Abstract
Objective:
Reports of concomitant diabetic ketoacidosis (DKA) and acute pancreatitis (AP) are lacking among emerging forms of diabetes. This longitudinal study characterized ketosis-prone diabetes (KPD) in patients presenting with concomitant AP and DKA.
Methods:
Multi-ethnic KPD patients (N = 755) were followed prospectively for 1 year from the time of index DKA using repeated metabolic and beta cell functional reserve measures. Baseline and longitudinal characteristics were compared between KPD patients whose index DKA was associated with (n = 54) or without (n = 701) AP.
Results:
The AP group had significantly higher baseline serum amylase, lipase, and triglyceride levels and significantly lower bicarbonate levels than the non-AP group. AP patients had significantly greater C-peptide area-under-the-curve with glucagon stimulation shortly after the index DKA, and higher fasting C-peptide (FCP) levels 6 to 12 months later. Using the validated “Aβ” KPD classification, 85% of AP patients had β+ status (preserved beta cell functional reserve), compared to 60% of non-AP patients (P = .04). Multivariate analysis revealed that among the β+ KPD subgroup with an identifiable precipitating factor for DKA (“provoked” DKA), patients with AP had worse long-term glycemic outcomes than patients whose DKA was associated with other factors.
Conclusion:
Despite greater clinical severity at presentation, KPD patients with AP have better preserved beta cell function than those without AP. β+ KPD patients presenting with AP have worse long-term glycemic control than those with other causes of provoked DKA. Factors other than beta cell function negatively impact glycemic control in KPD patients presenting with AP.
INTRODUCTION
A well-documented association exists between acute pancreatitis (AP) and diabetic ketoacidosis (DKA) (1-4). AP may precipitate DKA; however, levels of amylase and lipase are frequently elevated during DKA, even in the absence of other criteria of acute exocrine pancreatitis, making it unclear in many instances whether AP is the cause or consequence of DKA (1-4). It is also possible that both AP and DKA could be coincident outcomes of a common pathophysiologic mechanism. Moreover, the phenotypes of patients who develop DKA have changed markedly in recent decades, leading to the characterization of distinct syndromes of ketosis-prone diabetes (KPD) (5). It is therefore important to understand the characteristics of DKA presenting with and without AP in the context of emerging forms of KPD.
KPD is a heterogeneous syndrome defined by presentation with DKA (5,6). We previously validated a highly accurate and predictive classification scheme for KPD (7). This “Aβ” scheme distinguishes four subgroups based on the presence or absence of islet cell autoantibodies (A+ or A−), and quantitative differences in beta cell functional reserve (β+ or β−) (6). In the present study, we sought to specify comprehensively and longitudinally the characteristics of KPD patients and subgroups based on presentation of the index DKA episode with concomitant AP. We therefore prospectively followed KPD patients who presented with or without AP at the time of the index DKA episode, and compared epidemiologic, biochemical, and clinical differences between these groups at presentation and during long-term follow-up in a dedicated research clinic.
METHODS
The study protocol was approved by the Institutional Review Boards for Human Studies of Baylor College of Medicine, and the Harris County Hospital District. A total of 755 patients admitted to Ben Taub General Hospital, Houston, Texas, with DKA from June 1, 1999, to April 13, 2010, were enrolled in the study. The subjects gave informed consent to be followed prospectively in the KPD research clinic with a standard outpatient management and database protocol, as previously described (6,8).
Patients were classified according to the Aβ classification scheme for KPD 3 to 4 weeks after the index DKA episode, based on fasting and stimulated C-peptide level and circulating islet cell autoantibody level, as previously described (5,6). Acute pancreatitis was defined as the presence of serum amylase and/or lipase levels greater than three times the upper limit of normal (ULN), or imaging findings consistent with pancreatitis (9,10). At admission, age, height, weight, body mass index (BMI), sex, ethnicity, age at diagnosis of diabetes, duration of diabetes, family history of diabetes, prior DKA events, and history of alcohol use were recorded. In addition, the following clinical parameters were measured: serum amylase, lipase, blood urea nitrogen, triglycerides, bicarbonate, glucose, arterial pH, anion gap, and serum or urine ketone levels.
Beta cell functional reserve was determined by fasting C-peptide (FCP) or area-under-the-curve (AUC) for C-peptide during a glucagon stimulation test (GST) with validated cutoffs, as previously described (6). These tests were performed at baseline (3 to 4 weeks after recovery from the acute DKA episode) and 6 to 12 months following the index DKA episode. Glycemic control was measured by hemoglobin A1c (HbA1c) levels determined by high performance liquid chromatography analysis at baseline and 6 and 12 months after the index DKA.
Following resolution of the index DKA episode, a standard outpatient diabetes management protocol was followed, as previously described (6). If insulin was discontinued, the patient was monitored closely for development of hyperglycemia. If hyperglycemia developed, the patient was placed on metformin. Second- and third-line agents used included sulfonylureas, thiazolidinediones, acarbose, and meglitinides. Glucagon-like peptide (GLP)-1 agonists and dipeptidyl peptidase (DPP)-IV inhibitors were not used.
Using longitudinal, multivariate analyses of an extensive, prospectively acquired dataset for a large subgroup of KPD patients with preserved beta cell functional reserve and no autoantibodies (A− β+ KPD, also termed ketosis-prone type 2 diabetes), we previously showed that long-term beta cell functional reserve, glycemic control, and insulin requirement (11,12) are strongly predicted by whether the index DKA episode was precipitated by a clinically identifiable stressor (i.e., whether the index DKA episode was “provoked” by acute illness or trauma, or whether it was “unprovoked”) (8). Hence, as part of the analysis in the present study, we compared long-term glycemic control and beta cell functional reserve across three subsets of the A− β+ KPD subgroups of patients: those whose index DKA episode was precipitated or accompanied by AP (Provoked-AP), those whose index DKA episode was precipitated by an acute stressful event other than AP (Provoked-other), and those whose index DKA episode had no identifiable precipitating cause (Unprovoked).
Univariate statistical comparisons were performed using a two-tailed Student’s t test with assumption of unequal variance. The level of significance was set at P <.05. We performed analysis of variance, multivariate, and logistic regression analyses with mean values for HbA1c, GST-AUC, C-peptide, and FCP with age, age at diagnosis of diabetes, duration of diabetes, whether diabetes was new-onset or not, BMI, gender, and ethnicity as covariates.
RESULTS
Epidemiologic and Clinical Characteristics
Fifty-four patients presented with DKA and a concomitant clinical diagnosis of AP, while 701 patients presented with DKA but without AP. AP was likely precipitated by alcohol abuse in 55% of cases, by hypertriglyceridemia in 22% of cases, and was idiopathic in 28% of cases. All 54 of the AP patients had significantly elevated levels of serum lipase and/or amylase (greater than three times the ULN), while 20 of the AP patients also had radiologic imaging findings consistent with AP. The AP group had shorter duration of known diabetes prior to the index DKA episode, an average of 3 years compared to 5 years for the non-AP group (P = .001) (Table 1). In addition, patients in the AP group suffered fewer DKA episodes prior to the index DKA than patients in the non-AP group (0.5 episodes per person versus 2 episodes per person, respectively; P = .001). The AP group also had a higher proportion of patients with new-onset diabetes at the time of the index DKA episode than did the non-AP group (57% versus 37%, respectively; P = .005). A history of alcohol use was more frequent in the AP group than in the non-AP group (55% versus 28%, respectively; P = .009). The male-female ratio was significantly higher in the AP group than the non-AP group (3.2:1 versus 1.4:1, respectively; P = .04). Eighty percent of the patients in the AP group were overweight or obese, compared to 58% of the patients in the non-AP group (P = .007).
Table 1.
Demographic, Biochemical, and Clinical Characteristics of Patients Presenting With Diabetic Ketoacidosis Associated or Not Associated With Acute Pancreatitis
| AP (n = 54) |
Non-AP (n = 701) |
P value | |
|---|---|---|---|
| Age (years) | 39 ± 1.4 | 40 ± 0.5 | .8 |
| Age at diagnosis of diabetes (years) | 36.0 ± 1.4 | 33.0 ± 0.5 | .06 |
| Duration of diabetes (years) | 3 ± 0.8 | 5 ± 0.3 | .001 |
| Prior DKA episodes | 0.5 ± 0.1 | 2.0 ± 0.4 | .001 |
| New onset diabetes (%) | 31 (57) | 262 (37) | .005 |
| Gender ratio (M:F) | 3.2 :1 | 1.4 :1 | .04 |
| Weight category (%) | |||
| Lean | 3 (20) | 282 (42) | .007 |
| Overweight | 17 (37) | 170 (25) | |
| Obese | 20 (43) | 221 (33) | |
| BMI (kg/m2) | 30.3 ± 0.8 | 28.5 ± 0.3 | .09 |
| Ethnicity (no-%) | |||
| White | 4 (7) | 35 (13) | .07 |
| Hispanic | 36 (67) | 287 (41) | |
| African American | 14 (26) | 311 (45) | |
| Asian | 0 (0) | 7 (1) | |
| Ethanol use (%) | 27 (55) | 56 (28) | .009 |
| Amylase (× ULN) | 3.9 ± 0.5 | 0.2 ± 0.02 | <.0001 |
| Lipase (× ULN) | 11.3 ± 2.0 | 0.3 ± 0.03 | <.0001 |
| Serum triglyceride (mg/dL) | 1299.7 ± 275.7 | 218.9 ± 12.0 | .001 |
| Serum bicarbonate (mEq/L) | 9.6 ± 0.9 | 11.6 ± 0.2 | .04 |
| BUN (mg/dL) | 23.3 ± 3.3 | 21.5 ± 0.7 | .6 |
| Serum pH | 6.9 ± 0.1 | 7.2 ± 0.01 | .05 |
| Anion gap (mEq/L) | 21.2 ± 0.9 | 21.7 ± 0.3 | .6 |
| Serum glucose (mg/dL) | 466.6 ± 23.6 | 491.6 ± 7.3 | .3 |
| HbA1c (%) | 12.9 ± 0.4 | 13.3 ± 0.1 | .04 |
| GST-AUC C-peptide (ng/mL over 10 minutes) | 32.9 ± 3.4 | 18.0 ± 0.7 | .003 |
| Fasting C-peptide (ng/mL) | 1.1 ± 0.1 | 1.2 ± 0.2 | .2 |
Abbreviations: AP: acute pancreatitis; BMI = body mass index; BUN = blood urea nitrogen; GST-AUC C-peptide = area-under-the-curve (AUC) for C-peptide during a glucagon stimulation test (GST); HbA1c = hemoglobin A1c; non-AP = non-acute pancreatitis; ULN = upper limit of normal.
Laboratory Results and Beta Cell Functional Characteristics
At the time of admission with the index DKA episode, compared to patients in the non-AP group, patients in the AP group had (by definition) higher serum levels of the pancreatic inflammatory markers amylase (3.9 ± 0.5 times the ULN versus 0.2 ± 0.02 times the ULN; P <.0001) and lipase (11.3 ± 2.0 times the ULN versus 0.3 ± 0.03 times the ULN; P <.0001) (Table 1). Patients in the AP group also had higher levels of serum triglycerides (1299.7 ± 275.7 mg/dL versus 218.9 ± 12.0 mg/dL; P = .001), lower serum bicarbonate (9.6 ± 0.9 mEq/L versus 11.6 ± 0.2 mEq/L; P = .04), and lower arterial pH (6.9 ± 0.1 versus 7.2 ± 0.01; P =.05) than patients in the non-AP group.
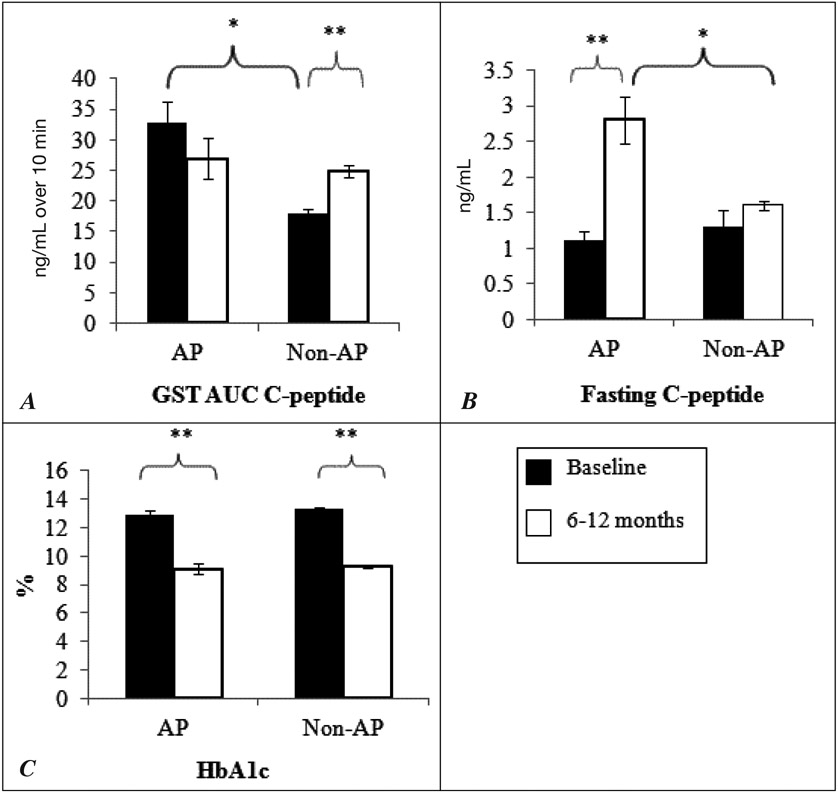
Patients in the AP group had higher mean stimulated C-peptide level and thus a greater beta cell functional reserve at baseline (3 to 4 weeks after the acute DKA episode) than patients in the non-AP group, with a GST-AUC C-peptide of 32.9 ± 3.4 ng/mL versus 18.0 ± 0.7 ng/mL over 10 min (P = .003) (Fig. 1). Patients in the AP group maintained a steady level of stimulated C-peptide for 6 to 12 months. The C-peptide response of patients in the non-AP group was blunted at baseline; however, these patients had a significant increase in stimulated C-peptide 6 to 12 months later, with the GST-AUC C-peptide rising from 18.0 ± 0.7 to 24.8 ± 1.0 ng/mL (P = .001). There was no significant difference in stimulated C-peptide levels between the groups in the measurements performed 6 to 12 months after the baseline measurements. Fasting C-peptide levels were not significantly different between the AP and non-AP groups at baseline. There was a significant rise in the FCP level 6 to 12 months later in patients in the AP group (1.1 ± 0.1 to 2.8 ± 0.3 ng/dL; P <.001), while the FCP level remained steady over the same period in patients in the non-AP group. Thus, 6 to 12 months after the index DKA event, patients in the AP group had a significantly higher fasting C-peptide level than non-AP patients (2.8 ± 0.3 ng/dL versus 1.6 ± 0.1 ng/dL; P = .01).
Fig. 1.
Comparison of beta cell functional reserve (A, GST-AUC C-peptide, B, fasting C-peptide) and C, glycemic control (HbA1c) at baseline and after 6 to 12 months of follow-up among AP and non-AP patients. *P <.05; **P <.001. GST-AUC C-peptide = Area-under-the-curve (AUC) for C-peptide during a glucagon stimulation test (GST); AP = acute pancreatitis.
Both AP and non-AP patients showed significant improvement in HbA1c level 6 to 12 months after the baseline measurement (12.9 ± 0.4 to 9.1 ± 0.4%; P <.001, and 13.3 ± 0.1 to 9.3 ± 0.1%; P <.001, respectively). However, there was no difference in HbA1c level between the groups at baseline or 6 to 12 months later.
A multivariate analysis of outcomes 6 to 12 months after the index DKA episode was conducted. The analysis controlled for age, age at diagnosis of diabetes, duration of diabetes, whether or not diabetes was new-onset, BMI, gender, and ethnicity. The results showed that the FCP level remained significantly higher in patients in the AP group than in patients in the non-AP group (P = .004) (Table 2). There were no group differences over time with respect to stimulated C-peptide or HbA1c levels. Logistic regression analysis revealed that patients in the AP group were more likely to have new-onset diabetes at the time of the index DKA episode (P = .05) and to be overweight or obese (P = .05).
Table 2.
Multivariate Analysis of Outcomes 6 to 12 Months After Index Diabetic Ketoacidosis Episode in Acute Pancreatitis versus Non-Acute Pancreatitis Groupsa
| AP | Non-AP | P value | |
|---|---|---|---|
| Fasting C-peptide (ng/mL) | 2.8 | 1.6 | .004 |
| GST-AUC C-peptide (ng/mL over 10 min) | 26.9 | 24.8 | .8 |
| HbA1c (%) | 9.1 | 9.3 | .5 |
Abbreviations: AP = acute pancreatitis; GST-AUC C-peptide = area-under-the-curve (AUC) for C-peptide during a glucagon stimulation test (GST); HbA1c = hemoglobin A1c; non-AP = non-acute pancreatitis.
Covariates in the analysis were: age, age at diagnosis of diabetes, duration of diabetes, whether diagnosis of diabetes was first made at the time of the index diabetic ketoacidosis (“new-onset” diabetes), body mass index, gender, and ethnicity.
Acute Pancreatitis Subgroup Analysis: Alcohol Users versus Nonusers
As there were significantly more consumers of alcohol in the AP group than the non-AP group, we compared alcohol users and nonusers within the AP group with respect to all of the baseline and outcome parameters described above. There were no significant differences between alcohol users and nonusers in any parameter except gender; alcohol users had a higher ratio of men to women (8:1 versus 1.4:1; P = .02).
A− β+ Ketosis-prone Diabetes Subgroup Analysis
Patients with β− forms of KPD almost never recover beta cell functional reserve, and are unable to achieve excellent glycemic control over the long term. In contrast, patients with β+ forms of KPD have variable long-term beta cell functional preservation and are frequently able to come off insulin treatment altogether while maintaining excellent glycemic control. We previously showed that long-term glycemic control and beta cell functional reserve within a large subgroup of A− β+ KPD patients are strongly linked to whether the index DKA episode was associated with a clinically identifiable precipitating factor (8). A− β+ KPD patients presenting with provoked DKA have significantly worse long-term glycemic outcomes and beta cell functional reserve, and are significantly more dependent on insulin than A− β+ KPD patients with unprovoked DKA.
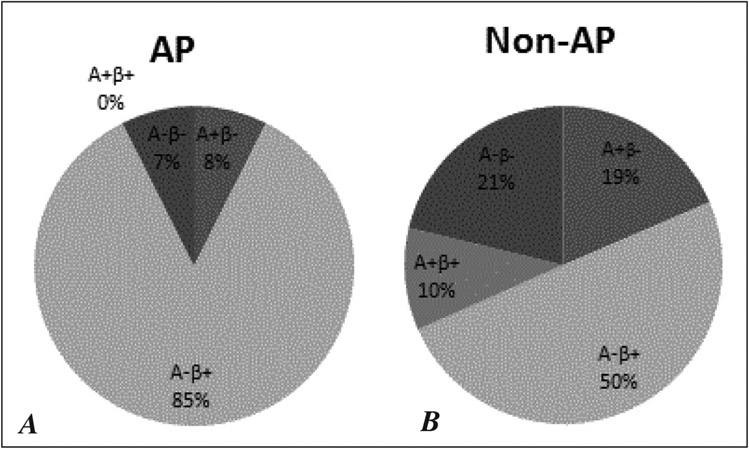
Univariate and multivariate analyses of data pertaining to the AP group indicated these patients had better preserved beta cell function both at presentation and over the long-term than did non-AP group patients, and also indicated that the AP group had a higher proportion of patients with new-onset diabetes. Hence, we were interested in investigating the distribution of KPD subtypes in the AP and non-AP groups, since these subtypes are distinguished by beta cell functional status and some subtypes are characterized by a predominance of new-onset diabetes. The analysis revealed that representation among the phenotypic categories of KPD was more restricted in the AP group, with a higher frequency of patients belonging to the A− β+ KPD category than was the case in the non-AP group. A− β+ KPD patients comprised 85% of the AP group versus 50% of the non-AP group (P = .04) (Fig. 2).
Fig. 2.
Distribution of the four KPD subgroups among (A) AP and (B) non-AP patients. Provoked-AP: n = 23 (14%); Provoked-other: n = 68 (40%); Unprovoked: n = 79 (46%).
We then compared patients with A− β+ KPD whose index DKA event was precipitated or accompanied by AP (Provoked-AP, n = 23, 14%) to those whose DKA event was precipitated by any other stressor (Provoked-other, n = 68, 40%) and to those whose index DKA event had no identifiable precipitating stressor (Unprovoked, n = 79, 46%) (Table 3). Significant differences between these subgroups were noted in regard to three of the main outcomes 6 to 12 months after the baseline measurements: GST-AUC C-peptide level, FCP level, and HbA1c level. The rank order of the changes observed among the subgroups 6 to 12 months after the baseline measurements were as follows: Provoked-other < Provoked-AP < Unprovoked (P <.001) for the GST-AUC C-peptide level, and Provoked-other < Unprovoked < Provoked-AP (P = .05) for the FCP level. However, the mean change in HbA1c level 6 to 12 months after the baseline measurement showed a different rank order: Unprovoked < Provoked-other < Provoked-AP (P <.001).
Table 3.
Demographic Parameters and Outcomes of A− β+ Ketosis-prone Diabetes Patients: Provoked by Acute Pancreatitis, Reasons Other than Acute Pancreatitis, and Unprovoked
| Provoked-AP | Provoked-other | Unprovoked | P value | |
|---|---|---|---|---|
| Age at diagnosis of diabetes (years) | 39.1 ± 10.33 | 38.5 ± 13.77 | 41.4 ± 13.06 | .38 |
| Duration of diabetes (years) | 0.8 ± 1.68 | 5.2 ± 5.11 | 0.1 ± 0.94 | <.001 |
| BMI (kg/m2) | 32.2 ± 6.67 | 30.5 ± 8.63 | 33.0 ± 8.46 | .19 |
| Fasting C-peptide (ng/mL at 6-12 months) | 3.0 ± 1.68 | 2.3 ± 1.14 | 2.8 ± 1.57 | .05 |
| GST-AUC C-peptide (ng/mL over 10 min) at 6-12 months | 36.8 ± 11.73 | 31.3 ± 14.34 | 44.0 ± 18.20 | <.001 |
| HbA1c (% at 6-12 months) | 9.5 ± 2.20 | 8.5 ± 2.35 | 7.5 ± 1.98 | <.001 |
| Change in fasting C-peptide (baseline to 6-12 months) | 1.4 ± 1.78 | 0.18 ± 1.97 | 1.0 ± 2.21 | <.001 |
| Change in GST-AUC C-peptide (baseline to 6-12 months) | −1.9 ± 25.09 | ±0.4 ± 22.08 | 17.6 ± 24.18 | <.001 |
| Change in HbA1c (baseline to 6-12 months) | −3.6 ± 3.53 | −5.0 ± 2.92 | −6.6 ± 3.07 | <.001 |
Abbreviations: AP = acute pancreatitis; BMI = body mass index; GST-AUC C-peptide = area-under-the-curve (AUC) for C-peptide during a glucagon stimulation test (GST); HbA1c = hemoglobin A1c.
Multinomial logistic regression analysis revealed that patients in these three A− β+ KPD subgroups were similar with respect to age and BMI, and also with respect to FCP and GST-AUC C-peptide levels 6 to 12 months after the index DKA episode. However, Provoked-AP patients were 1.4 times more likely to have a higher HbA1c level 6 to 12 months after the baseline measurements than were patients in the Provoked-other subgroup. Furthermore, patients in the Unprovoked A− β+ KPD subgroup were 1.04 times more likely to have a higher GST-AUC C-peptide level (P <.04) and more likely to have a shorter duration of known diabetes (odds ratio [OR] = 0.31; 95% confidence interval [CI], 0.17-0.58) than patients in the Provoked-other subgroup (Table 4).
Table 4.
Adjusted Odds Ratios of Outcomes 6 to 12 Months After Index DKA Episode Three Categories of A− β+ KPD Patientsa,c
| A− β+ KPD Patient Groups | Odds Ratiob | 95% Confidence Interval | P value |
|---|---|---|---|
| Unprovoked-AP | |||
| Age (years) | 0.97 | [0.94, 1.01] | .29 |
| BMI (kg/m2) | 1.01 | [0.96, 1.07] | .57 |
| Duration of diabetes (years) | 0.31 | [0.17, 0.58] | <.001 |
| HbA1c (%) | .906 | [0.71, 1.15] | .43 |
| Fasting C-peptide (ng/mL) | .854 | [0.59, 1.23] | .40 |
| GST-AUC C-peptide (ng/mL over 10 min) | 1.04 | [1.02, 1.07] | .04 |
| Provoked-AP | |||
| Age (years) | .984 | [0.93, 1.03] | .50 |
| BMI (kg/m2) | 1.01 | [0.94, 1.08] | .83 |
| Duration of diabetes (years) | 0.63 | [0.46, 0.85] | .003 |
| HbA1c (%) | 1.36 | [1.04, 1.76] | .02 |
| Fasting C-peptide (ng/mL) | 1.22 | [0.79, 1.88] | .37 |
| GST-AUC C-peptide (ng/mL over 10 min) | 1.01 | [0.98, 1.05] | .41 |
Abbreviations: AP = acute pancreatitis; BMI = body mass index; GST-AUC C-peptide = area-under-the-curve (AUC) for C-peptide during a glucagon stimulation test (GST); HbA1c = hemoglobin A1c; KPD = ketosis-prone diabetes.
The reference category is the Provoked-Other A−β+ KPD group.
Odds ratios were calculated from multinomial logistic regression analysis.
A lack of significance (and odds ratio values) showed that participants were similar with respect to age, BMI, and fasting C-peptide level.
DISCUSSION
Concomitant presentation of AP and DKA has previously been reported in the literature; however, little is known regarding the natural history and long-term implications of this association, especially among emerging forms of KPD. Here, we report the findings of a prospective analysis of a large cohort of KPD patients followed in a dedicated KPD clinic, with the aim of elucidating the epidemiologic and biochemical characteristics of patients who had clinical evidence of AP at the time of the initial DKA episode.
It is often difficult to diagnose pancreatitis in a DKA setting because of the possibility of nonspecific elevations in amylase and lipase levels in DKA without other clinical criteria or manifestations of exocrine pancreatitis (3). However, a study of 100 consecutive cases of DKA revealed that cases in which the serum amylase level was greater than three times the ULN had 97% specificity for image-confirmed pancreatitis, and cases in which the serum lipase level was greater than three times the ULN had a specificity for image-confirmed pancreatitis of 91% (2). In the present study, our criteria for pancreatitis were serum amylase and/or lipase levels greater than three times the ULN, and 20 patients had imaging findings that confirmed the diagnosis of pancreatitis.
KPD patients with AP had greater severity of clinical illness at presentation with the index DKA episode, with serum biochemical values that were more significantly deranged than those of KPD patients without AP. However, KPD patients with AP had a greater capacity for recovery, demonstrated by a higher beta cell functional reserve that persisted for 6 to 12 months. This correlated well with the distribution of KPD categories within each group, as 85% of patients in the AP group were β+, compared to 60% of patients in the non-AP group.
We previously showed that within a large subgroup of A− β+ KPD patients, those with a clinically identifiable precipitating factor for their DKA (i.e., those with provoked DKA) have significantly worse long-term glycemic outcomes and beta cell functional reserve than do patients with unprovoked DKA (8), regardless of whether the index DKA episode signaled new-onset diabetes. Since the AP group had better preserved beta cell functional reserve and included a higher proportion of patients with the A− β+ KPD subtype than the non-AP group, we examined the specific effects of AP by comparing Provoked-AP, Provoked-other, and Unprovoked A− β+ patients with respect to the key prognostic measures of beta cell functional reserve and glycemic control. Our results corroborated previous findings (8), in that the Unprovoked A− β+ KPD subtype is characterized by more robust beta cell functional reserve than either of the Provoked subtypes, and shows the greatest improvement in glycemic control over time, with the lowest HbA1c percentage after 6 to 12 months. Furthermore, multivariate analysis revealed that patients in the Provoked-AP group fell between the Provoked-other and Unprovoked A− β+ KPD groups in regard to beta cell functional reserve. Despite having better long-term beta cell function than patients with Provoked-other KPD, over time the Provoked-AP patients achieved the worst glycemic control of the 3 subtypes, having the highest HbA1c level after 6 to 12 months. These data suggest that factors other than beta cell function have a negative impact on long-term glycemic control in KPD patients presenting with AP, mitigating the advantage they possess of having excellent long-term beta cell functional reserve following the DKA episode. Patients with KPD reportedly have higher basal concentrations of glucagon and attenuated suppression of glucagon release, suggesting that alpha cell dysregulation may be important in the pathophysiology (13); however, it is unclear why this would affect the Provoked-AP group in particular. Additional factors could include dietary indiscretion associated with the higher prevalence of alcohol use in this group, lack of medical insurance or low socioeconomic status, treatment noncompliance, and psychosocial or substance abuse issues that we did not systematically investigate.
We found that patients with AP were more likely to be overweight or obese, which might be associated with more severe insulin resistance and poorer glycemic control. We did not explicitly use insulin sensitivity as a covariate, but in our comparative analyses of Provoked-AP, Provoked-other, and Unprovoked A− β+ KPD patients, we de facto normalized all subjects with regard to this factor because all of the patients had A− β+ KPD, which we showed previously is strongly associated with a patient’s likelihood of being overweight/obese and of suffering from a high frequency of other components of metabolic syndrome (6). However, differences in insulin sensitivity could have affected the comparisons between AP and non-AP patients, since 85% of the AP patients (versus only 50% of the non-AP patients) belonged to the A+ β− category.
A potential reason for concurrent AP and DKA, especially in patients without a history of alcohol abuse, is mutation in the carboxyl ester lipase (CEL) gene. Mutations in the CEL gene are known to cause a monogenic syndrome of diabetes with exocrine dysfunction (14). Reports from Japan have noted a link between autoimmune exocrine pancreatitis and fulminant type 1 diabetes in association with elevated levels of autoantibodies to amylase alpha-2A (15). Autoantibodies against exocrine pancreatic antigens such as carbonic anhydrase II and lactoferrin have also been linked to combined diabetes and pancreatitis (16). Abnormal development of the pancreas in utero—perhaps due to defects in the Wnt/B-catenin signaling pathway—could manifest in adults as combined exocrine-endocrine dysfunction (17). Patients with cystic fibrosis (CF) commonly develop diabetes due to endocrine pancreatic insufficiency associated with chronic exocrine pancreatitis. It is possible that CF-associated progressive pancreatic destruction and resultant decrease in beta cell mass could precipitate acute episodes of combined exocrine and endocrine pancreatic dysfunction. KPD patients whose first manifestation of diabetes is DKA in association with “idiopathic” nonalcoholic pancreatitis would be ideal candidates for studies investigating genetic or autoimmune pathophysiologic factors such as these, and are currently being investigated for these factors.
CONCLUSION
In conclusion, β+ KPD patients presenting with concomitant exocrine pancreatitis and DKA constitute a unique subgroup, with a distinct natural history of beta cell function. From a clinical standpoint, they differ from other Provoked A− β+ KPD patients in that their glycemic control is worse over time, despite better preserved beta cell function. Efforts to improve clinical outcomes in patients who present with DKA and AP should focus on identifying the factors associated with their long-term poor glycemic control and targeting them for intervention.
ACKNOWLEDGMENT
We thank Ivonne Coraza for maintaining the KPD database, Mary Lou Arvizu, RN, for expert nursing care of the patients, and Drs. Maria Redondo, Mike Metzker, Diane Scaduto, and Richard Sifers for helpful discussions. This work was supported by NIH-R21 grant DK082827 (to AB) and by the Baylor College of Medicine Diabetes Research Center (P30DK079638).
Abbreviations:
- AP
acute pancreatitis
- AUC
area-under-the-curve
- BMI
body mass index
- DKA
diabetic ketoacidosis
- FCP
fasting C-peptide
- GST
glucagon stimulation test
- HbA1c
hemoglobin A1c
- KPD
ketosis-prone diabetes
- ULN
upper limit of normal
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Nair S, Pitchumoni CS. Diabetic ketoacidosis, hyperlipidemia, and acute pancreatitis: the enigmatic triangle. Am J Gastroenterol. 1997;92:1560–1561. [PubMed] [Google Scholar]
- 2.Nair S, Yadav D, Pitchumoni CS. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol. 2000;95:2795–2800. [DOI] [PubMed] [Google Scholar]
- 3.Yadav D, Nair S, Norkus EP, Pitchumoni CS. Nonspecific hyperamylasemia and hyperlipasemia in diabetic ketoacidosis: incidence and correlation with biochemical abnormalities. Am J Gastroenterol. 2000;95:3123–3128. [DOI] [PubMed] [Google Scholar]
- 4.Tully GT, Lowenthal JJ. The diabetic coma of acute pancreatitis. Ann Intern Med. 1958;48:310–319. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev. 2008;29:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab. 2003;88:5090–5098. [DOI] [PubMed] [Google Scholar]
- 7.Balasubramanyam A, Garza G, Rodriguez L, et al. Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care. 2006;29:2575–2579. [DOI] [PubMed] [Google Scholar]
- 8.Nalini R, Ozer K, Maldonado M, et al. Presence or absence of a known diabetic ketoacidosis precipitant defines distinct syndromes of A− β+ ketosis-prone diabetes based on long-term β-cell function, human leukocyte antigen class II alleles, and sex predilection. Metabolism. 2010;59:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chase CW, Barker DE, Russell DL, Burns RP. Serum amylase and lipase in the evaluation of acute abdominal pain. Am Surg. 1996;62:1028–1033. [PubMed] [Google Scholar]
- 10.Ranson JH. Diagnostic standards for acute pancreatitis. World J Surg. 1997;21:136–142. [DOI] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144:350–357. [DOI] [PubMed] [Google Scholar]
- 12.Smiley D, Chandra P, Umpierrez GE. Update on diagnosis, pathogenesis and management of ketosis-prone type 2 diabetes mellitus. Diabetes Manag (Lond). 2011;1:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choukem SP, Sobngwi E, Boudou P, et al. β− and α-cell dysfunctions in Africans with ketosis-prone atypical diabetes during near-normoglycemic remission. Diabetes Care. 2013;36:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson BB, Torsvik J, Bjørkhaug L, et al. Diabetes and pancreatic exocrine dysfunction due to mutations in the carboxyl ester lipase gene-maturity onset diabetes of the young (CEL-MODY): a protein misfolding disease. J Biol Chem. 2011;286:34593–34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo T, Takizawa S, Tanaka S, et al. Amylase alpha-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes. 2009;58:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardt PD, Ewald N, Bröckling K, et al. Distinct autoantibodies against exocrine pancreatic antigens in European patients with type 1 diabetes mellitus and non-alcoholic chronic pancreatitis. JOP. 2008;9:683–689. [PubMed] [Google Scholar]
- 17.Murtaugh LC. The what, where, when and how of Wnt/β-catenin signaling in pancreas development. Organogenesis. 2008;4:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]