Abstract
Background
To investigate whether metformin monotherapy or adjunctive therapy improves the prognosis in patients with any type of cancer compared to non-metformin users (age ≥18).
Methods
Databases (Medline, Embase, and the Cochrane Central Register of Controlled Trials) and clinical trial registries (ClinicalTrials.gov; the World Health Organization International Clinical Trials Registry Platform) were screened for randomized, controlled trials (RCT) reporting at least progression-free survival (PFS) and/or overall survival (OS). Main outcome measures included hazard ratios (HR), and combined HRs and 95% confidence intervals (CI) were calculated using random-effects models.
Results
Of the 8419 records screened, 22 RCTs comprising 5943 participants were included. Pooled HRs were not statistically significant in both PFS (HR 0.97, 95% CI 0.82–1.15, I2 = 50%) and OS (HR 0.98, 95% CI 0.86–1.13, I2 = 33%) for patients with cancer between the metformin and control groups. Subgroup analyses demonstrated that metformin treatment was associated with a marginally significant improvement in PFS in reproductive system cancers (HR 0.86, 95% CI 0.74–1.00) and a significantly worse PFS in digestive system cancers (HR 1.45, 95% CI 1.03–2.04). The PFS or OS was observed consistently across maintenance dose, diabetes exclusion, median follow-up, risk of bias, and combined antitumoral therapies.
Conclusion
Metformin treatment was not associated with cancer-related mortality in adults compared with placebo or no treatment. However, metformin implied beneficial effects in the PFS of the patients with reproductive system cancers but was related to a worse PFS in digestive system cancers.
Systematic review registration
PROSPERO registration number CRD42022324672.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02599-4.
Keywords: Metformin, Cancer, RCTs, Meta-analysis, Cancer-related mortality
Background
Cancer death accounts for 21% of all cases in both men and women in the USA, and cancer is the second leading cause of death worldwide [1]. Of all incident cases, lung and bronchus cancer, prostate cancer, and colorectal cancer (CRC) account for the largest percentages in men. New diagnoses for women mostly include breast cancer (BC), lung cancer, and CRC. The statistics in 2020 showed that the risk of cancer death was accumulating regardless of the social development level [2]. Moreover, it is estimated that 19.3 million new cancer cases and almost 10.0 million deaths from cancer will occur in 2020.
Metformin is the first-line drug for type 2 diabetes (T2D) patients, which induces a hypoglycemic effect by targeting and activating the enzyme AMP-activated protein kinase (AMPK) and inhibiting hepatic glucose production. The activation of the AMPK-pathway may reduce the activity of insulin in promoting tumor progression and can inhibit the mammalian target of rapamycin (mTOR), which is closely connected to tumor cell proliferation [3–6]. In 2005, Evans et al. [7] retrospectively identified that metformin is related to a lower risk of developing cancer in patients with T2D, generating considerable publicity over the anticancer effect of metformin. In recent years, metformin has been advocated as a potential economic strategy to improve the prognosis in both diabetic and nondiabetic cancer patients.
However, the available results are controversial. Several studies and meta-analyses have indicated that metformin therapy is associated with reduced cancer-related mortality [8–12], while others point out that concomitant medication with metformin showed no significant effect on cancer-related mortality [13–18] or even led to inferior outcomes [19]. In the last decade, several randomized controlled trials (RCTs) have been conducted to assess the effectiveness of metformin monotherapy or adjunctive therapy in antitumor medications. We carried out a meta-analysis of RCTs to evaluate whether metformin reduces cancer-related mortality in adults compared with placebo or no treatment.
Methods
This prospective study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [20]. The protocol was registered with PROSPERO (CRD42022324672).
Eligibility criteria
The inclusion and exclusion criteria were prespecified. The inclusion criteria contained several essential factors, including (1) RCTs if metformin was one of the randomized therapies; (2) investigation of the efficacy of metformin monotherapy or as an adjunctive therapy comparing the treatment group with a control group (placebo or no treatment); (3) investigation of adults (age ≥ 18 years) with any type of cancer; and (4) presence of reported results on progression-free survival (PFS) and/or overall survival (OS). If the studies did not report PFS or OS, we contacted the investigators by e-mail, requesting them to provide survival data. Studies were excluded if they (1) were case reports, retrospective studies, observational studies, or post hoc analyses of RCTs; (2) synchronously used other antidiabetic drugs; or (3) had no available results related to survival.
Search strategy
Electronic searches of databases (Medline, Embase, and the Cochrane Central Register of Controlled Trials) and clinical trial registries (ClinicalTrials.gov; the World Health Organization International Clinical Trials Registry Platform) were conducted from their inception to June 1, 2022. To maximize the search for relevant trials, we hand-searched the bibliographies of identified studies and systematic reviews. Language restrictions were not applied to the search. Additional file 1 shows the detailed search strategy.
Study selection
All retrieved studies were screened by two independent researchers (ZY and JW) for titles and abstracts to evaluate their eligibility. Full-text publications or presentations were retrieved for further assessment when the information was insufficient. When studies had multiple publications or overlapping patients, the most recent publication was chosen.
Data collection
Data on the study designs, patient characteristics, interventions, and outcomes were collected from the included studies into a standard sheet by two independent researchers (JW and YZ). The hazard ratios (HR) included associated data that were either directly collected from the studies or assessed from Kaplan–Meier curves [21]. The adjusted HRs were extracted in preference to unadjusted HRs if provided by the studies.
Risk of bias assessment
The risk of bias in each trial was evaluated using the Cochrane Risk of Bias Assessment Tool (version 2) [22]. We scored every trial as low risk, with some concerns, or high risk based on the following criteria: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported result [22]. Two researchers (JW and FL) independently assessed the potential study bias of the included studies. Disagreements were resolved by consensus.
Subgroup analyses
We performed several subgroup analyses to evaluate the interactions according to the maintenance dose ([500, 1000), [1000, 1500), [1500, 2000), [2000, 2500) mg), diabetes exclusion (yes or no), risk of bias (low risk, some concerns, high risk), and combination with chemotherapy, radiotherapy (yes or no), and targeted therapy (yes or no). Previous studies have shown that cancers within the same system owned similar molecular characteristics [23–25]; therefore, we conducted retrospective subgroup analyses of the cancer type based on the systems that they originated from (reproductive, respiratory, or digestive system cancers).
Statistical analysis
The primary endpoints were the PFS and OS of cancer patients, measured by HRs. We performed statistical analyses based on the intention-to-treat results using the meta package in R (version 4.1.3). HRs and their 95% confidence intervals (CI) were used to assess outcomes, and P < 0.05 was considered statistically significant. Heterogeneity was estimated with the I2 test [26]. The assumption of heterogeneity was deemed valid for I2 > 25% and P < 0.10 as in a previous study [27]. If heterogeneity was not significant, we used fixed-effects models to pool outcomes. When heterogeneity was significant, we used random-effects models. Meta-regression and sensitivity analyses were performed to investigate potential sources of heterogeneity. Qualitative and quantitative assessments of small-study effects were performed with the funnel plot and Egger’s test [28, 29].
Results
Eligible studies and study characteristics
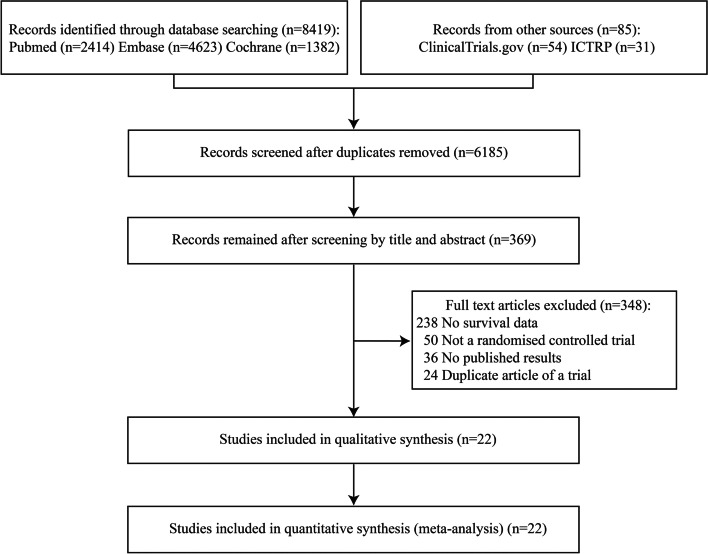
We screened 8419 records and identified 22 eligible trials (5943 participants) in the final meta-analysis (Fig. 1) [30–52]. All the studies were RCTs published between 2015 and 2022. The number of recruited participants in the included trials ranged from 25 to 3649. The mean age of the metformin and control groups was 58.6 and 58.9 years, respectively. The female proportion in the metformin and control groups was 64% and 65%, respectively. The population characteristics of the included trials are summarized in Additional file 2.
Fig. 1.
Search and selection of eligible studies for inclusion
All eligible studies comprised patients with reproductive (breast, ovary, endometrium, and prostate), respiratory (lung), and digestive (pancreas and liver) system cancers. Seventeen of the 22 studies were performed on those with advanced or metastatic cancer. All studies administered antitumor therapies to the patients, including chemotherapy, radiotherapy, targeted therapy, hormone therapy, and immunotherapy. Fifteen studies excluded patients with diabetes at the inclusion stage of the trials. Six studies included patients with and without diabetes and one included only patients with metabolic syndrome. A diagnosis of diabetes was noted in 310 (5%) of 5943 patients. All studies reported daily maintenance doses of metformin ranging from 500 to 2000 mg. All studies controlled or evaluated potential confounders; they conducted stratified randomization, reported balanced confounding factors in the metformin and control groups, or adjusted HRs by multivariable analysis. Table 1 summarizes the main characteristics of the studies. The methodological quality of the eligible studies was generally moderate to good (shown in Additional file 3: Figs. S1 and S2). The main source of bias was a lack of reporting if the allocation sequence was concealed until enrollment and assignment.
Table 1.
Main characteristics of included studies
| Author | Year | Country | Study design | Cancer location | Stage/other restriction | Combined therapy |
DM status (DM/non-DM) |
Sample size (metformin/control) |
Maintenance dose |
Median follow-up (months) |
Outcome type | Potential confounders control | Risk of bias | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sa | Ra | Mc | |||||||||||||
| Goodwin | 2022 | Multiple centers | Double-blinded | Breast | High-risk nonmetastatic | Chemotherapy + radiotherapy + hormone therapy + targeted therapyd | No |
3649 (1824/1825) |
850mg bid | 96.2 for ER/PgR+94.1 for ER/PgR- | OS | √ | √ | √ | Low risk |
| Pimentel | 2019 | Canada | Double-blinded | Breast | Metastatic or unrectable locally advanced | Chemotherapy + hormone therapy | No |
40 (22/18) |
850mg bid | Not given | PFS, OS | √ | √ | √ | Low risk |
| Nanni | 2019 | Italy | Open-label | Breast | Stage IV/ metastatic, HER2-negative | Chemotherapy | No |
122 (57/65) |
1000mg bid | 39.6 | PFS, OS | √ | √ | √ | Some concerns |
| Zhao | 2017 | China | Open-label | Breast | Metastatic or locally advanced | Hormone therapy | No |
60 (30/30) |
500mg bid | 22.3 | PFS, OS | × | √ | × | Some concerns |
| Salah | 2021 | Egypt | Open-label | Breast | Stage IV/ metastatic | Chemotherapy | No | 50(25/25) | 1000mg bid | 6 | PFS, OS | × | √ | × | Some concerns |
| Liubota | 2018 | Ukraine | Not given | Breast | Stage II-III | Chemotherapy + hormone therapy | Yes | 72(36/36) | 500mg tid | 39 | PFS, OS | × | √ | × | Some concerns |
| EL-Haggar | 2016 | Egypt | Not given | Breast | Newly diagnosed | Chemotherapy + hormone therapy | No | 102(51/51) | 850mg bid | Not given | PFS | × | √ | √ | Some concerns |
| Hamedi | 2018 | Iran | Open-label | Ovary | Epithelial | Chemotherapy | No |
70 (30/40) |
500mg tid | Not given | PFS | × | √ | × | Low risk |
| Zheng | 2019 | China | Open-label | Ovary | Epithelial | Chemotherapy | No |
44 (20/24) |
850mg qd | Not given | PFS | × | √ | × | Some concerns |
| Bae-Jump | 2020 | USA | Double-blinded | Endometrium | Stage III-IV/ recurrent | Chemotherapy |
Mixed (102/367) |
469 (234/235) |
850mg bid | 28 | PFS, OS | √ | √ | √ | Some concerns |
| Alghandour | 2021 | Egypt | Double-blinded | Prostate | High localized or node invasion or metastatic hormone sensitive | Chemotherapy + radiotherapy + hormone therapy |
Mixed (15/99) |
124 (62/62) |
850mg bid | 22 | PFS, OS | √ | √ | √ | Low risk |
| Martin | 2021 | France | Double-blinded | Prostate | Metastatic or hormone resistant | Chemotherapy | No |
99 (50/49) |
850mg bid | 86 | PFS, OS | × | √ | × | Some concerns |
| Li | 2019 | China | Double-blinded | Lung | Stage IIIB-IV/ EGFR mutated NSCLC | Targeted therapy | No |
202 (97/105) |
1000mg bid | 19.15 | PFS, OS | × | √ | × | Low risk |
| Arrieta | 2019 | Mexico | Open-label | Lung | Stage IIIB-IV/ EGFR mutated lung adenocarcinoma | Targeted therapy | No |
139 (69/70) |
500mg bid | 16.9 | PFS, OS | × | √ | √ | Some concerns |
| Lee | 2021 | South Korea | Open-label | Lung | Stage IIIB-IV/ EGFR-ALK wild NSCLC | Chemotherapy |
Mixed (36/129) |
165(82/83) | 1000mg bid | 32.4 | PFS, OS | √ | √ | √ | Some concerns |
| Marrone | 2018 | USA | Open-label | Lung | Stage IIIB-IV/ nonsquamous NSCLC | Chemotherapy + targeted therapy | No |
24 (18/6) |
1000mg bid | Not given | PFS, OS | × | √ | × | Some concerns |
| Sayed | 2015 | Egypt | Open-label | Lung | Stage IV/ NSCLC | Chemotherapy | No |
30 (15/15) |
500mg qd | Not given | OS | √ | √ | × | Low risk |
| Skinner | 2021 | USA | Open-label | Lung | Stage III/ unresectable NSCLC | Chemotherapy + radiotherapy | No |
167 (86/81) |
1000mg bid | 27.7 | PFS, OS | √ | √ | √ | Some concerns |
| Tsakiridis | 2021 | Canada | Open-label | Lung | Stage III/ Unresected locally advanced NSCLC | Chemotherapy + radiotherapy + immunotherapye | No |
54 (26/28) |
1000mg bid | Not given | PFS, OS | √ | √ | √ | Low risk |
| Kordes | 2015 | Netherlands | Double-blinded | Pancreas | Metastatic or unresectable locally advanced | Chemotherapy + targeted therapy |
Mixed (14/107) |
121 (60/61) |
1000mg bid | 28.1 | PFS, OS | √ | √ | √ | Low risk |
| Reni | 2016 | Italy | Open-label | Pancreas | Metastatic | Chemotherapy |
Mixed (23/37) |
60 (31/29) |
2000mg daily | Not given | PFS, OS | × | √ | √ | Some concerns |
| Shorbagy | 2020 | Egypt | Not given | Liver | Advanced | Targeted therapy |
Mixed (48/32) |
80 (40/40) |
500mg bid | Not given | PFS, OS | × | √ | × | Some concerns |
ER/PgR estrogen receptor and/or progesterone receptor, HER human epidermal growth factor receptor 2, PFS progression-free survival, OS overall survival, NSCLC non-small-cell lung cancer
aS=Stratified randomization
aR=Reported and no significant difference between metformin and control group
cM=Conducted multivariate analysis
d74.1%, 88.9%, 61.4%, 17.2% patients taking radiotherapy, chemotherapy, hormone therapy, targeted therapy, respectively
e20.3% patients taking immunotherapy
Efficacy of metformin in patients with cancers
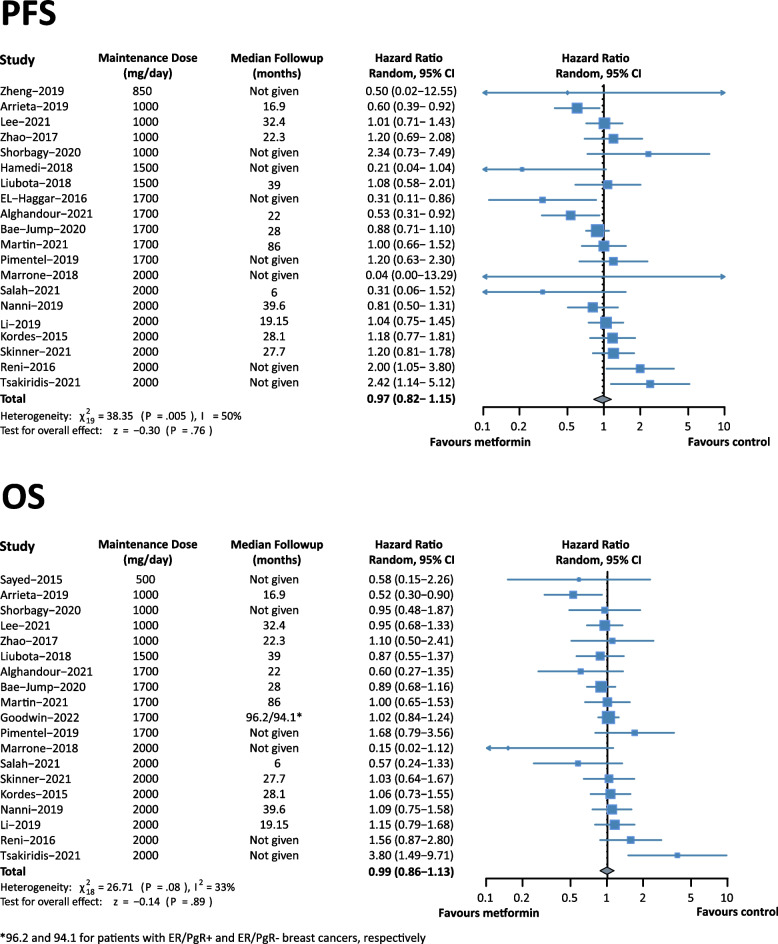
All 22 trials reported survival data, of which 20 and 18 reported PFS and OS, respectively. Both PFS (HR 0.97, 95% CI 0.82–1.15, I2 = 50%) and OS (HR 0.99, 95% CI 0.86–1.13, I2 = 33%) showed no significant difference between the metformin and control groups for patients with cancers (Fig. 2). Due to the heterogeneity, we applied a random-effects model to pool the HRs results. Our sensitivity analyses revealed that excluding any single study did not significantly affect the pooled estimate (Additional file 3: Figs. S3 and S4).
Fig. 2.
Forest plot of PFS and OS of trials evaluating metformin use
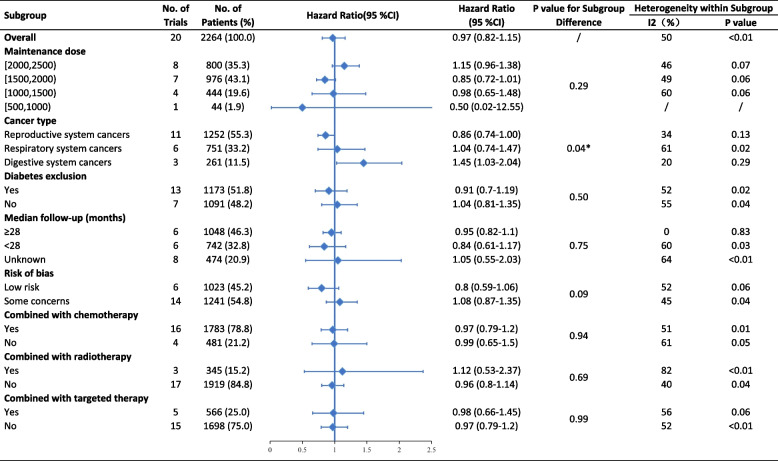
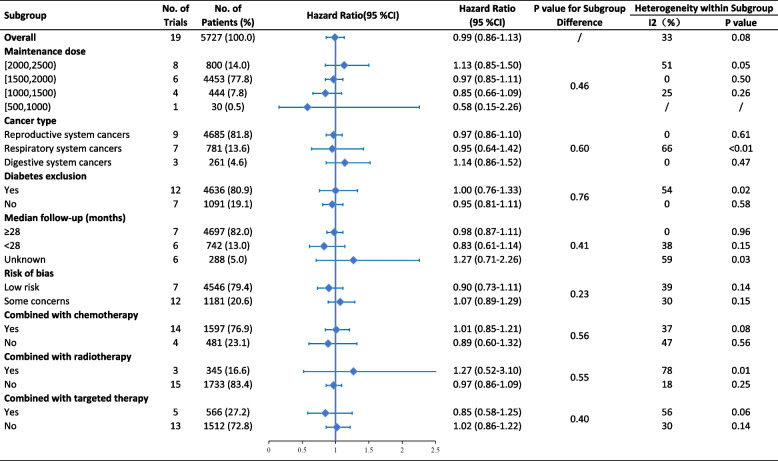
Subgroup analyses indicated that metformin use resulted in marginally significant improvement in PFS for patients with reproductive system cancers (HR 0.86, 95% CI 0.74–1.00). For digestive system cancers, metformin use showed significantly worse PFS (HR 1.45, 95% CI 1.03–2.04) (Fig. 3). The difference between subgroups based on cancer type was statistically significant in PFS (p = 0.04) but not in OS (p = 0.60) (Fig. 4). There was no clear evidence of between-subgroup differences based on maintenance dose, diabetes exclusion, median follow-up, risk of bias, and combined antitumoral therapies, neither in PFS nor in OS. Meta-regression revealed that the maintenance dose is not significantly correlated with improved OS (p = 0.07, coefficient = 0.0003, Additional file 3: Fig. S5). The subgroups’ meta-regression of the maintenance dose for PFS (p = 0.38) and median follow-up revealed no significant differences (p = 0.45 for PFS, p = 0.32 for OS).
Fig. 3.
Subgroup analyses for PFS
Fig. 4.
Subgroup analyses for OS
The funnel plot analysis did not show substantial asymmetry (Additional file 3: Fig. S6). We did not observe evidence of small-study effects, with Egger p values of 0.58 for PFS and 0.66 for OS.
Discussion
With individual participant data from 22 high-quality randomized controlled trials for more than 5943 patients with cancer, our meta-analysis revealed that metformin treatment was not associated with cancer-related mortality in adults compared with placebo or no treatment. Subgroup analysis suggests that metformin therapy is potentially beneficial for reproductive system cancers, including breast, ovary, endometrium, and prostate, but may be related to a worse prognosis for digestive system cancers, including pancreas and liver.
The effect of metformin in the prevention of reproductive system cancer progression may be related to its impact on the gonadal hormone levels. Metformin was reported to be effective in preventing hormone-related tumor progression, including breast [53], prostate, ovarian, and endometrial [54] cancers. Previous studies have reported that progestin can activate the PI3K/Akt pathway without progesterone receptor (PgR) mediation [55], and metformin suppresses both estrogen receptor (ER)/PgR signaling and PI3K/AKT/mTOR signaling to inhibit estradiol and progesterone-associated abnormal cell proliferation and hormone therapy resistance [56–58]. Recently, the largest RCT (MA.32), which enrolled 3649 patients with early BC, suggested prognostic benefits of metformin among HER2 + subtypes [39]. The addition of metformin did not reveal significant improvement in the total study population. However, the trial used invasive disease-free survival (IDFS) as a primary outcome instead of PFS, which placed more emphasis on cancer invasiveness. In addition, metformin exposure can affect human and mouse fetal testicular cells, thus reducing the production of androgens and testosterone [59]. Androgen signaling directly regulates Tcf7 and induces CD8+ T cell depletion, and higher mortality in men is observed with the development and progression of tumors in various organs [60]. A recent randomized trial of metformin treatment for 1 month found significantly lower testosterone concentrations in T2D men regardless of changes in blood glucose and weight [61]. For prostate cancer, androgen deprivation therapy (ADT) alone remains the first-line treatment in most cases. Pre-surgical administration of metformin in prostate cancer reduced the Ki-67 proliferation index by 29% compared with pretreatment biopsy [62]. A similar effect of metformin pre-surgical treatment in reducing tumor Ki-67 expression was also reported in endometrial cancer [63]. Further, metformin treatment was shown to reverse endometrial hyperplasia in a rat model [63, 64] and women with polycystic ovary syndrome (PCOS) [65], indicating metformin’s potential role in cancer prevention. Metformin has also shown anticancer effects in human ovarian cancer cells through ASK1-mediated mitochondrial damage and ER stress [66]. Our results concur with the findings of previous studies and support the more in-depth clinical investigations of the effect of metformin on hormone-related cancers.
Metformin monotherapy or combination therapy is associated with a worse prognosis in digestive system cancers, including pancreatic and liver cancers. Evidence from retrospective studies also indicates that chronic metformin treatment is related to enhanced tumor aggressiveness and sorafenib resistance in hepatocellular carcinoma [67, 68]. In two metastatic and advanced pancreatic cancer cohorts, the increased toxic effects of metformin were observed, such as esophagitis and lung infections, which limited their tolerance to originally prescribed doses of chemoradiotherapy and worsened the prognosis.
A significant association in the meta-regression between a low maintenance dose and prolonged OS was identified in our results. One possible explanation is that metformin may induce biphasic actions in various cell types, mostly showing a desirable effect at low concentrations and an undesirable or even toxic effect at high concentrations [69–81]. Furthermore, adverse effects of metformin, particularly diarrhea, have been reported to be dose-dependent [82, 83], may influence medication adherence and lead to poor treatment effects.
Limitations
Our findings are based on large samples from high-quality RCTs with relatively long-term follow-up and with between-study heterogeneity as low or medium, indicating that our conclusions are relatively reliable. However, there are several significant limitations. First, there was evidence indicating that the effect of metformin use on the survival of patients with diabetes depends on the cumulative metformin dose [84, 85]. However, we could not obtain baseline cumulative dose values and the duration of medication for each individual; therefore, we were unable to analyze the effects of cumulative metformin dose on PFS and OS. Future studies should pay more attention to the effect of the cumulative metformin dose on the survival of cancer patients. Second, as cancer treatment has entered the epoch of precision medicine, the number of included studies was limited, and more research is required to further classify cancers, such as classification on the organ level and even pathological diagnoses on the molecular level.
Conclusions
Metformin treatment was not associated with cancer-related mortality in adults compared with placebo or no treatment. However, metformin showed potentially beneficial effects on the PFS of the patients with reproductive system cancers but was related to a worse PFS in patients with digestive system cancers. The positive or desired effects may be maximal in low-dose conditions. Further studies are required to elucidate the effects and underlying mechanisms in specific cancer subtypes.
Supplementary Information
Additional file 1: Table S1. Search strategy.
Additional file 2: Table S1. Population characteristics of included studies.
Additional file 3: Fig. S1. Risk of bias summary. Fig. S2. Risk of bias graph for each included study. Fig. S3. Sensitivity analyses for PFS. Fig. S4. Sensitivity analyses for OS. Fig. S5. Meta-regression for OS by maintenance dose. Fig. S6. Funnel plots for PFS and OS.
Acknowledgments
Not applicable.
Abbreviations
- RCT
Randomized controlled trials
- PFS
Progression-free survival
- OS
Overall survival
- HR
Hazard ratios
- CI
Confidence interval
- BC
Breast cancer
- CRC
Colorectal cancer
- T2D
Type 2 diabetes
- AMPK
AMP-activated protein kinase
- mTOR
Mammalian target of rapamycin
- PgR
Progesterone receptor
- ER
Estrogen receptor
Authors’ contributions
JW, FL, and JL developed the initial idea for the study. JW, FL, ZL, and JL designed the scope and planned the methodological approach. JW and ZY coordinated the meta-analysis process, wrote the meta-analysis protocol, and completed the PROSPERO registration, with contributions from JH, LZ, YZ, QC, and WY. ZY coordinated the meta-analysis update. JW, ZY, and FL defined the search strings, executed the search, exported results, and removed duplicate records. JW, ZY, and FL screened abstracts and texts for the systematic review, extracted relevant data from the systematic review articles, and did quality assessment. JW and ZY extracted the data for further analysis. JW, YC, and XM wrote the computer code and performed the meta-analysis. JW, ZY, and FL wrote the first draft of the manuscript and all authors contributed to critically revising the manuscript. FL and JL are senior and corresponding authors who contributed equally to this study. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant No 82172685, 82001223, 81873635 and 81901401).
Availability of data and materials
All data generated or analyzed during this study are available in the article, additional files, or from the corresponding author upon reasonable request. The study protocol can be accessed on PROSPERO (CRD42022324672).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Wen and Zhenjie Yi contributed equally to this work.
Fangkun Liu and Jingfang Liu are senior and corresponding authors who contributed equally to this study.
Contributor Information
Fangkun Liu, Email: liufangkun@csu.edu.cn.
Jingfang Liu, Email: jinfang_liu@csu.edu.cn.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 4.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J Clin Oncol. 2016;34(35):4261–4269. doi: 10.1200/JCO.2016.67.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. Bmj. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Yao W, Chu Q, Han R, Wang Y, Sun J, et al. Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non-small cell lung cancer and type 2 diabetes. Cancer Lett. 2015;369(1):97–102. doi: 10.1016/j.canlet.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27(12):2184–2195. doi: 10.1093/annonc/mdw410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Wu J, He Q, Liang W, He J. The prognostic value of metformin for advanced non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res. 2018;7(3):389–396. doi: 10.21037/tlcr.2018.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong TT, Wu QJ, Lin B, Ruan SK, Kushima M, Takimoto M. Observational studies on the association between post-diagnostic metformin use and survival in ovarian cancer: a systematic review and meta-analysis. Front Oncol. 2019;9:458. doi: 10.3389/fonc.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wensink MJ, Lu Y, Tian L, Shaw GM, Rizzi S, Jensen TK, et al. Preconception antidiabetic drugs in men and birth defects in offspring : a nationwide cohort study. Ann Intern Med. 2022;175(5):665–673. doi: 10.7326/M21-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens RJ, Ali R, Bankhead CR, Bethel MA, Cairns BJ, Camisasca RP, et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55(10):2593–2603. doi: 10.1007/s00125-012-2653-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZJ, Yuan J, Bi Y, Wang C, Liu Y. The effect of metformin on biomarkers and survivals for breast cancer- a systematic review and meta-analysis of randomized clinical trials. Pharmacol Res. 2019;141:551–555. doi: 10.1016/j.phrs.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Nayan M, Punjani N, Juurlink DN, Finelli A, Austin PC, Kulkarni GS, et al. Metformin use and kidney cancer survival outcomes. Am J Clin Oncol. 2019;42(3):275–284. doi: 10.1097/COC.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Chen H, Chen S, Li Z, Chen J, Li W. The effect of concomitant use of statins, NSAIDs, low-dose aspirin, metformin and beta-blockers on outcomes in patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Oncoimmunology. 2021;10(1):1957605. doi: 10.1080/2162402X.2021.1957605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morio K, Kurata Y, Kawaguchi-Sakita N, Shiroshita A, Kataoka Y. Efficacy of metformin in patients with breast cancer receiving chemotherapy or endocrine therapy: systematic review and meta-analysis. Ann Pharmacother. 2022;56(3):245–255. doi: 10.1177/10600280211025792. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Ma X, Long J, Du X, Pan B, Mao H. Metformin and survival of women with breast cancer: a meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2022;47(3):263–269. doi: 10.1111/jcpt.13500. [DOI] [PubMed] [Google Scholar]
- 19.Roy S, Malone S, Grimes S, Morgan SC. Impact of concomitant medications on biochemical outcome in localised prostate cancer treated with radiotherapy and androgen deprivation therapy. Clin Oncol (R Coll Radiol) 2021;33(3):181–190. doi: 10.1016/j.clon.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tierney J, Stewart L, Ghersi D, Burdett S, Sydes M. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Yin J, Zhou W, Bai J, Xie Y, Xu K, et al. Complex impact of DNA methylation on transcriptional dysregulation across 22 human cancer types. Nucleic Acids Res. 2020;48(5):2287–2302. doi: 10.1093/nar/gkaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Li L, Wang Z, Pan T, Sahni N, Jin X, et al. LncMAP: Pan-cancer atlas of long noncoding RNA-mediated transcriptional network perturbations. Nucleic Acids Res. 2018;46(3):1113–1123. doi: 10.1093/nar/gkx1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Sahni N, Pancsa R, McGrail DJ, Xu J, Hua X, et al. Revealing the determinants of widespread alternative splicing perturbation in cancer. Cell Rep. 2017;21(3):798–812. doi: 10.1016/j.celrep.2017.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. doi: 10.1136/bmj.k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 30.Alghandour R, Ebrahim MA, Elshal AM, Ghobrial F, Elzaafarany M, MA EL. Repurposing metformin as anticancer drug: Randomized controlled trial in advanced prostate cancer (MANSMED) Urol Oncol. 2021;39(12):831.e1–831e10. doi: 10.1016/j.urolonc.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 31.El Shorbagy S, abuTaleb F, Labib HA, Ebian H, Harb OA, Mohammed MS, et al. Prognostic significance of VEGF and HIF-1 α in hepatocellular carcinoma patients receiving sorafenib versus metformin sorafenib combination. J Gastrointest Cancer. 2021;52(1):269–279. doi: 10.1007/s12029-020-00389-w. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin PJ, Ennis M, Cescon DW, Elser C, Haq R, Hamm CM, et al. Phase II randomized clinical trial (RCT) of metformin (MET) vs placebo (PLAC) in combination with chemotherapy (CXT) in refractory locally advanced (LABC) or metastatic breast cancer (MBC) Cancer Res. 2019;79(4):P1–16. [Google Scholar]
- 33.Pujalte Martin M, Borchiellini D, Thamphya B, Guillot A, Paoli JB, Besson D, et al. TAXOMET: A French prospective multicentric randomized phase ii study of docetaxel plus metformin versus docetaxel plus placebo in metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2021;19(6):501–509. doi: 10.1016/j.clgc.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Nanni O, Amadori D, De Censi A, Rocca A, Freschi A, Bologna A, et al. Metformin plus chemotherapy versus chemotherapy alone in the first-line treatment of HER2-negative metastatic breast cancer. The MYME randomized, phase 2 clinical trial. Breast Cancer Res Treat. 2019;174(2):433–442. doi: 10.1007/s10549-018-05070-2. [DOI] [PubMed] [Google Scholar]
- 35.Bae-Jump VL, Sill M, Gehrig PA, Moxley K, Hagemann AR, Waggoner SE, et al. A randomized phase II/III study of paclitaxel/carboplatin/metformin versus paclitaxel/carboplatin/placebo as initial therapy for measurable stage III or IVA, stage IVB, or recurrent endometrial cancer: an NRG Oncology/GOG study. Gynecol Oncol. 2020;159:7. [Google Scholar]
- 36.Pimentel I, Lohmann AE, Ennis M, Dowling RJO, Cescon D, Elser C, et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast. 2019;48:17–23. doi: 10.1016/j.breast.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Gong C, Wang Z, Zhang J, Wang L, Zhang S, et al. A randomized phase II study of aromatase inhibitors plus metformin in pre-treated postmenopausal patients with hormone receptor positive metastatic breast cancer. Oncotarget. 2017;8(48):84224–84236. doi: 10.18632/oncotarget.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liubota R, Cheshuk V, Zotov O, Vereshchako R, Anikusko M, Liubota I, et al. Metformin in neoadjuvant systemic therapy of breast cancer patients with metabolic syndrome. Arch Oncol. 2018;24(1):1–5. [Google Scholar]
- 39.Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, Lemieux J, et al. Effect of metformin vs placebo on invasive disease-free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA. 2022;327(20):1963–1973. doi: 10.1001/jama.2022.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsakiridis T, Pond GR, Wright J, Ellis PM, Ahmed N, Abdulkarim B, et al. Metformin in combination with chemoradiotherapy in locally advanced non-small cell lung cancer: the OCOG-ALMERA randomized clinical trial. JAMA Oncol. 2021;7(9):1333–1341. doi: 10.1001/jamaoncol.2021.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skinner H, Hu C, Tsakiridis T, Santana-Davila R, Lu B, Erasmus JJ, et al. Addition of metformin to concurrent chemoradiation in patients with locally advanced non-small cell lung cancer: the NRG-LU001 phase 2 randomized clinical trial. JAMA Oncol. 2021;7(9):1324–1332. doi: 10.1001/jamaoncol.2021.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salah H. Metformin as an adjuvant treatment in non diabetic metastatic breast cancer. J Am Coll Clin Pharm. 2021;4(9):1230. [Google Scholar]
- 43.Lee Y, Joo J, Lee YJ, Lee EK, Park S, Kim TS, et al. Randomized phase II study of platinum-based chemotherapy plus controlled diet with or without metformin in patients with advanced non-small cell lung cancer. Lung Cancer. 2021;151:8–15. doi: 10.1016/j.lungcan.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Zheng Y, Zhu J, Zhang H, Liu Y, Sun H. Metformin plus first-line chemotherapy versus chemotherapy alone in the treatment of epithelial ovarian cancer: a prospective open-label pilot trial. Cancer Chemother Pharmacol. 2019;84(6):1349–1357. doi: 10.1007/s00280-019-03963-7. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Jiang L, Wang Y, Zhao Y, Zhang XJ, Wu G, et al. Combination of metformin and gefitinib as first-line therapy for nondiabetic advanced NSCLC patients with EGFR mutations: a randomized, double-blind phase II trial. Clin Cancer Res. 2019;25(23):6967–6975. doi: 10.1158/1078-0432.CCR-19-0437. [DOI] [PubMed] [Google Scholar]
- 46.Arrieta O, Barrón F, Padilla MS, Avilés-Salas A, Ramírez-Tirado LA, Arguelles Jiménez MJ, et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(11):e192553. doi: 10.1001/jamaoncol.2019.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marrone KA, Zhou X, Forde PM, Purtell M, Brahmer JR, Hann CL, et al. A randomized phase II study of metformin plus paclitaxel/carboplatin/bevacizumab in patients with chemotherapy-naïve advanced or metastatic nonsquamous non-small cell lung cancer. Oncologist. 2018;23(7):859–865. doi: 10.1634/theoncologist.2017-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamedi B, Khalili A, Roozmeh S, Namazi G, Saraf Z. Combination of metformin and chemotherapy decreases the recurrence rates of epithelial ovarian cancers: a randomized clinical trial. Int J Cancer Manag. 2018;11(7):e11621.
- 49.Reni M, Dugnani E, Cereda S, Belli C, Balzano G, Nicoletti R, et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: an open-label, randomized phase II trial. Clin Cancer Res. 2016;22(5):1076–1085. doi: 10.1158/1078-0432.CCR-15-1722. [DOI] [PubMed] [Google Scholar]
- 50.El-Haggar SM, El-Shitany NA, Mostafa MF, El-Bassiouny NA. Metformin may protect nondiabetic breast cancer women from metastasis. Clin Exp Metastasis. 2016;33(4):339–357. doi: 10.1007/s10585-016-9782-1. [DOI] [PubMed] [Google Scholar]
- 51.Sayed R, Saad AS, El Wakeel L, Elkholy E, Badary O. Metformin addition to chemotherapy in stage IV non-small cell lung cancer: an open label randomized controlled study. Asian Pac J Cancer Prev. 2015;16(15):6621–6626. doi: 10.7314/apjcp.2015.16.15.6621. [DOI] [PubMed] [Google Scholar]
- 52.Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(7):839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 53.Royce M, Bachelot T, Villanueva C, Özgüroglu M, Azevedo SJ, Cruz FM, et al. Everolimus plus endocrine therapy for postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: a clinical trial. JAMA Oncol. 2018;4(7):977–984. doi: 10.1001/jamaoncol.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu C, Zhang Z, Yu Y, Liu Y, Zhao F, Yin L, et al. Inhibiting the PI3K/Akt pathway reversed progestin resistance in endometrial cancer. Cancer Sci. 2011;102(3):557–564. doi: 10.1111/j.1349-7006.2010.01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407(6803):538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins G, Mesiano S, DiFeo A. Effects of metformin on cellular proliferation and steroid hormone receptors in patient-derived, low-grade endometrial cancer cell lines. Reprod Sci. 2019;26(5):609–618. doi: 10.1177/1933719118779734. [DOI] [PubMed] [Google Scholar]
- 57.Hu M, Zhang Y, Feng J, Xu X, Zhang J, Zhao W, et al. Uterine progesterone signaling is a target for metformin therapy in PCOS-like rats. J Endocrinol. 2018;237(2):123–137. doi: 10.1530/JOE-18-0086. [DOI] [PubMed] [Google Scholar]
- 58.Xiong F, Xiao J, Bai Y, Zhang Y, Li Q, Lishuang X. Metformin inhibits estradiol and progesterone-induced decidualization of endometrial stromal cells by regulating expression of progesterone receptor, cytokines and matrix metalloproteinases. Biomed Pharmacother. 2019;109:1578–1585. doi: 10.1016/j.biopha.2018.10.128. [DOI] [PubMed] [Google Scholar]
- 59.Tartarin P, Moison D, Guibert E, Dupont J, Habert R, Rouiller-Fabre V, et al. Metformin exposure affects human and mouse fetal testicular cells. Hum Reprod. 2012;27(11):3304–3314. doi: 10.1093/humrep/des264. [DOI] [PubMed] [Google Scholar]
- 60.Kwon H, Schafer JM, Song NJ, Kaneko S, Li A, Xiao T, et al. Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci Immunol. 2022;7(73):eabq2630. [DOI] [PMC free article] [PubMed]
- 61.Hu Y, Ding B, Shen Y, Yan RN, Li FF, Sun R, et al. Rapid changes in serum testosterone in men with newly diagnosed type 2 diabetes with intensive insulin and metformin. Diabetes Care. 2021;44(4):1059–1061. doi: 10.2337/dc20-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joshua AM, Zannella VE, Downes MR, Bowes B, Hersey K, Koritzinsky M, et al. A pilot ‘window of opportunity’ neoadjuvant study of metformin in localised prostate cancer. Prostate Cancer Prostatic Dis. 2014;17(3):252–258. doi: 10.1038/pcan.2014.20. [DOI] [PubMed] [Google Scholar]
- 63.Tas M, Kutuk MS, Serin IS, Ozgun MT, Oner G, Ozturk F. Comparison of antiproliferative effects of metformine and progesterone on estrogen-induced endometrial hyperplasia in rats. Gynecol Endocrinol. 2013;29(4):311–314. doi: 10.3109/09513590.2012.743010. [DOI] [PubMed] [Google Scholar]
- 64.Guo M, Zhou JJ, Huang W. Metformin alleviates endometrial hyperplasia through the UCA1/miR-144/TGF-β1/AKT signaling pathway. Int J Mol Med. 2020;45(2):623–633. doi: 10.3892/ijmm.2019.4438. [DOI] [PubMed] [Google Scholar]
- 65.Wang T, Zhang J, Hu M, Zhang Y, Cui P, Li X, et al. Differential Expression patterns of glycolytic enzymes and mitochondria-dependent apoptosis in PCOS patients with endometrial hyperplasia, an early hallmark of endometrial cancer, in vivo and the impact of metformin in vitro. Int J Biol Sci. 2019;15(3):714–725. doi: 10.7150/ijbs.31425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma L, Wei J, Wan J, Wang W, Wang L, Yuan Y, et al. Low glucose and metformin-induced apoptosis of human ovarian cancer cells is connected to ASK1 via mitochondrial and endoplasmic reticulum stress-associated pathways. J Exp Clin Cancer Res. 2019;38(1):77. doi: 10.1186/s13046-019-1090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casadei Gardini A, Marisi G, Scarpi E, Scartozzi M, Faloppi L, Silvestris N, et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin Pharmacother. 2015;16(18):2719–2725. doi: 10.1517/14656566.2015.1102887. [DOI] [PubMed] [Google Scholar]
- 68.Casadei Gardini A, Faloppi L, De Matteis S, Foschi FG, Silvestris N, Tovoli F, et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: validation study and biological rationale. Eur J Cancer. 2017;86:106–114. doi: 10.1016/j.ejca.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Calabrese EJ, Agathokleous E, Kapoor R, Dhawan G, Kozumbo WJ, Calabrese V. Metformin-enhances resilience via hormesis. Ageing Res Rev. 2021;71:101418. doi: 10.1016/j.arr.2021.101418. [DOI] [PubMed] [Google Scholar]
- 70.Emelyanova L, Bai X, Yan Y, Bosnjak ZJ, Kress D, Warner C, et al. Biphasic effect of metformin on human cardiac energetics. Transl Res. 2021;229:5–23. doi: 10.1016/j.trsl.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vytla VS, Ochs RS. Metformin increases mitochondrial energy formation in L6 muscle cell cultures. J Biol Chem. 2013;288(28):20369–20377. doi: 10.1074/jbc.M113.482646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alshawi A, Agius L. Low metformin causes a more oxidized mitochondrial NADH/NAD redox state in hepatocytes and inhibits gluconeogenesis by a redox-independent mechanism. J Biol Chem. 2019;294(8):2839–2853. doi: 10.1074/jbc.RA118.006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35(2):446–454. doi: 10.2337/dc11-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar VB, Bernardo AE, Vyas K, Franko M, Farr S, Lakshmanan L, et al. Effect of metformin on nitric oxide synthase in genetically obese (ob/ob) mice. Life Sci. 2001;69(23):2789–2799. doi: 10.1016/s0024-3205(01)01359-5. [DOI] [PubMed] [Google Scholar]
- 75.Zhao X, Zeng Z, Gaur U, Fang J, Peng T, Li S, et al. Metformin protects PC12 cells and hippocampal neurons from H(2) O (2) -induced oxidative damage through activation of AMPK pathway. J Cell Physiol. 2019. 10.1002/jcp.28337. [DOI] [PubMed]
- 76.Zhao Y, Wang Z, Mao Y, Li B, Zhu Y, Zhang S, et al. NEAT1 regulates microtubule stabilization via FZD3/GSK3β/P-tau pathway in SH-SY5Y cells and APP/PS1 mice. Aging (Albany NY) 2020;12(22):23233–23250. doi: 10.18632/aging.104098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ravera S, Cossu V, Tappino B, Nicchia E, Dufour C, Cavani S, et al. Concentration-dependent metabolic effects of metformin in healthy and Fanconi anemia lymphoblast cells. J Cell Physiol. 2018;233(2):1736–1751. doi: 10.1002/jcp.26085. [DOI] [PubMed] [Google Scholar]
- 78.Houshmand B, Tabibzadeh Z, Motamedian SR, Kouhestani F. Effect of metformin on dental pulp stem cells attachment, proliferation and differentiation cultured on biphasic bone substitutes. Arch Oral Biol. 2018;95:44–50. doi: 10.1016/j.archoralbio.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 79.Chen D, Wang Y, Wu K, Wang X. Dual effects of metformin on adipogenic differentiation of 3T3-L1 preadipocyte in AMPK-dependent and independent manners. Int J Mol Sci. 2018;19(6):1547. doi: 10.3390/ijms19061547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khallaghi B, Safarian F, Nasoohi S, Ahmadiani A, Dargahi L. Metformin-induced protection against oxidative stress is associated with AKT/mTOR restoration in PC12 cells. Life Sci. 2016;148:286–292. doi: 10.1016/j.lfs.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 81.Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012;13:11. doi: 10.1186/1471-2202-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hermann LS. Metformin: a review of its pharmacological properties and therapeutic use. Diabetes Metab. 1979;5(3):233–245. [PubMed] [Google Scholar]
- 83.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 84.Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, et al. Metformin: taking away the candy for cancer? Eur J Cancer. 2010;46(13):2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 85.Kang J, Jeong SM, Shin DW, Cho M, Cho JH, Kim J. The associations of aspirin, statins, and metformin with lung cancer risk and related mortality: a time-dependent analysis of population-based nationally representative data. J Thorac Oncol. 2021;16(1):76–88. doi: 10.1016/j.jtho.2020.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search strategy.
Additional file 2: Table S1. Population characteristics of included studies.
Additional file 3: Fig. S1. Risk of bias summary. Fig. S2. Risk of bias graph for each included study. Fig. S3. Sensitivity analyses for PFS. Fig. S4. Sensitivity analyses for OS. Fig. S5. Meta-regression for OS by maintenance dose. Fig. S6. Funnel plots for PFS and OS.
Data Availability Statement
All data generated or analyzed during this study are available in the article, additional files, or from the corresponding author upon reasonable request. The study protocol can be accessed on PROSPERO (CRD42022324672).