the Penn Acoustic Neuroma QoL scale (n = 24, 20.7%) and Dizziness Handicap Inventory (n = 16, 13.8%). Seventy-two studies reported less-than 100% participation in QoL evaluation. The commonest reason for non-participation was a lack of response (n = 1,718, 60.8%), incomplete questionnaires (n = 268, 9.4%) and cognitive dysfunction (n = 258, 9.1%).
Conclusion
Informed clinical decision-making in PFT patients requires the development of specific QoL outcomes. Core outcome sets, and minimal clinically important differences (MCID) are essential for these metrics to show clinically significant improvements in patient QoL.
Keywords: brain tumour, medulloblastoma, vestibular schwannoma, quality of life, astrocytoma, meningioma
Introduction
Advanced surgical techniques, chemotherapy and refined postoperative care have markedly improved the survival of patients with posterior fossa tumours (PFTs) (1, 2, 3, 4). As a result, many patients are living into adulthood (5, 6). Given the supposed rarity of intrinsic PFTs in the adult population, there is a paucity in the literature as it pertains to the prognostic factors and therapeutic management of these tumours, with quality of life (QoL) measures becoming important in measuring treatment efficacy (7).
Patient-reported health-related quality of life (HRQoL) refers to the patient's perception of their physical and occupational function, psychological state, level of independence, social relationships and somatic sensation influenced by their medical condition and/or therapeutic consequences (8). Patient-reported outcome measures (PROMs) accurately capture the patient's condition and can effectively aid in quality of care, compared to clinician-reported outcomes which parallel poorly with the patient's own perceptions (9).
Clinical endpoints such as complications, overall survival (OS), and progression-free survival (PFS) are typically used when assessing the effectiveness of treatment and overall clinical outcome. However, they fail to accurately convey individual patient QoL, which is increasingly becoming an important part of clinical decision-making (10). With the improved survival, there is a need for providing an optimum “onco-functional” balance between mitigating mortality and preserving QoL, hence making these QoL metrics essential in evaluating therapeutic efficacy.
Although there is an existing review on HRQoL of specific extra-axial PF tumours (11); to the best of our knowledge there is yet to be a review on HRQoL in PF neoplasms collectively. This study will review the current methods used to assess HRQoL in PFTs and evaluate the methods for assessing QoL by PF tumour histology.
Aim and objectives
This systematic review covers the following objectives:
-
(1)
Outline the usage and reporting of HRQoL in PFTs
-
(2)
Measure the levels of participation and dropout in studies using HRQoL measures
Materials and methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and registered on the International Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD42020224005).
Search strategy
Preliminary searches of PubMed and Google Scholar using key words “posterior fossa tumour” and “quality of life” were carried out to determine Medical Subject Heading (MeSH) terms to be used in the following systematic search. A structured search string was developed to identify studies outlining the use of QoL measures in patients with PFTs. Synonyms relating to the two subject areas were formulated into a comprehensive search strategy (Supplementary Figure S1). The search was applied to Medline via Ovid, Embase, Cochrane Library, Scopus, Web of Science and PsychINFO between the 1st and 8th December 2020.
Selection criteria
Adult patients (>18 years of age) with histologically confirmed PFTs were included in the study. Patients with childhood PFTs who had undergone QoL evaluation in adulthood were also included. Conservative, surgical and adjuvant PFT interventions were considered. Outcomes included QoL, OS, PFS and common posterior fossa surgery complications. Randomised-controlled trials (RCTs), controlled clinical trials, cohort and case-controlled observational studies, cross-sectional studies and case series and cross-sectional studies were included. Articles published in English from 1990 to date were considered. Only studies with a sample size of 10 or more were included. Performance measures such as Karnofsky Performance Status (KPS) were not considered QoL measures.
After deduplication, title and abstract screening was performed against pre-defined eligibility criteria by two independent reviewers (M.K. and C.J.). An “initial calibration phase” was undertaken, whereby a random sample of 30 studies were initially tested for their eligibility based on the title and abstract. The reviewers independently screened the studies and compared their results in the presence of a third reviewer (G.A.). By doing so, a mutual understanding of the inclusion criteria was ascertained. Data management was carried out on COVIDENCE Systematic Review software (Veritas Health Innovation, Melbourne, Australia). Potentially eligible studies were further screened for full-text review. Disagreements as to eligibility of studies were discussed and resolved between reviewers; in the case of no resolution an appeal was made to a third reviewer (G.A.).
Data extraction and synthesis
Data extraction was performed on a Microsoft Office Excel (Version 16, Office 365) proforma. A short data extraction pilot of 5 studies was undertaken independently by three reviewers (M.K., C.J. and N.C.). Following this exercise, the proforma was enhanced to accurately capture themes in the study pool not previously stated in the extraction proforma. Additionally, backward citation tracing was adopted during data extraction which yielded 2 additional papers not found in the original database search. Methodological endpoints relating to (i) study design (ii) demographic data (iii) PFT management (iv) QoL measure (v) OS and PFS (vi) complications (vii) participation rate (viii) reason for non-participation and (ix) quality assessment were extracted from the dataset. Given the diverse tumour population and to allow for ease of comparison, the tumours were stratified by grade (benign or malignant) and anatomical site (intra or extra-axial).
Quality assessment
Risk of bias assessment was carried out by three independent reviewers (M.K., C.J. and N.C.) using the National Institute of Health Quality Assessment for the respective study designs. Conflict resolution was conducted between the two reviewers, and in the case of no resolution an appeal was made to a senior reviewer (G.A.).
Statistical analysis
Intrinsic posterior fossa tumours were analysed separately from vestibular schwannoma (VS), Glomus Jugulare Tumours (GJT) and meningiomas (MG). Descriptive statistics were generated using SPSS (IBM SPSS Statistics for Macintosh, Version 27.0.). Q-Q plots were developed to establish the normality of the data set. Median and interquartile range (IQR) were subsequently reported.
Results
Scope of review
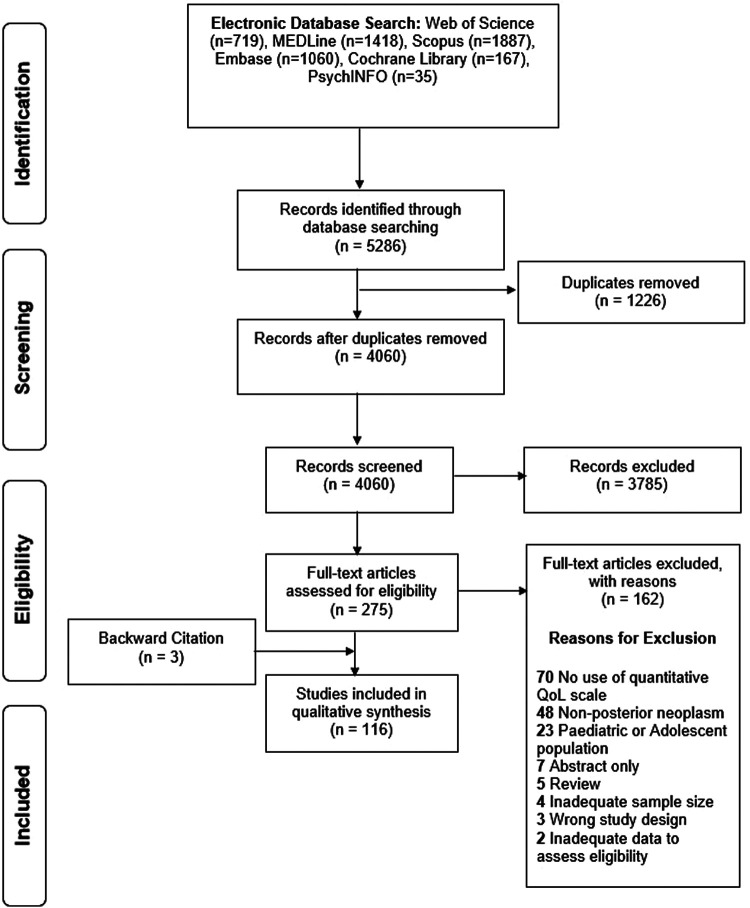
The search string returned 5,286 articles, of which 4,060 were considered for title and abstract screening after deduplication. 275 articles underwent full-text screening of which 162 were excluded. Three additional articles were included via backward citation tracing, resulting in 116 articles were included for qualitative analysis undergoing full qualitative synthesis (Figure 1, Supplementary Table S1).
Figure 1.
PRISMA diagram.
Study characteristics
The studies recruited a median of 104 patients (IQR = 168), 38 males (IQR = 70) and 39 females (IQR = 78). Cross-sectional studies were the most common study design (56.9, n = 66), followed by observational cohort (36.2%; n = 42) and case-control (5.2%; n = 6). Majority of studies came from the United States (USA) (25.0%; n = 29), Germany (13.8%; n = 16), and the United Kingdom (UK) (8.6%; n = 10). Additionally, multi-country studies were also present, with three studies being produced by a Norway and USA multicentre collaborative. A retrospective recruitment method of study samples was most adopted (603.%, n = 70).
Tumour pathology
Collectively, 25,801 PFTs were identified in the study pool. VS was the most frequent tumour (92.6%, n = 23,886), followed by pilocytic astrocytoma (PA) (2.5%, n = 657), MG (1.7%, n = 437), medulloblastoma (MB) (1.6%, n = 400), ependymoma (EP) (1.2%, n = 313), hemangioblastoma (HB) (0.2%, n = 40), GJT (0.2%, n = 40) and other unspecified PFTs (0.1%, n = 28).
Health-Related quality of life
Apart from self-designed questionnaires (SDQs), a total of 25 different HRQoL measures were used in the study pool (Table 1). Short Form Survey-36 (SF-36) was the most used QoL measure in the whole study pool (47.4%, n = 55), followed by the Penn Acoustic Neuroma QoL (PANQoL) scale (20.7%, n = 24), and Dizziness Handicap Inventory (DHI) (13.8%, n = 16). QoL measures were most commonly administered post-operatively (74.1%, n = 86), followed by both pre and post-operatively (16.4%, n = 19) and solely pre-operatively (0.9%, n = 1). Patients were followed up for a median of 18 months (IQR = 59).
Table 1.
Quality of life measures summary.
| Quality of life measure | Site-specific | Disease-specific | Multidimensional? | Number of studies | Patient or clinician | Domains |
|---|---|---|---|---|---|---|
| SF-36 | No | No | Yes | 55 | Patient | Physical functioning |
| Physical role | ||||||
| Bodily pain | ||||||
| General health | ||||||
| Vitality | ||||||
| Social functioning | ||||||
| Emotional role | ||||||
| Mental health | ||||||
| SF-12 | No | No | Yes | 1 | Patient | Mental Functioning |
| Physical Functioning | ||||||
| PANQOL | No | Yes | Yes | 24 | Patient | Anxiety |
| Facial functioning | ||||||
| General health | ||||||
| Balance | ||||||
| Hearing loss | ||||||
| Energy | ||||||
| Pain | ||||||
| GBI | No | No | Yes | 12 | Patient | Overall |
| General health | ||||||
| Physical functioning | ||||||
| Social functioning | ||||||
| DHI | No | No | Yes | 15 | Patient | Physical |
| Emotional | ||||||
| Functional | ||||||
| FaCE | No | No | Yes | 2 | Patient | Facial movement |
| Facial comfort | ||||||
| Oral function | ||||||
| Eye comfort | ||||||
| Lacrimal control | ||||||
| Social function | ||||||
| NFTI-QOL | No | Yes | Yes | 1 | Patient | Hearing |
| Dizziness and Balance | ||||||
| Facial palsy | ||||||
| Sight | ||||||
| Mobility and Walking | ||||||
| Role and Outlook on life | ||||||
| Pain | ||||||
| Anxiety and Depression | ||||||
| PROMIS-10 | No | No | Yes | 2 | Patient | Overall health |
| Pain | ||||||
| Fatigue | ||||||
| Social health | ||||||
| Mental health | ||||||
| Physical health | ||||||
| PCMIS | No | Yes | Yes | 1 | Patient | Diplopia |
| Facial sensation | ||||||
| Facial palsy | ||||||
| Hearing | ||||||
| Swallowing and speaking | ||||||
| Motor disturbance | ||||||
| Sensory disturbance | ||||||
| Consciousness and | ||||||
| communication | ||||||
| QLQ-C30 | No | Yes | Yes | 4 | Patient | Physical Role |
| Cognitive | ||||||
| Emotional | ||||||
| Social | ||||||
| Global QoL | ||||||
| GHSI | No | No | Yes | 2 | Patient | General |
| Social | ||||||
| Physical Health | ||||||
| QLQ-BN20 | No | Yes | Yes | 2 | Patient | Headaches |
| Seizures | ||||||
| Drowsiness | ||||||
| Hair loss | ||||||
| Itchy skin | ||||||
| Leg weakness | ||||||
| Bladder control | ||||||
| Future uncertainty | ||||||
| Visual disorder | ||||||
| Motor dysfunction | ||||||
| Communication deficit | ||||||
| Heidelberg SYQOL Inventory | No | No | Yes | 1 | Patient | Cranial Deficits |
| Headaches | ||||||
| Fatigue | ||||||
| APHAB | No | No | Yes | 1 | Patient | Ease of communication |
| Reverberation | ||||||
| Background noise | ||||||
| Aversiveness of sounds | ||||||
| BBS | No | No | No | 1 | Patient | Speech perception |
| Spatial hearing | ||||||
| Quality of sound | ||||||
| THI | No | No | Yes | 6 | Patient | Functional |
| Emotional | ||||||
| Catastrophic | ||||||
| HHI | No | No | Yes | 5 | Patient | Emotional |
| Social | ||||||
| MDASI-BT | No | No | Yes | 2 | Patient | Pain |
| Fatigue | ||||||
| Nausea | ||||||
| Disturbed sleep | ||||||
| Distress | ||||||
| Shortness of breath | ||||||
| Difficulty remembering | ||||||
| Lack of appetite | ||||||
| Drowsiness | ||||||
| Dry mouth | ||||||
| Sadness | ||||||
| Vomiting | ||||||
| Numbness | ||||||
| Symptom interference | ||||||
| HSQ | No | No | Yes | 2 | Patient | Health perception |
| Physical functioning | ||||||
| Physical role | ||||||
| Emotional role | ||||||
| Social functioning | ||||||
| Mental health | ||||||
| Bodily pain | ||||||
| Energy/Fatigue | ||||||
| Physical component scale | ||||||
| Mental component scale | ||||||
| IPQ-R | No | No | Yes | 2 | Patient | Timeline |
| Consequences | ||||||
| Personal control | ||||||
| Treatment control | ||||||
| Illness coherence | ||||||
| Emotional representations | ||||||
| HADS | No | No | No | 2 | Patient | Anxiety |
| Depression | ||||||
| WHOQOL-Bref | No | No | Yes | 1 | Patient | Physical health |
| Psychological | ||||||
| Social relationships | ||||||
| Environment | ||||||
| ABC | No | No | No | 1 | Patient | Activities of Daily Living |
| Motor | ||||||
| FACT-Br | No | Yes | Yes | 1 | Patient | Physical |
| Social/Family | ||||||
| Emotional | ||||||
| Functional | ||||||
| General Wellbeing | ||||||
| EQ-5D | No | No | Yes | 1 | Patient | Mobility |
| Self-Care | ||||||
| Usual Activities | ||||||
| Pain / Discomfort | ||||||
| Anxiety / Depression |
Benign, intra-axial
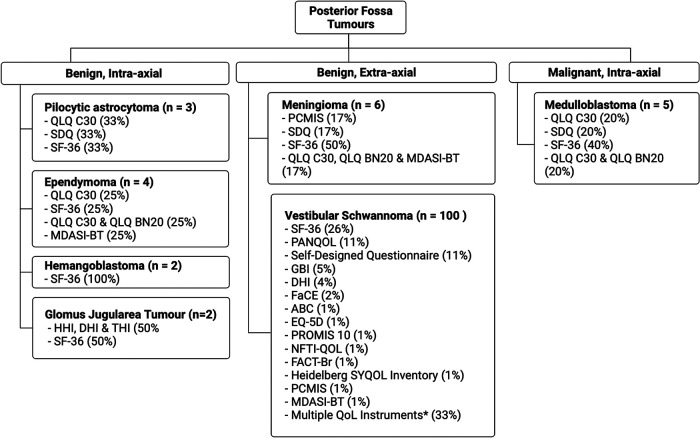
The benign, intra-axial tumour population includes PA, EP, HB and GJT. Eleven studies in total addressed these tumour types. SF-36, QLQ-C30 and a SDQ were all used once respectively in the PA study population (n = 3). Armstrong et al. noted an increased incidence of memory problems with radiation dose as well as poorer physical ability and social functioning as measured by SF-36 (53). Using QLQ-C30, a later study confirmed this, underscoring the intensity of radiotherapy as a major determinant in poorer somatic status and perceived QoL (109).
In both HB studies, SF-36 was the only QoL instrument used (Figure 2). Surgical resection of HB tumours has a limited impact on QoL, whereby numbers are comparable to a healthy population. However, resective surgery on brainstem tumours results in poor QoL outcomes. In this instance, radiosurgery is recommended as an alternative (33).
Figure 2.
Percentage QoL measure usage in posterior fossa tumours (*indicates studies where multiple QoL metrics were used).
Two studies discussed GJT. One study in the cohort used a combination of DHI, Tinnitus Handicap Inventory (THI), SF-12 and Hearing Handicap Inventory (HHI), while the other study used SF-36. Stereotactic radiotherapy (SRT) use in GJT tumours has significant affects on hearing, with 40% more patients experiencing hearing difficulties compared to the conservatively-treated population (60). SRT patients also report worse physical and emotional health (60). A separate German study showed no difference in physical and mental health between SRT cohort and the healthy population according to SF-36 (124).
In the EP cohort (n = 4), QLQ-C30 was most common (50%, n = 2) with SF-36, QLQ-BN20 and MDASI being used once. QLQ-BN20 and QLQ-C30 were both used in one study. An inability to work was a common feature in EP patients (19, 52), with many requiring help with activities of daily living (52). Dutzmann et al. found EP patients had a worse QoL when comparing to alternative brain neoplasms, particularly in motor function (48). As seen in other neoplasms, radiation therapy had a significant impact on patients' QoL compared to non-irradiated (48).
Benign, extra-axial
VS tumours formed the bulk of benign, extra-axial lesions, forming 86.2% (n = 100) of the entire study cohort. In studies with a primary focus on VS, 488 patients underwent serial magnetic resonance imaging (MRI), and 3,562 patients underwent a “wait and see” observational approach. Resective surgery was reported in 13,344 patients in 91 (79.3%) studies. In the VS study pool (86.2%, n = 100), the retro-sigmoid approach to the PF was the most notable (17.0%, n = 17), followed by a trans labyrinth (15.0%, n = 15) and middle cranial fossa approach (11.0%, n = 11). The three most common stand-alone QoL measures in the VS subgroup were SF-36 (26.0%, n = 26), PANQOL (11.0%, n = 11) and SDQs (11.0%, n = 11). When considered in combination with other QoL metrics SF-36 (49.0%, n = 49), PANQOL (24.0%, n = 24) and GBI (12.0%, n = 12) were the most common (Figure 2).
Meningiomas comprised a small proportion of this strata (n = 5) with SF-36 being the most used metric in this subgroup (40%). QLQ-C30, QLQ-BN20, MDASI and a SDQ were all used once. One study used a composite HRQoL outcome, based on QLQ-C3O, QLQ-BN20 and MDASI. Aggressive surgical resection resulted in improved QoL using QLQ-C30 and BN20 (41). Grauvogel et al. noted a correlation between tumour size and degree of patient-perceived vertigo impairment. (72) Although surgical resection can improve QoL, there are mentions of decrease in general health and social functioning due to postoperative complications, particularly hemiparesis, swallowing impairments and hypoacusis (111).
Malignant, intra-axial
In the MB study sample (n = 5), SF-36 and QLQ-C30 were both used twice, followed by SDQ, QLQ-BN20 and an SDQ that were all used once. One study used a combination of QLQ-C30 and QLQ-BN20. Improvement in QoL and neurocognitive functioning was recognised in combination treatment of radiotherapy and maintenance cisplatin, lomustine and vincristine, despite considerable toxicity during treatment (10). In the long term, MB survivors suffer significant intellectual impairments which have an impact on independence in adulthood. This is said to be affected by parental educational achievement and occurrence of post-operative cerebellar mutism (38). Armstrong et al. noted irradiation of temporal region resulted in poorer emotional functioning whereas general health was more affected when the posterior fossa region was irradiated (53).
Participation rate
Seventy-two studies (62%) reported less-than 100% participation, of which 33 studies gave specific reasons for non-participation (Table 2). The median participation rate was 78% (Range: 29%–97%). The commonest reason for non-participation was a lack of response to respective questionnaires (n = 1,718), followed by incomplete questionnaires (n = 268) and cognitive dysfunction (n = 258). Other reasons included participants declining to take part in the study (n = 178), disability (n = 104), not meeting the study inclusion criteria (n = 84), inaccessibility (n = 79), death(n = 70), language barriers (n = 48), personal difficulties (n = 15), and loss to follow-up (n = 3).
Table 2.
Study participation.
| Study | Participation (%) | Reasons for non-participation |
|---|---|---|
| Leong 2015 | 45.2 | No response (n = 482) |
| Kessel 2017 | 66.8 | No response (n = 61) |
| Inoue 2001 | 86.0 | Questionnaires could not be delivered (n = 32) |
| No response (n = 28) | ||
| Browne 2008 | 71.4 | No response (n = 28) |
| Did not meet inclusion criteria (n = 6) | ||
| Bateman 2000 | 76.0 | No response (n = 17) |
| Andersson 1997 | 90.0 | No response (n = 16) |
| Sun 2015 | 87.5 | Inaccessible (n = 3) |
| Subramaniam 2005 | 93.0 | Declined (n = 2) |
| Disability (n = 1) | ||
| Lost to follow-up (n = 1) | ||
| Blom 2020 | 75.8 | Declined (n = 11) |
| Link 2018 | 79.0 | No response (n = 30) |
| Lodder 2018 | 40.8 | No response (n = 483) |
| Incomplete Data (n = 38) | ||
| Dirven 2020 | 93.0 | No response (n = 2) |
| Sandooram 2010 | 94.2 | Lost to follow up (n = 2) |
| Scheich 2014 | 78.0 | No response (n = 26) |
| LeReste 2013 | 81.5 | Death (n = 7) |
| Kim 2015 | 75.5 | No response (n = 35) |
| Combs 2013 | 42.0 | No response (n = 114) |
| Deceased (n = 51) | ||
| Wirsching 2020 | 60.8 | Did not consent (n = 88) |
| Foreign mother tongue (n = 48) | ||
| Cognitive impairment (n = 249) | ||
| Van Leeuwen 1996 | 77.0 | Relocation (n = 14) |
| Refused to participate (n = 17) | ||
| Deceased (n = 9) | ||
| Yang 2018 | 29.0 | Declined (n = 36) |
| MacAndie 2004 | 84.0 | No response (n = 5) |
| Change of address (n = 3) | ||
| Armstrong 2010 | 87.4 | Paralysis (n = 103) |
| Timmer 2010 | 91.0 | Dementia (n = 9) |
| Brooker 2010 | 78.0 | Did not meet inclusion criteria (n = 40) |
| Pan 2012 | 92.0 | Did not meet inclusion criteria (n = 3) |
| Breivik 2012 | 97.4 | Declined (n = 5) |
| Vogel 2008 | 87.8 | Personal issues (n = 6) |
| No response (n = 4) | ||
| Hruba 2019 | 67.3 | Did not meet inclusion criteria (n = 35) |
| Foley 2017 | 87.0 | Declined (n = 10) |
| Reading difficulties (n = 1) | ||
| Emotional difficulties to survey content (n = 1) | ||
| Van Leeuwen 2013 | 76.8 | Personal problems (n = 7) |
| No response (n = 29) | ||
| Soulier 2017 | 76.0 | Living abroad (n = 14) |
| Missing contact information (n = 6) | ||
| No response (n = 289) | ||
| Tufarelli 2006 | 85.0 | No response (n = 69) |
| Baumann 2005 | 70.0 | Declined (n = 8) |
| Relocated (n = 7) | ||
| Deceased (n = 3) | ||
| Miller 2019 | 36.8 | Incomplete survey (n = 230) |
Quality assessment
Majority of the cohort and cross-sectional studies were deemed “Good” (59.4%, n = 69), followed by “Fair” (39.7%, n = 46) and “Poor” (1.7%, n = 2). Case-controlled studies were regarded as “Good” (66.6%, n = 2) and “Poor” (33.3%, n = 1). The single case series and controlled intervention study were deemed “Fair” and “Good” respectively.
Discussion
Herein, we present the first systematic review to date outlining QoL reporting in adult patients with PFTs. This search was conducted to map the current usage of HRQoL measures for tumours situated in the posterior cranial fossa, as well as establish reasoning behind patient non-participation in QoL surveys. Our systematic review identified 25 self-administered QoL measures in 115 different studies, with some groups developing their own questionnaires. The number of measures being used, despite only 7 major tumour types in the study population, highlights the heterogeneity of PFT QoL measurement. Without the site-specific or disease-specific measurement of core metrics in adult PFTs, QoL reporting remains insufficient to assess clinically significant changes after treatment.
Quality of life reporting
Althougha plethora of available metrics appears beneficial, there is a lack of clinically useful data, due to a reduction in specificity (126, 127). To the best of our knowledge, PANQOL and the Petroclival Meningioma Impairment Scale (PCMIS) were the only disease-specific measures used in PFTs, measuring outcomes in sporadic VS and petroclival meningiomas respectively. Generic metrics allow for assessment of broader domains and comparisons between different studies and conditions, however they lack the specificity achieved by tailored disease questionnaires (128). Additionally, as specific metrics evaluate the areas of wellbeing that are important to patients with distinct histology, they are sensitive enough to assess change and inform clinical decision-making (129).
SF-36 was the most common QoL metric used overall and in the VS study subgroup (49.0%). As a metric, SF-36 is one of the most widely used and reliable instruments in a neuro-oncology setting (152). Albeit beyond the scope of this paper, an argument can be made for SF-36 as the most useful metric in PFTs and brain tumours in general. SF-36 has been shown to adequately discriminate between benign and malignant brain tumours in physical and social functioning domains. Additionally, there is consistency in agreement between physical and emotional health status with other functional status measures such as Beck Depression Inventory II and Barthel Index (152). However, when evaluating use specifically for VS, there are major flaws to consider.
Hearing loss is a common symptom following VS surgery affecting over 90% of patients (130) and plays a prominent role in patient QoL (18, 76). However, Godefroy et al. (106) highlighted the inability of SF-36 to assess changes in hearing. Additional criticism comes from the SF-36's social relationships scale, whereby this is measured by how much physical and emotional sequalae interrupted social activities. However, most literature regard social relationships as a measure of social and emotional loneliness (131, 132). Given it is reported that VS patients rely heavily on family support post-treatment (50%) and the increased time taken to adjust to relationships (15%), the impact of this limitation is important (130).
The Brain cancer-specific Quality of Life Questionnaire (QLQ-BN20) was developed by the European Organisation for Research and Treatment of Cancer (EORTC) to assess QoL in brain tumours. The primary benefit of this compared to a more broad metric is its ability to capture frequently encountered problems in the patient group and its brevity (150). Distinctly for VS, Shaffer et al. developed the Penn Acoustic Neuroma QoL metric, which is posed to provide more clinically-useful information to clinicians and can be useful in assessing differences in QoL in VS patient groups undergoing different treatment regimens (71, 78).
There is a complementary effect in using both a generic and specific QoL measures; whilst a generic measure can determine changes to a patient's physical status in relation to the population, disease-specific metrics can aid in monitoring the specific cause of a patient's reduced functionality (133). Hence, in the example the SF-36 measure could be complemented by PANQoL or HHI to increase its changes to patient symptoms. As well as the incorporation of specific symptoms in generic QoL questionnaires (134, 135), lessons may be learned from initiatives such as the Computerized Adaptive Assessment of Disease Impact (DICAT) project, which has developed standardised disease specific QoL metrics to gauge the impact of discrete disease symptomatology whilst also allowing for comparable metrics between studies (136).
Minimal clinically important difference and core outcome sets
QoL instruments are increasingly being used as primary outcomes in RCTs. It is important to ascertain whether there are significant differences observed in QoL to determine clinically important change as opposed to simply statistically significant change. Guyatt et al. developed the notion of minimal clinically important difference (MCID) defined as “the lowest change in PRO in a specific domain of interest that patients perceive as important that would lead the clinician to consider a change in patient management” (137, 138). The use of patient-centred MCIDs is important to conveying change in QoL studies, as improvement may not be obvious to clinicians when evaluating treatment. A popular, patient-centred method of determining MCID is the anchor-based approach, as opposed to the Delphi method which is mainly expert consensus led (137, 139, 148). The anchor-based approach is associates numerical values to subjective assessments of improvement. For example, patients are asked if they felt “the same”, “a little better” or “quite better” after receiving treatment. These responses are then linked to a measure scale which is more in line with the patient's subjective state. MCID data is malleable, changing for a specific QoL instrument used in a specific patient population (137, 139). To the best of our knowledge, MCID studies have only been conducted for VS tumours (45, 115), emphasising the need for additional MCID studies for PFTs to determine clinical significance especially in common malignant tumours such as MB.
A core outcome set (COS) is a group of defined outcomes that should be measured and reported in any given trial as a minimum (140). In developing core metrics for disease- or PF-specific QoL outcomes a similar approach in methodology should be taken whereby all stakeholders i.e., oncologists, nurses, neurosurgeons, QoL experts, carers and most importantly, patients form a consensus to develop a more robust and specific metric to assess QoL of patients with PFTs. By doing so, it will allow for more robust evaluation of patient QoL and give valuable insight into the managing PFTs in the future.
Participation and dropout rates
Majority of the studies reported patient non-participation, exposing the study pool to selection bias. Generally, the rate of participation was well-reported with the top three being lack of response (n = 1,718), incomplete questionnaires and cognitive dysfunction (n = 258) which is similar to rates in other brain neoplasms (141). However, there is a lack of sufficient measures to reduce drop-out rates in these studies. Firstly, reducing the amount of data collection has been suggested to play a role in mitigating drop-out rates (142). Additional attempts to increase participation may be to create a simplified version of QoL measures with cognitive dysfunction, outlining specific ways in which the QoL measure should be completed to reduce incomplete questionnaires. Another reason for non-participation that is easily fixable is the language barrier, by translating the questionnaire into different languages (83, 88, 113). Finally, the role of digital forms as well as automated reminders should not be underestimated (143). However, this may serve to discriminate against individuals who lack technological aptitude.
In the presence of a notable disability, an argument can be raised for the use of personal carers as proxies to fill out questionnaires. Previous work has suggested good agreement between patient-reported and carer-reportedoutcomes, particularly in recurrent disease (9, 144). However, similarity in ratings appear to decrease as patients become increasingly disabled which defeats the purpose of a proxy measure in that circumstance (145).
PFTs are routinely being treated in adulthood and QoL is increasingly playing a role in clinical decision-making (125). Using OS and PFS as proxy-measures is no longer a gold-standard indicator of the quality of the care provided to patients, particularly in slow-growing tumours like HBs (33). As well as allowing for individualised patient care, QoL tools assist in developing targeted treatment methods depending on patient morbidity and establishing the superiority of a specific treatment regimen or surgical approach (146). To accurately capture patient QoL, appropriate questions and measuring tools are required. Given the heterogeneity of brain tumour symptomatology, prognosis, and post-operative sequelae, it is a given that disease and site-specific measures should be utilised. If not, imprecise patient data will ultimately lead to uninformed changes in care. Based on our study, one could argue for the sole use of disease-specific QoL metrics when evaluating treatments in PFT patients as they are more accurate in assessing change over time. However, it is important not to preclude generic instruments as they allow for broad comparisons between studies. Hence, our study would agree with authors that recommend the simultaneous use of both types when assessing change in PFT patients (151).
This study contains a number of limitations. Only articles in English were included in this study, excluding useful literature on the topic in other languages. Secondly, majority of included studies that incorporated patients with non-PF neoplasms, did not specify the patients with PFTs or provide a subgroup analysis of this cohort. This decreased the specificity in understanding distinct PF tumour pathology, prognosis and QoL. Thirdly, the heterogeneity that exists within distinct PFTs (notably MB) was not accounted for, hence differences in the distinct tumour subtype pathology, symptomatology and prognosis were not noted. Finally, due to heterogeneous cohort of PFTs the data may be skewed towards more common neoplasms such as VS. To account for this, the tumours have been stratified based on grading and anatomical location in order to draw out more meaningful conclusions.
Conclusion
With improved survival in adult PFTs, there is a growing need for QoL outcomes to assess the efficacy of interventions and institutional care. This study has mapped the current landscape of QoL reporting in adult PFTs as well as the rate of participation and reasoning. There is a low number of disease-specific QoL metrics for PFTs, with the majority using generic methods such as SF-36 which has flaws in its specificity for unique symptomatology in PFTs. Future studies should focus on developing disease-specific metrics using a consensus of patients, carers, neurosurgeons and oncologists to increase instrument sensitivity. These metrics can be used in combination with more generic instruments to allow for broad data comparison whilst accurately assessing change over time.
Funding
Ciaran Hill is supported by Cancer Research UK (CrUK), Advanced Medical Services (AMS), National Institute for Health and Care Research (NIHR) and National Brain Appeal.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
GA and CH contributed to conception and design of the study. GA, CJ, MK and NC organised the database. GA performed formal statistical analysis and wrote the first draft of the manuscript. GA, CJ, MK and NC wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.970889/full#supplementary-material.
References
- 1.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. (2019) 21(Suppl 5):v1–v100. 10.1093/neuonc/noz150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack IF. Brain tumors in children. N Engl J Med. (1994) 331(22):1500–7. 10.1056/NEJM199412013312207 [DOI] [PubMed] [Google Scholar]
- 3.Frič R, Due-Tønnessen BJ, Lundar T, Egge A, Kronen Krossnes B, Due-Tønnessen P, et al. Long-term outcome of posterior fossa medulloblastoma in patients surviving more than 20 years following primary treatment in childhood. Sci Rep. (2020) 10(1):9371. 10.1038/s41598-020-66328-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollack IF. Multidisciplinary management of childhood brain tumors: a review of outcomes, recent advances, and challenges. J Neurosurg Pediatr. (2011) 8(2):135–48. 10.3171/2011.5.PEDS1178 [DOI] [PubMed] [Google Scholar]
- 5.Lundar T, Due-Tønnessen BJ, Frič R, Brandal P, Due-Tønnessen P. Adult outcome after treatment of pediatric posterior fossa ependymoma: long-term follow-up of a single consecutive institutional series of 22 patients with more than 5 years of survival. J Neurosurg Pediatr. (2020) 26(1):22–6. 10.3171/2020.1.peds19700 [DOI] [PubMed] [Google Scholar]
- 6.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. (2015) 17(Suppl 4):iv1–iv62. 10.1093/neuonc/nov189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossman R, Ram Z. Posterior Fossa intra-axial tumors in adults. World Neurosurg. (2016) 88:140–5. 10.1016/j.wneu.2015.12.066 [DOI] [PubMed] [Google Scholar]
- 8.Kaplan RM, Bush JW. Health-related quality of life measurement for evaluation research and policy analysis. APA PsycArticles. (1982) 1(1):61–80. 10.1037/0278-6133.1.1.61 [DOI] [Google Scholar]
- 9.Gil Z, Abergel A, Spektor S, Khafif A, Fliss DM. Patient, caregiver, and surgeon perceptions of quality of life following anterior skull base surgery. Arch Otolaryngol Head Neck Surg. (2004) 130(11):1276–81. 10.1001/archotol.130.11.1276 [DOI] [PubMed] [Google Scholar]
- 10.Dirven L, Luerding R, Beier D, Bumes E, Reinert C, Seidel C, et al. Neurocognitive functioning and health-related quality of life in adult medulloblastoma patients: long-term outcomes of the NOA-07 study. J Neurooncol. (2020) 148(1):117–30. 10.1007/s11060-020-03502-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauden A, Weir P, Hawthorne G, Kaye A. Systematic review of quality of life in the management of vestibular schwannoma. J Clin Neurosci. (2011) 18(12):1573–84. 10.1016/j.jocn.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 12.Pompili A, Caperle M, Pace A, Ramazzotti V, Raus L, Jandolo B, et al. Quality-of-life assessment in patients who had been surgically treated for cerebellar pilocytic astrocytoma in childhood. J Neurosurg. (2002) 96(2):229–34. 10.3171/jns.2002.96.2.0229 [DOI] [PubMed] [Google Scholar]
- 13.Leong SC, Lesser TH. A national survey of facial paralysis on the quality of life of patients with acoustic neuroma. Otol Neurotol. (2015) 36(3):503–9. 10.1097/MAO.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 14.Kessel KA, Fischer H, Vogel MM, Oechsner M, Bier H, Meyer B, et al. Fractionated vs. single-fraction stereotactic radiotherapy in patients with vestibular schwannoma: hearing preservation and patients’ self-reported outcome based on an established questionnaire. Strahlenther Onkol. (2017) 193(3):192–9. 10.1007/s00066-016-1070-0 [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, Ogawa K, Kanzaki J. Quality of life of vestibular schwannoma patients after surgery. Acta Otolaryngol. (2001) 121(1):59–61. 10.1080/000164801300006281 [DOI] [PubMed] [Google Scholar]
- 16.Browne S, Distel E, Morton RP, Petrie KJ. Patients’ quality of life, reported difficulties, and benefits following surgery for acoustic neuroma. J Otolaryngol Head Neck Surg. (2008) 37(3):417–22. 10.2310/7070.2008.0023 [DOI] [PubMed] [Google Scholar]
- 17.Bateman N, Nikolopoulos TP, Robinson K, O'Donoghue GM. Impairments, disabilities, and handicaps after acoustic neuroma surgery. Clin Otolaryngol Allied Sci. (2000) 25(1):62–5. 10.1046/j.1365-2273.2000.00326.x [DOI] [PubMed] [Google Scholar]
- 18.Andersson G, Ekvall L, Kinnefors A, Nyberg G, Rask-Andersen H. Evaluation of quality of life and symptoms after translabyrinthine acoustic neuroma surgery. Am J Otol. (1997) 18(4):421–6. [PubMed] [Google Scholar]
- 19.Acquaye AA, Vera E, Gilbert MR, Armstrong TS. Clinical presentation and outcomes for adult ependymoma patients. Cancer. (2017) 123(3):494–501. 10.1002/cncr.30355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun D, Shi Z, Li P, Shi S, Cai Y. Psychological status and quality of life in acoustic neuroma patients with facial palsy after microsurgery: a 1-year postoperative follow-up study. Acta Neurol Belg. (2015) 115(3):311–6. 10.1007/s13760-014-0382-z [DOI] [PubMed] [Google Scholar]
- 21.Subramaniam K, Eikelboom RH, Eager KM, Atlas MD. Unilateral profound hearing loss and the effect on quality of life after cerebellopontine angle surgery. Otolaryngol Head Neck Surg. (2005) 133(3):339–46. 10.1016/j.otohns.2005.05.017 [DOI] [PubMed] [Google Scholar]
- 22.Frič R, Eide PK. The presigmoid approach for removal of tumours causing ventral compression of the brainstem. Surgical results and postoperative quality of life. Br J Neurosurg. (2011) 25(1):86–93. 10.3109/02688697.2010.525266 [DOI] [PubMed] [Google Scholar]
- 23.Blom SSAH, Aarts H, Wever CC, Kunst HPM, Semin GR. Quality of life, social function, emotion, and facial paresis in Dutch vestibular schwannoma patients. Laryngoscope Investig Otolaryngol. (2020) 5(3):477–84. 10.1002/lio2.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin EJ, Bigelow DC, Lee JY, Ruckenstein MJ. Quality of life in acoustic neuroma patients. Otol Neurotol. (2015) 36(4):653–6. 10.1097/MAO.0000000000000674 [DOI] [PubMed] [Google Scholar]
- 25.Link MJ, Lund-Johansen M, Lohse CM, Driscoll CLW, Myrseth E, Tveiten OV, et al. Quality of life in patients with vestibular schwannomas following gross total or less than gross total microsurgical resection: should we be taking the entire tumor out? Neurosurgery. (2018) 82(4):541–7. 10.1093/neuros/nyx245 [DOI] [PubMed] [Google Scholar]
- 26.Lodder WL, van der Laan BFAM, Lesser TH, Leong SC. The impact of acoustic neuroma on long-term quality-of-life outcomes in the United Kingdom. Eur Arch Otorhinolaryngol. (2018) 275(3):709–17. 10.1007/s00405-018-4864-0 [DOI] [PubMed] [Google Scholar]
- 27.Wagner JN, Glaser M, Wowra B, Muacevic A, Goldbrunner R, Cnyrim C, et al. Vestibular function and quality of life in vestibular schwannoma: does size matter? Front Neurol. (2011) 2:55. 10.3389/fneur.2011.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klersy PC, Arlt F, Hofer M, Meixensberger J. Quality of life in patients with unilateral vestibular schwannoma on wait and see – strategy. Neurol Res. (2018) 40(1):34–40. 10.1080/01616412.2017.1390184 [DOI] [PubMed] [Google Scholar]
- 29.Hebb ALO, Erjavec N, Morris DP, Mulroy L, Bance M, Shoman N, et al. Quality of life related to symptomatic outcomes in patients with vestibular schwannomas: a Canadian centre perspective. Am J Otolaryngol. (2019) 40(2):236–46. 10.1016/j.amjoto.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 30.Sandooram D, Hornigold R, Grunfeld B, Thomas N, Kitchen ND, Gleeson M. The effect of observation versus microsurgical excision on quality of life in unilateral vestibular schwannoma: a prospective study. Skull Base. (2010) 20(1):47–54. 10.1055/s-0029-1242985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheich M, Ginzkey C, Reuter E, Harnisch W, Ehrmann D, Hagen R. Quality of life after microsurgery for vestibular schwannoma via the middle cranial fossa approach. Eur Arch Otorhinolaryngol. (2014) 271(7):1909–16. 10.1007/s00405-013-2671-1 [DOI] [PubMed] [Google Scholar]
- 32.Čada Z, Balatková Z, Chovanec M, Čakrt O, Hrubá S, Jeřábek J, et al. Vertigo perception and quality of life in patients after surgical treatment of vestibular schwannoma with pretreatment prehabituation by chemical vestibular ablation. Biomed Res Int. (2016) 2016:6767216. 10.1155/2016/6767216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Reste PJ, Henaux PL, Morandi X, Carsin-Nicol B, Brassier G, Riffaud L. Sporadic intracranial haemangioblastomas: surgical outcome in a single institution series. Acta Neurochir (Wien. (2013) 155(6):1003–9; discussion 9. 10.1007/s00701-013-1681-5 [DOI] [PubMed] [Google Scholar]
- 34.Morisako H, Goto T, Ohata K. Petroclival meningiomas resected via a combined transpetrosal approach: surgical outcomes in 60 cases and a new scoring system for clinical evaluation. J Neurosurg. (2015) 122(2):373–80. 10.3171/2014.8.JNS132406 [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, Jin Roh K, Oh HS, Chang WS, Moon IS. Quality of life in patients with vestibular schwannomas according to management strategy. Otol Neurotol. (2015) 36(10):1725–9. 10.1097/MAO.0000000000000885 [DOI] [PubMed] [Google Scholar]
- 36.Plotkin SR, Duda DG, Muzikansky A, Allen J, Blakeley J, Rosser T, et al. Multicenter, prospective, phase II and biomarker study of high-dose bevacizumab as induction therapy in patients with neurofibromatosis type 2 and progressive vestibular schwannoma. J Clin Oncol. (2019) 37(35):3446–54. 10.1200/JCO.19.01367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tos T, Caye-Thomasen P, Stangerup SE, Tos M, Thomsen J. Long-term socio-economic impact of vestibular schwannoma for patients under observation and after surgery. J Laryngol Otol. (2003) 117(12):955–64. 10.1258/002221503322683830 [DOI] [PubMed] [Google Scholar]
- 38.Kieffer V, Chevignard MP, Dellatolas G, Puget S, Dhermain F, Grill J, et al. Intellectual, educational, and situation-based social outcome in adult survivors of childhood medulloblastoma. Dev Neurorehabil. (2019) 22(1):19–26. 10.1080/17518423.2018.1424262 [DOI] [PubMed] [Google Scholar]
- 39.Combs SE, Welzel T, Kessel K, Habermehl D, Rieken S, Schramm O, et al. Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients’ self-reported outcome. Radiother Oncol. (2013) 106(2):175–80. 10.1016/j.radonc.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 40.Ryzenman JM, Pensak ML, Tew JM. Facial paralysis and surgical rehabilitation: a quality of life analysis in a cohort of 1,595 patients after acoustic neuroma surgery. Otol Neurotol. (2005) 26(3):516–21; discussion 21. 10.1097/01.mao.0000169786.22707.12 [DOI] [PubMed] [Google Scholar]
- 41.Wirsching HG, Morel C, Roth P, Weller M. Socioeconomic burden and quality of life in meningioma patients. Qual Life Res. (2020) 29(7):1801–8. 10.1007/s11136-020-02461-1 [DOI] [PubMed] [Google Scholar]
- 42.van Leeuwen JP, Braspenning JC, Meijer H, Cremers CW. Quality of life after acoustic neuroma surgery. Ann Otol Rhinol Laryngol. (1996) 105(6):423–30. 10.1177/000348949610500602 [DOI] [PubMed] [Google Scholar]
- 43.Samii M, Metwali H, Gerganov V. Efficacy of microsurgical tumor removal for treatment of patients with intracanalicular vestibular schwannoma presenting with disabling vestibular symptoms. J Neurosurg. (2017) 126(5):1514–9. 10.3171/2016.4.JNS153020 [DOI] [PubMed] [Google Scholar]
- 44.Chweya CM, Tombers NM, Lohse CM, Link MJ, Carlson ML. Disease-Specific quality of life in vestibular schwannoma: a national cross-sectional study comparing microsurgery, radiosurgery, and observation. Otolaryngol Head Neck Surg. (2021) 164(3):639–44. 10.1177/0194599820941012 [DOI] [PubMed] [Google Scholar]
- 45.Kerezoudis P, Yost KJ, Tombers NM, Celda MP, Carlson ML, Link MJ. Defining the minimal clinically important difference for patients with vestibular schwannoma: are all quality-of-life scores significant? Neurosurgery. (2019) 85(6):779–85. 10.1093/neuros/nyy467 [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Wang Z, Huang M, Chai Y, Jia H, Wu Y, et al. Bonebridge implantation in patients with single-sided deafness resulting from vestibular schwannoma resection: objective and subjective benefit evaluations. Acta Otolaryngol. (2018) 138(10):877–85. 10.1080/00016489.2018.1469789 [DOI] [PubMed] [Google Scholar]
- 47.Tveiten OV, Carlson ML, Link MJ, Lund-Johansen M. Audiovestibular handicap and quality of life in patients with vestibular schwannoma and “excellent” hearing. Neurosurgery. (2017) 80(3):386–92. 10.1227/NEU.0000000000001238 [DOI] [PubMed] [Google Scholar]
- 48.Dützmann S, Schatlo B, Lobrinus A, Murek M, Wostrack M, Weiss C, et al. A multi-center retrospective analysis of treatment effects and quality of life in adult patients with cranial ependymomas. J Neurooncol. (2013) 114(3):319–27. 10.1007/s11060-013-1187-2 [DOI] [PubMed] [Google Scholar]
- 49.Rameh C, Magnan J. Quality of life of patients following stages III-IV vestibular schwannoma surgery using the retrosigmoid and translabyrinthine approaches. Auris Nasus Larynx. (2010) 37(5):546–52. 10.1016/j.anl.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 50.MacAndie C, Crowther JA. Quality of life in patients with vestibular schwannomas managed conservatively. Clin Otolaryngol Allied Sci. (2004) 29(3):215–8. 10.1111/j.1365-2273.2004.00806.x [DOI] [PubMed] [Google Scholar]
- 51.Jufas N, Flanagan S, Biggs N, Chang P, Fagan P. Quality of life in vestibular schwannoma patients managed by surgical or conservative approaches. Otol Neurotol. (2015) 36(7):1245–54. 10.1097/MAO.0000000000000789 [DOI] [PubMed] [Google Scholar]
- 52.Armstrong TS, Vera-Bolanos E, Gilbert MR. Clinical course of adult patients with ependymoma: results of the adult ependymoma outcomes project. Cancer. (2011) 117(22):5133–41. 10.1002/cncr.26181 [DOI] [PubMed] [Google Scholar]
- 53.Armstrong GT, Jain N, Liu W, Merchant TE, Stovall M, Srivastava DK, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. (2010) 12(11):1173–86. 10.1093/neuonc/noq104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kristin J, Glaas MF, Schipper J, Klenzner T, Eysel-Gosepath K, Jansen P, et al. Patient quality of life after vestibular schwannoma removal: possibilities and limits to measuring different domains of patients’ wellbeing. Eur Arch Otorhinolaryngol. (2019) 276(9):2441–7. 10.1007/s00405-019-05499-1 [DOI] [PubMed] [Google Scholar]
- 55.Timmer FC, van Haren AE, Mulder JJ, Hanssens PE, van Overbeeke JJ, Cremers CW, et al. Quality of life after gamma knife radiosurgery treatment in patients with a vestibular schwannoma: the patient's perspective. Eur Arch Otorhinolaryngol. (2010) 267(6):867–73. 10.1007/s00405-009-1140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myrseth E, Møller P, Wentzel-Larsen T, Goplen F, Lund-Johansen M. Untreated vestibular schwannomas: vertigo is a powerful predictor for health-related quality of life. Neurosurgery. (2006) 59(1):67–76; discussion 67–76. 10.1227/01.NEU.0000219838.80931.6B [DOI] [PubMed] [Google Scholar]
- 57.Parving A, Tos M, Thomsen J, Møller H, Buchwald C. Some aspects of life quality after surgery for acoustic neuroma. Arch Otolaryngol Head Neck Surg. (1992) 118(10):1061–4. 10.1001/archotol.1992.01880100053013 [DOI] [PubMed] [Google Scholar]
- 58.Henzel M, Hamm K, Sitter H, Gross MW, Surber G, Kleinert G, et al. Comparison of stereotactic radiosurgery and fractionated stereotactic radiotherapy of acoustic neurinomas according to 3-D tumor volume shrinkage and quality of life. Strahlenther Onkol. (2009) 185(9):567–73. 10.1007/s00066-009-1959-y [DOI] [PubMed] [Google Scholar]
- 59.Broomfield SJ, O'Donoghue GM. Self-reported symptoms and patient experience: a British acoustic neuroma association survey. Br J Neurosurg. (2016) 30(3):294–301. 10.3109/02688697.2015.1071323 [DOI] [PubMed] [Google Scholar]
- 60.Hebb ALO, Erjavec N, Morris DP, Shoman NM, Mulroy L, Walling SA. Treatment of patients with glomus jugulare tumours (GJT) and its subjective effect on quality of life (QoL) measures. Am J Otolaryngol. (2020) 41(6):102559. 10.1016/j.amjoto.2020.102559 [DOI] [PubMed] [Google Scholar]
- 61.Lin V, Jacobson M, Dorion J, Chen J, Nedzelski J. Global assessment of outcomes after varying reinnervation techniques for patients with facial paralysis subsequent to acoustic neuroma excision. Otol Neurotol. (2009) 30(3):408–13. 10.1097/MAO.0b013e31819a8e26 [DOI] [PubMed] [Google Scholar]
- 62.Glaas MF, Schäfer R, Jansen P, Franz M, Stenin I, Klenzner T, et al. Quality of life after translabyrinthine vestibular schwannoma resection-reliability of the German PANQOL questionnaire. Otol Neurotol. (2018) 39(6):e481–8. 10.1097/MAO.0000000000001819 [DOI] [PubMed] [Google Scholar]
- 63.Brooker JE, Fletcher JM, Dally MJ, Briggs RJ, Cousins VC, Smee RI, et al. Quality of life among acoustic neuroma patients managed by microsurgery, radiation, or observation. Otol Neurotol. (2010) 31(6):977–84. 10.1097/MAO.0b013e3181e8ca55 [DOI] [PubMed] [Google Scholar]
- 64.Lynn SG, Driscoll CL, Harner SG, Beatty CW, Atkinson EJ. Assessment of dysequilibrium after acoustic neuroma removal. Am J Otol. (1999) 20(4):484–94. [PubMed] [Google Scholar]
- 65.Fahy C, Nikolopoulos TP, O'Donoghue GM. Acoustic neuroma surgery and tinnitus. Eur Arch Otorhinolaryngol. (2002) 259(6):299–301. 10.1007/s00405-002-0473-y [DOI] [PubMed] [Google Scholar]
- 66.Pan HC, Sheehan J, Sheu ML, Chiu WT, Yang DY. Intracapsular decompression or radical resection followed by gamma knife surgery for patients harboring a large vestibular schwannoma. J Neurosurg. (2012) 117(Special_Suppl):69–77. 10.3171/2012.6.GKS12697 [DOI] [PubMed] [Google Scholar]
- 67.Ning F, Zuo H, Guo L, Jiao C, Xu X, Kong B, et al. An investigation of life quality of patients after two different acoustic neuroma resections. Acta Otolaryngol. (2019) 139(7):547–51. 10.1080/00016489.2019.1606437 [DOI] [PubMed] [Google Scholar]
- 68.Varughese JK, Wentzel-Larsen T, Pedersen PH, Mahesparan R, Lund-Johansen M. Gamma knife treatment of growing vestibular schwannoma in Norway: a prospective study. Int J Radiat Oncol Biol Phys. (2012) 84(2):e161–6. 10.1016/j.ijrobp.2012.03.047 [DOI] [PubMed] [Google Scholar]
- 69.Martin HC, Sethi J, Lang D, Neil-Dwyer G, Lutman ME, Yardley L. Patient-assessed outcomes after excision of acoustic neuroma: postoperative symptoms and quality of life. J Neurosurg. (2001) 94(2):211–6. 10.3171/jns.2001.94.2.0211 [DOI] [PubMed] [Google Scholar]
- 70.Turel MK, Thakar S, Rajshekhar V. Quality of life following surgery for large and giant vestibular schwannomas: a prospective study. J Neurosurg. (2015) 122(2):303–11. 10.3171/2014.10.JNS14534 [DOI] [PubMed] [Google Scholar]
- 71.Shaffer BT, Cohen MS, Bigelow DC, Ruckenstein MJ. Validation of a disease-specific quality-of-life instrument for acoustic neuroma: the penn acoustic neuroma quality-of-life scale. Laryngoscope. (2010) 120(8):1646–54. 10.1002/lary.20988 [DOI] [PubMed] [Google Scholar]
- 72.Grauvogel J, Kaminsky J, Rosahl SK. The impact of tinnitus and vertigo on patient-perceived quality of life after cerebellopontine angle surgery. Neurosurgery. (2010) 67(3):601–9; discussion 9–10. 10.1227/01.NEU.0000374725.19259.EA [DOI] [PubMed] [Google Scholar]
- 73.Myrseth E, Møller P, Pedersen PH, Vassbotn FS, Wentzel-Larsen T, Lund-Johansen M. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. (2005) 56(5):927–35; discussion 35. 10.1055/s-2005-916493 [DOI] [PubMed] [Google Scholar]
- 74.Miller LE, Brant JA, Chen J, Kaufman AC, Ruckenstein MJ. Hearing and quality of life over time in vestibular schwannoma patients: observation compared to stereotactic radiosurgery. Otol Neurotol. (2019) 40(8):1094–100. 10.1097/MAO.0000000000002334 [DOI] [PubMed] [Google Scholar]
- 75.Breivik CN, Nilsen RM, Myrseth E, Pedersen PH, Varughese JK, Chaudhry AA, et al. Conservative management or gamma knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery. (2013) 73(1):48–56; discussion 7. 10.1227/01.neu.0000429862.50018.b9 [DOI] [PubMed] [Google Scholar]
- 76.Di Maio S, Akagami R. Prospective comparison of quality of life before and after observation, radiation, or surgery for vestibular schwannomas. J Neurosurg. (2009) 111(4):855–62. 10.3171/2008.10.JNS081014 [DOI] [PubMed] [Google Scholar]
- 77.Prummer CM, Kerezoudis P, Tombers NM, Peris-Celda M, Link MJ, Carlson ML. Influence of selection bias in survey studies derived from a patient-focused organization: a comparison of response data from a single tertiary care center and the acoustic neuroma association. Otol Neurotol. (2019) 40(4):504–10. 10.1097/MAO.0000000000002151 [DOI] [PubMed] [Google Scholar]
- 78.Carlson ML, Tveiten OV, Driscoll CL, Goplen FK, Neff BA, Pollock BE, et al. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg. (2015) 122(4):833–42. 10.3171/2014.11.JNS14594 [DOI] [PubMed] [Google Scholar]
- 79.Carlson ML, Tombers NM, Kerezoudis P, Celda MP, Lohse CM, Link MJ. Quality of life within the first 6 months of vestibular schwannoma diagnosis with implications for patient counseling. Otol Neurotol. (2018) 39(10):e1129–e36. 10.1097/MAO.0000000000001999 [DOI] [PubMed] [Google Scholar]
- 80.Pollock BE, Driscoll CL, Foote RL, Link MJ, Gorman DA, Bauch CD, et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. (2006) 59(1):77–85; discussion 77–85. 10.1227/01.NEU.0000219217.14930.14 [DOI] [PubMed] [Google Scholar]
- 81.Nishiyama T, Oishi N, Kojima T, Kasuya K, Noguchi M, Ishikawa T, et al. Validation and multidimensional analysis of the Japanese penn acoustic neuroma quality-of-life scale. Laryngoscope. (2020) 130(12):2885–90. 10.1002/lary.28488 [DOI] [PubMed] [Google Scholar]
- 82.Berkowitz O, Han YY, Talbott EO, Iyer AK, Kano H, Kondziolka D, et al. Gamma knife radiosurgery for vestibular schwannomas and quality of life evaluation. Stereotact Funct Neurosurg. (2017) 95(3):166–73. 10.1159/000472156 [DOI] [PubMed] [Google Scholar]
- 83.Oddon PA, Montava M, Salburgo F, Collin M, Vercasson C, Lavieille JP. Conservative treatment of vestibular schwannoma: growth and penn acoustic neuroma quality of life scale in French language. Acta Otorhinolaryngol Ital. (2017) 37(4):320–7. 10.14639/0392-100X-1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deberge S, Meyer A, Le Pabic E, Peigne L, Morandi X, Godey B. Quality of life in the management of small vestibular schwannomas: observation, radiotherapy and microsurgery. Clin Otolaryngol. (2018) 43(6):1478–86. 10.1111/coa.13203 [DOI] [PubMed] [Google Scholar]
- 85.Breivik CN, Varughese JK, Wentzel-Larsen T, Vassbotn F, Lund-Johansen M. Conservative management of vestibular schwannoma–a prospective cohort study: treatment, symptoms, and quality of life. Neurosurgery. (2012) 70(5):1072–80; discussion 80. 10.1227/NEU.0b013e31823f5afa [DOI] [PubMed] [Google Scholar]
- 86.Wangerid T, Bartek J, Svensson M, Förander P. Long-term quality of life and tumour control following gamma knife radiosurgery for vestibular schwannoma. Acta Neurochir (Wien). (2014) 156(2):389–96. 10.1007/s00701-013-1924-5 [DOI] [PubMed] [Google Scholar]
- 87.Vogel JJ, Godefroy WP, van der Mey AG, le Cessie S, Kaptein AA. Illness perceptions, coping, and quality of life in vestibular schwannoma patients at diagnosis. Otol Neurotol. (2008) 29(6):839–45. 10.1097/MAO.0b013e3181820246 [DOI] [PubMed] [Google Scholar]
- 88.Medina MD, Carrillo A, Polo R, Fernandez B, Alonso D, Vaca M, et al. Validation of the penn acoustic neuroma quality-of-life scale (PANQOL) for spanish-speaking patients. Otolaryngol Head Neck Surg. (2017) 156(4):728–34. 10.1177/0194599816688640 [DOI] [PubMed] [Google Scholar]
- 89.Del Río L, Lassaletta L, Díaz-Anadón A, Alfonso C, Roda JM, Gavilán J. Tinnitus and quality of life following vestibular schwannoma surgery. B-ENT. (2012) 8(3):167–71. [PubMed] [Google Scholar]
- 90.Stavas MJ, Carlson ML, Attia A, Jacobson GP, Rivas A, Morales-Paliza M, et al. Does radiation dose to the vestibule predict change in balance function and patient perceived dizziness following stereotactic radiotherapy for vestibular schwannoma? Am J Otolaryngol. (2014) 35(5):565–71. 10.1016/j.amjoto.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 91.Hrubá S, Chovanec M, Čada Z, Balatková Z, Fík Z, Slabý K, et al. The evaluation of vestibular compensation by vestibular rehabilitation and prehabilitation in short-term postsurgical period in patients following surgical treatment of vestibular schwannoma. Eur Arch Otorhinolaryngol. (2019) 276(10):2681–9. 10.1007/s00405-019-05503-8 [DOI] [PubMed] [Google Scholar]
- 92.Kelleher MO, Fernandes MF, Sim DW, O'Sullivan MG. Health-related quality of life in patients with skull base tumours. Br J Neurosurg. (2002) 16(1):16–20. 10.1080/02688690120114183 [DOI] [PubMed] [Google Scholar]
- 93.Cheng S, Naidoo Y, da Cruz M, Dexter M. Quality of life in postoperative vestibular schwannoma patients. Laryngoscope. (2009) 119(11):2252–7. 10.1002/lary.20217 [DOI] [PubMed] [Google Scholar]
- 94.Nicoucar K, Momjian S, Vader JP, De Tribolet N. Surgery for large vestibular schwannomas: how patients and surgeons perceive quality of life. J Neurosurg. (2006) 105(2):205–12. 10.3171/jns.2006.105.2.205 [DOI] [PubMed] [Google Scholar]
- 95.Ribeyre L, Spitz E, Frère J, Gauchard G, Parietti-Winkler C. Correlations between postural control and psychological factors in vestibular schwannoma patients. J Vestib Res. (2016) 26(4):387–94. 10.3233/VES-160588 [DOI] [PubMed] [Google Scholar]
- 96.van Leeuwen BM, Herruer JM, Putter H, van der Mey AG, Kaptein AA. The art of perception: patients drawing their vestibular schwannoma. Laryngoscope. (2015) 125(12):2660–7. 10.1002/lary.25386 [DOI] [PubMed] [Google Scholar]
- 97.Lassaletta L, Alfonso C, Del Rio L, Roda JM, Gavilan J. Impact of facial dysfunction on quality of life after vestibular schwannoma surgery. Ann Otol Rhinol Laryngol. (2006) 115(9):694–8. 10.1177/000348940611500908 [DOI] [PubMed] [Google Scholar]
- 98.Iyer AP, Gunn R, Sillars H. Quality of life after vestibular schwannoma surgery: does hearing preservation make a difference? J Laryngol Otol. (2010) 124(4):370–3. 10.1017/S0022215109992040 [DOI] [PubMed] [Google Scholar]
- 99.Myrseth E, Møller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. (2009) 64(4):654–61; discussion 61–3. 10.1227/01.NEU.0000340684.60443.55 [DOI] [PubMed] [Google Scholar]
- 100.Lee J, Fung K, Lownie SP, Parnes LS. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg. (2007) 133(1):56–60. 10.1001/archotol.133.1.56 [DOI] [PubMed] [Google Scholar]
- 101.Sandooram D, Grunfeld EA, McKinney C, Gleeson MJ. Quality of life following microsurgery, radiosurgery and conservative management for unilateral vestibular schwannoma. Clin Otolaryngol Allied Sci. (2004) 29(6):621–7. 10.1111/j.1365-2273.2004.00881.x [DOI] [PubMed] [Google Scholar]
- 102.Presutti L, Magnaguagno F, Pavesi G, Cunsolo E, Pinna G, Alicandri-Ciufelli M, et al. Combined endoscopic-microscopic approach for vestibular schwannoma removal: outcomes in a cohort of 81 patients. Acta Otorhinolaryngol Ital. (2014) 34(6):427–33. [PMC free article] [PubMed] [Google Scholar]
- 103.Lloyd SK, Kasbekar AV, Baguley DM, Moffat DA. Audiovestibular factors influencing quality of life in patients with conservatively managed sporadic vestibular schwannoma. Otol Neurotol. (2010) 31(6):968–76. 10.1097/MAO.0b013e3181e8c7cb [DOI] [PubMed] [Google Scholar]
- 104.da Cruz MJ, Moffat DA, Hardy DG. Postoperative quality of life in vestibular schwannoma patients measured by the SF36 health questionnaire. Laryngoscope. (2000) 110(1):151–5. 10.1097/00005537-200001000-00027 [DOI] [PubMed] [Google Scholar]
- 105.Broomfield SJ, Mandavia AK, Nicholson JS, Mahmoud O, King AT, Rutherford SA, et al. Long-term quality of life following vestibular schwannoma excision via the translabyrinthine approach. Otol Neurotol. (2017) 38(8):1165–73. 10.1097/MAO.0000000000001507 [DOI] [PubMed] [Google Scholar]
- 106.Godefroy WP, Kaptein AA, Vogel JJ, van der Mey AG. Conservative treatment of vestibular schwannoma: a follow-up study on clinical and quality-of-life outcome. Otol Neurotol. (2009) 30(7):968–74. 10.1097/MAO.0b013e3181b4e3c9 [DOI] [PubMed] [Google Scholar]
- 107.Foley RW, Maweni RM, Jaafar H, McConn Walsh R, Javadpour M, Rawluk D. The impact of primary treatment strategy on the quality of life in patients with vestibular schwannoma. World Neurosurg. (2017) 102:111–6. 10.1016/j.wneu.2017.02.087 [DOI] [PubMed] [Google Scholar]
- 108.van Leeuwen BM, Borst JM, Putter H, Jansen JC, van der Mey AG, Kaptein AA. Emotional intelligence in association with quality of life in patients recently diagnosed with vestibular schwannoma. Otol Neurotol. (2014) 35(9):1650–7. 10.1097/MAO.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 109.Scholtes C, Baust K, Weinhold L, Creutzig U, Gnekow A, Hinz A, et al. Health status, health-related quality of life, and socioeconomic outcome in childhood brain tumor survivors: a German cohort study. Neuro Oncol. (2019) 21(8):1069–81. 10.1093/neuonc/noz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Godefroy WP, Hastan D, van der Mey AG. Translabyrinthine surgery for disabling vertigo in vestibular schwannoma patients. Clin Otolaryngol. (2007) 32(3):167–72. 10.1111/j.1365-2273.2007.01427.x [DOI] [PubMed] [Google Scholar]
- 111.Pintea B, Kandenwein JA, Lorenzen H, Boström JP, Daher F, Velazquez V, et al. Factors of influence upon the SF-36-based health related quality of life of patients following surgery for petroclival and lateral posterior surface of pyramid meningiomas. Clin Neurol Neurosurg. (2018) 166:36–43. 10.1016/j.clineuro.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 112.Park SS, Grills IS, Bojrab D, Pieper D, Kartush J, Maitz A, et al. Longitudinal assessment of quality of life and audiometric test outcomes in vestibular schwannoma patients treated with gamma knife surgery. Otol Neurotol. (2011) 32(4):676–9. 10.1097/MAO.0b013e3182138fc5 [DOI] [PubMed] [Google Scholar]
- 113.van Leeuwen BM, Herruer JM, Putter H, Jansen JC, van der Mey AG, Kaptein AA. Validating the penn acoustic neuroma quality of life scale in a sample of Dutch patients recently diagnosed with vestibular schwannoma. Otol Neurotol. (2013) 34(5):952–7. 10.1097/MAO.0b013e31828bb2bb [DOI] [PubMed] [Google Scholar]
- 114.Dhayalan D, Lund-Johansen M, Finnkirk M, Tveiten Ø. Fatigue in patients with vestibular schwannoma. Acta Neurochir (Wien). (2019) 161(9):1809–16. 10.1007/s00701-019-04003-2 [DOI] [PubMed] [Google Scholar]
- 115.Carlson ML, Tveiten Ø, Yost KJ, Lohse CM, Lund-Johansen M, Link MJ. The minimal clinically important difference in vestibular schwannoma quality-of-life assessment: an important step beyond P < 0.05. Otolaryngol Head Neck Surg. (2015) 153(2):202–8. 10.1177/0194599815585508 [DOI] [PubMed] [Google Scholar]
- 116.Soulier G, van Leeuwen BM, Putter H, Jansen JC, Malessy MJA, van Benthem PPG, et al. Quality of life in 807 patients with vestibular schwannoma: comparing treatment modalities. Otolaryngol Head Neck Surg. (2017) 157(1):92–8. 10.1177/0194599817695800 [DOI] [PubMed] [Google Scholar]
- 117.Betchen SA, Walsh J, Post KD. Self-assessed quality of life after acoustic neuroma surgery. J Neurosurg. (2003) 99(5):818–23. 10.3171/jns.2003.99.5.0818 [DOI] [PubMed] [Google Scholar]
- 118.Tufarelli D, Meli A, Alesii A, De Angelis E, Badaracco C, Falcioni M, et al. Quality of life after acoustic neuroma surgery. Otol Neurotol. (2006) 27(3):403–9. 10.1097/00129492-200604000-00018 [DOI] [PubMed] [Google Scholar]
- 119.Robinett ZN, Walz PC, Miles-Markley B, Moberly AC, Welling DB. Comparison of long-term quality-of-life outcomes in vestibular schwannoma patients. Otolaryngol Head Neck Surg. (2014) 150(6):1024–32. 10.1177/0194599814524531 [DOI] [PubMed] [Google Scholar]
- 120.Nikolopoulos TP, Johnson I, O'Donoghue GM. Quality of life after acoustic neuroma surgery. Laryngoscope. (1998) 108(9):1382–5. 10.1097/00005537-199809000-00024 [DOI] [PubMed] [Google Scholar]
- 121.Baumann I, Polligkeit J, Blumenstock G, Mauz PS, Zalaman IM, Maassen MM. Quality of life after unilateral acoustic neuroma surgery via middle cranial fossa approach. Acta Otolaryngol. (2005) 125(6):585–91. 10.1080/00016480510026935 [DOI] [PubMed] [Google Scholar]
- 122.Al-Shudifat AR, Kahlon B, Höglund P, Lindberg S, Magnusson M, Siesjo P. A patient-assessed morbidity to evaluate outcome in surgically treated vestibular schwannomas. World Neurosurg. (2016) 94:544–50.e2. 10.1016/j.wneu.2016.07.043 [DOI] [PubMed] [Google Scholar]
- 123.Riffaud L, Saikali S, Leray E, Hamlat A, Haegelen C, Vauleon E, et al. Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg. (2009) 111(3):478–87. 10.3171/2009.1.JNS081004 [DOI] [PubMed] [Google Scholar]
- 124.Henzel M, Hamm K, Gross MW, Surber G, Kleinert G, Failing T, et al. Fractionated stereotactic radiotherapy of glomus jugulare tumors. Local control, toxicity, symptomatology, and quality of life. Strahlenther Onkol. (2007) 183(10):557–62. 10.1007/s00066-007-1701-6 [DOI] [PubMed] [Google Scholar]
- 125.King S, Exley J, Parks S, Ball S, Bienkowska-Gibbs T, MacLure C, et al. The use and impact of quality of life assessment tools in clinical care settings for cancer patients, with a particular emphasis on brain cancer: insights from a systematic review and stakeholder consultations. Qual Life Res. (2016) 25(9):2245–56. 10.1007/s11136-016-1278-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. (1995) 273(1):59–65. 10.1001/jama.1995.03520250075037 [DOI] [PubMed] [Google Scholar]
- 127.Wiebe S, Guyatt G, Weaver B, Matijevic S, Sidwell C. Comparative responsiveness of generic and specific quality-of-life instruments. J Clin Epidemiol. (2003) 56(1):52–60. 10.1016/S0895-4356(02)00537-1 [DOI] [PubMed] [Google Scholar]
- 128.Frendl DM, Ware JE. Patient-reported functional health and well-being outcomes with drug therapy: a systematic review of randomized trials using the SF-36 health survey. Med Care. (2014) 52(5):439–45. 10.1097/MLR.000000000000010311 [DOI] [PubMed] [Google Scholar]
- 129.Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP. Reporting and interpretation of SF-36 outcomes in randomised trials: systematic review. Br Med J. (2009) 338:a3006. 10.1136/bmj.a3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wiegand DA, Fickel V. Acoustic neuroma–the patient's perspective: subjective assessment of symptoms, diagnosis, therapy, and outcome in 541 patients. Laryngoscope. (1989) 99(2):179–87. 10.1288/00005537-198902000-00010 [DOI] [PubMed] [Google Scholar]
- 131.Hawthorne G. Perceived social isolation in a community sample: its prevalence and correlates with aspects of peoples’ lives. Soc Psychiatry Psychiatr Epidemiol. (2008) 43(2):140–50. 10.1007/s00127-007-0279-8 [DOI] [PubMed] [Google Scholar]
- 132.Gierveld JDJ, Tilburg TV. A 6-item scale for overall, emotional and social loneliness: confirmatory tests on survey data. Research on Aging. (2006) 28(5):82–98. 10.1177/0164027506289723 [DOI] [Google Scholar]
- 133.Ware JE, Gandek B, Guyer R, Deng N. Standardizing disease-specific quality of life measures across multiple chronic conditions: development and initial evaluation of the QOL disease impact scale (QDIS®). Health Qual Life Outcomes. (2016) 14:84. 10.1186/s12955-016-0483-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. (1989) 27(3 Suppl):S217–32. 10.1097/00005650-198903001-00018 [DOI] [PubMed] [Google Scholar]
- 135.Sprangers MA, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European organization for research and treatment of cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC study group on quality of life. Qual Life Res. (1993) 2(4):287–95. 10.1007/BF00434800 [DOI] [PubMed] [Google Scholar]
- 136.Ware JE, Guyer R, Harrington M, Strom M, Boulanger R. Evaluation of a more comprehensive survey item bank of standardizing disease-specific impact comparisons across chronic conditions. Qual Life Res. (2012) 21:27–8. [Google Scholar]
- 137.King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res. (2011) 11(2):171–84. 10.1586/erp.11.9 [DOI] [PubMed] [Google Scholar]
- 138.Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis. (1987) 40(2):171–8. 10.1016/0021-9681(87)90069-5 [DOI] [PubMed] [Google Scholar]
- 139.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. (2005) 28(2):172–91. 10.1177/0163278705275340 [DOI] [PubMed] [Google Scholar]
- 140.Gargon E, Gorst SL, Williamson PR. Choosing important health outcomes for comparative effectiveness research: 5th annual update to a systematic review of core outcome sets for research. PLoS One. (2019) 14(12):e0225980. 10.1371/journal.pone.0225980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fountain DM, Allen D, Joannides AJ, Nandi D, Santarius T, Chari A. Reporting of patient-reported health-related quality of life in adults with diffuse low-grade glioma: a systematic review. Neuro Oncol. (2016) 18(11):1475–86. 10.1093/neuonc/now107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alexander W. The uphill path to successful clinical trials: keeping patients enrolled. Pharm Ther. (2013) 38(4):225–7. [PMC free article] [PubMed] [Google Scholar]
- 143.Erharter A, Giesinger J, Kemmler G, Schauer-Maurer G, Stockhammer G, Muigg A, et al. Implementation of computer-based quality-of-life monitoring in brain tumor outpatients in routine clinical practice. J Pain Symptom Manage. (2010) 39(2):219–29. 10.1016/j.jpainsymman.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 144.Deschler DG, Walsh KA, Friedman S, Hayden RE. Quality of life assessment in patients undergoing head and neck surgery as evaluated by lay caregivers. Laryngoscope. (1999) 109(1):42–6. 10.1097/00005537-199901000-00009 [DOI] [PubMed] [Google Scholar]
- 145.Sneeuw KC, Aaronson NK, Osoba D, Muller MJ, Hsu MA, Yung WK, et al. The use of significant others as proxy raters of the quality of life of patients with brain cancer. Med Care. (1997) 35(5):490–506. 10.1097/00005650-199705000-00006 [DOI] [PubMed] [Google Scholar]
- 146.Gil Z, Fliss DM. Quality of life in patients with skull base tumors: current status and future challenges. Skull Base. (2010) 20(1):11–8. 10.1055/s-0029-1242979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thomeer H, Bonnard D, Franco-Vidal V, Porez F, Darrouzet P, Liguoro D, et al. Prognostic factors of balance quality after transpetrosal vestibular schwannoma microsurgery. Otol Neurotol. (2015) 36(5):886–91. 10.1097/MAO.0000000000000740 [DOI] [PubMed] [Google Scholar]
- 148.Bellamy N, Carette S, Ford PM, Kean WF, Le Riche NG, Lussier A, et al. Osteoarthritis antirheumatic drug trials: iII, setting the delta for clinical trials: results of a consensus development (Delphi) exercise. J Rheumatol. (1992) 19(3):451–7. [PubMed] [Google Scholar]
- 149.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. (2005) 64(1):29–33. 10.1136/ard.2004.022905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Osoba D, Aaronson NK, Muller M, Sneeuw K, Hsu MA, Yung WK, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. (1996) 5:139–50. 10.1007/BF00435979 [DOI] [PubMed] [Google Scholar]
- 151.Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. 3rd ed. Oxford, UK: Oxford University Press; (2003). [Google Scholar]
- 152.Bunevicius A. Reliability and validity of the SF-36 health survey questionnaire in patients with brain tumors: a cross-sectional study. Health Qual Life Outcomes. (2017) 15(1):92. 10.1186/s12955-017-0665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.