Abstract
This perspective considers the benefits of the potential future use of the cell permeant calpain inhibitor, calpeptin, as a drug to treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Recent work has reported calpeptin’s capacity to inhibit entry of the virus into cells. Elsewhere, several drugs, including calpeptin, were found to be able to inhibit extracellular vesicle (EV) biogenesis. Unsurprisingly, because of similarities between viral and EV release mechanisms, calpeptin has also been shown to inhibit viral egress. This approach, identifying calpeptin, through large-scale screening studies as a candidate drug to treat COVID-19, however, has not considered the longer term likely benefits of calpain inhibition, post-COVID-19. This perspective will reflect on the capacity of calpeptin for treating long COVID by inhibiting the overproduction of neutrophil extracellular traps potentially damaging lung cells and promoting clotting, together with limiting associated chronic inflammation, tissue damage and pulmonary fibrosis. It will also reflect on the tolerated and detrimental in vivo side-effects of calpain inhibition from various preclinical studies.
Keywords: calpeptin, COVID-19, Extracellular Vesicles, therapy
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), on entering the upper respiratory tract and infecting type II alveolar cells, causes severe inflammation [1,2]). This hyperinflammation (so-called cytokine storm or cytokine release syndrome) from recruited immune cells forms a cycle of chronic inflammation, ultimately damaging lung tissue [3]. As the angiotensin-converting enzyme 2 (ACE2) receptor is widely expressed in many organs, infection of the gastrointestinal, renal and cardiovascular systems is also common alongside acute systemic inflammatory symptoms [4]. As a blood pressure regulator in the lungs, ACE2 controls the renin–angiotensin system. By balancing the activity of ACE, ACE2 offers protection of the lungs from acute injury, but this is disturbed upon the viral spike protein (S protein) binding ACE2, leading to acute injury with associated chronic inflammation and resultant lung fibrosis [5]. Despite the development of vaccines against COVID-19, because of the delay in vaccinating the world’s population, people are still getting infected and becoming seriously ill. There is therefore an ongoing need to develop drugs able to inhibit SARS-CoV-2 infection but also with antifibrotic and anti-inflammatory capacity [6].
This perspective will discuss the future use of calpeptin, the cell permeant cathepsin/calpain inhibitor, as a possible anti-SARS-CoV-2 drug. It will focus on calpeptin’s capacity to inhibit: (i) viral entry and (ii) extracellular vesicle (EV) release and viral egress. However, these reports have not commented on additional benefits of calpain inhibition, especially important in post-COVID-19. This article will therefore also reflect on calpeptin’s inhibition of (iii) neutrophil extracellular trap (NET) formation [7] and (iv) inflammation [8], tissue damage and pulmonary fibrosis (PF) [9].
Calpeptin as an inhibitor of SARS-CoV-2 uptake
Inhibition of SARS-CoV-2 entry
SARS-CoV-2 has two possible entry mechanisms, thus broadening its tissue tropism: (i) in transmembrane serine protease 2+ (TMPRSS2+) cells, a rapid entry is achieved in a pH-independent manner, cells being activated rapidly at the cell surface. (ii) In cells lacking or with low-level expression of TMPRSS2, the virus is endocytosed and sorted to endolysosomes where activation is pH-dependent.
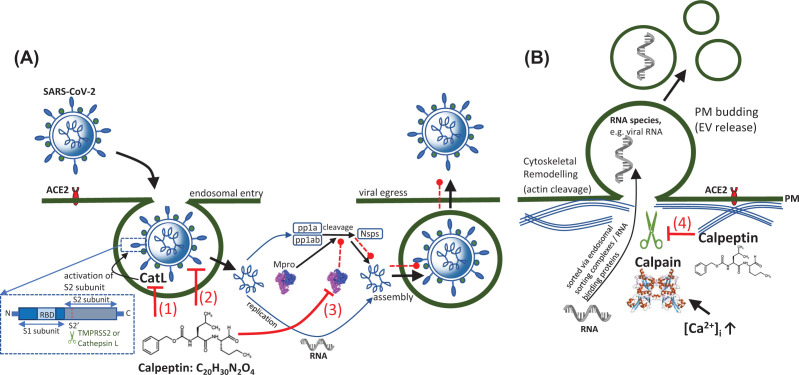
Both pathways require activation of the S protein. After the receptor binding domain (RBD) within the S1 subunit has bound ACE2 on target cells, the conformationally altered S2 subunit mediates membrane fusion following proteolytic cleavage away of S1, at the S1/S2 boundary (S2′ site) (Figure 1A(1)). If following the rapid, pH-independent pathway, TMPRSS2 performs this cleavage and activation of the viral S protein. However, in TMPRSS2− cells (Figure 1A(1)), the slower acid-activated route is followed utilizing the host protease, cathepsin L (CatL), found in acidic endo/lysosomal compartments. Where SARS-CoV-2 enters TMPRSS2− cells through endocytosis, numerous studies have shown that CatL inhibitors can inhibit viral entry [10–12], thus pointing to the use of CatL inhibitor, calpeptin (Figure 1A(1)).
Figure 1. Calpeptin-mediated inhibition of calpain and its effect on SARS-CoV-2 entry and egress.
(A) During SARS-CoV-2 endosomal entry, in TMPRSS- or low expressing cells, SARS-CoV-2 follows a slow, pH-dependent pathway. Here, calpeptin inhibits cathepsin L- (CatL-) mediated activation of the S2 subunit of the S protein, thereby blocking viral entry (1). Calpeptin can also block viral entry by high-affinity binding to the S protein RBD thereby blocking S protein: ACE2 interaction (2). Calpeptin binds with high affinity to Mpro thereby preventing cleavage of polyproteins pp1a and pp1ab into the nonstructural proteins 1–16, resulting in inhibition of assembly and viral egress (3). In (B), calpeptin inhibits calpain-mediated remodeling of the actin cytoskeleton, thereby inhibiting the release of shedding EVs which may incorporate various viral macromolecules (4). Any such regulation of EV release may help reduce EV-mediated fibroblast proliferation and pulmonary fibrosis.
In work in which libraries were screened for small molecule inhibitors for repurposing as entry inhibitor drugs, calpeptin was identified as having activity in in vitro infectivity assays. In Vero E6 cells with either low or high ACE2 expression, calpeptin (‘SR-914’) showed an EC50 of 174 and 163 nM, respectively [13]. This study suggested several mechanisms of action, including blocking entry by preventing ACE2:S protein RBD interaction through high affinity binding of calpeptin to S protein RBD on the S1 subunit (Figure 1A(2)).
Inhibition of SARS-CoV-2 main proteases
In a study to screen, using X-ray crystallography, 5000 approved drugs or those in clinical trials, that bind to SARS-CoV-2 Main protease, Mpro, also known as 3C-like protease 3CLpro, calpeptin was found to be the most potent of these post-entry inhibitors. It bound in the active site, demonstrating high antiviral activity (EC50 = 72 nM) [14]. Using a SARS-CoV-2 pseudotyped particles (PP) entry assay to evaluate binding, and entry inhibitors, calpeptin was identified as a potent entry inhibitor [15], thus also confirming previous studies [16,17]. The latter study used the calpain/cathepsin B inhibitor, MDL 28710.
The structures of Mpro complexed with calpain inhibitors II and XII, recently solved [18], revealed binding sites that support the empirically observed inhibition of the protease activity of SARS-CoV-2 Mpro [19]. This potentially reveals a strategy for inhibiting both Mpro (Figure 1A(3)) and CatL [18]. These inhibitors have a broader spectrum of activity, also demonstrating antiviral activities against other coronaviruses, including MERS-CoV [20].
Calpeptin-mediated inhibition of extracellular vesicle/SARS-CoV-2-mediated release from infected cells
Extracellular vesicle biogenesis
EVs are membrane-bound intercellular communicative vesicles [21]. Carrying receptor proteins, cytokines, miRNA, mRNA, bioactive lipids and various metabolites, they are released from a wide range of cells and found in all body fluids and interstitial spaces [22]. Classified according to their mechanism of biogenesis, EVs comprise exosomes, microvesicles (or microparticles/ectosomes, MVs) and apoptotic bodies (ApoBs). Exosomes (50–100 nm) have an endosomal origin, resulting from the intraluminal budding of early endosomes to generate multivesicular bodies (MVB) containing intraluminal vesicles, released as exosomes upon fusion of these MVBs with the PM. MVs (50 nm to 1 µm) are released by budding and fission of the PM. Membrane curvature is initiated by ceramide, generated from sphingomyelin by sphingomyelinase; MV release is also accompanied by a breakdown in the asymmetry of the lipid bilayer and exposition of phosphatidylserine on the outer leaflet. During apoptosis, and rearrangement of the cytoskeleton, ApoBs (1–5 μm) are released. EVs in this article will refer to MVs and exosomes.
Targeting extracellular vesicle biogenesis pathways as a means of limiting viral infection
In infectious diseases, EVs play a plethora of roles in enhancing infection and immune evasion [23]. For some time, it has been known that EVs and viruses share elements of their biogenesis pathways [24,25]. EVs released from virally infected cells, besides carrying molecules from their parent cells, also harbor viral genetic elements and proteins [24] and may be considered as defective viruses. In studies of the β-coronavirus family, using the prototypic mouse hepatitis virus, as well as SARS-CoV-2, these viruses egress infected cells by lysosomal exocytosis [26], having been trafficked to lysosomes from Golgi apparatus and trans-Golgi network via late endosomes/MVBs. As both EV and virus biogenesis may occur at the PM or within endosomes using endosomal sorting complexes required for transport (ESCRT) machinery to complete membrane fission, this justifies the aim of inhibiting EV biogenesis from virally infected cells as a means of limiting infection.
EVs play significant roles in disease pathology. For example, procoagulant endothelial EVs are released due to endothelial damage, TNF-α [27], or complement activation [28] resulting in coagulation and venous thromboembolism, presented in COVID-19, as deep vein thrombosis or pulmonary embolism. Pharmacological regulation of EV release has already been investigated [29,30] and in the task of finding drugs able to limit viral infection, this is an obvious direction, as recently demonstrated [31]. Kongsomros et al. identified calpeptin to be the most effective EV inhibitor drug against SARS-CoV-2. As a Ca2+-activated neutral cysteine protease, calpain, once activated, binds cytoskeletal proteins which leads to not only deformation of the PM, promoting EV release, but also cell migration, cellular proliferation and apoptosis [32]. The inhibition of calpain suppresses the release of EVs [30,31,33,34] (Figure 1B). Showing dose-dependent inhibition of infectious SARS-CoV-2 particles (IC50 0.6 μM in Vero-E6 cells), in combination with antivirals, specifically remdesivir, calpeptin had increased effectivity [31]. Previously, calpeptin was demonstrated to inhibit SARS-CoV replication in vitro (EC50 2 μM; IC50 17 μM) [35]. A plethora of EV inhibitory drugs have been identified, targeting cytoskeletal organization, endocytosis and lipid-related mechanisms. Therefore, such combination therapies may pose an interesting strategy, with the proviso that as the pathways involved in EV biogenesis share certain molecular components, off-target effects of such EV inhibitors are also considered.
Calpeptin inhibition of NETs as a therapeutic target in pulmonary fibrosis
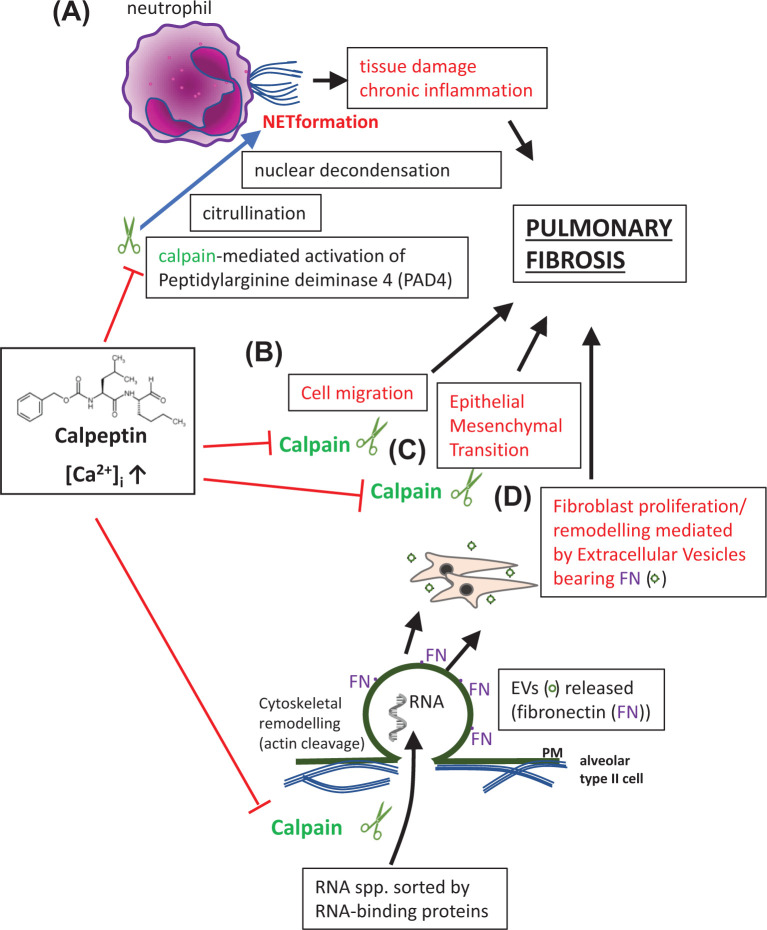
The excessive release of NETs, webs of DNA extruded from neutrophils, containing enzymes able to sequester pathogens, is associated with tissue damage, chronic inflammation and has been implicated in PF [36]. Indeed, using an in vitro alveolar model, NETosis-induced epithelial–mesenchymal transition (EMT) following SARS-CoV-2 infection was deemed an important step leading to PF [37]. NETs therefore probably play a major role in COVID-19 pathology [38]. Peptidyl arginine deiminase 4 (PAD4) is up-regulated in COVID-19 in the lung and is essential in NETosis [39,40]. A pathway for NET formation was recently proposed that may be relevant for developing new COVID-19 therapies. This proposed that PAD4-mediated citrullination which induces nuclear decondensation requires calpain-mediated activation of the PAD4 enzyme (Figure 2A). In turn this synergizes with the calpain-mediated proteolysis of nuclear lamina and chromatin-bound proteins in the nucleus [41]. As a result, both PAD4 and calpain inhibition diminished the calcium ionophore-mediated, nuclear decondensation in neutrophils. This points to a further possible benefit of calpeptin in ameliorating PF, post-COVID-19.
Figure 2. Calpeptin-mediated inhibition of calpain reduces inflammation and PF in COVID-19.
(A) Calpleptin inhibits calpain activation of PAD4 and in turn citrullination, nuclear decondensation and NETosis-mediated tissue damage, inflammation and lung fibrosis. (B) Calpeptin inhibits calpain-mediated inflammatory cell migration and (C) EMT-mediated lung fibrosis, both leading to lung fibrosis. In (D), calpeptin inhibits calpain-mediated plasma membrane budding and fibroblast remodeling due to the FN bearing EVs.
Calpain inhibition to reduce chronic inflammation and subsequent pulmonary fibrosis
According to current data, approximately 42% of COVID-19 patients develop acute respiratory distress syndrome (ARDS) [42]. As in the earlier SARS and MERS epidemics, ARDS in the COVID-19 pandemic was deemed a risk factor for fibrosis, but with added risk factors including old age and admission to intensive care. Even after removal of SARS-CoV-2, PF may continue to develop [43]. The proinflammatory state of ARDS, likely to be exacerbated in the elderly, is mediated by endothelial and epithelial injury from uncontrolled release of matrix metalloproteinases [44] and fibroproliferation. Together with proinflammatory cytokines TGF-β, VEGF, IL-6 and TNF-α, this may lead to PF in COVID-19 [45]. Fibrotic damage to lung tissue as occurs in PF is followed by release of a spectrum of cytokines identical to that described for COVID-19. The risk factors shared by both conditions include being male, elderly and having comorbidities such as diabetes and hypertension. The likely similar pathology of the lung disease could thus guide effective repurposing of drugs to treat severe COVID-19 [46].
Calpeptin inhibition of cell migration and pulmonary fibrosis
Fibrosis occurs following a persistent insult to the lung or dysregulation of any of the four steps leading to wound healing [47]. Any of these stages therefore represent potential targets for antifibrotic therapy. Looking at lung inflammation, for some time we have known that calpain inhibitors show anti-inflammatory properties [48,49]. In COVID-19, the timing of any anti-inflammatory intervention, such as with corticosteroids or IL-1/IL-6 inhibitors is critical. Considering the three stages of COVID-19 proposed by Siddiqi and Mehra [50], anti-inflammatory therapies would be detrimental to administer during stage I (early infection) with high viral loads. It may be more appropriate, however, during the second stage of pulmonary involvement without hypoxia (IIa) and through phase IIb (by the end of which viral invasion has reached its minimum) through to the hyperinflammatory phase (stage III). Besides viral-mediated injury, bystander pathology of cells may be due to the influx of inflammatory neutrophils and monocytes. Calpeptin, as a calpain inhibitor can block integrin-mediated cell detachment [51]. It could therefore further block the infiltration of inflammatory cells (Figure 2B). Of note, calpain inhibition modulated cell migration (in tumor metastasis) by decreasing retraction of the rear of the cell by stabilizing linkages between integrins and the cytoskeleton [52].
Calpeptin-mediated inhibition of EMT in pulmonary fibrosis
Following infection (or injury) to epithelial cells and subsequent inflammation and cell migration, fiboblast proliferation and differentiation into myofibroblasts by EMT is another potential target for antifibrotic therapy. An important recent investigation into potential therapies for TGF-β-induced fibrosis found that whilst translation of calpain 9 (CAPN9) induced by TGF-β caused myofibroblast differentiation in wild-type mice, Capn9−/− mice, lacking CAPN9, were protected from fibrosis induced in heart, liver and lung [53]. Calpains, as cysteine proteinases that mediate Ca2+-dependent proteolysis of E-cadherin, are important contributors to organ fibrosis. In a mouse model of bleomycin (BLM)-induced PF, calpeptin inhibited IL-6, angiopoietin-1 and TGF-β1 production and fibrosis (attributed to collagen deposition) [46]. In other work, inhibition of calpain activity and ERK1/2 signaling in mice, reduced BLM-induced PF, supposedly through inhibition of EMT [54] (Figure 2C). This followed similar work where calpeptin treatment of BLM-induced PF in mice had been found to be antifibrotic through reduced EMT and TGF-β1-Smad2/3 signaling [55].
Extracellular vesicle release in severe COVID-19 as a contributory factor in pulmonary fibrosis
A more recently considered factor contributing to fibrosis is that of EVs through disruptions in wound healing. Indeed, a recently described contributor to the pathology of PF was WNT-5a-mediated signaling via EVs, which stimulated fibroblast proliferation [56]. Furthermore, fibronectin (FN) expressed on the surface of these EVs, stimulates, at least in vitro, integrin α5β1 signaling and pathological fibroblast remodeling (Figure 2D). This is manifest as invasion and activation [17]. Part of the increased level of EVs, which is characteristic of severe COVID-19 and associated ARDS, is due to endothelial injury, whether released from the pulmonary capillary vasculature (angiotensin-converting enzyme+ [ACE+] [57]; von Willebrand Factor− [vWF−] [58]) or systemic vasculature (ACE−; vWF+). EVs play a crucial role in the pathogenesis of PF. In vivo work has shown EVs release from injured endothelial cells to help develop PF [59]. This has also been supported by EV release from proinflammatory M2 alveolar macrophages [60]. Targeted pharmacological inhibition of EV biogenesis, as referred to above, may thus contribute to the growing arsenal of therapeutic interventions against COVID-19.
Possible side-effects of calpeptin therapy, from in vivo studies
Calpains promote inflammation by a number of mechanisms leading to NF-κB activation and production of proinflammatory cytokines. They also result in recruitment of inflammatory cells and migration (as evidenced by calpain inhibition blocking integrin-mediated detachment of cells [51]). Furthermore, calpains increase leukocyte–endothelium interaction and thus plasma extravasation and diapedesis of inflammatory cells and this chronic inflammatory response eventually promotes fibrotic lesions. As mentioned earlier, there is considerable evidence in support of calpain inhibition as a means of protecting against tissue damage due to chronic inflammation. However, besides other positive effects, there is also evidence from preclinical studies of various detrimental effects [61].
By way of example of tolerated side-effects of calpeptin therapy, inhibition of calpain in preclinical models revealed itself to be neuroprotective following cancer chemotherapy [62], with no long-term detrimental side-effects. Furthermore, in mice, calpain-1 and -2 deficiency due to a tissue-specific or ubiquitous gene knockdown of CAPNS1 was tolerated.
In terms of detrimental side-effects, CAPNS1 knockout in aged mice and calpain-1 and -2 deficiency in muscle, resulted in dystrophy [63]. In mouse knockouts, muscular dystrophy was also caused by inhibition of calpain-3 [64]. Calpain-1 deficiency in mice and humans (due to CAPN1 mutation) helped bring about ataxia [65]. In other work, CAPN1 knockdown in mice affected platelet aggregation but with no adverse effect on bleeding times [66].
Conclusions and perspectives
This article has described the inhibition of EV biogenesis as a way of limiting viral cell-to-cell transmission. Depending on the EV biogenesis pathway being targeted, there may be added benefits, especially in ameliorating PF in COVID-19, as mentioned above. Treating COVID-19 with calpain inhibitors such as calpeptin, a potent inhibitor or EV release [29,30] will provide not only antiviral activity but also potentially attenuate NET formation, inhibit EMT [55], chronic inflammation and PF. Although the focus has been on calpeptin, other EV inhibitors such as GW4869, which inhibits nSMase- (neutral sphingomyelinase-) mediated deformation of the PM (and was effective in limiting Zika viral infection [67]), may also be considered. This is because GW4869 can also reduce TNF-α release from macrophages [68] important in post-COVID-19 where TNF-α is a key inflammatory cytokine in associated ARDS and PF [69].
This perspective has summarized four significant roles of calpain inhibition in COVID-19, using the peptidomimetic calpain inhibitor, calpeptin. As a prospective drug, calpeptin has low toxicity having been tolerated in mice for up to 4 weeks [70]. Although calpeptin is not currently in clinical trials as a treatment for COVID-19, BLD-2660, a synthetic, small molecule inhibitor against calpain 1, 2 and 9 is in Phase 2 clinical trials to reduce IL-6 levels and attenuate fibrotic damage [71]. Moreover, calpeptin or other calpain inhibitors have been in clinical trials for a host of other conditions [72] or will be, having shown recent efficacy in preclinical studies [73]. Another potential therapy for post-COVID-19 PF is the use of mesenchymal stem cell-derived EVs [74]. However, since much effort has been put into finding new, isoform-specific calpain inhibitors [75], with many of these also in clinical trials [13,73], drug repurposing of such selective inhibitors seems particularly advantageous and should be pursued to treat not just acute COVID-19 but also to manage the long-term effects of post-COVID-19.
Abbreviations
- ACE
angiotensin-converting enzyme
- ARDS
acute respiratory distress syndrome
- BLM
bleomycin
- CAPN9
calpain 9
- EVs
extracellular vesicles
- FN
fibronectin
- MVB
multivesicular bodies
- NET
neutrophil extracellular trap
- NF-κβ
nuclear factor-kappa B
- PAD4
peptidyl arginine deiminase 4
- PF
pulmonary fibrosis
- RBD
receptor binding domain
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- TNF-α
Tumour Necrosis Factor-alpha
Data Availability
Data sharing is not applicable to this paper as it is a perspective article and there are no data.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
CRediT Author Contribution
Jameel Inal: Conceptualization, Formal analysis, Supervision, Investigation, Writing—original draft, Project administration, Writing—review & editing. Ainura Paizuldaeva: Investigation, Writing—original draft, Writing—review & editing. Esmeralda Terziu: Investigation, Writing—original draft, Writing—review & editing.
References
- 1.Das A., Roy S., Swarnakar S. and Chatterjee N. (2021) Understanding the immunological aspects of SARS-CoV-2 causing COVID-19 pandemic: A therapeutic approach. Clin. Immunol. 231, 108804 10.1016/j.clim.2021.108804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavillegrand J.R., Garnier M., Spaeth A., Mario N., Hariri G., Pilon A.et al. (2021) Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients. Ann. Intensive Care 11, 9 10.1186/s13613-020-00798-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustine J.N. and Jones D. (2021) Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 191, 4–17 10.1016/j.ajpath.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M. and Lindskog C. (2020) The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 16, e9610 10.15252/msb.20209610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinnon K.H., Leist S.R., Okuda K., Dang H., Fritch E.J., Gully K.L.et al., SARS-CoV-2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci. Transl. Med. 0, eabo5070 10.1126/scitranslmed.abo5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara F., Granata G., Pelliccia C., La Porta R. and Vitiello A. (2020) The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2: Anti-inflammatory and anti-fibrotic therapy could solve the lung complications of the infection? Eur. J. Clin. Pharmacol. 76, 1615–1618 10.1007/s00228-020-02947-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahilog Z., Zhao H., Wu L., Alam A., Eguchi S., Weng H.et al. (2020) The role of neutrophil NETosis in organ injury: novel inflammatory cell death mechanisms. Inflammation 43, 2021–2032 10.1007/s10753-020-01294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzas E.I., György B., Nagy G., Falus A. and Gay S. (2014) Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10, 356–364 10.1038/nrrheum.2014.19 [DOI] [PubMed] [Google Scholar]
- 9.Lanyu Z. and Feilong H. (2019) Emerging role of extracellular vesicles in lung injury and inflammation. Biomed. Pharmacother. 113, 108748 10.1016/j.biopha.2019.108748 [DOI] [PubMed] [Google Scholar]
- 10.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L. and Bates P. (2005) Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. PNAS 102, 11876–11881 10.1073/pnas.0505577102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao M.M., Yang W.L., Yang F.Y., Zhang L., Huang W.J., Hou W.et al. (2021) Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Targeted Ther. 6, 134 10.1038/s41392-021-00558-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T., Luo S., Libby P. and Shi G.P. (2020) Cathepsin L-selective inhibitors: a potentially promising treatment for COVID-19 patients. Pharmacol. Therapeut. 213, 107587 10.1016/j.pharmthera.2020.107587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mediouni S., Mou H., Otsuka Y., Jablonski J.A., Adcock R.S., Batra L.et al. (2022) Identification of potent small molecule inhibitors of SARS-CoV-2 entry. SLAS Discov. 27, 8–19 10.1016/j.slasd.2021.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Günther S., Reinke P.Y.A., Fernández-García Y., Lieske J., Lane T.J., Ginn H.M.et al. (2021) X-ray screening identifies active site and allosteric inhibitors of SARS-CoV-2 main protease. Science (New York, N.Y.) 372, 642–646 10.1126/science.abf7945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu M., Pradhan M., Gorshkov K., Petersen J.D., Shen M., Guo H.et al. (2022) A high throughput screening assay for inhibitors of SARS-CoV-2 pseudotyped particle entry. SLAS Discov. 27, 86–94 10.1016/j.slasd.2021.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C.Z., Xu M., Pradhan M., Gorshkov K., Petersen J.D., Straus M.R.et al. (2020) Identifying SARS-CoV-2 entry inhibitors through drug repurposing screens of SARS-S and MERS-S pseudotyped particles. ACS Pharmacol. Transl. Sci. 3, 1165–1175 10.1021/acsptsci.0c00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L.et al. (2020) Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586, 113–119 10.1038/s41586-020-2577-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacco M.D., Ma C., Lagarias P., Gao A., Townsend J.A., Meng X.et al. (2020) Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M(pro) and cathepsin L. Sci. Adv. 6, eabe0751 10.1126/sciadv.abe0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C., Sacco M.D., Hurst B., Townsend J.A., Hu Y., Szeto T.et al. (2020) Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 30, 678–692 10.1038/s41422-020-0356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y., Ma C., Szeto T., Hurst B., Tarbet B. and Wang J. (2021) Boceprevir, calpain inhibitors II and XII, and GC-376 have broad-spectrum antiviral activity against coronaviruses. ACS Infect. Dis. 7, 586–597 10.1021/acsinfecdis.0c00761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R.et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Sousa K.P., Rossi I., Abdullahi M., Ramirez M.I., Stratton D. and Inal J.M. (2022) Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. e1835 10.1002/wnan.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inal J.M., Ansa-Addo E.A. and Lange S. (2013) Interplay of host-pathogen microvesicles and their role in infectious disease. Biochem. Soc. Trans. 41, 258–262 10.1042/BST20120257 [DOI] [PubMed] [Google Scholar]
- 24.Nolte-'t Hoen E., Cremer T., Gallo R.C. and Margolis L.B. (2016) Extracellular vesicles and viruses: are they close relatives? PNAS 113, 9155–9161 10.1073/pnas.1605146113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inal J.M. and Jorfi S. (2013) Coxsackievirus B transmission and possible new roles for extracellular vesicles. Biochem. Soc. Trans. 41, 299–302 10.1042/BST20120272 [DOI] [PubMed] [Google Scholar]
- 26.Ghosh S., Dellibovi-Ragheb T.A., Kerviel A., Pak E., Qiu Q., Fisher M.et al. (2020) β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell 183, 1520.e14–1535.e14 10.1016/j.cell.2020.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inal J. (2020) COVID-19 comorbidities, associated procoagulant extracellular vesicles and venous thromboembolisms: a possible link with ethnicity? Br. J. Haematol. 190, e218–e220 10.1111/bjh.17011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inal J. (2020) Complement-mediated extracellular vesicle release as a measure of endothelial dysfunction and prognostic marker for COVID-19 in peripheral blood - Letter to the Editor. Clin. Hemorheol. Microcirc. 75, 383–386 10.3233/CH-200958 [DOI] [PubMed] [Google Scholar]
- 29.Catalano M. and O'Driscoll L. (2020) Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. Vesicles 9, 1703244 10.1080/20013078.2019.1703244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosgodage U.S., Trindade R.P., Thompson P.R., Inal J.M. and Lange S. (2017) Chloramidine/Bisindolylmaleimide-I-mediated inhibition of exosome and microvesicle release and enhanced efficacy of cancer chemotherapy. Int. J. Mol. Sci. 18, 1007 10.3390/ijms18051007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kongsomros S., Suksatu A., Kanjanasirirat P., Manopwisedjaroen S., Prasongtanakij S., Jearawuttanakul K.et al. (2021) Anti-SARS-CoV-2 activity of extracellular vesicle inhibitors: screening, validation, and combination with remdesivir. Biomedicines 9, 1230 10.3390/biomedicines9091230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco S.J. and Huttenlocher A. (2005) Regulating cell migration: calpains make the cut. J. Cell Sci. 118, 3829–3838 10.1242/jcs.02562 [DOI] [PubMed] [Google Scholar]
- 33.Mallick R.L., Kumari S., Singh N., Sonkar V.K. and Dash D. (2015) Prion protein fragment (106-126) induces prothrombotic state by raising platelet intracellular calcium and microparticle release. Cell Calcium 57, 300–311 10.1016/j.ceca.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 34.Jorfi S., Ansa-Addo E.A., Kholia S., Stratton D., Valley S., Lange S.et al. (2015) Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci. Rep. 5, 13006 10.1038/srep13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnard D.L., Hubbard V.D., Burton J., Smee D.F., Morrey J.D., Otto M.J.et al. (2004) Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and beta-D-N4-hydroxycytidine. Antiviral Chem. Chemother. 15, 15–22 10.1177/095632020401500102 [DOI] [PubMed] [Google Scholar]
- 36.Suzuki M., Ikari J., Anazawa R., Tanaka N., Katsumata Y., Shimada A.et al. (2020) PAD4 deficiency improves bleomycin-induced neutrophil extracellular traps and fibrosis in mouse lung. Am. J. Respir. Cell Mol. Biol. 63, 806–818 10.1165/rcmb.2019-0433OC [DOI] [PubMed] [Google Scholar]
- 37.Pandolfi L., Bozzini S., Frangipane V., Percivalle E., De Luigi A., Violatto M.B.et al. (2021) Neutrophil extracellular traps induce the epithelial-mesenchymal transition: implications in post-COVID-19 fibrosis. Front. Imchengmunol. 12, 663303 10.3389/fimmu.2021.663303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A.et al. (2020) Neutrophil extracellular traps in COVID-19. JCI Insight 5, e138999 10.1172/jci.insight.138999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arisan E.D., Uysal-Onganer P. and Lange S. (2020) Putative roles for peptidylarginine deiminases in COVID-19. Int. J. Mol. Sci. 21, 4662 10.3390/ijms21134662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Y., Chen X. and Liu X. (2022) NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and beyond. Front. Immunol. 13, 838011 10.3389/fimmu.2022.838011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gößwein S., Lindemann A., Mahajan A., Maueröder C., Martini E., Patankar J.et al. (2019) Citrullination licenses calpain to decondense nuclei in neutrophil extracellular trap formation. Front. Immunol. 10, 2481 10.3389/fimmu.2019.02481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S.et al. (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 180, 934–943 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spagnolo P., Balestro E., Aliberti S., Cocconcelli E., Biondini D., Casa G.D.et al. (2020) Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respiratory Med. 8, 750–752 10.1016/S2213-2600(20)30222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zemans R.L., Colgan S.P. and Downey G.P. (2009) Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am. J. Respir. Cell Mol. Biol. 40, 519–535 10.1165/rcmb.2008-0348TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George P.M., Wells A.U. and Jenkins R.G. (2020) Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respiratory Med. 8, 807–815 10.1016/S2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabata C., Tabata R. and Nakano T. (2010) The calpain inhibitor calpeptin prevents bleomycin-induced pulmonary fibrosis in mice. Clin. Exp. Immunol. 162, 560–567 10.1111/j.1365-2249.2010.04257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wynn T.A. (2011) Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 208, 1339–1350 10.1084/jem.20110551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunz S., Niederberger E., Ehnert C., Coste O., Pfenninger A., Kruip J.et al. (2004) The calpain inhibitor MDL 28170 prevents inflammation-induced neurofilament light chain breakdown in the spinal cord and reduces thermal hyperalgesia. Pain 110, 409–418 10.1016/j.pain.2004.04.031 [DOI] [PubMed] [Google Scholar]
- 49.Muniappan L., Javidan A., Jiang W., Mohammadmoradi S., Moorleghen J.J., Katz W.S.et al. (2017) Calpain inhibition attenuates adipose tissue inflammation and fibrosis in diet-induced obese mice. Sci. Rep. 7, 14398 10.1038/s41598-017-14719-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siddiqi H.K. and Mehra M.R. (2020) COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung Transpl. 39, 405–407 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huttenlocher A., Palecek S.P., Lu Q., Zhang W., Mellgren R.L., Lauffenburger D.A.et al. (1997) Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 272, 32719–32722 10.1074/jbc.272.52.32719 [DOI] [PubMed] [Google Scholar]
- 52.Schoenwaelder S.M., Yuan Y., Cooray P., Salem H.H. and Jackson S.P. (1997) Calpain cleavage of focal adhesion proteins regulates the cytoskeletal attachment of integrin alphaIIbbeta3 (platelet glycoprotein IIb/IIIa) and the cellular retraction of fibrin clots. J. Biol. Chem. 272, 1694–1702 10.1074/jbc.272.3.1694 [DOI] [PubMed] [Google Scholar]
- 53.Kim D.H., Beckett J.D., Nagpal V., Seman-Senderos M.A., Gould R.A., Creamer T.J.et al. (2019) Calpain 9 as a therapeutic target in TGFβ-induced mesenchymal transition and fibrosis. Sci. Transl. Med. 11, eaau2814 10.1126/scitranslmed.aau2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou M., Zhang G., Zou J., Liu Y., Liu B., Hu X.et al. (2020) Inhibition of the ERK1/2-ubiquitous calpains pathway attenuates experimental pulmonary fibrosis in vivo and in vitro. Exp. Cell. Res. 391, 111886 10.1016/j.yexcr.2020.111886 [DOI] [PubMed] [Google Scholar]
- 55.Liu Y., Liu B., Zhang G.Q., Zou J.F., Zou M.L. and Cheng Z.S. (2018) Calpain inhibition attenuates bleomycin-induced pulmonary fibrosis via switching the development of epithelial-mesenchymal transition. Naunyn-Schmiedeberg’s Arch. Pharmacol. 391, 695–704 10.1007/s00210-018-1499-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin-Medina A., Lehmann M., Burgy O., Hermann S., Baarsma H.A., Wagner D.E.et al. (2018) Increased extracellular vesicles mediate WNT5A signaling in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 198, 1527–1538 10.1164/rccm.201708-1580OC [DOI] [PubMed] [Google Scholar]
- 57.Gordon C., Gudi K., Krause A., Sackrowitz R., Harvey B.G., Strulovici-Barel Y.et al. (2011) Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am. J. Respir. Crit. Care Med. 184, 224–232 10.1164/rccm.201012-2061OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Müller A.M., Hermanns M.I., Skrzynski C., Nesslinger M., Müller K.M. and Kirkpatrick C.J. (2002) Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp. Mol. Pathol. 72, 221–229 10.1006/exmp.2002.2424 [DOI] [PubMed] [Google Scholar]
- 59.Xie H., Gao Y.M., Zhang Y.C., Jia M.W., Peng F., Meng Q.H.et al. (2020) Low let-7d exosomes from pulmonary vascular endothelial cells drive lung pericyte fibrosis through the TGFβRI/FoxM1/Smad/β-catenin pathway. J. Cell. Mol. Med. 24, 13913–13926 10.1111/jcmm.15989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao M.Y., Zhang W.H., Ma W.T., Liu Q.H., Xing L.H. and Zhao G.F. (2019) microRNA-328 in exosomes derived from M2 macrophages exerts a promotive effect on the progression of pulmonary fibrosis via FAM13A in a rat model. Experiment. Mol. Med. 51, 1–16 10.1038/s12276-019-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji J., Su L. and Liu Z. (2016) Critical role of calpain in inflammation. Biomed. Rep. 5, 647–652 10.3892/br.2016.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cetinkaya-Fisgin A., Luan X., Reed N., Jeong Y.E., Oh B.C. and Hoke A. (2020) Cisplatin induced neurotoxicity is mediated by Sarm1 and calpain activation. Sci. Rep. 10, 21889 10.1038/s41598-020-78896-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piper A.K., Sophocleous R.A., Ross S.E., Evesson F.J., Saleh O., Bournazos A.et al. (2020) Loss of calpains-1 and -2 prevents repair of plasma membrane scrape injuries, but not small pores, and induces a severe muscular dystrophy. Am. J. Physiol. Cell Physiol. 318, C1226–C1237 10.1152/ajpcell.00408.2019 [DOI] [PubMed] [Google Scholar]
- 64.Richard I., Broux O., Allamand V., Fougerousse F., Chiannilkulchai N., Bourg N.et al. (1995) Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 81, 27–40 10.1016/0092-8674(95)90368-2 [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Hersheson J., Lopez D., Hammer M., Liu Y., Lee K.H.et al. (2016) Defects in the CAPN1 gene result in alterations in cerebellar development and cerebellar ataxia in mice and humans. Cell Rep. 16, 79–91 10.1016/j.celrep.2016.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azam M., Andrabi S.S., Sahr K.E., Kamath L., Kuliopulos A. and Chishti A.H. (2001) Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol. Cell. Biol. 21, 2213–2220 10.1128/MCB.21.6.2213-2220.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Y., Li Y., Zhang H., Zhao R., Jing R., Xu Y.et al. (2018) Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov. 4, 19 10.1038/s41421-018-0017-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Essandoh K., Yang L., Wang X., Huang W., Qin D., Hao J.et al. (2015) Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim. Biophys. Acta 1852, 2362–2371 10.1016/j.bbadis.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malaviya R., Laskin J.D. and Laskin D.L. (2017) Anti-TNFα therapy in inflammatory lung diseases. Pharmacol. Therapeut. 180, 90–98 10.1016/j.pharmthera.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang C., Xu B., Ma Z., Liu C., Deng Y., Liu W.et al. (2017) Inhibition of calpains protects Mn-induced neurotransmitter release disorders in synaptosomes from mice: involvement of SNARE complex and synaptic vesicle fusion. Sci. Rep. 7, 3701 10.1038/s41598-017-04017-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Apaydın Ç.B., Çınar G. and Cihan-Üstündağ G. (2021) Small-molecule antiviral agents in ongoing clinical trials for COVID-19. Curr. Drug Targets 22, 1986–2005 10.2174/1389450122666210215112150 [DOI] [PubMed] [Google Scholar]
- 72.Zanetta C., Nizzardo M., Simone C., Monguzzi E., Bresolin N., Comi G.P.et al. (2014) Molecular therapeutic strategies for spinal muscular atrophies: current and future clinical trials. Clin. Ther. 36, 128–140 10.1016/j.clinthera.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 73.Robinson K.J., Yuan K., Plenderleith S.K., Watchon M. and Laird A.S. (2021) A novel calpain inhibitor compound has protective effects on a zebrafish model of spinocerebellar ataxia type 3. Cells 10, 2592 10.3390/cells10102592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bazdyrev E., Rusina P., Panova M., Novikov F., Grishagin I. and Nebolsin V. (2021) Lung fibrosis after COVID-19: treatment prospects. Pharmaceuticals (Basel, Switzerland) 14, 807 10.3390/ph14080807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donkor I.O. (2015) An updated patent review of calpain inhibitors (2012 - 2014). Expert Opin. Ther. Pat. 25, 17–31 10.1517/13543776.2014.982534 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this paper as it is a perspective article and there are no data.