ABSTRACT
Antibiotic use drives antimicrobial resistance (AMR). The Antimicrobial Review Kit (ARK) study is a complex intervention based on national antibiotic stewardship guidance. We describe the implementation of ARK at a 760-bed teaching hospital that uses electronic prescribing. An online education module was disseminated to healthcare workers, and the ARK decision tool was incorporated into the medical clerking pro forma. From July 2018, junior doctors audited the frequency, the outcomes of pre-72-hour antibiotic reviews and the use of the ARK tool. The data were used to formulate specialty-level feedback and bench marking. First-phase data were plotted on statistical process control (SPC) charts to distinguish between common and special cause variation. There was significant improvement in antibiotic review rates (81% to 93%) and stop rates (10% to 15%). The stop rate reached 25% in the most recent data. Given the promising trends, it may be possible to achieve the target stop rate of 30%.
KEYWORDS: antimicrobial, stewardship, resistance, ARK, antibiotic
Introduction
Antimicrobial resistance (AMR) is a global threat to modern healthcare with the estimated number of sepsis cases caused by pathogens resistant to one or more key antibiotics increasing by 35% in England between 2013 and 2017.1,2 In response to the threat of AMR, the UK government has an ambition to reduce antimicrobial use in humans by 15% by 2024.3
In 2012, the Department of Health published Start smart - then focus (SSTF), which provides UK hospitals with a framework for prudent antibiotic prescribing.4 SSTF recommends an antibiotic review within the first 72 hours of initiation and recommends stopping antibiotics at this stage if infection is an unlikely cause for the patient's symptoms.5 Early review and cessation of antibiotic therapy may reduce antibiotic use in hospitals and contribute to reducing the risk of antimicrobial resistance.6
The Antimicrobial Review Kit (ARK) was informed by SSTF principals, providing a simplified approach to rationalise antibiotic decision making and includes a decision tool (simple communication tool of infection diagnosis certainty/uncertainty), an education module describing the aims of ARK, patient information leaflets, audit data collection tools and recommendations for developing a team of healthcare workers to implement the ARK intervention.7,8
The ARK feasibility study, using a paper prescribing system, successfully increased the pre-72-hour antibiotic cessation rates (time from antibiotic initiation to stop) from 9% to 36%.8 The 72-hour time frame was chosen as it is a reasonable time to have completed investigations and to have received medical imaging reports, biochemistry and microbiology results in order to make a final diagnosis and to stop antibiotics if infection is an unlikely cause of symptoms. We adopted these principles in a hospital that uses an electronic prescribing and medication administration system (EPMA; WellSky, JAC Computer Services, Basildon, UK). Local audit data showed the pre-72-hour antibiotic review stop rate to be 8% at baseline in the Royal Cornwall Hospitals NHS Trust (RCHT), comparable to the rates reported in other English hospitals.9
This local quality improvement project was part of a multi-centre stepped-wedge randomised controlled trial.8 Herein, we discuss the methods and findings of the ARK project at RCHT from its launch in May 2018 to December 2019 (Fig 1).
Fig 1.
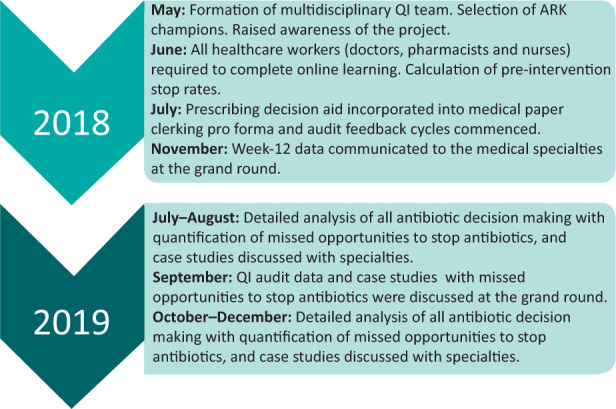
Stages of Antimicrobial Review Kit project at Royal Cornwall Hospitals NHS Trust from its launch in May 2018 to December 2019. ARK = Antimicrobial Review Kit; QI = quality improvement.
Aims
There are three specific aims of ARK for the Royal Cornwall Hospital based on the study protocol and the outcomes of the ARK feasibility study.8
Aim 1: The decision tool (‘possible or probable’) completed for 80% of patients started on antibiotics in medical specialties (process measure).
Aim 2: >90% of patients with evidence of a pre-72-hour antibiotic review in the medical notes (process measure).
Aim 3: Pre-72-hour antibiotic cessation rates of 30% (outcome measure).
Materials and methods
Context
The study hospital is a 760-bed district general hospital serving a population of 450,000 people. The EPMA is deployed in all inpatient areas. The hospital has a mature antibiotic stewardship programme, which requires all antibiotic prescriptions to include an indication as well as a review or stop date (although this is not mandated on the electronic prescribing system). Compliance is regularly audited and maintained at approximately 65% for both metrics. When measured in defined daily doses (DDD) of antibiotics adjusted for hospital admission rate, the study hospital (when compared with other, non-teaching hospitals in England) is ranked in the lowest quintile.10 Since the launch of ARK in July 2018, the hospital has also implemented the National Institute for Health and Care Excellence (NICE) guidelines for common infections in medical specialties and the emergency department after an audit identified opportunities for improvement in antibiotic use through course length optimisation.11,12 The NICE sepsis guidelines were implemented at the study hospital in January 2019.13
Measures
Pre-intervention data were collected on 22 June 2018, 1 week prior to the launch date for ARK (02 July 2018), to calculate the baseline pre-72-hour review rates and stop rates. The following data were collected by the audit team: patient hospital number, date of birth, gender, date antibiotic prescription started, the indication for the antibiotic, the assigned ARK decision tool category, whether the antibiotic was reviewed within 72 hours, the outcome of the antibiotic review (stop, change route, switched agent or no change), the medical specialty responsible for completion of the decision tool and the antibiotic review. Thereafter, point prevalence data collection was repeated on the 09 July 2018 (1 week post-launch of ARK), then weekly for 4 weeks, then fortnightly for two audit cycles and then approximately monthly thereafter. During the point prevalence audits, the audit team reviewed all inpatients on participating wards who were receiving antibiotics at the time of the audit or had received antibiotics at any time during their inpatient stay (1 day snapshot). The audit data were checked by the local ARK study principal investigator for accuracy and consistency and grouped by specialty.
Process measures collected during the audit were the proportion of completion of the decision tool (‘possible or probable’) in the post-take ward round notes (post-intervention only) and the proportion of patients with evidence of a pre-72-hour antibiotic review in the medical notes. The outcome measure collected was the proportion of antibiotics stopped at the pre-72-hour antibiotic review. These measures were matched with the original ARK feasibility study.8
The ARK study required only one baseline audit prior to intervention implementation (completed on 22 June 2018). However, in order to produce the statistical process control (SPC) run charts, we required at least eight pre-intervention data points. Medical patients discharged from medical specialties during the first week of a month between January 2017 and June 2018 and who had received at least one dose of antibiotic were identified using the EPMA system. The medical notes for a pragmatic sample of 30 patients were requested in order to retrospectively determine the antibiotic review rates and antibiotic stop rates for these time points.
Interventions
In May 2018, a team of healthcare professionals from a variety of backgrounds was convened (Fig 1). The team included the principal investigator (PI) who was the lead antibiotic pharmacist in the hospital, an acute medical consultant, a medical microbiologist, the lead infection control nurse and a foundation year doctor. The team began by identifying a group of 48 ARK ‘champions’ in each of the medical specialties, which included medical consultants, senior nurses and pharmacists.
Stage 1: Raising awareness, study set-up and completion of the online education module
The team began by raising awareness of the project within the hospital via an introductory letter sent through internal email. The project was presented at meetings that reached a wide professional audience, including the grand round, the acute medical governance meeting and the senior nurse governance meeting in May 2018. This allowed participants to vocalise any concerns and to make suggestions to strengthen the project.
All medical healthcare staff (including nursing, medical and pharmacy) were requested, via email in June 2018, to complete the ARK 10-minute online learning, with a reminder sent 2 weeks later. At the time of the ARK launch, 40/48 (83%) champions and 247 other healthcare workers had completed the ARK online learning.
ARK was designed so that each antibiotic prescription at initiation is accompanied by the decision support tool. The hospital EPMA team were not able to incorporate this decision support tool into the electronic prescribing system. Instead, the decision tool was incorporated into the paper clerking pro forma and used simple tick boxes to communicate whether antibiotics were being prescribed for a possible or probable infection as in the ARK study (Fig 2). This decision tool was introduced on 02 July 2018. The ARK feasibility study also recommended a ‘hard stop’ on antibiotics at 72 hours, meaning the antibiotic automatically stopped after 72 hours and required a re-prescription if it was still required. We did not adopt this hard stop due to concerns about unintentional missed antibiotic doses.
Fig 2.

The decision tool incorporated into the paper clerking pro forma. Simple tick boxes to communicate whether antibiotics were being prescribed for a probable or a possible infection.
In June 2018, foundation year doctors and members of the antibiotic pharmacy team were recruited to undertake the pre-intervention and the post-implementation data collection. This involved a review of the physical paper patient notes and data for the three processes collected.
Whether the consultant indicated their level of diagnostic certainty/uncertainty of the infection by ticking the possible box or the probable box in the medical paper clerking pro forma (post-ARK implementation only).
Whether the antibiotic was reviewed within the first 72 hours with a documented antibiotic prescribing decision in the medical notes.
The outcome of that pre-72-hour review (primary aim to stop antibiotics if they were no longer indicated).
Stage 2: Audit feedback cycles
After each audit cycle, specialty-specific data were sent via email to consultants, senior nurses and pharmacists in each medical specialty, and the overall study data were emailed to the foundation doctors. The email highlighted the importance of early antibiotic review and, where possible, early cessation as well as promoting the ARK ‘possible or probable’ decision tool. The monthly audit data feedback to specialties continued after the 12-week point but the emails to junior doctors ceased at 12 weeks. This first intervention allowed for continued feedback and conversation between the ARK team and the target specialties, with the aim of optimising antibiotic cessation.
Stage 3: Open discussion of ARK study audit data at the grand round
In November 2018, the ARK study PI presented the first 12 weeks of ARK data to the grand round, highlighting the differences in the pre-72-hour stop rates between the medical specialties. Three medical specialties had not stopped any of the reviewed antibiotics over the first 12 weeks' audit cycle. Possible reasons for the differences between specialties were explored in an open forum during the grand round.
Stage 4: Individual case review to determine further opportunity to optimise antibiotic therapy
The inpatients receiving antibiotics on the medical wards between 24 July 2019 and 14 August 2019 were reviewed to quantify missed opportunities to stop antibiotics. The primary focus of the review was to determine whether antibiotics were still indicated after the 72 hours had elapsed, taking in to account the presenting, and the ongoing, signs and symptoms of infection, the inflammatory markers, medical imaging and National Early Warning Score 2 (NEWS2) value. One-hundred and fifty-five patients were reviewed, of which, 140 (90%) were deemed to have convincing evidence of bacterial infection at the pre-72-hour review, 10 (6%) were deemed unnecessary and could have been stopped, and for five (3%) the evidence of ongoing infection was weak or it was more difficult to determine whether the antibiotics should have been stopped. This provided evidence that there was potential to stop up to 9% more antibiotics than were being stopped at the pre-72-hour review. There was evidence of missed opportunities to stop antibiotics at the pre-72-hour review in all eight medical specialties. These data were discussed at the grand round in September 2019.
Stage 5: Repeat data collection and review case studies
In both October 2019 and December 2019, individual inpatient cases were reviewed to again determine missed opportunities to stop antibiotics at the pre-72-hour review. Case studies where antibiotics were continued when they could have been stopped were summarised, incorporated into a report and e-mailed to specialty leads. There remained a significant opportunity to further increase the pre-72-hour antibiotic stop rate. In October 2019, the actual stop rate was 14%, compared with a potential stop rate of 25%. In December 2019, the actual stop rate was 21%, compared with a potential stop rate of 42%.
Analysis
The data were analysed by SPC using Life QI (See Data, Exeter, UK). The statistical analysis, using Nelson's rules, would only trigger if the likelihood of a real change was over 99%.14 Data were then presented graphically as SPC charts.
Ethical considerations
This study was covered by the ethics approvals for the ARK study.8
Results
Between July 2018 and May 2020, we undertook 21 point-prevalence audits of 1,807 adult patients who were admitted to medical specialties and commenced antibiotics for a suspected bacterial infection. Data for age were missing in 24 patients. The median age for the remaining patients was 77.0 years (interquartile range (IQR) 50.0). Of the 1,807 patients, 898 (52.4%) were men, 783 (45.7%) were women and there were data missing for 34 (2.0%) patients.
The most common infection diagnoses were respiratory tract infections, which accounted for 816 (45.4%) patients' diagnoses, followed by unknown or unspecified source of infection for 264 (14.7%) patients, genitourinary infection for 247 (13.7%) patients, skin and soft tissue infection for 118 (6.6%) patients, gastrointestinal infection for 75 (4.2%) patients, hepatobiliary infection for 65 (3.6%) patients, no infection documented in audit form for 64 (3.6%) patients, cardiovascular system infection for 54 (3.0%) patients, bacteraemia without source for 37 (2.0%) patients, central nervous system infection for 28 (1.6%) patients, bone and joint infection for 22 (1.2%) patients, and other (eyes; ear, nose and throat; or dental) infection for 8 (0.4%) patients.
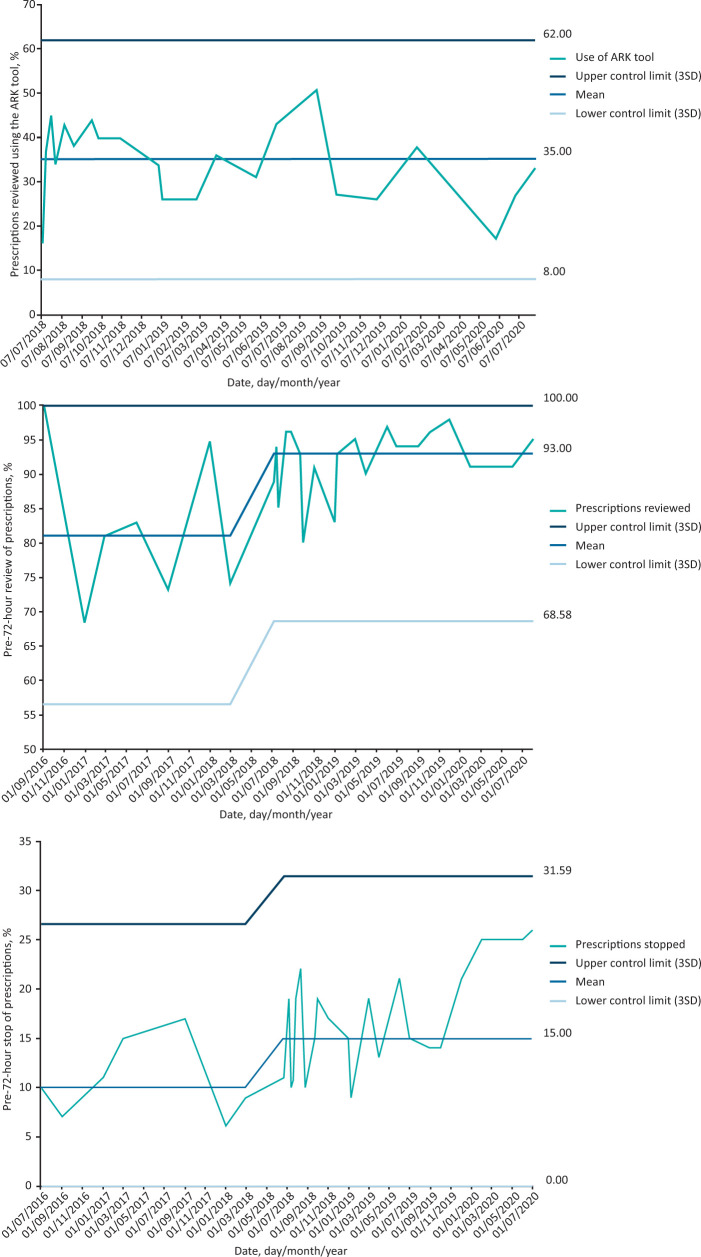
Aim 1 (decision tool): On average 35% of patients had evidence of completion of the ‘possible or probable’ decision support tool with no change in the uptake throughout the study period (Fig 3a).
Fig 3.
Statistical process control charts. a) Antibiotic prescriptions reviewed using the Antimicrobial Review Kit decision tool in the paper clerking pro forma. b) Pre-72-hour antibiotic review rates. c) Pre-72-hour antibiotic cessation rates. ARK = Antimicrobial Review Kit; SD = standard deviation.
Aim 2 (review rates): The percentage of antibiotics reviewed improved from 81% in the pre-intervention period to 93% post-August 2018 (Fig 3b).
Aim 3 (stop rates): The pre-72-hour antibiotic stop rate increased from 10% pre-intervention to 15% in the post-intervention period (Fig 3c). This rate increased to over 20% for the last 4 months, but not with a statistically significant change.
Discussion
Summary
Aim 1: The decision tool completed for 80% of patients started on antibiotics in medical specialties
Completion of the ARK ‘possible or probable’ decision support tool remained low, averaging 35%, and below the target completion rate of 80%. The decision tool was originally designed to be part of the paper prescription chart, thereby forcing the prescriber to indicate diagnostic uncertainty at the time of prescribing. We were unable to fully implement the decision tool into our electronic prescribing system, nor safely incorporate the hard stop.
Aim 2: >90% of patients with evidence of a pre-72-hour antibiotic review in the medical notes
Implementation of the ARK intervention significantly increased the pre-72-hour antibiotic review rate from 81% to 93% and the pre-72-hour antibiotic stop rate from 10% to 15% (which increased to over 20% for the last 4 months but not with a statistically significant change; further follow-up data would be needed to demonstrate whether this is a real improvement).
Aim 3: Pre-72-hour antibiotic cessation rates of 30%
Pre-72-hour antibiotic cessation rates were 15%–25%. This did not meet the pre-defined aim of 30%.
Interpretation
We successfully deployed several key elements of the ARK study toolkit that included the online education and the regular sustained audit feedback cycles. We were unsuccessful with embedding the ‘possible or probable’ decision tool due to incompatibilities with our EPMA system. Implementing ARK resulted in significant improvements in antibiotic stewardship in medical specialties despite poor uptake of the decision support tool. In response to teleconferences with several ARK study PIs, the chief investigator and staff members of Well Sky, the EPMA system has been amended to enable the incorporation of ARK into our EPMA. With the release of this updated version of the EPMA system, we expect to be able to optimise the ARK intervention at the study hospital.
Limitations
This is a single-centre study, therefore, our results may not be generalisable to other hospitals. The multi-centre ARK study will show whether our findings are similar across different UK hospitals.
We retrospectively collected pre-intervention pre-72-hour review and stop rates, and in a smaller sample size than the prospective audits: only 30 patients compared with 86 patients per cycle in the prospective ARK audits. Collecting pre-intervention data via a different method, and in a reduced number of patients at each data point, may influence the findings. Additionally, there was a 3-month preparation period at the study hospital before the launch of ARK. This included raising awareness of the study and completion of online training, both potentially increasing the pre-intervention antibiotic review and stop rates. We do not have a denominator for the number of healthcare professionals requested to complete training, so we cannot determine the reach of online training. Reassuringly the 10% stop rate pre-ARK is in keeping with other antibiotic stewardship audits during this period which revealed an antibiotic stop rate of 8%–10%.
Although we found an opportunity to further increase the pre-72-hour antibiotic stop rate, determining appropriateness of antibiotic prescribing is potentially subjective. The process could be made more objective by using a standardised audit tool and by triangulating the opinions of specialists from a variety of backgrounds, not just exclusively infection specialists.15
We were not able to control for other concurrent interventions during the study period. The NICE sepsis guidelines were implemented in our hospital during the ARK intervention period.12 Antibiotic course length optimisation in line with NICE guidelines also started during the study period following an audit that demonstrated opportunities to optimise course lengths.15 We did not determine whether the intervention impacted on patient outcome measures, such as antibiotic use and mortality, due to the potential for multiple confounders for which we would be unable to correct. The multi-centre ARK study is designed to show whether the ARK intervention impacts patient outcomes.
The EPMA system did not accommodate the ‘possible or probable’ decision support tool, instead, we adopted a paper version of the tool. Also, the ARK team were not confident of the safety of the 72-hour hard stop within the current version of EPMA. Non-adoption of these two components of the ARK intervention may have impeded optimisation of the intervention. Quality improvement is an iterative process, and we will reconsider these with the new version of EPMA that has been updated to facilitate ARK (due for release in summer 2022).
Conclusion
The ARK intervention was associated with a significant increase in both the pre-72-hour review rate and the pre-72-hour stop rate. However, no change was seen in the use of the ‘possible or probable’ decision tool. The implementation of the ARK intervention is complex and requires further optimisation at the study hospital. Optimisation would require a focus on embedding use of the ‘possible or probable’ decision tool and reconsidering the adoption of the 72-hour hard stop, both potentially facilitated by the forthcoming EPMA upgrade.
References
- 1.Public Health England . AMR local indicators – produced by the UKHSA. PHE, 2018. https://fingertips.phe.org.uk/profile/amr-local-indicators [Accessed 21 March 2022]. [Google Scholar]
- 2.World Health Organization . Antimicrobial resistance: global report on surveillance. WHO, 2014. https://apps.who.int/iris/bitstream/handle/10665/112647/WHO_HSE_PED_AIP_2014.2_eng.pdf?sequence=1 [Accessed 21 March 2022]. [Google Scholar]
- 3.HM Government . Tackling antimicrobial resistance 2019–2024. GOV.UK, 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf [Accessed 21 March 2022]. [Google Scholar]
- 4.Public Health England . Start smart - then focus: Antimicrobial stewardship toolkit for English hospitals. PHE, 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/417032/Start_Smart_Then_Focus_FINAL.PDF [Accessed 21 March 2022]. [Google Scholar]
- 5.Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J. Improving the quality of antibiotic prescribing in the NHS by developing a new antimicrobial stewardship programme: Start Smart - Then Focus. J Antimicrob Chemother 2012;67(Suppl 1):i51–63. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . The evolving threat of antimicrobial resistance: options for action. WHO, 2012. https://apps.who.int/iris/bitstream/handle/10665/44812/9789241503181_eng.pdf. [Accessed 28 March 2022]. [Google Scholar]
- 7.Santillo M, Sivyer K, Krusche A, et al. Intervention planning for Antibiotic Review Kit (ARK): a digital and behavioural intervention to safely review and reduce antibiotic prescriptions in acute and general medicine. J Antimicrob Chemother 2019;74:3362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross ELA, Sivyer K, Islam J, et al. Adaptation and implementation of the ARK (Antibiotic Review Kit) intervention to safely and substantially reduce antibiotic use in hospitals: A feasibility study. J Hosp Infect 2019;103:268–75. [DOI] [PubMed] [Google Scholar]
- 9.Public Health England . AMR local indicators – produced by the UKHSA. PHE, 2017. https://fingertips.phe.org.uk/profile/amr-local-indicators [Accessed 28 March 2022]. [Google Scholar]
- 10.Public Health England . AMR local indicators – produced by the UKHSA: antibiotic prescribing. PHE, 2019. https://fingertips.phe.org.uk/profile/amr-local-indicators/data#page/3/gid/1938132909/pat/158/par/NT_trust/ati/118/are/REF/iid/93555/age/1/sex/4 [Google Scholar]
- 11.National Institute for Health and Care Excellence. Summary of antimicrobial prescribing guidance – managing common infections. NICE, 2021. www.bnf.org/wp-content/uploads/2021/07/summary-antimicrobial-prescribing-guidance_july-21-for-BNF.pdf [Accessed 08 August 2022]. [Google Scholar]
- 12.Powell N, Stephens J, Rule R, et al. Potential to reduce antibiotic use in secondary care: Single-centre process audit of prescription duration using NICE guidance for common infections. Clin Med 2021;21:e39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Sepsis: recognition, diagnosis and early management: NICE guideline [NG51]. NICE, 2017. www.nice.org.uk/guidance/ng51 [Accessed 23 March 2022]. [PubMed] [Google Scholar]
- 14.Koutras MV, Bersimis S, Maravelakis PE. Statistical process control using Shewhart control charts with supplementary runs rules. Methodol Comput Appl Probab 2007;9:207–24. [Google Scholar]
- 15.Hood G, Hand KS, Cramp E, et al. Measuring appropriate antibiotic prescribing in acute hospitals: development of a national audit tool through a Delphi consensus. Antibiotics (Basel) 2019;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]