Abstract
Background
Steroids have been shown to reduce inflammation, hypoxic pulmonary vasoconstriction (HPV) and lung edema. Based on evidence from clinical trials, steroids are widely used in severe COVID-19. However, the effects of steroids on pulmonary gas volume and blood volume in this group of patients are unexplored.
Objective
Profiting by dual-energy computed tomography (DECT), we investigated the relationship between the use of steroids in COVID-19 and distribution of blood volume as an index of impaired HPV. We also investigated whether the use of steroids influences lung weight, as index of lung edema, and how it affects gas distribution.
Methods
Severe COVID-19 patients included in a single-center prospective observational study at the intensive care unit at Uppsala University Hospital who had undergone DECT were enrolled in the current study. Patients’ cohort was divided into two groups depending on the administration of steroids. From each patient’s DECT, 20 gas volume maps and the corresponding 20 blood volume maps, evenly distributed along the cranial–caudal axis, were analyzed. As a proxy for HPV, pulmonary blood volume distribution was analyzed in both the whole lung and the hypoinflated areas. Total lung weight, index of lung edema, was estimated.
Results
Sixty patients were analyzed, whereof 43 received steroids. Patients not exposed to steroids showed a more extensive non-perfused area (19% vs 13%, p < 0.01) and less homogeneous pulmonary blood volume of hypoinflated areas (kurtosis: 1.91 vs 2.69, p < 0.01), suggesting a preserved HPV compared to patients treated with steroids. Moreover, patients exposed to steroids showed a significantly lower lung weight (953 gr vs 1140 gr, p = 0.01). A reduction in alveolar–arterial difference of oxygen followed the treatment with steroids (322 ± 106 mmHg at admission vs 267 ± 99 mmHg at DECT, p = 0.04).
Conclusions
The use of steroids might cause impaired HPV and might reduce lung edema in severe COVID-19. This is consistent with previous findings in other diseases. Moreover, a reduced lung weight, as index of decreased lung edema, and a more homogeneous distribution of gas within the lung were shown in patients treated with steroids.
Trial registration: Clinical Trials ID: NCT04316884, Registered March 13, 2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04200-z.
Keywords: COVID-19, Dual-energy CT, Steroids, Hypoxic pulmonary vasoconstriction
Background
The spread of the COVID-19, caused by the beta coronavirus SARS-CoV-2, led to the declaration of a pandemic in March 2020 [1]. Since then, an “unprecedented flood of research on the coronavirus” [2] brought to the elucidation of many molecular mechanisms subtending the disease [3–5]. However, despite the prompt recognition of the consequences of SARS-CoV-2 on different organs and functions [6], the pathophysiological mechanisms affecting the respiratory function still remain to be thoroughly understood. This difficulty derives from the fact that the primary function of the respiratory system, i.e., the gas exchange, is based on the interplay of two different sub-systems [7], alveolar ventilation and pulmonary capillary perfusion that deal with different physiological mechanisms and are associated with different structural features.
Numerous drugs have been tested as anti-SARS-CoV-2 agents [8]. Among them, steroids (i.e., dexamethasone 6 mg for 10 days) have been demonstrated to improve survival [9, 10] and are therefore widely used in severe COVID-19. Despite their effectiveness and well-known pleiotropic effects [11], the pharmacodynamics of steroids on COVID-19 lungs have not yet been widely reported. Based on the previous literature, a steroid-induced dysregulation of hypoxic pulmonary vasoconstriction (HPV) [12, 13] as well as a reduction in lung edema [14] can be hypothesized to occur in COVID-19 patients.
Already in the early stages of the pandemic, lung perfusion abnormalities were reported based on dual-energy computed tomography (DECT) [15]. Contrast-enhanced DECT is a powerful lung imaging method based on two concurrent X-ray energy spectra. Taking advantage of the different attenuation profiles of different substances, including iodine contrast, DECT can simultaneously characterize regional distribution of gas and blood volume in the lung parenchyma [16, 17].
The main objective of this study was to investigate the effects of steroids on regional distribution of pulmonary gas and blood volume as indices of shunting affecting oxygenation in critically ill patients with severe COVID-19 using data from contrast-enhanced DECT.
The study had three main aims: firstly, to examine the possibility of visualize pharmacodynamical mechanisms of drugs on lung parenchyma using DECT; secondly, to investigate the association between the use of steroids and HPV in COVID-19 lungs and therefore examine the possible influence of steroids on ventilation/perfusion matching; and thirdly, to explore the association between steroids therapy and magnitude of lung edema and consequently the effects on regional distribution of gas volume and oxygenation.
Methods
Study population
We analyzed clinically indicated DECT performed on patients included in a prospective observational single-center study of critically ill COVID-19 patients admitted to the intensive care unit (ICU) at Uppsala University Hospital in Sweden between April 7, 2020, and January 18, 2021. The study was approved by the National Ethical Review Agency (EPM; No. 2020-01623) and performed according to the Declaration of Helsinki and its subsequent revisions. Patients older than 18 years with positive PCR test for SARS-CoV2 on nasal swab specimen and available chest DECT performed during the intensive care stay were analyzed. All patients were not vaccinated for SARS-CoV2. The scans were acquired on clinical indication in static lung conditions, covering the whole lung parenchyma in supine position (see Fig. 1). Comprehensive clinical data were collected at ICU admission and the day of DECT. A subgroup analysis was conducted, dividing the sampled population in two groups: the group of patients not treated with steroids (No-Steroids group) and the one treated with steroids (Steroids group). In June 2020, the early report of the RECOVERY dexamethasone trial [9, 10] was published and the treatment guidelines of the disease were changed accordingly. Consequently, all patients recruited after June 16, 2020, were treated with steroids, forming up the group labeled “Steroids.” Patients chronically treated with steroids for reasons different from COVID-19 but that received, during their ICU stay, a steroids dose comparable to or higher than Dexamethasone 6 mg/day were included in the Steroids group.
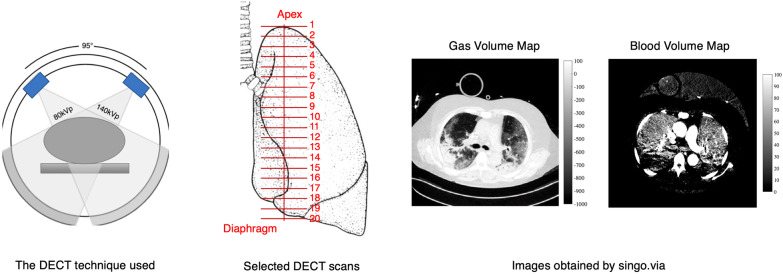
Fig. 1.
Dual-energy computed tomography (DECT) image acquisition and analysis. Left panel: DECT. A simplified representation of the DECT technique used, the SOMATOM Definition Flash, Siemens AG, Erlangen, Germany with two X-ray sources, characterized by two voltages (100 kVp and Sn 140 kVp), and two corresponding detectors rotating at a known constant angle. Central panel: Selected DECT scans. Coronal representation of a lung. Twenty DECT images, equally distributed between the apex and the diaphragmatic dome, were selected along the cranial–caudal axis. Both lungs were analyzed. Right panel: Images obtained by singo.via. Images used for further analysis. Representative example of pulmonary gas volume map and pulmonary blood volume map
DECT image analysis
Dual-energy computed tomography was always performed in supine position. For each raw DECT dataset, routine material-specific DECT maps were generated using a secondary workstation (Syngo.via Multimodality Workplace; Siemens Medical Solutions) that applied a three-material decomposition algorithm [18]. Two different series of images were selected and used for further analysis: the gas distribution (pulmonary gas volume) maps and the corresponding blood distribution (pulmonary blood volume) maps (see Fig. 1). Twenty images evenly spaced along the cranial–caudal axis and located between the apex and the diaphragmatic dome were analyzed (see Fig. 1). A manual segmentation of the lung parenchyma was applied, and big vessels, heart and mediastinal structures were excluded from the subsequent analysis. As regard to the pulmonary gas volume maps, the Hounsfield units (HU) range of − 1000 (gas) to + 100 (non-aerated lung) was considered, as done in previous studies (see Fig. 2). Four lung compartments were then defined [19]: hyperinflated (− 1000 to − 800 HU), normoinflated (− 800 to − 500 HU), poorly inflated (− 500 to − 100 HU) and non-inflated (− 100 to + 100 HU) lung. As regard to the pulmonary blood volume maps, we studied the range between − 100 and + 150 HU (see Fig. 2). Based on previous studies [20–22], zero HU was set as a limit between non-perfused (HU ≤ 0) and perfused lung compartment (HU > 0). Each compartment was separately computed and expressed as cm2 and as percentage of the total lung volume in each map [22]. From the bell-shaped curves characterizing the HU distribution of perfused blood volume maps, kurtosis was calculated as measure of both dispersion and morphology of the curves (see Fig. 3A) as done in previous studies [23–26]. Kurtosis belongs to the big chapter of the measures of variance, and it is commonly used to quantify both dispersion and morphology of density histograms derived from computed tomography images. A reduced kurtosis corresponds to a decreased homogeneity of pulmonary blood volume. The latter has been previously interpreted as indirect index of HPV efficiency [27]: a low homogeneity of pulmonary blood volume (low kurtosis) corresponding to a preserved HPV. To further assess the relation between and HPV, where a reduced pulmonary blood volume is expected in the hypoinflated lung, pulmonary blood volume was separately assessed for the whole lung and for the hypoinflated areas, the latter characterized by poorly inflated and non-inflated lung regions (i.e., HU ranging between − 500 and + 100 HU) (see Fig. 3).
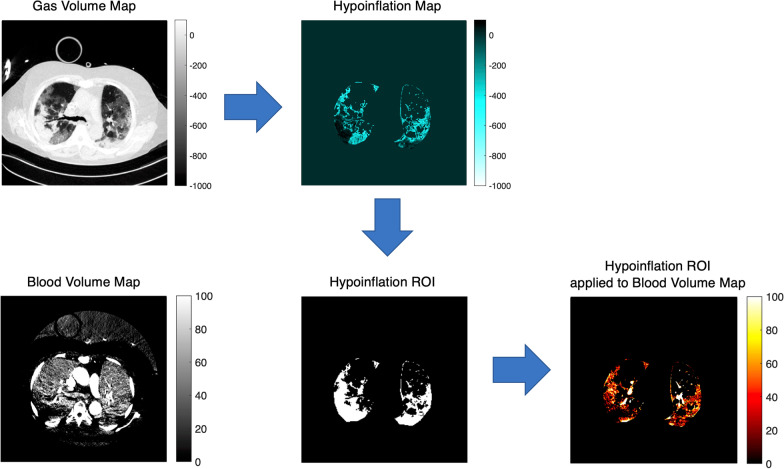
Fig. 2.
Dual-energy computed tomography image analysis. Pulmonary gas volume maps were characterized by a HU range of − 1000 to + 100 HU. Pulmonary blood volume maps were characterized by a HU range of − 100 to + 150 HU. To assess hypoxic pulmonary vasoconstriction, pulmonary blood volume distribution was assessed for the whole lung and for the hypoinflated lung, the latter characterized by poorly inflated and non-inflated lung regions (HU between − 500 and + 100). A region of interest for hypoinflated lung (Hypoinflation ROI) was obtained extrapolating hypoinflated areas (Hypoinflation Map) from the pulmonary gas volume map. Hence, the hypoinflated ROI was applied to pulmonary blood volume map to analyze blood volume distribution of the hypoinflated areas
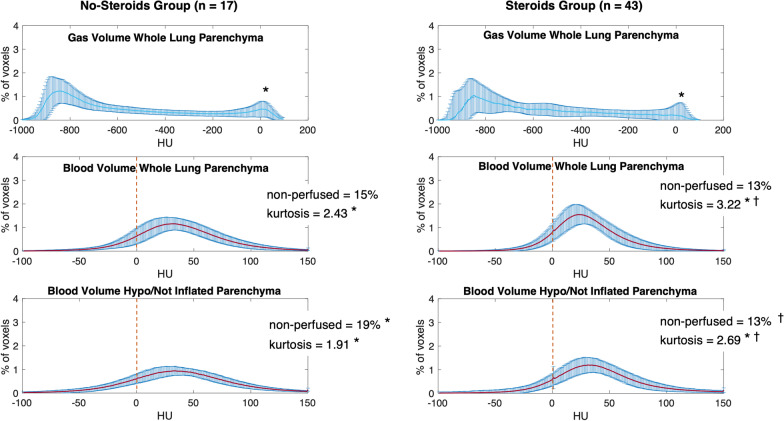
Fig. 3.
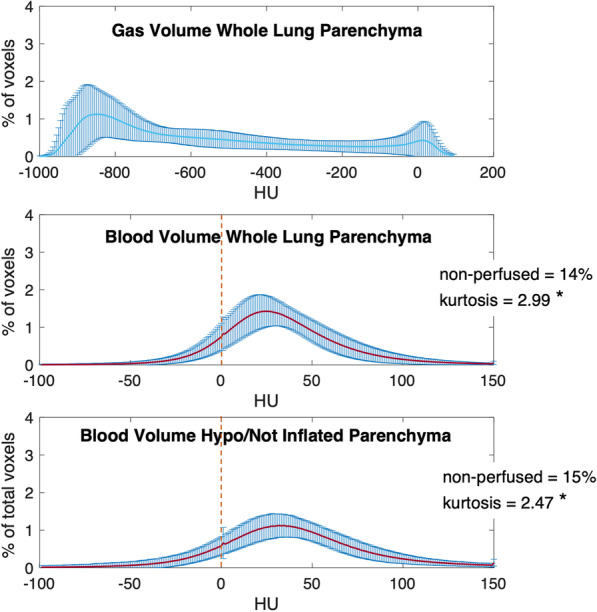
HU distribution for pulmonary gas volume map and blood volume maps, for the whole study population (n = 60 patients). Above: the HU distribution for pulmonary gas volume maps in the whole lung parenchyma; middle: HU distribution for pulmonary blood volume maps in the whole lung parenchyma; below: HU distribution for pulmonary blood volume maps in the hypoinflated lung parenchyma. x-axis: HU values. y-axis: percent of voxels compared to the total amount of voxels. Values represented as mean ± standard deviation (SD) or percent
Lung weight analysis
Total lung volume (ml), total gas volume (ml) and lung weight (g) were computed [28–31]. The analysis was performed for the whole population as well as separately for the No-Steroids and the Steroids group.
Statistical analysis
Continuous variables were reported as mean ± standard deviation (SD). The Wilcoxon signed-rank test (α = 0.05) was chosen as a nonparametric test for paired observations. The Wilcoxon rank-sum test (α = 0.05) was used as a nonparametric test in the case of independent observations. Fisher’s exact test (α = 0.05) was used to test statistically significant differences in the case of categorical variables. More details about Materials and methods are reported as Additional file 1: Data Supplement.
Results
Study population
Sixty patients affected by severe COVID-19 and in need of intensive care were analyzed in the study (see Table 1). The mean age was of 63 ± 9 years and 20% were women. At ICU admission, the ratio of partial pressure of arterial oxygen to inspired oxygen fraction at the day of the DECT was 127 ± 46 mmHg and the SAPS3 was 51 ± 9. During their ICU stay before DECT, 41% of patients were intubated and 22% experienced an acute thrombotic event. In this selected population, the 30-day mortality was 29%. Forty-three out of sixty patients (72%) received steroids as treatment of COVID-19-associated pneumonia (Dexamethasone 6 mg for 10 days) [9, 10]. In order to compare the severity of the disease in No-Steroid and Steroid patients, we analyzed a wide selection of clinical and biochemical markers (see Table 2). The Steroids group showed a lower incidence of thrombotic events: 14% compared to 41% (p = 0.03), a significantly lower alveolar–arterial difference of oxygen (AaDO2) the day of DECT compared to ICU admission (267 ± 99 mmHg vs 322 ± 106 mmHg, p = 0.04) as well as lower inflammation (e.g., IL-6 equal to 218 ± 155 pg/ml in the No-Steroids group vs 40 ± 19 pg/ml in the Steroids group, p = 0.01) compared to the No-Steroids group. The antithrombotic treatment (the dose of Dalteparin per kg) as well as the value of factor Xa (p = 0.11) did not differ between the two groups.
Table 1.
Descriptive statistics for all patients included in the study (sample size = 60)
| Mean | SD | |
|---|---|---|
| Age (years) | 63 | 9 |
| BMI | 31 | 6 |
| Female | n | % |
|---|---|---|
| 12 | 20 |
| Clinical history before ICU stay | n | % |
|---|---|---|
| Previous pulmonary disease | 16 | 27 |
| Thrombotic events before ICU | 6 | 10 |
| At ICU admission | Mean | SD |
|---|---|---|
| Respiratory rate (bpm) | 27 | 8 |
| SAPS 3 | 51 | 9 |
| Days of COVID-19 symptoms | 11 | 3 |
| FIO2 | 62 | 14 |
| pH | 7.45 | 0.06 |
| PaCO2 [mm Hg] | 35 | 5 |
| PaO2/FIO2 ratio [mm Hg] | 127 | 46 |
| SpO2 [%] | 94 | 2 |
| AaDO2 [mm Hg] | 325 | 105 |
| Weight [kg] | 97 | 18 |
| Dalteparin [IE] /kg | 144 | 42 |
| n | % | |
|---|---|---|
| Intubated patients | 21 | 35 |
| On DECT day | Mean | SD |
|---|---|---|
| Days of invasive ventilation | 7 | 7 |
| PEEP [cm H2O] | 11 | 4 |
| Static compliance | 46 | 20 |
| Tidal volume [mL]/kg PBW | 8 | 3 |
| pH | 7.42 | 0.08 |
| PaCO2 [mm Hg] | 42 | 1 |
| PaO2/FIO2 ratio [mm Hg] | 129 | 31 |
| SpO2 [%] | 94 | 3 |
| AaDO2 [mm Hg] | 272 | 97 |
| Weight (kg) | 96 | 18 |
| Ferritin [µg/l] | 2028 | 1398 |
| D-dimer [ng/ml] | 6 | 11 |
| Interleukin-6 (IL-6) [pg/ml] | 284 | 493 |
| Factor Xa [KIE/l] | 0.53 | 0.2 |
| n | % | |
|---|---|---|
| Intubated patients | 24 | 41 |
| Spontaneously breathing patients | 36 | 64 |
| Acute thrombotic events before DECT | 13 | 22 |
| At ICU discharge | Mean | SD |
|---|---|---|
| Days of invasive ventilation | 14 | 11 |
| Days of ICU stay | 14 | 11 |
| Highest ferritin [µg/l] | 2884 | 3054 |
| Highest D-dimer [ng/ml] | 13 | 26 |
| Highest interleukin-6 (IL-6) [pg/ml] | 228 | 380 |
| n | % | |
|---|---|---|
| Alive 30 days after ICU | 43 | 71 |
| Invasive mechanical ventilation | 32 | 53 |
| Thrombotic events | 16 | 27 |
Data were reported as mean ± standard deviation (SD) or numerosity (%)
Days of invasive ventilation, ventilatory settings and respiratory mechanics values refer only to the subgroup of mechanically ventilated patients. Spontaneously breathing patients refers to patients with preserved respiratory drive both with and without invasive mechanical ventilation
BMI, body mass index; SAPS 3, the simplified acute physiology score 3; FIO2, inspiratory fraction of oxygen; PaO2, partial pressure of arterial oxygen; SpO2, peripheral oxygen saturation; PCO2, partial pressure of arterial carbon dioxide; AaDO2, alveolar arterial difference of oxygen
Table 2.
Descriptive statistics for the two patients’ subgroups: No-Steroids group (left, simple size = 17) and Steroids group (right, simple size = 43)
| No-Steroids (n = 17) | Steroids (n = 43) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Wilcoxon rank-sum test (α = 0.05) | |
| Age (years) | 62 | 9 | 64 | 9 | 0.37 |
| BMI | 31 | 6 | 31 | 6 | 1 |
| n | % | n | % | Fisher’s exact test (α = 0.05) | |
|---|---|---|---|---|---|
| Female | 1 | 6 | 11 | 26 | 0.15 |
| Clinical history before ICU stay | n | % | n | % | |
|---|---|---|---|---|---|
| Previous pulmonary disease | 5 | 29 | 11 | 26 | 0.76 |
| Thrombotic events before ICU | 2 | 7 | 4 | 9 | 1 |
| At ICU admission | Mean | SD | Mean | SD | Wilcoxon rank-sum test (α = 0.05) |
|---|---|---|---|---|---|
| Respiratory rate (bpm) | 28 | 9 | 28 | 7 | 0.72 |
| SAPS 3 | 51 | 7 | 51 | 11 | 0.97 |
| Days of COVID-19 symptoms | 11 | 3 | 11 | 3 | 0.84 |
| FIO2 | 60 | 13 | 63 | 16 | 0.54 |
| pH | 7.46 | 0.05 | 7.45 | 0.06 | 0.56 |
| PaCO2 [mm Hg] | 36 | 5 | 35 | 6 | 0.22 |
| PaO2/FIO2 ratio [mm Hg] | 138 | 65 | 124 | 36 | 0.88 |
| SpO2 [%] | 93 | 3 | 94 | 2 | 0.42 |
| AaDO2 [mm Hg] | 326 | 102 | 332 | 106 | 0.9 |
| Weight [kg] | 100 | 20 | 96 | 17 | 0.49 |
| Dalteparin [IE] /kg | 133 | 45 | 148 | 41 | 0.11 |
| n | % | n | % | Fisher’s exact test (α = 0.05) | |
|---|---|---|---|---|---|
| Intubated patients | 5 | 29 | 16 | 37 | 0.77 |
| On DECT day | Mean | SD | Mean | SD | Wilcoxon rank-sum test (α = 0.05) |
|---|---|---|---|---|---|
| Days from start of symptoms | 16 | 8 | 13 | 6 | 0.45 |
| Days from steroids therapy start | 0 | 0 | 4 | 3 | < 0.01 |
| Days of invasive ventilation | 9 | 6 | 6 | 7 | 0.31 |
| PEEP [cm H2O] | 12 | 4 | 10 | 4 | 0.08 |
| Static compliance | 43 | 24 | 48 | 18 | 0.49 |
| Tidal volume [mL]/kg PBW | 8 | 2 | 9 | 4 | 0.37 |
| pH | 7.43 | 0.08 | 7.41 | 0.08 | 0.45 |
| PaCO2 [mm Hg] | 42 | 12 | 42 | 10 | 0.95 |
| PaO2/FIO2 ratio [mm Hg] | 123 | 25 | 132 | 34 | 0.43 |
| SpO2 [%] | 93 | 3 | 94 | 2 | 0.86 |
| AaDO2 [mm Hg] | 284 | 81 | 267 | 99 | 0.63 |
| Weight (kg) | 100 | 21 | 95 | 17 | 0.43 |
| Ferritin [µg/l] | 2315 | 1676 | 1694 | 950 | 0.27 |
| D-dimer [ng/ml] | 9 | 13 | 5 | 11 | 0.72 |
| Interleukin-6 (IL-6) [pg/ml] | 218 | 155 | 40 | 19 | 0.21 |
| Factor Xa [KIE/l] | 0.55 | 0.23 | 0.53 | 0.2 | 0.18 |
| n | % | n | % | Fisher’s exact test (α = 0.05) | |
|---|---|---|---|---|---|
| Intubated patients | 8 | 47 | 16 | 37 | 0.77 |
| Spontaneously breathing patients | 9 | 52 | 27 | 69 | 1 |
| Acute thrombotic events before DECT | 7 | 41 | 6 | 14 | 0.03* |
| At ICU discharge | Mean | SD | Mean | SD | Wilcoxon rank-sum test (α = 0.05) |
|---|---|---|---|---|---|
| Days of invasive ventilation | 12 | 8 | 5 | 3 | 0.14 |
| Days of ICU stay | 16 | 6 | 8 | 4 | 0.45 |
| Highest ferritin [µg/l] | 5067 | 4257 | 2046 | 1974 | 0.03* |
| Highest D-dimer [ng/ml] | 26 | 42 | 10 | 20 | 0.9 |
| Highest interleukin-6 (IL-6) [pg/ml] | 449 | 564 | 95 | 71 | 0.01* |
| n | % | n | % | Fisher’s exact test (α = 0.05) | |
|---|---|---|---|---|---|
| Alive 30 days after ICU | 12 | 71 | 31 | 72 | 0.49 |
| Invasive mechanical ventilation | 10 | 59 | 22 | 51 | 0.77 |
| Thrombotic events | 8 | 47 | 8 | 19 | 0.04* |
Data were reported as mean ± standard deviation (SD) or numerosity (%). The analyzed variables are the same as in Table 1. Wilcoxon rank-sum test to detect statistical differences for continuous variables; (α = 0.05); Fisher’s exact test to detect statistical differences for categorical variables; (α = 0.05); *: to mark statistically significant differences.
DECT image analysis
The HU analysis describing the gas distribution in the whole lung parenchyma showed two peaks corresponding to the hyperinflated and to the non-inflated HU compartments (see Fig. 3 and Additional file 2: Table E1). The analysis of pulmonary blood volume distribution showed 12% of non-perfused areas in the whole lung and 11% in the hypoinflated lung (p = 0.06). The HU distribution for pulmonary blood volume maps had a significantly lower kurtosis (lower homogeneity) in the hypoinflated lung compared to the whole lung parenchyma. This was true for the whole population (2.47 vs 2.99) as well as for the two analyzed subgroups: No-Steroids (1.91 vs 2.43) and Steroids group (2.69 vs 3.22) (see Figs. 3, 4, Additional file 2: Figure E1 and Tables E1–E2). Patients treated with steroids showed a smaller amount of non-inflated lung, compared to patients not exposed to steroids (10% vs 13%, p = 0.01). However, steroids did not affect the volume of hyperinflated lung (26% vs 27% of total lung volume, p = 0.24). Hypoinflated areas of the lung showed a significantly lower non-perfused lung parenchyma in the Steroids group compared to the No-Steroids group (13% vs 19% of total lung volume, p < 0.01). Exposure to steroids was associated with an increased kurtosis (increased homogeneity) of the HU distribution of pulmonary blood volume within the lung parenchyma, regardless of the degree of inflation (2.43 vs 3.22, p < 0.01 for the whole lung and 1.91 vs 2.69, p < 0.01 for the hypoinflated lung). The high kurtosis (high homogeneity of pulmonary blood volume distribution) found in the Steroids groups might suggest a jeopardized HPV [27] (see Figs. 4, Additional file 2: Figure E1 and Table E2).
Fig. 4.
HU distribution for pulmonary gas volume map and blood volume maps, separately for the No-Steroids group (n = 17 patients; Panel A) and the Steroids group (n = 43 patients; Panel B). Above: the HU distribution for pulmonary gas volume maps in the whole lung parenchyma; middle: HU distribution for pulmonary blood volume maps in the whole lung parenchyma; below: HU distribution for pulmonary blood volume maps in the hypoinflated lung parenchyma. x-axis: HU values. y-axis: percent of voxels compared to the total amount of voxels. Values represented as mean ± standard deviation (SD) or percent. *: for statistical differences between whole lung parenchyma (middle) and hypoinflated parenchyma (Below); †: for statistical differences between the No-Steroids group (Panel A) and the Steroids group (Panel B)
Lung weight analysis
The analysis of total lung volume, gas volume and lung weight is reported in Tables 3, 4, 5 and Additional file 2: Figure E2. The whole population showed a mean lung weight of 966 ± 303 g and a mean total gas volume of 1436 ± 718 ml. The total lung volume was 2248 ± 894 ml of which 30% was hyperinflated and 10% was non-inflated. By dividing the patients based on their exposure to steroids, significant differences were observed for lung volume and lung weight. The group of patients exposed to steroids showed a significantly lower lung weight (913 g vs 1123 g, p = 0.01) as well as a significantly lower volume of non-inflated lung parenchyma compared to patients not exposed to steroids (9% vs 11% of the total lung volume, p = 0.02). The total gas volume inside the lung was not affected by steroids (1425 vs 1465 ml, p = 0.71) (Table 4).
Table 3.
Lung volume (ml) calculated for the whole lung parenchyma as well as for each HU compartment (hyper-, normally, poorly and non-inflated)
| Lung volume (ml) | Lung volume (%) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Panel A | ||||
| All patients | ||||
| Total lung | 2248 | 894 | ||
| Hyperinflated | 676 | 614 | 30 | 18 |
| Normally inflated | 822 | 409 | 36 | 11 |
| Poorly inflated | 535 | 249 | 24 | 11 |
| Non-inflated | 216 | 200 | 10 | 10 |
| Lung volume (ml) | Lung volume (%) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Panel B | ||||
| No-Steroids | ||||
| Total lung | 2377 | 974 | ||
| Hyperinflated | 679 | 468 | 29 | 13 |
| Normally inflated | 862 | 400 | 36 | 9 |
| Poorly inflated | 567 | 262 | 24 | 8 |
| Non-inflated | 269 | 153 | 11 | 8 |
| Lung volume (ml) | Lung volume (%) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | α = 0.05 | Mean | SD | α = 0.05 | |
| Panel C | ||||||
| Steroids | ||||||
| Total lung | 2198 | 868 | 0.59 | |||
| Hyperinflated | 674 | 668 | 0.44 | 31 | 19 | 0.59 |
| Normally inflated | 806 | 416 | 0.60 | 36 | 11 | 0.78 |
| Poorly inflated | 522 | 245 | 0.67 | 24 | 12 | 0.45 |
| Non-inflated | 196 | 213 | 0.01 | 9 | 11 | 0.02 |
Panel A: whole study population (n = 60); Panel B: No-Steroids group (n = 17); Panel C: Steroids group (n = 43). Each HU compartment was reported as absolute values as well as percentage of the total lung parenchyma. Data were reported as mean ± standard deviation (SD). Wilcoxon rank-sum test was used to detect statistical differences (α = 0.05); *: to mark statistically significant differences.
Table 4.
Gas volume (ml) calculated for the whole lung parenchyma as well as for each HU compartment (hyper-, normally, poorly and non-inflated)
| Gas volume (ml) | Gas volume (%) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Panel A | ||||
| All patients | ||||
| Total lung | 1436 | 718 | ||
| Hyperinflated | 617 | 557 | 43 | 18 |
| Normally inflated | 580 | 281 | 40 | 13 |
| Poorly inflated | 203 | 93 | 14 | 9 |
| Non-inflated | 36 | 13 | 3 | 1 |
| Gas volume (ml) | Gas volume (%) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Panel B | ||||
| No-steroids | ||||
| Total lung | 1465 | 671 | ||
| Hyperinflated | 614 | 421 | 42 | 14 |
| Normally inflated | 609 | 275 | 42 | 11 |
| Poorly inflated | 207 | 99 | 14 | 7 |
| Non-inflated | 36 | 14 | 2 | 1 |
| Gas volume (ml) | Gas volume (%) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | α = 0.05 | Mean | SD | α = 0.05 | |
| Panel C | ||||||
| Steroids | ||||||
| Total lung | 1425 | 743 | 0.71 | |||
| Hyperinflated | 619 | 607 | 0.48 | 43 | 20 | 0.49 |
| Normally inflated | 568 | 286 | 0.62 | 40 | 14 | 0.66 |
| Poorly inflated | 201 | 91 | 0.88 | 14 | 10 | 0.39 |
| Non-inflated | 36 | 12 | 0.74 | 3 | 1 | 0.37 |
Panel A: whole study population (n = 60); Panel B: No-Steroids group (n = 17); Panel C: Steroids group (n = 43). Each HU compartment was reported as absolute values as well as percentage of the total lung parenchyma. Data were reported as mean ± standard deviation (SD). Wilcoxon rank-sum test was used to detect statistical differences (α = 0.05); *: to mark statistically significant differences
Table 5.
Lung weight (g) calculated for the whole lung parenchyma as well as for each HU compartment (hyper-, normally, poorly and non-inflated)
| Weight (g) | Weight (%) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Panel A | ||||
| All patients | ||||
| Total lung | 966 | 303 | ||
| Hyperinflated | 114 | 76 | 12 | 10 |
| Normally inflated | 301 | 148 | 31 | 11 |
| Poorly inflated | 353 | 170 | 37 | 10 |
| Non-inflated | 198 | 150 | 20 | 13 |
| Weight (g) | Weight (%) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Panel B | ||||
| No-Steroids | ||||
| Total lung | 1123 | 328 | ||
| Hyperinflated | 122 | 66 | 11 | 7 |
| Normally inflated | 310 | 145 | 27 | 9 |
| Poorly inflated | 417 | 184 | 37 | 7 |
| Non-inflated | 291 | 151 | 25 | 11 |
| Weight (g) | Weight (%) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | α = 0.05 | Mean | SD | α = 0.05 | |
| Panel C | ||||||
| Steroids | ||||||
| Total lung | 913 | 276 | 0.01* | |||
| Hyperinflated | 108 | 80 | 0.57 | 12 | 11 | 0.93 |
| Normally inflated | 290 | 151 | 0.85 | 32 | 12 | 0.34 |
| Poorly inflated | 323 | 165 | 0.60 | 35 | 11 | 0.44 |
| Non-inflated | 192 | 152 | 0.01* | 21 | 14 | 0.03 |
Panel A: whole study population (n = 60); Panel B: No-Steroids group (n = 17); Panel C: Steroids group (n = 43). Each HU compartment was reported as absolute values as well as percentage of the total lung parenchyma. Data were reported as mean ± standard deviation (SD). Wilcoxon rank-sum test was used to detect statistical differences (α = 0.05); *: to mark statistically significant differences
Discussion
To date, despite established evidence of a reduced mortality in severe COVID-19 patients treated with steroids compared to a control population [9, 10], no studies investigated the effects of steroids on the lungs in this patients’ population. In the current study, for the first time, we introduced the use of dual-energy computed tomography to study the effects of steroids on lung ventilation and perfusion as well as on lung weight in patients affected by severe COVID-19 and in need of intensive care. The main findings of the current study can be summarized as follows: (1) the use of steroids is associated with a high kurtosis of lung perfusion in the hypoventilated regions of the lung. This suggests that steroids might counteract hypoxic pulmonary vasoconstriction; (2) steroids reduced lung weight, possibly originated by a decreased edema; (3) steroids improve regional gas distribution; (4) despite potential effects on hypoxic pulmonary vasoconstriction, gas change was improved as evidenced by a reduction in AaDO2, suggesting that the effects of steroids to reduced edema and improve gas distribution predominated.
In clinical contexts different from COVID-19, as high-altitude pulmonary edema (HAPE), steroids have been demonstrated to reduce pulmonary arterial systolic pressure, presumably counteracting hypoxic pulmonary vasoconstriction [12, 13]. At the same time, steroids reduce inflammation and lung edema [14]. The possible known mechanisms of action of steroids on lungs pathophysiology are multifold. Steroid therapy is known for improving pulmonary function in preterm newborns by augmenting nitric oxide-mediated vessels relaxation as well as by stimulating surfactant production [32]. Steroids prevent capillary leak, stimulating alveolar sodium and water reabsorption [14], and enhance nitric oxide availability in the lung parenchyma [33]. There is a still ongoing debate about the possible benefits of their anti-inflammatory and antifibrotic properties on patients with acute respiratory distress syndrome [34, 35]. The current study is the first investigating the possible effects of steroids on lungs in critically ill patients affected by severe COVID-19.
Interestingly, in the current study, the Steroids and the No-Steroids groups were similar to each other in all respects except for the indexes of inflammation and the onset of thromboembolic events during their ICU stay (see Table 2). Patients treated with steroids showed significantly lower inflammatory markers and, at the same time, a lower occurrence of thromboembolic events (19% vs 47%, p = 0.04). The dose of antithrombotic drugs as well as the levels of factor Xa and D-dimers could not justify such differences. Although concomitant causes, potentially influencing the prothrombotic state of these patients, cannot be ruled out, the immunomodulatory effects and antithrombotic properties of steroids [36] can be a possible explanation for these differences [37]. Although not reaching statistical significance, patients treated with steroids showed an improved oxygenation (PaO2/FIO2: 132 vs 123, p = 0.43), a reduction in alveolar arterial difference of oxygen (AaDO2 326 mmHg vs 332 mmHg, p = 0.9) and ameliorate static compliance (48 vs 43, p = 0.49) as well as less days of mechanical ventilation and days of ICU stay. A significant reduction in AaDO2 followed the treatment with steroids (322 ± 106 mmHg at admission vs 267 ± 99 mmHg at DECT, p = 0.04). The sample was underpowered to investigate a difference in mortality between the two groups.
Effects of steroids on hypoxic pulmonary vasoconstriction
HPV is a homeostatic mechanism, peculiar of the pulmonary vasculature. Intrapulmonary arterioles constrict in response to alveolar hypoxemia, diverting blood from injured lung toward better ventilated lung regions. Thereby, HPV optimizes ventilation/perfusion match and improves oxygen delivery [38, 39]. A dysregulated HPV has been hypothesized in ARDS [40] and COVID-19 patients [15, 41]. The current study was neither designed to directly investigate the mechanisms of action of steroids on HPV in COVID-19 patients nor planned to grade the HPV derangement induced by COVID-19. However, our data suggest that in patients affected by severe COVID-19 the use of steroids might jeopardize HPV (see Figs. 4, 5 and Additional file 2: Table E2). A damped HPV is apparently not in line with the known beneficial effects of steroids observed in severe COVID-19 patients [9, 42]. However, this can mitigate the risk for right ventricular failure, one of the major determinants of poor clinical outcomes in ARDS [43, 44]. Moreover, other concomitant beneficial mechanisms induced by steroids as anti-inflammatory and immunomodulatory effects, as well as the reduction in lung edema, demonstrated in this study, might also compensate for a worsened HPV and an exacerbated ventilation/perfusion mismatch. Although HPV is the primary active mechanism influencing regional lung perfusion, other concomitant factors can have a role. Among others, superinfections by Gram-negative bacteria, potentially less hindered in patients treated with steroids, could possibly attenuate HPV [45]. At the same time, direct mechanical effects as well as undefined intracellular signaling can be listed among non-hypoxia-related mechanisms affecting regional lung perfusion [46, 47].
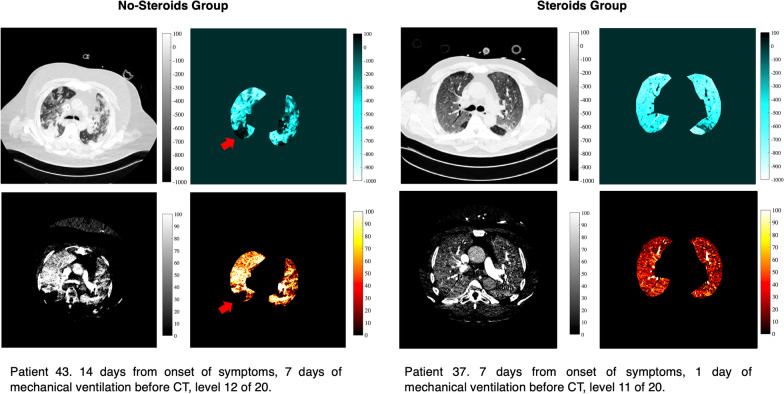
Fig. 5.
Representative example of DECT scans collected on the No-Steroids group (left) and the Steroids group (right). On the left: Patient not treated with steroids (No-Steroids group). On the right: Patient treated with steroids (Steroids group). For both No-Steroids and Steroids group, upper images show a pulmonary gas volume image (left) and the corresponding map (right); lower images show a pulmonary blood volume image (left) and the corresponding map (right). Different colormaps have been used to emphasize different degree of pulmonary gas and blood volume. For pulmonary gas volume maps, the range of HU is between − 1000 and + 100 HU. For pulmonary blood volume maps, the range of HU is between 0 and + 100 HU. 0 HU corresponding to non-perfused areas, 100 HU corresponding to highly perfused areas. The red arrow indicates the areas of preserved hypoxic pulmonary vasoconstriction where the areas of low pulmonary blood volume correspond to the areas of low inflation
Effects of steroids on lung edema
At the pulmonary level, steroids prevent inflammatory capillary leak and edema, stimulating alveolar sodium and water reabsorption [14]. We demonstrated a reduced lung weight as well as lower volume of non-inflated lung in patients treated with steroids compared to control (see Table 3, 5 and Additional file 2: Figure E2).
Although not affecting global gas volume (see Tables 3, 5), steroids improved the homogeneity of gas distribution within the lung parenchyma (see Fig. 4 and Additional file 2: Table E2). The exposure to steroids reduced the non-inflated, atelectatic lung compartment. This is reflected in a significantly reduced AaDO2 following treatment with steroids (322 ± 106 mmHg at admission vs 267 ± 99 mmHg at DECT, p = 0.04). These findings can also be interpreted as a consequence of a reduced lung edema and inflammation following treatment with steroids. Thus, although there was evidence for reduced HPV the beneficial effects of steroids acting to reduce edema and improve gas distribution on gas exchange predominated.
Alongside emerging understanding of the pulmonary vascular involvement in COVID-19 patients, the key mechanism of the most immediately dangerous consequence of COVID-19 (hypoxemia) has been ascribed to the concurrent disruption of the tri-compartmental model of lung oxygenation [48]. Consequently, research has progressively focused upon the ventilation/perfusion mismatch. Mauri et al. [49], in a small cohort of COVID-19 patients studied using electrical impedance tomography, reported that ventilation/perfusion mismatch was elevated and mainly due to non-perfused but ventilated units. Later, using a computational model, Busana et al. [50] attributed hypoxemia of COVID-19 to a marked hyperperfusion of poorly ventilated lung regions.
Given its availability in clinical practice, chest contrast-enhanced DECT is emerging as a promising tool for studying lung physiology [16, 17]. The strength of DECT is that it simultaneously provides information about both gas and blood distribution and, consequently, has great potential in describing ventilation/perfusion dysregulation in COVID-19 [41]. Recently, Ball et al. [20] performed the first quantitative study regarding the distribution of gas volume and blood in the lungs of patients affected by COVID-19 by using DECT, reporting several deviations from normal physiology, among which the blood volume distribution in non-aerated tissue mass.
This is the first study based on chest DECT investigating the functional effects of steroids on lungs in patients affected by severe COVID-19. Compared to the previous literature, the current study took a step forward, not only investigating the regional distribution of pulmonary gas volume and blood volume in COVID-19, but also characterizing the effects that steroids have on them. Our results suggested that a deranged HPV or a reduced lung edema might modify the ventilation/perfusion mismatch characterizing COVID-19 lungs.
Limitations of the study can be listed as follows. Being an observational study, the analysis is based on DECT performed on the basis of a clinical indication and this might imply a selection bias. However, all COVID-19 patients admitted to our ICU, with very few exceptions, underwent either computed tomography with contrast or DECT. All were performed in supine position. As reported in Table 2, the time between the symptoms start and DECT was not different between the two groups (16 ± 8 days for the No-Steroids group vs 13 ± 6 days for the Steroids group, p = 0.45). Moreover, in the Steroids group, the DECT examinations included in the study were all performed within 4 ± 3 days from the start of the steroid treatment (see Table 2). With few exceptions with clear contraindications, pronation was routinely applied for all patients, already before ICU stay. Therefore, the exact time spent in pronation by each patient cannot be reliably quantified. The execution of the DECT was performed not before one hour from the end of the eventual preceding pronation phase due to the necessity of observing whether the patient was sufficiently stable for the transport to the CT facility. All patients in the No-Steroids group were admitted before June 16, 2020; after that date, the COVID-19 patients admitted to the ICU were treated with steroids (Steroids group) as a consequence of a change of treatment guidelines. The fact that steroids have been introduced at a specific time of the pandemic might introduce a chronology bias into the study. As a matter of fact, the SARS-CoV2 pandemic during its course has been characterized by a continuous change of treatment strategies and clinical routines that affect the generalizability of many studies on COVID-19. However, in our study, all the recruited patients were not vaccinated for SARS-CoV2, they were all affected by the same SARS-CoV2 variant (the original Wuhan variant) and, in the studied time lapse, there were not concomitant antiviral/anti-inflammatory treatments specific to COVID-19 other than steroids. Given the demonstrated positive effects of steroids and their routine use in severe COVID-19 patients [9], a prospective study comparing patients exposed or not exposed to steroids would have entailed unsolvable ethical issues. Given the design of the study, the direct causality between steroids and a deranged HPV could not be directly investigated. We did not directly study how steroids influence the pathophysiological mechanisms of HPV. Moreover, a deranged HPV is not the only factor interfering with the regional distribution of perfused blood volume, e.g., pulmonary thrombotic events, lung heterogeneity or interstitial edema are concomitant causes also affecting blood distribution and shunt. However, of all factors potentially aggravating shunt, HPV is the only one influencing, in a targeted manner, hypo-/non-inflated lung regions. The DECT scanner does not reach the resolution necessary to observe the behavior of single capillaries. For this reason, the attenuation value of one voxel portrayed the behavior of many alveoli and of the complex mesh of capillary vessels surrounding them. In this respect, the choice of focusing the analysis to the subpopulation of voxels subtending hypo- or non-inflated areas reduces the possible error deriving from the mechanical interaction between the inflated alveoli and the neighboring capillary units, being them in a similar inflation status. Although intriguing and supported by indirect evidences (i.e., lung imaging), the hypothesized parallelism between the mechanisms of action of steroids in HAPE and COVID-19 needs further investigations [51]. Steroids have pleiotropic effects [11] and the current study investigated only some possible consequences of pharmacodynamic mechanisms characterizing steroids. Chest DECT provides static information about gas volume at one predefined moment. Pulmonary gas volume maps are widely used in the literature to infer ventilation distribution [19]. The same applies to DECT pulmonary blood volume maps [52]. DECT generates images describing blood volume distribution within the lung at one single time point: For this reason, these images should only be indirectly and cautiously used to infer lung perfusion. Despite its limitations, the present study is the first one shedding light on the effects of steroids on the regional distribution of pulmonary gas volume and blood volume in COVID-19 patients.
Conclusions
Chest DECT can provide unique functional information and visualize the effects of pharmacodynamic mechanisms of drugs on lung parenchyma. Taking advantage of chest DECT, we show changes in pulmonary gas volume and blood volume that might stand for an association between the use of steroids and hypoxic pulmonary vasoconstriction in severe COVID-19 patients in need of intensive care. Steroids can influence ventilation/perfusion mismatch. Moreover, treatment with steroids is associated with a reduced lung edema and consequently improves regional distribution of gas volume within the lungs of severe COVID-19 patients.
Supplementary Information
Additional file 1. Supplementary Material and Methods.
Additional file 2. Table E1. Descriptive statistics for HU distribution for pulmonary gas volume and blood volume maps. Whole population (n = 60). Comparison between the whole parenchyma and hypoinflated areas. Data were reported as mean ± standard deviation (SD) as well as percent. Wilcoxon rank-sum test was used to test statistical differences between the whole parenchyma and the hypoinflated one (α = 0.05); *: to mark statistically significant differences. Table E2. Descriptive statistics for HU distribution for pulmonary gas volume and blood volume maps. Separately for the No-Steroids group (n = 17, Panel A) and the Steroids group (n = 43, Panel A) Comparison between the whole parenchyma and hypoinflated areas (Panel A and B) as well as between No-Steroids and Steroids group (Panel C). Data were reported as mean ± standard deviation (SD) as well as percent. Wilcoxon rank-sum test was used to test statistical differences (α = 0.05); *: to mark statistically significant differences. Figure E1. Not Perfused Area % vs Kurtosis scatter plot in the No-Steroids (left) and the Steroids (right) group. Data reported for all the included patients, divided in No-Steroids (left) and Steroids (right), in the whole parenchyma (blue) and the hypo-/non-inflated lung (red). The stars represent the mean values for each of the four groups of data. The group treated with steroids (right /stars) has significantly higher values of kurtosis than patients in the No-Steroids group (left/stars). The Not Perfused parenchyma in the hypo/not inflated lung is larger in the No-Steroids group (left red) compared to the Steroids group (right red). No differences are detectable in the Whole lung parenchyma (blue) between Steroids and No-Steroids group. These findings can be interpreted as preserved hypoxic pulmonary vasoconstriction (HPV) in the No-Steroids group and jeopardized HPV in the Steroids group. Figure E2. Distribution of total lung weight in the two analyzed groups: N-Steroids (left) and Steroids (right). Each orange circle indicates the estimated lung weight in grams for each included patient. The mean values are indicated as black lines and reported as values.
Acknowledgements
The authors thank the study nurses Elin Söderberg and Joanna Wessbergh, the radiology assistant Monica Segelsjö and the biobank research assistants Amanda Svensson, Labolina Spång, Philip Karlsson and Erik Danielsson for their expert help with compiling study data and organizing sample analysis.
Abbreviations
- BMI
Body mass index
- COVID-19
Coronavirus disease 2019
- CT
Computed tomography
- DECT
Dual-energy computed tomography
- FIO2
Inspiratory fraction of oxygen
- HAPE
High-altitude pulmonary edema
- HU
Hounsfield units
- HPV
Hypoxic pulmonary vasoconstriction
- ICU
Intensive care unit
- IL-6
Interleukin 6
- IQR
Interquartile range
- PCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- PaO2/FIO2
Ratio of arterial oxygen partial pressure to fractional inspired oxygen
- PCR
Polymerase chain reaction
- SAPS 3
Simplified acute physiology score 3
- SpO2
Peripheral oxygen saturation
Author contributions
RF, ML and MH conceived the study on COVID-19 and inflammatory markers. MP and GP conceived the study on DECT and designed the method of analysis. All the authors analyzed and interpreted the data of the study. MP and GP drafted the manuscript, and all the other authors revised it critically for important intellectual content. All the authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
Open access funding provided by Uppsala University. The study was funded by the SciLifeLab/KAW national COVID-19 research program project grant to MH (KAW 2020.0182 and KAW 2020.0241); the Swedish Research Council grant to RF (2014-02569 and 2014-07606) and to GP (2018-02438); the Swedish Heart and Lung foundation to GP (20200877 and 20200825) and MH (2021-0089, 2019-0639, 2019-0637); the Swedish Society for Medical Research to MP (463402221); the Swedish Society of Medicine to MP (SLS-959793); and the Alvar Gullstrand research grant to GP (ALF-938050). Funding bodies had no role in the design of the study, collection and interpretation of data or in the writing of the manuscript.
Availability of data and materials
Data are available from the corresponding author on reasonable request after appropriate ethical and data safety requirements are met (https://doi.org/10.17044/scilifelab.14229410).
Declarations
Ethics approval and consent to participate
The presented data are part of a study approved by the National Ethical Review Agency (EPM; No. 2020-01623). The Declaration of Helsinki and its subsequent revisions were followed. Informed consent was obtained from the patient or from next of kin if the patient was unable to give consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO). Timeline of WHO’s response to COVID-19. WHO webpage. 2020.
- 2.Else H. How a torrent of COVID science changed research publishing—in seven charts. Nature. 2020;588:553. doi: 10.1038/d41586-020-03564-y. [DOI] [PubMed] [Google Scholar]
- 3.Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9:622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santacroce L, Charitos IA, Carretta DM, de Nitto E, Lovero R. The human coronaviruses (HCoVs) and the molecular mechanisms of SARS-CoV-2 infection. J Mol Med (Berl) 2021;99:93–106. doi: 10.1007/s00109-020-02012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavriatopoulou M, Korompoki E, Fotiou D, Ntanasis-Stathopoulos I, Psaltopoulou T, Kastritis E, et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020;20:493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glenny RW, Robertson HT. Determinants of pulmonary blood flow distribution. Comp Physiol. 2011;1:39–59. doi: 10.1002/cphy.c090002. [DOI] [PubMed] [Google Scholar]
- 8.Pluskota-Karwatka D, Hoffmann M, Barciszewski J. Reducing SARS-CoV-2 pathological protein activity with small molecules. J Pharm Anal. 2021;11:383–397. doi: 10.1016/j.jpha.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 12.Maggiorini M. High altitude-induced pulmonary oedema. Cardiovasc Res. 2006;72:41–50. doi: 10.1016/j.cardiores.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Bliss A, Mahajan S, Boehm KM. Systematic review of the effects of phosphodiesterase-5 inhibitors and dexamethasone on high altitude pulmonary edema (HAPE) Spartan Med Res J. 2019;3:1–6. doi: 10.51894/001c.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthay MA, Clerici C, Saumon G. Active fluid clearance from the distal air spaces of the lung. J Appl Physiol. 2002;93:1533–1541. doi: 10.1152/japplphysiol.01210.2001. [DOI] [PubMed] [Google Scholar]
- 15.Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;20:1365–1366. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu GM, Zhao Y, Zhang LJ, Schoepf UJ. Dual-energy CT of the lung. AJR Am J Roentgenol. 2012;199:S40–S53. doi: 10.2214/AJR.12.9112. [DOI] [PubMed] [Google Scholar]
- 17.Godoy MCB, Naidich DP, Marchiori E, Assadourian B, Leidecker C, Schmidt B, et al. Basic principles and postprocessing techniques of dual-energy CT illustrated by selected congenital abnormalities of the thorax. J Thorac Imaging. 2009;24:152–159. doi: 10.1097/RTI.0b013e31819ca7b2. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Yu L, Primak AN, McCollough CH. Quantitative imaging of element composition and mass fraction using dual-energy CT: three-material decomposition. Med Phys. 2009;36:1602–1609. doi: 10.1118/1.3097632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 20.Ball L, Robba C, Herrmann J, Gerard SE, Xin Y, Mandelli M, et al. Lung distribution of gas and blood volume in critically ill COVID-19 patients: a quantitative dual-energy computed tomography study. Crit Care. 2021;25:1–12. doi: 10.1186/s13054-021-03610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellemgaard K. The alveolar-arterial oxygen difference: its size and components in normal man. Acta Physiol Scand. 1966;67:10–20. doi: 10.1111/j.1748-1716.1966.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 22.Uhrig M, Simons D, Ganten MK, Hassel JC, Schlemmer HP. Histogram analysis of iodine maps from dual energy computed tomography for monitoring targeted therapy of melanoma patients. Future Oncol. 2015;11:591–606. doi: 10.2217/fon.14.265. [DOI] [PubMed] [Google Scholar]
- 23.Yamashiro T, Matsuoka S, Estépar RSJ, Bartholmai BJ, Diaz A, Ross JC, et al. Kurtosis and skewness of density histograms on inspiratory and expiratory CT scans in smokers. COPD. 2011;8:13–20. doi: 10.3109/15412555.2010.541537. [DOI] [PubMed] [Google Scholar]
- 24.Mascalchi M, Camiciottoli G, Diciotti S. Lung densitometry: why, how and when. J Thorac Dis. 2017;9:3319–3345. doi: 10.21037/jtd.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perchiazzi G, Rylander C, Derosa S, Pellegrini M, Pitagora L, Polieri D, et al. Regional distribution of lung compliance by image analysis of computed tomograms. Respir Physiol Neurobiol. 2014;201:60–70. doi: 10.1016/j.resp.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka S, Kurihara Y, Yagihashi K, Niimi H, Nakajima Y. Quantification of thin-section CT lung attenuation in acute pulmonary embolism: correlations with arterial blood gas levels and CT angiography. AJR Am J Roentgenol. 2006;186:1272–1279. doi: 10.2214/AJR.05.0047. [DOI] [PubMed] [Google Scholar]
- 27.Iyer KS, Newell JD, Jin D, Fuld MK, Saha PK, Hansdottir S, et al. Quantitative dual-energy computed tomography supports a vascular etiology of smoking-induced inflammatory lung disease. Am J Respir Crit Care Med. 2016;193:652–661. doi: 10.1164/rccm.201506-1196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reske A, Reske A, Gast H, Seiwerts M, Beda A, Gottschaldt U, et al. Extrapolation from ten sections can make CT-based quantification of lung aeration more practicable. Intensive Care Med. 2010;36:1836–1844. doi: 10.1007/s00134-010-2014-2. [DOI] [PubMed] [Google Scholar]
- 29.Ball L, Braune A, Corradi F, Brusasco C, Garlaschi A, Kiss T, et al. Ultra-low-dose sequential computed tomography for quantitative lung aeration assessment—a translational study. Intensive Care Med Exp. 2017;5:19. doi: 10.1186/s40635-017-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rylander C, Högman M, Perchiazzi G, Magnusson A, Hedenstierna G. Oleic acid lung injury: a morphometric analysis using computed tomography. Acta Anaesthesiol Scand. 2004;48:1123–1129. doi: 10.1111/j.1399-6576.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 31.Pellegrini M, Larina A, Mourtos E, Frithiof R, Lipcsey M, Hultström M, et al. A quantitative analysis of extension and distribution of lung injury in COVID-19: a prospective study based on chest computed tomography. Crit Care. 2021;25:1–12. doi: 10.1186/s13054-021-03685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;2021. [DOI] [PMC free article] [PubMed]
- 33.Murata T, Hori M, Sakamoto K, Karaki H, Ozaki H. Dexamethasone blocks hypoxia-induced endothelial dysfunction in organ-cultured pulmonary arteries. Am J Respir Crit Care Med. 2004;170:647–655. doi: 10.1164/rccm.200309-1311OC. [DOI] [PubMed] [Google Scholar]
- 34.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhuri D, Sasaki K, Karkar A, Sharif S, Lewis K, Mammen MJ, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47:521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipcsey M, Persson B, Eriksson O, Blom AM, Fromell K, Hultström M, et al. The outcome of critically Ill COVID-19 patients is linked to thromboinflammation dominated by the Kallikrein/Kinin system. Front Immunol. 2021;12:62. doi: 10.3389/fimmu.2021.627579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B, Beigmohammadi MT, Farrokhpour M, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: The INSPIRATION Randomized Clinic. J Am Med Assoc. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richter T, Bergmann R, Musch G, Pietzsch J, Koch T. Reduced pulmonary blood flow in regions of injury 2 hours after acid aspiration in rats. BMC Anesthesiol. 2015;15:1–9. doi: 10.1186/s12871-015-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LRG, Mewburn JD, et al. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest. 2017;151:181–192. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price LC, Wort SJ. Pulmonary hypertension in ARDS: inflammation matters! Thorax. 2017;72:396–397. doi: 10.1136/thoraxjnl-2016-209199. [DOI] [PubMed] [Google Scholar]
- 41.Lang M, Som A, Carey D, Reid N, Mendoza DP, Flores EJ, et al. Pulmonary vascular manifestations of COVID-19 pneumonia. Radiol Cardiothorac Imaging. 2020;2:3. doi: 10.1148/ryct.2020200277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;2021. [DOI] [PMC free article] [PubMed]
- 43.Ryan D, Frohlich S, McLoughlin P. Pulmonary vascular dysfunction in ARDS. Ann Intensive Care. 2014;4:1–11. doi: 10.1186/s13613-014-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zochios V, Parhar K, Tunnicliffe W, Roscoe A, Gao F. The right ventricle in ARDS. Chest. 2017;152:181–193. doi: 10.1016/j.chest.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Gierhardt M, Pak O, Walmrath D, Seeger W, Grimminger F, Ghofrani HA, et al. Impairment of hypoxic pulmonary vasoconstriction in acute respiratory distress syndrome. Eur Respir Rev. 2021;30:161. doi: 10.1183/16000617.0059-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuster DP, Marklin GF. The effect of regional lung injury or alveolar hypoxia on pulmonary blood flow and lung water measured by positron emission tomography. Am Rev Respir Dis. 1986;133:1037–1042. doi: 10.1164/arrd.1986.133.6.1037. [DOI] [PubMed] [Google Scholar]
- 47.Kelly VJ, Hibbert KA, Kohli P, Kone M, Greenblatt EE, Venegas JG, et al. Hypoxic pulmonary vasoconstriction does not explain all regional perfusion redistribution in asthma. Am J Respir Crit Care Med. 2017;196:834–844. doi: 10.1164/rccm.201612-2438OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGonagle D, Bridgewood C, Meaney JFM. A tricompartmental model of lung oxygenation disruption to explain pulmonary and systemic pathology in severe COVID-19. Lancet Respir Med. 2021;9:665–672. doi: 10.1016/S2213-2600(21)00213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019∗. Crit Care Med. 2020;48:1129–1134. doi: 10.1097/CCM.0000000000004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busana M, Giosa L, Cressoni M, Gasperetti A, di Girolamo L, Martinelli A, et al. The impact of ventilation–perfusion inequality in COVID-19: a computational model. J Appl Physiol. 2021;130:865–876. doi: 10.1152/japplphysiol.00871.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karmouty-Quintana H, Thandavarayan RA, Keller SP, Sahay S, Pandit LM, Akkanti B. Emerging mechanisms of pulmonary vasoconstriction in SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) and potential therapeutic targets. Int J Mol Sci. 2020;21:1–21. doi: 10.3390/ijms21218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ameli-Renani S, Rahman F, Nair A, Ramsay L, Bacon JL, Weller A, et al. Dual-energy CT for imaging of pulmonary hypertension: challenges and opportunities. Radiographics. 2014;34:1769–1790. doi: 10.1148/rg.347130085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Material and Methods.
Additional file 2. Table E1. Descriptive statistics for HU distribution for pulmonary gas volume and blood volume maps. Whole population (n = 60). Comparison between the whole parenchyma and hypoinflated areas. Data were reported as mean ± standard deviation (SD) as well as percent. Wilcoxon rank-sum test was used to test statistical differences between the whole parenchyma and the hypoinflated one (α = 0.05); *: to mark statistically significant differences. Table E2. Descriptive statistics for HU distribution for pulmonary gas volume and blood volume maps. Separately for the No-Steroids group (n = 17, Panel A) and the Steroids group (n = 43, Panel A) Comparison between the whole parenchyma and hypoinflated areas (Panel A and B) as well as between No-Steroids and Steroids group (Panel C). Data were reported as mean ± standard deviation (SD) as well as percent. Wilcoxon rank-sum test was used to test statistical differences (α = 0.05); *: to mark statistically significant differences. Figure E1. Not Perfused Area % vs Kurtosis scatter plot in the No-Steroids (left) and the Steroids (right) group. Data reported for all the included patients, divided in No-Steroids (left) and Steroids (right), in the whole parenchyma (blue) and the hypo-/non-inflated lung (red). The stars represent the mean values for each of the four groups of data. The group treated with steroids (right /stars) has significantly higher values of kurtosis than patients in the No-Steroids group (left/stars). The Not Perfused parenchyma in the hypo/not inflated lung is larger in the No-Steroids group (left red) compared to the Steroids group (right red). No differences are detectable in the Whole lung parenchyma (blue) between Steroids and No-Steroids group. These findings can be interpreted as preserved hypoxic pulmonary vasoconstriction (HPV) in the No-Steroids group and jeopardized HPV in the Steroids group. Figure E2. Distribution of total lung weight in the two analyzed groups: N-Steroids (left) and Steroids (right). Each orange circle indicates the estimated lung weight in grams for each included patient. The mean values are indicated as black lines and reported as values.
Data Availability Statement
Data are available from the corresponding author on reasonable request after appropriate ethical and data safety requirements are met (https://doi.org/10.17044/scilifelab.14229410).