Abstract
The detection of Cryptosporidium oocysts in drinking water is critically dependent on the quality of immunofluorescent reagents. Experiments were performed to develop a method for producing highly specific antibodies to Cryptosporidium oocysts that can be used for water testing. BALB/c mice were immunized with six different antigen preparations and monitored for immunoglobulin G (IgG) and IgM responses to the surface of Cryptosporidium oocysts. One group of mice received purified oocyst walls, a second group received a soluble protein preparation extracted from the outside of the oocyst wall, and the third group received whole inactivated oocysts. Three additional groups were immunized with sequentially prepared oocyst extracts to provide for a comparison of the immune response. Mice injected with the soluble protein extract demonstrated an IgG response to oocysts surface that was not seen in the whole-oocyst group. Mice injected with whole oocysts showed an IgM response only, while mice injected with purified oocyst walls showed little increase in IgM or IgG levels. Of the additional reported preparations only one, BME (2-mercaptoethanol treated), produced a weak IgM response to the oocyst wall. A mouse from the soluble oocyst extract group yielding a high IgG response was utilized to produce a highly specific IgG1 monoclonal antibody (Cry104) specific to the oocyst surface. Comparative flow cytometric analysis indicated that Cry104 has a higher avidity and specificity to oocysts in water concentrates than other commercially available antibodies.
Cryptosporidium parvum is a parasitic protozoan (coccidium) which is among the most common causes of diarrheal disease in humans (14). Cryptosporidium oocysts are environmentally robust and can survive in aquatic environments for months (17). These oocysts are also resistant to standard chlorination disinfection used for drinking water treatment (9, 17). It is a common waterborne disease in western countries, where it accounts for 1 to 2% of all cases (21), with as few as 30 ingested Cryptosporidium oocysts causing (4, 20) a profuse watery diarrhea. Infection in immunocompromised individuals is severe and prolonged (3).
The detection of low levels of Cryptosporidium in environmental waters is extremely difficult. Most routinely used detection methods rely on antibodies to separate oocysts from debris using techniques such as flow cytometry (22) or immunomagnetic separation (1). The fluorescently labeled oocysts are then enumerated using epifluorescent microscopy or flow cytometry. However, the separation and detection of oocysts is limited by the specificity of available monoclonal antibodies (MAbs) (23).
All currently available Cryptosporidium-specific MAbs are either of the immunoglobulin M (IgM) or IgG3 subclasses (25), which have been developed for detection in fecal material rather than for water analysis. IgM MAbs are known to be larger and more “sticky” than MAbs of the IgG1 subclass (18). In water these MAbs bind nonspecifically to algal and mineral particles, resulting in substantial background fluorescence and false-positive results (24). MAbs of the IgG1 subclass are usually higher affinity and less sticky, thus greatly reducing nonspecific binding and cross-reactivity with other organisms (18). Another advantage of MAbs of the IgG1 subclass is the ease of purification by methods such as protein A precipitation (5).
In order to obtain an IgG1 MAb, it is essential to obtain a strong IgG response to the oocyst surface. In previous studies, immunization with whole oocysts produced MAbs predominantly to the sporozoite and a few to the oocyst wall (12). Removal of sporozoites from antigen preparations may reduce this predominant IgM response. The structure of the oocyst wall is rich in complex polysaccharides (11), and this may cause an IgM response. Antigen structure as well as concentration plays a key role in inducing an immune response (7). Therefore, removal of sporozoites and reduction of antigen size and structure were considered key factors to be taken into account when preparing antigens. In addition, sequentially extracted oocyst antigens (8) were used to give an idea of the immunogenicity of different oocyst wall preparations.
In this study the immune response to six oocyst preparations was evaluated. After the induction of a strong secondary IgG response in one group (soluble oocyst extract), a subsequent fusion produced a highly specific IgG1 MAb (Cry104) to the oocyst wall of C. parvum.
MATERIALS AND METHODS
Oocyst purification.
Fecal samples positive for C. parvum were obtained from naturally infected calves in Sydney, Australia. The feces were diluted approximately 1:4 in water and centrifuged at 5,000 × g for 10 min. The liquid layer was then discarded, the pellet was resuspended again in water, and the procedure was repeated. Fatty materials were then removed by resuspending the pellet in ice-cold 1% NaHCO3 solution, adding an ice-cold ether layer and centrifuging the mixture at 5,000 × g for 10 min. After centrifugation, the supernatant containing the fat plug was discarded, the pellet was resuspended in ice-cold 1% (wt/vol) NaHCO3 solution and passed through a layer of prewetted nonabsorbent cotton wool, and the ether extraction step was repeated. After final centrifugation, the pellet was resuspended in 40 ml of ice-cold 55% (wt/vol) sucrose solution. Then, 10 ml of ice-cold H2O was slowly added, assuring two layers were formed, and the sample was centrifuged at 4,000 × g for 20 min. Oocysts were collected from the surface interface, and the sucrose flotation step was repeated until no visible contaminating material could be detected. Purified oocysts were surface sterilized with ice-cold 70% (vol/vol) ethanol for 30 min, washed once in phosphate-buffered saline (PBS; Oxoid), and stored in PBS at 4°C.
Purified oocyst wall.
C. parvum oocyst walls were purified from excysted oocysts using immunomagnetic separation (IMS). Freshly purified oocysts were excysted (16), and the percentage of excystation was determined by flow cytometry (25). Only samples with >99.5% empty oocysts were processed further.
Anti-mouse IgM IMS beads (Dynal, Oslo, Norway) were coated with an IgM MAb (Cry26) specific to the oocyst wall of C. parvum (23) according to the manufacturer's instructions. Then, 1 ml of beads (108) was mixed with 1 ml of excysted oocysts (109) and incubated at 4°C for 30 min. The beads coated with oocysts were then concentrated using a magnetic concentrator (Dynal), and the supernatant containing sporozoites was removed. The beads were gently washed in 1 ml of PBS plus 1% (wt/vol) bovine serum albumin (BSA) for 30 min. The beads were magnetically concentrated once more and vigorously vortexed to dissociate the oocyst wall bead complexes, and the beads were magnetically removed. The supernatant contained the oocyst cells. The procedure was repeated until no contaminating particles (e.g., sporozoites) could be detected by flow cytometry. A total of 5 × 108 oocyst walls were collected, aliquoted, and frozen at −20°C.
Soluble oocyst extract.
Oocysts suspended at 109/ml in 0.5% (wt/vol) sodium dodecyl sulfate (SDS) were boiled for 1 h. The sample was centrifuged 13,000 × g for 10 min, and the supernatant was precipitated with 5 volumes of acetone at −20°C overnight. After centrifugation (10 min at 13,000 × g), a small white precipitate was resuspended in sterile PBS. A total of 109 oocysts yielded roughly 600 to 1,200 μg of acetone precipitate, measured using the Bio-Rad (Hercules, Calif.) DC protein assay with BSA as a standard.
Additional preparations.
To give a comparative measure of the immune response, three extracts of oocysts as described by Hornok et al. (8) originally used to orally inoculate chickens were also used for immunization. Briefly, three sequential extraction preparations were prepared. Oocysts were first subjected to freeze-thawing in liquid nitrogen to produce “oocyst cytosol” antigen (OCA). The insoluble material was then treated with Triton X-114 to dissolve membrane-bound proteins (TRE). The remaining insoluble oocyst material was then solubilized in SDS and 2-mercaptoethanol (BME).
Immunization.
All mice were initially tail bled to obtain preimmune control serum. Groups of five BALB/c (ARC) female mice 8 to 12 weeks old were immunized by intraperitoneal injection (i.p.) with either an oocyst wall preparation, whole oocysts, soluble protein extract, or the three preparations described by Hornok et al. (8). Each preparation in PBS was emulsified with an equal volume of Freund complete adjuvant. The whole-oocyst control group received 4 × 106 whole heat-inactivated oocysts (80°C, 30 min), and the oocyst wall mice received oocysts walls at 4 × 104/ml. Groups of mice receiving the soluble protein extracts received between 50 and 80 μg of protein.
A second i.p. injection (100 μl) with the same preparations, emulsified in Freund incomplete adjuvant (FIA) were given after 3 weeks. Mice were bled 3 weeks after the second injection to check for immune response. Mice showing strong IgG immune responses were selected for fusions and given two final boosts, one given i.p. 5 days and another given intravenously in PBS 3 days prior to the fusion.
Mouse serum IgM and IgG levels.
Blood collected from tail bleeds was centrifuged at 13,000 × g for 1 min, and the top layer of serum was stored at −20°C.
Serum was tested for immunofluorescence at 1:103, 1:104, and 1:105 dilutions in PBS containing 1% (wt/vol) BSA.
An aliquot (100 μl) of each serum dilution was added to 10 μl of intact oocyst suspension containing 107 oocysts/ml and incubated for 15 min at room temperature. To determine IgG and IgM concentrations, 100 μl of prediluted labeled, anti-IgG (Zymed 61-6011) (1:100) or FITC-labeled anti-IgM (Sigma F-9259) (1:50) was added to the samples for 15 min at room temperature and then analyzed by flow cytometry. The fluorescence intensity of 2,000 oocysts was measured for both IgG and IgM levels at each serum dilution. Negative controls of PBS or preimmune serum and a positive control of a MAb (Cry26) specific to the surface of Cryptosporidium oocysts was analyzed with each batch of samples.
Fusion procedure.
The fusion procedure employed was that as described by Pererva (15).
Hybridoma screening.
Approximately 7 to 14 days after the fusion, the hybridomas visible at ×400 were marked, and 100 μl of tissue culture supernatant was aseptically removed.
To each hybridoma supernatant, 10 μl of 107 oocysts/ml was added and incubated for 15 min at room temperature. A second FITC-labeled anti-mouse antibody (Amrad) was then added (100 μl at 1:50 dilution in 1% [wt/vol] BSA in PBS) and incubated at room temperature for 15 min. Oocysts were analyzed by flow cytometry with high fluorescence indicating supernatant containing anti-Cryptosporidium antibodies. Hybridomas were tested for anti-Cryptosporidium antibodies of IgM or IgG classes, as described for mouse serum antibodies. All positive hybridomas were tested for isotype using a commercial hemagglutination assay (Serotec).
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
Oocysts (5 × 107 to 5 × 108/ml) were added to an equal volume of reducing buffer (0.125 M Tris HCl, 3% [wt/vol] SDS, 10% [vol/vol] glycerol, 5% [vol/vol] 2-mercaptoethanol, and 0.02% [wt/vol] bromophenol blue) and boiled for 3 min at 100°C. This reduced sample was then run on a 12% polyacrylamide separating gel with a 5% stacking gel in a Bio-Rad Miniprotean II cell apparatus at 200 V. High- and low-molecular-weight markers (Novex) were run alongside the sample.
SDS-PAGE gels were electroblotted onto nitrocellulose (Microfiltration Systems) employing a wet blotting system (10) at 12 V for 1 h. The nitrocellulose was cut into strips, and each strip was blocked in 2% (wt/vol) milk in PBS. Strips were incubated with mouse serum (diluted 1:1,000) or anti-Cryptosporidium antibodies (i.e., Cry26 at 10 μg/ml) for 1 h at room temperature. After the strips were washed three times in PBS, they were incubated in an alkaline phosphatase-conjugated anti-mouse antibody (Cappel) diluted 1:20 in 2% (wt/vol) milk in PBS and then developed with 0.5 ml of 4-chloro-1-naphthol and 10 μl of H2O2 in 2 ml of PBS.
Oocyst staining in water samples.
The effectiveness of MAbs for analysis of water samples was evaluated by flow cytometry. Samples (10 liters) of untreated surface water were collected throughout Australia and then concentrated by flocculation (22). A composite water sample was prepared by mixing aliquots from a range of different sites. C. parvum seeded samples consisted of 50 μl of untreated water concentrate and 10 μl of 108 oocyst seeds. Then, 100 μl of hybridoma supernatant (2 μg/ml) was incubated with the seeded samples at room temperature for 20 min, and 100 μl of anti-mouse FITC-conjugated antibody (Silenus; 1:100) was added for a further 20 min. Samples were analyzed by flow cytometry to determine which antibody produced the greatest separation between the immunofluorescent oocyst population and the background fluorescent particles within the water concentrates.
Functional measurement of avidity.
Avidity of binding of the FITC-labeled Cryptosporidium-specific antibodies Cry104, Cry26, and Immucell (Immucell, Portland, Oreg.) were estimated as follows. Pure antibodies (20 μg/ml) were serially diluted for 20 double dilutions. Each dilution was then incubated with 107 oocysts for 20 min at room temperature. A negative control of unlabeled oocysts in PBS was also prepared to provide an endpoint for binding. Fluorescence values for each dilution were recorded and plotted to obtain the value for 50% maximal binding to the oocysts. Assumptions were made that the total input antibody is very nearly the same as free antibody; therefore, the dissociation constant (Kd) is proportional to this 50% concentration. The relative affinity (Ka) is then calculated as the reciprocal value.
RESULTS
Antibody response to C. parvum oocyst surface epitopes.
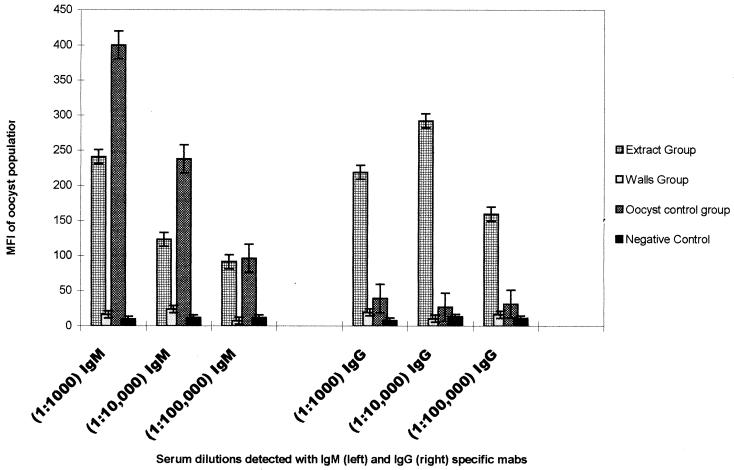
Mouse sera were tested by flow cytometry for IgG and IgM antibodies to the surface of oocysts (Fig. 1). Mice receiving two injections of purified oocyst walls showed little or no increase in either IgM or IgG against the oocyst wall. The whole-oocyst control mice responded with increased IgM levels but produced little or no IgG response. In contrast, the soluble-protein-extract group produced higher IgG levels than that of the whole-oocyst control group, but with less IgM response. Additional immunization of mice receiving the protein extract further increased IgG levels, with a drop in IgM levels (data not shown). Further immunization of the whole-oocyst control group still produced a predominant IgM response, with no increase in IgG levels, and the oocyst wall group produced no IgG or IgM response. Of the three extracts (8), only one (BME) gave a weak IgM response to the oocyst wall. The highest-response soluble-protein extract mouse was chosen for the fusion procedure. This analysis resulted in the production of nine MAbs against the oocyst wall: eight IgMs and one the IgG1 MAb Cry104.
FIG. 1.
Comparison of the fluorescence intensities of C. parvum oocysts stained with sera of three dilutions (1:1,000, 1:10,000, and 1:100,000) from the soluble-extract, oocyst wall, or whole-oocyst control groups that were then stained with an anti-IgG or an anti-IgM fluorescently labeled antibody. Data are calculated by subtracting the fluorescent value (arb) obtained from mouse serum samples after two immunizations from the preimmune serum samples from serum samples from individual mice. MFI data obtained from each group were then averaged, and the standard deviations were calculated.
Analysis of antibodies for oocyst staining in water samples.
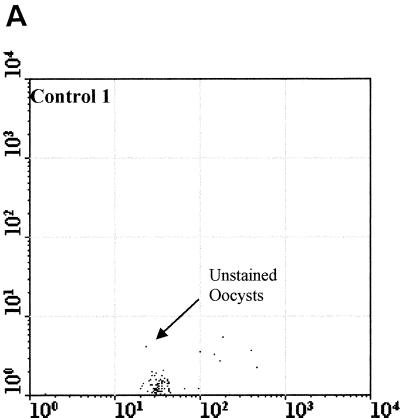
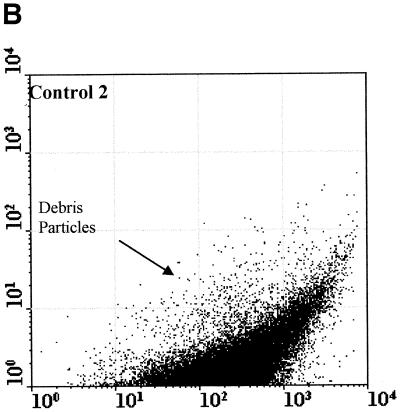
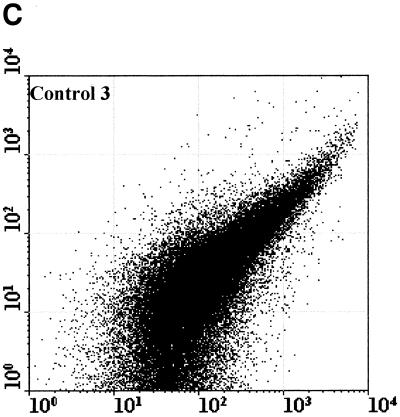
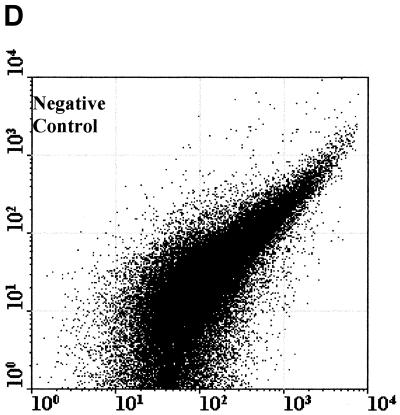
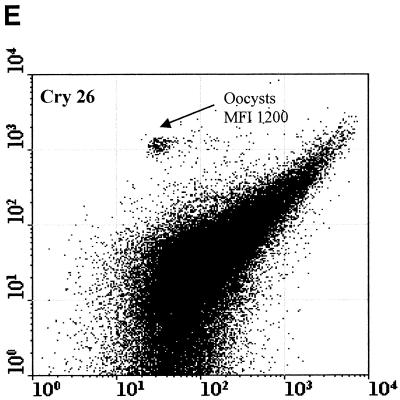
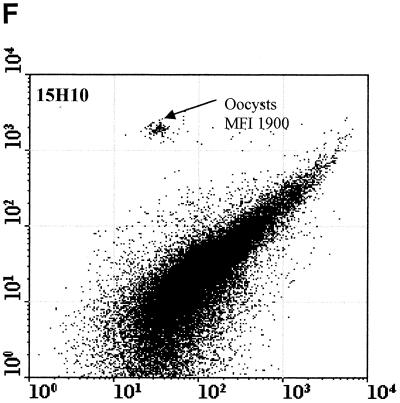
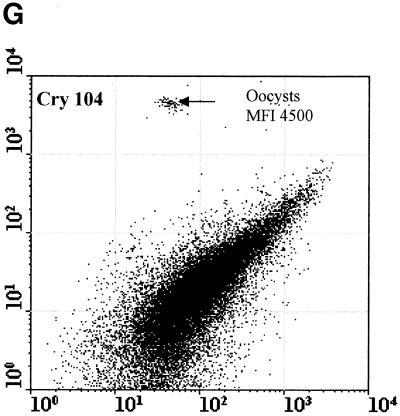
Figure 2 compared three MAbs (2 μg/ml) for the differentiation of C. parvum oocysts and particles in concentrates from environmental water samples. Cry104, the IgG1 antibody produced for this report, gave the greatest separation of oocysts from particles present in water concentrates and the highest mean fluorescence intensity (MFI) of the oocyst population (i.e., 4,500). Oocysts stained with Cry104 also had formed a tight group with little scatter, indicating constant levels of this antigen on each oocyst and reproducible fluorescence staining. The MAb Cry26 is an IgM routinely used for examination of water samples (15), and 15H10 is another IgM MAb generated in our laboratory. Both of the IgM MAbs, Cry26 and 15H10, showed a more dispersed fluorescence population of oocysts than did Cry104 and less separation of the oocyst population from debris in water. This reduced separation corresponds to lower MFI values for the oocyst populations of 1,200 (Cry26) and 1,900 (15H10) compared with an MFI of 4,500 for Cry104.
FIG. 2.
Comparison of antibodies for staining oocysts in water samples. The axes show side-scatter (x axis) versus green-fluorescence (y axis) data. The MFI oocyst population was also recorded. Control 1 (A) is oocysts only, and control 2 (B) is unstained oocyst in a water concentrate. Control 3 (C) is Cry104 with no oocysts in a water concentrate, and the negative control (D) is stained with an anti-Giardia antibody. Note the differences in the separation between the oocysts and the debris particles obtained with different antibodies. Cry26 (E) and 15H10 (F) data are also presented. (G) Cry104 shows the greatest separation of the oocyst population from debris in water samples, with the highest MFI of the oocyst population.
Western blot examination of serum samples and MAbs.
Western blots of sera from mice immunized with whole oocysts (control group), oocyst walls, and soluble extract (Fig. 3) demonstrated an immune response to a large number of bands. The whole-oocyst control group and the soluble-protein-extract sera reacted with multiple bands of from >100 kDa down to 30 kDa as described by Tilley et al. (20).
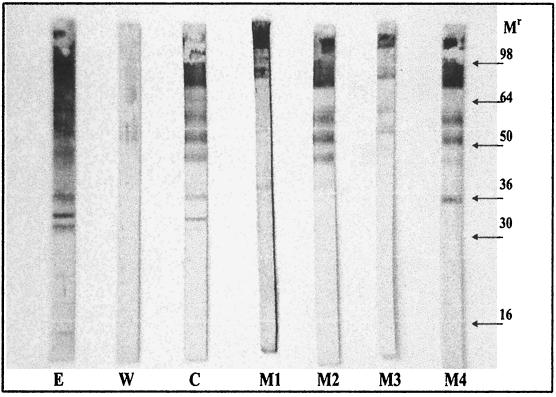
FIG. 3.
Western blot of C. parvum protein extracts probed with mouse serum from the following mouse groups: E, soluble-extract mice; W, oocyst wall mice; and C, oocyst control group (whole oocysts). Results obtained with MAbs are presented as follows: M1, Cry26 (IgM); M2, Cry104 (IgG1); M3, Cry212 (IgM); and M4, Crypto-a-Glo (IgM). Cry26, Cry104, and Cry212 were from Macquarie University Centre for Analytical Biotechnology, and Crypto-a-Glo was from Waterborne, New Orleans, La.
The only distinct difference between the banding patterns was that the soluble-protein-extract group picked up four smaller bands of <30 kDa which were not recognized by the oocyst control group. The soluble-extract group (Fig. 3) showed a more sharply defined banding pattern, which is indicative of high-avidity serum (2). In contrast, the mice receiving purified oocyst walls reacted with only two bands at approximately 52 and 58 kDa. Serum from the three groups, OCA, TRE, and BME, showed banding patterns similar to those previously reported (not shown). The BME banding pattern was very similar to that of the whole-oocyst group. MAbs analyzed by Western blot also reacted with multiple bands, although the patterns varied for each MAb. All MAbs recognized antigens of >100 kDa, and all MAbs tested excluding Cry26 recognized all three of the distinct bands between 64 and 46 kDa. Cry104 and Cry26 recognize a 42-kDa band and Crypto-a-glo band at 36 kDa. These results are consistent with the antibodies recognizing common epitopes present on numerous proteins. Smaller bands located at between 36 to 14 kDa, which reacted with the soluble-extract mouse serum, were not recognized by any MAb, and at present there is no evidence that these proteins are present on the oocyst surface.
All MAbs showed uniform staining of the oocyst wall when tested by fluorescence microscopy. This finding suggests that there is an abundance of epitopes across the oocyst surface. There also maybe subtle differences in the epitopes which the MAbs recognize, as seen in the differences in banding patterns in the Western blots.
Functional measurement of avidity.
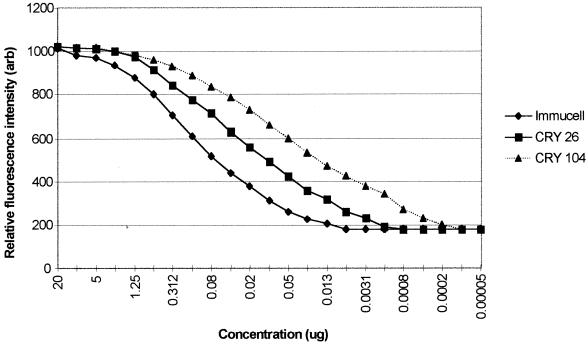
The binding curves of the three MAbs Cry26, Cry104, and Immucell in response to 107 oocysts is shown in Fig. 4. Data obtained from these plots were then used to calculate the relative avidity of the whole MAbs (Table 1). MAb Cry104 possessed the highest avidity, with a 50% binding at less than half the concentration of Cry26. This indicates that Cry104 has the highest avidity to C. parvum oocysts. Twice as much Cry26 was required for 50% binding, and more than 10 times as much of the Immucell antibody was required.
FIG. 4.
Binding curves of the three FITC-labeled MAbs Cry26, Cry104, and Immucell. The oocyst concentration of 107/ml was constant for every antibody dilution. Relative fluorescence intensity values for each of the 20 serial dilution were recorded and plotted. The value for 50% maximal binding was then calculated for each MAb by reading the value off the curve.
TABLE 1.
Functional measure of avidity (Ka) values
Sources: Cry104 and Cry26, Macquarie University Centre for Analytical Biotechnology; Immucell, Immucel, Portland, Oreg.
Ka, affinity constant of whole antibody.
DISCUSSION
During immunization, mice were monitored for both IgM and IgG antibodies against oocyst surface antigens. The whole-oocyst control mice produced a high IgM response, with little or no IgG. This would suggest that there was little T-cell-dependent immune response (i.e., shift to IgG) and that immunization directly stimulated B cells, resulting directly in less isotype switching and reduced affinity maturation in the MAbs produced (7). Hence, IgM antibodies dominate the immune response. This may be due to C. parvum oocysts having a complex polysaccharide at their surface. The presence of sporozoites in this preparation may have also reduced the immungenicity. McDonald et al. (12) showed that the sporozoite is highly immunogenic compared to the oocyst wall, with <10% of the hybridomas against whole oocysts reacting with the oocyst wall. Therefore, sporozoites were removed when preparing both the purified oocyst wall and the soluble extract to maximize the response to the oocyst wall.
The mice receiving purified oocyst walls showed no immune response to surface epitopes and in Western blots reacted only weakly with a restricted number of antigens. Despite the use of Freund adjuvant, the purified oocyst walls were substantially less immunogenic than the inactivated whole oocysts. The purified walls were intact apart from the longitudinal slit that releases the internal contents, so presumably the difference is due to the absence of immunogenic internal antigens. This also suggests that without sporozoites present there is limited immunogenicity, as suggested by Tzipori (21). There is no evidence that excystation could alter or denature the outer epitopes of the oocyst walls, thus making them different from those of naturally occurring oocysts. This is evident since excysted oocysts can still be stained with MAbs against the oocyst wall (25).
The mice receiving soluble oocyst extract demonstrated a moderate IgM and a strong secondary IgG response. After the large oocyst wall structure is broken up into smaller complexes by SDS extraction and then precipitated out the oocyst wall, proteins become T-cell-dependent antigens. After further immunizations with soluble extract (data not shown), the IgG response further increased, while the IgM response decreased. This was not observed in the whole-oocyst control or purified wall group, which showed a predominant IgM response or no response, respectively, after each immunization. A possible explanation for these results is the large (5-μm) size of the oocysts wall being too large for T-cell processing, thus allowing stimulation of low-affinity B cells (i.e., B cells producing IgM).
Antigens used by other workers (8) were also evaluated for the production of antibodies to the oocyst surface. The mice immunized with OCA and TRE preparations showed no response to the oocyst wall. The antisera reacted with internal membrane on excysted oocysts and not the oocyst surface. The BME antigen produced a very weak IgM response against the oocyst wall even though it was solubilized in SDS (2 min) similarly to the soluble oocyst extract. The difference may be due to the sequential preparation of these antigens. Removing the internal proteins could have affected the structure of the BME antigen either by a change in structure or integrity. The lack of immune response to the BME and purified-oocyst-wall antigens demonstrates that the oocyst wall carbohydrate structure is not very immunogenic.
In the Western blot analysis, bands covering a size range of >100 kDa to approximately 36 kDa all carried the same epitopes, as defined by MAb binding. The results are consistent with the epitopes being an oligosaccharide carried on several different proteins. Previous work (13) has demonstrated that epitopes on the oocyst wall are sensitive to sodium meta-periodate treatment, indicating that they have carbohydrate components.
In this study evaluation by fluorescence microscopy showed uniform staining of the oocyst wall, suggesting that a common antigen covers the oocyst wall. Competition studies between the MAbs Cry26 and Cry104 (results not shown) utilizing different fluorescent labels on each MAb demonstrated that these MAbs do recognize a common epitope and compete for binding sites. However, MAbs analyzed by Western blot analysis showed differences in banding patterns, suggesting that they recognize slightly different structures.
The functional avidity of Cry104 was compared with other anti-Cryptosporidium antibodies. The 50% binding level for the IgG1 (Cry104) whole antibody was calculated to be 7 × 108 mol−1, i.e., less than half that of the other antibodies tested (Table 1).
The high avidity of Cry104 is also reflected in the water sample analysis (Fig. 2). Cry104 gave the tightest cluster of oocysts and the best separation from debris in concentrated environmental water samples. Similar findings were reported previously (6). This high signal-to-noise ratio is desirable for water testing by flow cytometry. Increased differentiation of oocysts from debris means fewer nonspecific fluorescent debris particles are detected and analyzed by the cytometer, allowing increased analysis speeds and so reducing analysis time. This will allow analytical laboratories to more accurately and reliably test concentrated water samples for Cryptosporidium spp.
ACKNOWLEDGMENTS
This study was supported by a collaborative Commonwealth Department of Industry, Science and Tourism grant (DIST), with additional financial support from Australian Water Technologies and Becton Dickinson.
We thank Australian Water Technologies for the supply of concentrated water samples. The IgG1 MAb Cry104 is the subject of International Patent Application number PCT/AV98/00368.
REFERENCES
- 1.Bukhari Z, McCuin R M, Fricker C R, Clancy J L. Immunomagnetic separation of Cryptosporidium parvum from source water samples of various turbidities. Appl Environ Microbiol. 1998;64:4495–4499. doi: 10.1128/aem.64.11.4495-4499.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catty D. Antibodies: a practical approach. Oxford, England: IRL Press; 1988. pp. 7–58. [Google Scholar]
- 3.Connolly G M, Dryden M S, Shanson D C, Gazzard B G. Cryptosporidial diarrhoea in AIDS and its treatment. Gut. 1988;29:593–597. doi: 10.1136/gut.29.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupont H L, Chappell C L, Sterling C R, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 5.Ey P L, Prowse S J, Jenkin C R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Biochemistry. 1978;15:129–144. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari B C, Vesey G, Weir C J, Williams K L, Veal D A. Comparison of Cryptosporidium-specific and Giardia-specific monoclonal antibodies for monitoring water samples. Water Res. 1999;33:1611–1617. [Google Scholar]
- 7.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. pp. 53–136. [Google Scholar]
- 8.Hornok S, Szell Z, Nieuwenhuijs J, Nieuwland M G B, Cornelissen A W C A, Varga I. Immunogenicity of three oocyst extracts of Cryptosporidium baileyi in experimentally infected chickens. Parasitol Res. 1998;85:71–77. doi: 10.1007/s004360050509. [DOI] [PubMed] [Google Scholar]
- 9.Karanis P, Schoenen D, Maier W A, Seitz H M. Drinking water and parasites. Immun Infekt. 1993;21:132–136. [PubMed] [Google Scholar]
- 10.Khyse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 11.Luft B J, Payne D, Woodmansee D, Kim C W. Characterization of the Cryptosporidium antigens from sporulated oocysts of Cryptosporidium parvum. Infect Immun. 1987;55:2436–2441. doi: 10.1128/iai.55.10.2436-2441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald V, Deer R M A, Nina J M S, Wright S, Chiodini P L, McAdam K P W J. Characteristics and specificity of hybridoma antibodies against oocyst antigens of Cryptosporidium parvum from man. Parasite Immunol. 1991;13:251–259. doi: 10.1111/j.1365-3024.1991.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 13.Moore A G, Vesey G, Champion A, Scandizzo P, Deere D, Veal D, Williams K L. Viable Cryptosporidium parvum oocysts exposed to chlorine or other oxidising conditions may lack identifying epitopes. Int J Parasitol. 1998;28:1205–1212. doi: 10.1016/s0020-7519(98)00070-8. [DOI] [PubMed] [Google Scholar]
- 14.O'Donoghue P J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:1–55. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 15.Pererva N. Cryptosporidium parvum antigens and ultrastructure. Ph.D. thesis. Sydney, Australia: Macquarie University; 1998. [Google Scholar]
- 16.Robertson L J, Campbell A T, Smith H V. In vitro excystation of Cryptosporidium parvum. Parasitology. 1993;106:13–19. doi: 10.1017/s003118200007476x. [DOI] [PubMed] [Google Scholar]
- 17.Robertson L J, Campbell A T, Smith H V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl Environ Microbiol. 1992;58:494–500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roitt I, Brostoff J, Male D. Immunology. 4th ed. London, England: Mosby Publications; 1996. pp. 107–129. [Google Scholar]
- 19.Smith H V, Rose J B. Waterborne cryptosporidiosis. Parasitol Today. 1990;6:8–12. doi: 10.1016/0169-4758(90)90378-h. [DOI] [PubMed] [Google Scholar]
- 20.Tilley N, Fayer R, Guidry A, Upton S J, Blagburn B L. Cryptosporidium parvum (Apicomplexa: Cryptosporidiae) oocysts and sporozoite antigens recognized by bovine colostral antibodies. Infect Immun. 1990;58:2966–2971. doi: 10.1128/iai.58.9.2966-2971.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzipori S. Cryptosporidiosis in perspective. Adv Parasitol. 1988;27:63–129. doi: 10.1016/S0065-308X(08)60353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesey G, Slade J S, Byrne M, Shepherd K, Dennis P J, Fricker C R. Routine monitoring of Cryptosporidium oocysts in water using flow cytometry. J Appl Bacteriol. 1993;75:87–90. doi: 10.1111/j.1365-2672.1993.tb03413.x. [DOI] [PubMed] [Google Scholar]
- 23.Vesey G. Application of flow cytometry to the detection of Cryptosporidium in water. Ph.D. thesis. Sydney, Australia: Macquarie University; 1996. [Google Scholar]
- 24.Vesey G, Deere D, Weir C J, Ashbolt N, Williams K L, Veal D A. A simple method for evaluating Cryptosporidium-specific antibodies for monitoring environmental water samples. Lett Appl Microbiol. 1997;25:316–320. doi: 10.1046/j.1472-765x.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 25.Vesey G, Griffiths K R, Gauci M R, Deere D, Williams K L, Veal D A. A simple and rapid measurement of Cryptosporidium excystation using flow cytometry. Int J Parasitol. 1997;27:1353–1359. doi: 10.1016/s0020-7519(97)00085-4. [DOI] [PubMed] [Google Scholar]