Abstract
We measured antibody binding to diverse norovirus virus-like particles over 12 months in 16 children. All had maternal antibodies at 2 months, with estimated lowest levels at 5 months of age. Antibody increases after 3 months suggested natural infections. This information could guide the timing of future norovirus vaccines.
Keywords: acute gastroenteritis, antibodies, immunity, infants, multiplex Luminex assay, norovirus, vaccination
Infants are born with IgG antibodies to multiple norovirus genotypes; waning immunity appears consistent with infection risk. Pediatric norovirus vaccines should be administered when titers are low enough to avoid interference from maternally acquired antibodies, but before antibodies have completely waned.
Norovirus causes approximately 70 000 child deaths per year, mainly in low-resource settings [1]. Despite its high burden of disease, little is known about the breadth and duration of maternally acquired immunoglobulin (IgG) antibodies in infants and the subsequent development of active immunity to norovirus. Identifying when infants are most vulnerable to infection can guide the timing of future pediatric norovirus vaccines, avoiding interference from maternally acquired antibodies yet providing maximal protection against disease.
It is difficult to obtain norovirus antigens to measure humoral immunity, as norovirus cannot be cultured in standard cell culture systems. Instead, virus-like particles (VLPs) are used for norovirus strain-specific antigen in immunological assays [2]. Multiplex Luminex immunoassays test binding to antigens attached to magnetic microspheres. This approach has been used in previous studies to efficiently measure binding to multiple antigens using a single sample [3]. The goal of this study was to assess the breadth and dynamics of serum antibodies specific to diverse norovirus strains in the first year of life.
MATERIALS AND METHODS
Study Design and Sample Collection
Remnant blood samples were obtained from 16 children in León, Nicaragua, who participated in an observational study of rotavirus vaccine immunogenicity between November 2014 and March 2015 [4]. The base study collected blood at 2 and 3 months of age. Children in the base study later entered a clinical trial of dietary supplementation with rice bran (1-5 g daily) vs the usual diet starting at 6 months of age, which included an additional blood collection at 12 months of age (NCT02615886) [5]. Blood was collected via venipuncture in a serum separation tube (HumaTube Serum Gel-C/A 73030, Human Diagnostics Worldwide, Germany), centrifuged to separate for serum, and stored at −80°C until analysis. Exclusions for both studies included prior hospitalization, known immunocompromising condition, and lack of rotavirus immunization per national guidelines. A diarrhea episode or receipt of antibiotics between 4 and 6 months of age was an additional exclusion for the rice bran study. The Institutional Review Boards (IRBs) at Colorado State University, Universidad Nacional Autónoma de Nicaragua, León (UNAN–León), and the University of North Carolina at Chapel Hill (UNC-CH) approved this study. We used additional samples following known infections to verify that the assay detects norovirus-specific antibodies from the SAGE Study, approved by IRBs at UNC-CH and UNAN–León.
Laboratory Methods
See the Supplementary Material for details on the reagents and methods used to measure norovirus antibody responses in infant blood samples. Briefly, heat-inactivated serum samples were diluted 1:100 and then serially diluted 1:4 to a final dilution of 1:1 638 400 in duplicate. Quality control serum obtained commercially (BioIVT, Westbury, NY) was run on each plate. Eighteen magnetic fluorescent Luminex microspheres (Luminex Corp; Austin, TX) covalently coupled with a distinct norovirus VLP were added to each sample dilution in a 96-well plate. Plates were incubated at 2-8°C overnight or at room temperature for 90 minutes, and then washed 2 times with wash buffer. Diluted anti-IgG-PE, anti-IgA-PE, or anti-IgM-PE detection antibody (SouthernBiotech, Birmingham, AL) was then added to each well and incubated on a plate shaker for 1 hour at room temperature. After another 2 wash cycles, beads were resuspended in sheath fluid (Luminex) and read on the Luminex FlexMap3D instrument to measure the median fluorescent intensity (MFI) from each of the VLP-coupled beads.
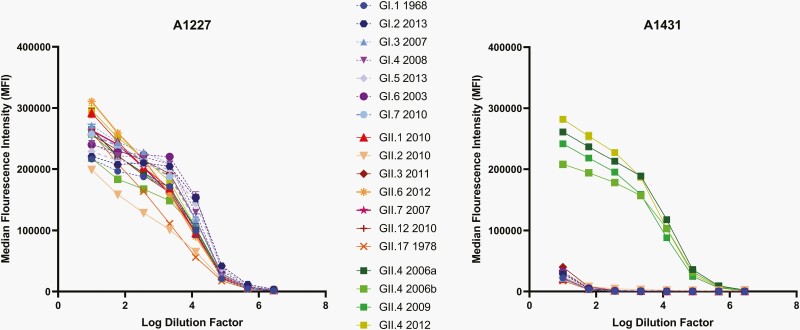
To calculate sample titers, 4-parameter sigmoidal logistic (4PL) dose-response curves were fit to plots of MFI duplicates vs the log of the sample dilution using Graphpad Prism 8.1 (Graphpad Software, San Diego, CA) (Figure 1). The top plateaus of the 4PL curves were constrained to 300 000 MFI in the IgG and IgM assays and 200 000 MFI in the IgA assay to facilitate curve fits for weak samples. A 50% effective concentration (EC50) was interpolated from the curves and reported as the sample titer.
Figure 1.
Test of multiplex Luminex assay specificity for 18 norovirus strains. A1227 is broadly cross-reactive to all norovirus genotypes, while A1431 is specific for the GII.4 norovirus genotype and shows cross-reactivity to multiple GII.4 strains.
Statistical Analysis
We summarized norovirus antibody titers at 2 (M2), 3 (M3), and 12 (M12) months for each child. Titers above the strain-specific lower limit of quantitation (LLOQ) at a given time were considered seropositive, otherwise seronegative. We identified presumed norovirus infections where a ≥4-fold increase in titer was observed between M3 and M12. Titers below the LLOQ at M3 were set to ½ the LLOQ.
We regressed the log2-transformed EC50s at M2 and M3 against the child’s age (days) when serum samples were collected. Child- and strain-specific regression model coefficients represented daily antibody decay rates. Decay rates were not estimated for norovirus strains that showed an increase in titer between M2 and M3, indicating an early norovirus infection, nor for strains with unquantifiable titer at M2. We did not include M12 titers in the models, as we could not rule out the possibility of unobserved norovirus infections between M3 and M12. We calculated maternal antibody half-life as the inverse of the absolute value of each strain-specific decay rate [6].
RESULTS
In the base study, 9 children received rice bran supplementation and 7 did not. Fifteen children were breastfed at M2: 2 were breastfed exclusively until the second month of life, 1 until the third month of life, and 2 until the fifth month of life. On average, mixed (breast and bottle) feeding was initiated before 2 months of age, and most children were not exclusively breastfed at M2 (Table 1). Presumed norovirus infections occurred in 6 children, of whom 4 received rice bran supplementation and 1 was exclusively breastfed until 5 months of age (Supplementary Figure 1).
Table 1.
Characteristics and Antibody Kinetics of 16 Children Enrolled in Rice Bran Supplementation Study
| Study ID | Received Rice Bran Supplementation | Breastfed at 2 mo of Age | Age Bottle Feeding Introduced (mo) | Presumed Norovirus Infection Between 3 and12 moa |
|---|---|---|---|---|
| A-11 | Y | Y | 5 | None |
| A-12 | Y | Y | 1 | None |
| A-14 | Y | Y | 2 | None |
| A-15 | N | Y | 3 | GII.3 |
| A-17 | N | Y | 1 | None |
| A-18 | N | Y | 1 | None |
| A-22 | N | Y | 1 | None |
| A-24 | N | Y | 1 | None |
| A-27 | Y | Y | 1 | GI.7 |
| B-02 | N | Y | 1 | GII.3, GII.17, GII.4 |
| B-04 | Y | Y | 5 | GII.3, GII.4 |
| B-06 | Y | Y | 2 | GII.6 |
| B-09 | Y | Y | 1 | None |
| B-12 | N | Y | 1 | None |
| B-15 | Y | N | 1 | None |
| B-17 | Y | Y | 1 | GII.4 |
Based on 4-fold or greater increase in genotype-specific titer between months 3 and 12, substituting ½ the lower limit of quantitation for unquantifiable titers.
Samples were tested for VLP binding by serum IgG, IgA, and IgM antibodies (Supplementary Tables 3 and 4). We found a broad IgG distribution at M2 and M3, which would be expected of maternally derived antibodies transferred across the placenta [7].
All 16 children provided serum samples at M2 and M3, and 13 children provided additional samples at M12. Most children (56%-100%) were seropositive for each norovirus strain at M2 (Supplementary Table 5). By M3, seroprevalence for most strains declined, and a relatively constant decay rate was observed across children and strains between M2 and M3 (Supplementary Figure 2).
To estimate antibody decay, we excluded one child (B-04) with unquantifiable titers at M3, and one child (B-12) with stable titers between M2 and M3 that heavily inflated the half-life. Across all 14 children, the mean antibody half-life was 25 days, and the mean duration of quantifiable titer was 161 days (5.3 months) (Supplementary Table 6).
DISCUSSION
We found that infants are born with presumed maternally acquired antibodies to multiple norovirus strains, which decay rapidly in the first months of life. The kinetics of presumed maternally acquired norovirus antibodies are similar to those from studies in the United Kingdom [8] and in India [9]. Six of 16 children experienced ≥4-fold increases in IgG antibodies to specific norovirus VLPs between 3 and 12 months of age, consistent with new infections. This finding aligns with an estimated 23 norovirus gastroenteritis episodes per 100 child-years reported in a large birth cohort at the same research site [10].
Infants showed unique VLP binding patterns early in life, likely reflecting the mother’s norovirus exposure history. Newly generated antibody responses between M3 and M12 were fairly specific to distinct genotypes, in contrast to broader responses to multiple genotypes observed in adults [11]. This finding supports those from previous studies showing that the breadth of norovirus immunity increases with repeated viral exposures, and thus with age.
Although norovirus exposure history is not available for this study, the MLA appears capable of identifying norovirus infections to the genotype level, and the IgG increases found at 12 months would indicate infections expected in children at this age. A limitation of this study is that the MLA measures antibody binding, rather than antibody neutralization. When compared with binding assays, surrogate neutralization assays that measure antibody-blockade of VLP-ligand binding better correlate with protection against norovirus disease [12].
While breastfeeding provides antibodies that may have provided mucosal protection against norovirus infections, serum antibodies acquired during pregnancy are possibly more informative correlates of protection in children who are not exclusively breastfed for 6 months as recommended by the World Health Organization. Rice bran supplementation starting at 6 months did not appear to correlate with protection against presumed norovirus infection in this small study. Larger studies and more precise measurements of infant feeding practices are needed to elucidate their protective mechanisms against enteric infections.
On average, maternally acquired serum antibodies would be expected to fall to unquantifiable levels by 5 months of age. Five months may be the optimal timing for pediatric norovirus vaccination, as the infant is at maximal risk of norovirus infection, and there should be minimal interference from maternally acquired serum antibodies on vaccine response. While these results use data from only 2 time points and lack the precision of more frequent sampling, the overall trends in antibody decay are consistently negative and of similar magnitude across children and strains. Additionally, the MLA, which can assess immunity to many VLPs using small serum volumes, could be considered for efficient measurement of infectious outcomes in future pediatric norovirus vaccine trials. In conclusion, this study adds to what is known about the breath and kinetics of norovirus-specific immunity in infancy and provides needed information to guide the timing of future pediatric norovirus vaccine schedules.
Supplementary Material
Notes
Acknowledgments . Assay development and sample testing were performed by Takeda Vaccines.
Financial support . Y. R. is supported by grant (D43TW010923) from the Fogarty International Center. S. B.-D. is supported by grants (R01AI127845 and K24AI141744) from the National Institute of Allergy and Infectious Diseases (NIAID). L. C. L. and R. S. B. are supported by National Institute of Allergy and Infectious Disease (grant number R01AI148260).
Potential conflicts of interest . L. C. L. and R. S. B. hold patents on norovirus vaccine design and ongoing collaborations with VaxArt, Takeda Vaccines, and HilleVax. R. S. B. is a member of the advisory committee for VaxArt. S. B.-D. has received investigator-initiated research awards from Takeda Vaccines unrelated to this report. The remaining authors reported no conflicts of interest.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Nadja A Vielot, Department of Family Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Amanda Brinkman, Takeda Vaccines, Inc., Cambridge, Massachusetts, USA.
Christina DeMaso, Takeda Vaccines, Inc., Cambridge, Massachusetts, USA.
Samuel Vilchez, Center of Infectious Diseases, Department of Microbiology and Parasitology, Faculty of Medical Sciences, National Autonomous University of Nicaragua, León, León, Nicaragua.
Lisa C Lindesmith, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Filemon Bucardo, Center of Infectious Diseases, Department of Microbiology and Parasitology, Faculty of Medical Sciences, National Autonomous University of Nicaragua, León, León, Nicaragua.
Yaoska Reyes, Center of Infectious Diseases, Department of Microbiology and Parasitology, Faculty of Medical Sciences, National Autonomous University of Nicaragua, León, León, Nicaragua.
Ralph S Baric, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Elizabeth P Ryan, Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, Colorado, USA.
Ralph Braun, Takeda Vaccines, Inc., Cambridge, Massachusetts, USA.
Sylvia Becker-Dreps, Department of Family Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
References
- 1. Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013; 8:e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrington PR, Yount B, Johnston RE, Davis N, Moe C, Baric RS. Systemic, mucosal, and heterotypic immune induction in mice inoculated with Venezuelan equine encephalitis replicons expressing Norwalk virus-like particles. J Virol 2002; 76:730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wade TJ, Griffin SM, Egorov AI, et al. Application of a multiplex salivary immunoassay to detect sporadic incident norovirus infections. Sci Rep 2019; 9:19576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker-Dreps S, Vilchez S, Bucardo F, et al. The association between fecal biomarkers of environmental enteropathy and rotavirus vaccine response in Nicaraguan infants. Pediatr Infect Dis J 2017; 36:412–6. [DOI] [PubMed] [Google Scholar]
- 5. Borresen EC, Guajardo MC, Zambrana LE, et al. Association between infant feeding practices and nutritional status in healthy Nicaraguan infants. J Food Nutr Diet. 2016; 1:110. [Google Scholar]
- 6. Nyiro JU, Sande C, Mutunga M, et al. Quantifying maternally derived respiratory syncytial virus specific neutralising antibodies in a birth cohort from coastal Kenya. Vaccine 2015; 33:1797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowling BJ, Perera RAPM, Fang VJ, et al. Maternal antibodies against influenza in cord blood and protection against laboratory-confirmed influenza in infants. Clin Infect Dis 2020; 71:1741–8. [DOI] [PubMed] [Google Scholar]
- 8. Parker SP, Cubitt WD, Jiang XJ, Estes MK. Seroprevalence studies using a recombinant Norwalk virus protein enzyme immunoassay. J Med Virol 1994; 1994:146–50. [DOI] [PubMed] [Google Scholar]
- 9. Menon VK, George S, Shanti AA, et al. Exposure to human and bovine noroviruses in a birth cohort in southern India from 2002 to 2006. J Clin Microbiol 2013; 51:2391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reyes Y, González F, Gutierrez L, et al. Secretor status strongly influences the incidence of symptomatic norovirus infection in a genotype-dependent manner in a Nicaraguan birth cohort. J Infect Dis 2022; 225:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindesmith LC, McDaniel JR, Changela A, et al. Sera antibody repertoire analyses reveal mechanisms of broad and pandemic strain neutralizing responses after human norovirus vaccination. Immunity 2019; 50:1530–41.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reeck A, Kavanagh O, Estes MK, et al. Serologic correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 2010; 202:1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.