Abstract
A new meningococcal group C-CRM197 conjugate vaccine (MnCC; Meningitec) has been evaluated in multiple clinical trials in the United States and most recently has been approved for routine administration in the United Kingdom. Meningococcal serogroup C (MnC)-specific immunoglobulin G (IgG) antibodies in pre- and postimmunization sera obtained from healthy U.S. adults, toddlers, and infants were quantitated by enzyme-linked immunosorbent assay (ELISA) and by an antibody-dependent, complement-mediated serum bactericidal assay (SBA). Serogroup-specific IgG antibody (micrograms per milliliter) in adults immunized either with the quadrivalent polysaccharide (A, C, Y, and W-135) vaccine or with MnCC showed a strong correlation (r = 0.848 and 0.934, respectively) by linear regression analysis with SBA. Sera from infants immunized with the MnCC (n = 30) and an age-matched unimmunized control group (n = 15) were also analyzed. Linear regression analysis of serum bactericidal and IgG ELISA data from sera obtained at 2 months of age (preimmunization) showed no correlation; however, a high degree of correlation was observed at time points after two (r = 0.877) and three (r = 0.951) immunizations, where significant rises in anti-MnC polysaccharide antibodies occurred relative to the age-matched control group. Infants previously primed with 3 doses of MnCC were given a booster dose of conjugate vaccine at 12 to 15 months of age. The correlation coefficient of ELISA to SBA for combined pre- and postbooster data was r = 0.836 (n = 48 pairs). In conclusion, increases in serum bactericidal activity in immunized adult, toddler, and infant populations were found to correlate very well with increases in serogroup-specific IgG concentrations, whereas the correlation between these two assays in nonimmunized 2-month-old infants was poor. Characterizing the relationship between these methods is important for understanding the significance of antigen-specific antibody concentrations relative to vaccine performance and protection from disease.
Polysaccharide vaccines for Neisseria meningitidis serogroups A, C, Y, and W-135 have been in use for approximately 20 years and have been effective in preventing invasive disease in high-risk populations and in controlling disease outbreak situations (5, 7, 8, 17–19, 21, 25, 27). However, the vaccine is not immunogenic in children under the age of 2 years, the age group at the highest risk for disease (3). With the success of Haemophilus influenzae type b conjugate vaccines in infants, similarly prepared conjugate vaccines with the polysaccharide from N. meningitidis group C (MnC) are showing promise for at-risk subjects. Recently, MnC conjugate vaccines have been introduced in the United Kingdom for routine use.
Efficacy trials have not been conducted with these MnC conjugate vaccines due to the sporadic and unpredictable nature of disease outbreaks; thus, reliance on characterization of the immune response is of paramount importance (7). Two serologic methods have been used extensively in the evaluation of protective status and vaccine performance, the serum bactericidal assay (SBA) and the enzyme-linked immunosorbent assay (ELISA) for immunoglobulin G (IgG) antibodies specific to the group C polysaccharide (8, 24).
These two methods are utilized to determine their relationship with sera from multiple trials in the United States evaluating an MnC-CRM197 conjugate (MnCC; Meningitec) vaccine. Previously standardized methods are utilized (8, 24); the reagents and materials used in this ELISA and SBA are rigorously screened to ensure consistent and specific assay performance. The relationship of ELISA to SBA is evaluated with randomly selected sera covering the broad range of anti-MnC polysaccharide antibody concentrations observed in adults, toddlers, and infants before and after immunization with MnCC.
MATERIALS AND METHODS
Vaccines.
Antibodies to meningococcal C serogroup were quantitated in preimmune and immune sera obtained from human subjects who were administered some of the following vaccines: licensed quadrivalent (A, C, Y, W-135) meningococcal polysaccharide vaccine (MnCPs) (Menomune; Connaught Laboratories, Swiftwater, Pa.), one dose contained 50 μg of each serogroup; MnCC (Wyeth-Lederle Vaccines, Pearl River, N.Y.), each dose contained 10 μg of meningococcal group C saccharide; a heptavalent pneumococcal saccharide-CRM197 conjugate vaccine (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) (Prevnar, Wyeth-Lederle Vaccines).
Other vaccines were administered concomitantly at age-appropriate schedules (3): oral polio (Orimune, Wyeth-Lederle Vaccines); diphtheria, tetanus, pertussis, and Haemophilus b (DTP/HbOC) (TETRAMUNE; Wyeth-Lederle Vaccines); and measles, mumps, and rubella (Merck & Co., Inc., West Point, Pa.).
Serum samples.
Sera were randomly selected from subjects who participated in several clinical trials (conducted by Wyeth-Lederle Vaccines) evaluating investigational vaccines. Sera from 30 healthy adult subjects enrolled in the trial through a center located near Philadelphia, Pa., where the subjects were equally randomized to receive a single dose of either quadrivalent (A, C, Y, W-135) MnCPs or MnCC, were collected prior to immunization and 4 to 6 weeks postimmunization. Sera from healthy infants who were enrolled in a trial through four centers located in Atlanta, Ga., Baltimore, Md., Nashville, Tenn., and Pittsburgh, Pa., and who received four consecutive doses of either MnCC or a heptavalent pneumococcal saccharide-CRM197 conjugate vaccine at 2, 4, 6, and 12 to 15 months of age were collected prior to each immunization and 4 to 6 weeks postimmunization (30). Concomitantly administered routine vaccines were oral polio vaccine and DTP/HbOC at 2, 4, and 6 months, and measles, mumps, and rubella and HbOC at 12 to 15 months. The study was approved by institutional review boards at each center, and written informed consent was obtained from parents or guardians prior to enrollment.
Sera from healthy adults immunized with MnCPs vaccine served as quality control specimens to determine bactericidal and ELISA performance and repeatability. All serum specimens were bar coded at the clinical site, and ELISA and bactericidal analyses were performed without knowledge as to which specimens were collected pre- or postimmunization or to protocol. In addition, serum specimens submitted for ELISA and bactericidal determinations were distributed independently for analysis by different individuals. After completion of all analyses, the specimens were decoded for statistical analysis.
SBA.
The bactericidal assay target organism, N. meningitidis serogroup C (strain C11), was obtained from G. Carlone at the Centers for Disease Control and Prevention and was originally obtained by E. Gotschlich in 1965 (11). Bacterial cells were grown overnight on brain heart infusion agar plates (Difco Laboratories, Detroit, Mich.), containing 1% horse serum (Gibco BRL Life Technologies, Inc., Gaithersburg, Md.) at 37°C with 5% CO2, harvested with sterile swabs, resuspended in Gey's solution, and stored at −70°C in 0.5-ml aliquots. A fresh aliquot was obtained for each experiment to eliminate differences in the passage history of the strain.
Bactericidal assays were performed essentially as described by Maslanka et al. (24). Briefly, serial dilutions of heat-inactivated test and control sera (56°C for 30 min) are prepared in microtiter plates (25 μl/well). Bacterial cells (12.5 μl of a suspension containing approximately 4 × 103 CFU/ml) and freshly thawed rabbit complement (12.5 μl) (Pel-Freeze Biologicals, Brown Deer, Wis.) were mixed and added to appropriate rows in the microtiter plate. Microtiter plates were then incubated at 37°C for 60 min. At the end of the incubation, 100 μl of tryptic soy agar overlay medium, equilibrated to 45°C, was added to all microtiter wells. Plates were incubated overnight at 37°C under 5% CO2 and the resulting colonies were enumerated under magnification using a dissecting microscope. The bactericidal antibody titer was expressed as the reciprocal of the final serum dilution yielding ≥50% killing compared to the average for control wells (bacteria plus complement) from each assay plate. The lowest dilution of sera tested for bactericidal activity was a twofold dilution of the test specimen. All sera were tested by serially diluting (twofold) each serum through nine assay wells. If no bactericidal activity was detected in the twofold-diluted sera (i.e., bactericidal activity of less than 2), the bactericidal titer is reported as 1. Sera with bactericidal activity exceeding the initial dilution scheme were tested, beginning with a higher-dilution scheme. Each serum was tested a minimum of two times, on different days. The resulting titers from the two days were averaged if the individual titers were within a single dilution of the mean of the two titers. Those specimens with more than a single dilution difference from the mean titer were repeated additional times to determine the appropriate assigned SBA titer. Each assay incorporated the following four controls: (i) bacterial T0 control (determines number of CFU of N. meningitidis added to the assay mixture at the time of assay initiation), (ii) complement control, i.e., bacterial cells plus complement only (determines complement-dependent killing), (iii) serum control, i.e., bacterial cells plus heat-inactivated test serum only (determines complement-independent killing), and (iv) a known positive control serum, included on each plate and treated as an unknown. The titer of the positive control serum must be reproducible between assays to within a single twofold dilution for acceptance of the data.
Anti-N. meningitidis serogroup C ELISA.
The anti-N. meningitidis serogroup C polysaccharide ELISA previously described was modified slightly to determine serum IgG antibodies (8). Medium-binding ELISA plates (Nalge Nunc International, Naperville, Ill.) were used in place of the Immulon II plates; the meningococcal serogroup C polysaccharide was prepared by Wyeth-Lederle Vaccines instead of using antigen available from the Centers for Disease Control and Prevention (prepared by Connaught Laboratories), and coating of ELISA plates with antigen was performed with sterile buffers prepared using pyrogen-free bottled water to minimize the possibility of introducing endotoxins into the ELISA system (8). Human serum, starting at a 50-fold dilution and serially diluted 2-fold, was added to wells of the antigen-coated plates. Bound antibodies were detected by using a goat anti-human IgG alkaline phosphatase-labeled antibody followed by p-nitrophenyl phosphate substrate. Antibody assignments were based on endpoint dilutions, at 0.3 absorbance unit, of a logarithmic regression of the reciprocal of dilution versus absorbance. The known concentration of the reference preparation (CDC1992), with an assigned value of 24.10 μg/ml) was used to assign antibody concentrations of each test serum or control serum (15). The lower quantitation limit of the assay was 0.10 μg/ml. Values of less than 0.10 were reported as 0.05 μg/ml.
Statistical analysis.
Analysis of data was performed using the Pearson product-moment correlation coefficient (r), calculated by using the log10-transformed bactericidal titer and log10-transformed ELISA antibody concentration for each serum sample (19). The significance (P) was calculated using a two-tailed Student's t test. Simple linear regression analysis of the log10-transformed data was performed for the calculation of the regression coefficient (β) (20).
RESULTS
Analysis of sera from adult subjects.
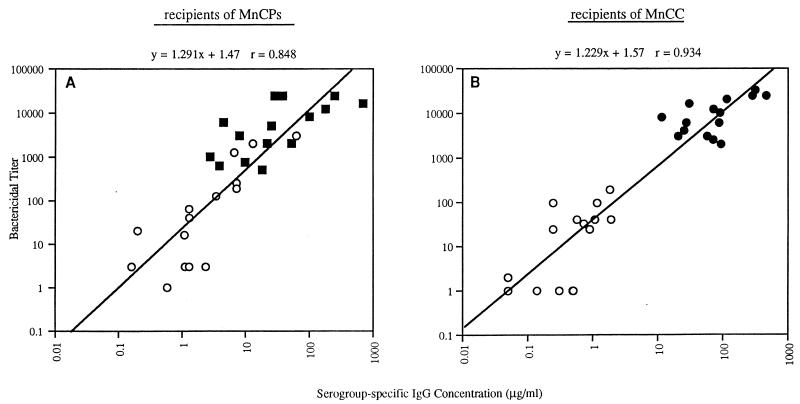
To determine the relationship between serogroup-specific IgG concentration and bactericidal activity, sera obtained from 30 healthy adults who were randomized to receive MnCPs or MnCC were tested in each assay. When the combined results for pre- and postimmunization samples were analyzed, there was little difference in the correlation (r = 0.848 and 0.934) and regression coefficients (slopes = 1.291 and 1.229) between the group receiving MnCPs and the group receiving MnCC (Fig. 1).
FIG. 1.
Meningococcal serogroup C bactericidal titer versus serogroup-specific IgG concentrations (ELISA) for adult subjects immunized with one dose of MnCC (n = 15) or a licensed quadrivalent polysaccharide (A, C, Y, W-135) vaccine (n = 15). Sera were collected for analysis prior to immunization (○) or approximately 1 month postimmunization with the licensed quadrivalent polysaccharide vaccine (■ [A]) or the conjugate vaccine (● [B]).
Analysis of sera from infant subjects.
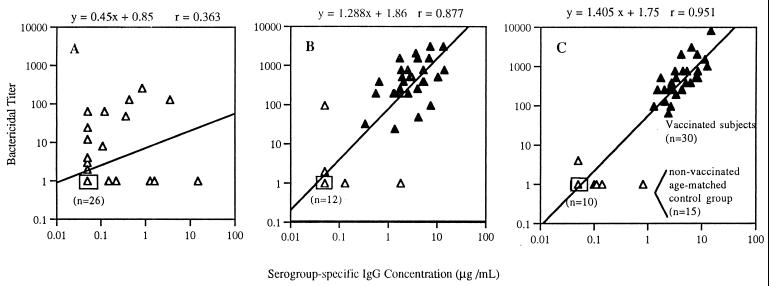
ELISA was performed on sera obtained from infants immunized at 2, 4, and 6 months of age with MnCC. From the study population, a representative subset of immunized (n = 30) and age-matched control subjects (n = 15) were selected for bactericidal titer analysis. Results for preimmune sera from 2-month-old subjects showed no relationship between the two test methods (Fig. 2). However, the results for sera collected from the immunized and control groups following the second (Fig. 2B) or third dose (Fig. 2C) of MnCC demonstrated good correlation coefficients. Following administration of the third dose of MnCC, all infant subjects had serogroup-specific IgG concentrations exceeding 1.0 μg/ml and corresponding serum bactericidal titers of 64 or greater. In contrast, the age-matched control group of infants at 7 months of age all had serogroup-specific IgG concentrations of less than 1.0 μg/ml and corresponding serum bactericidal titers of 4 or less (Fig. 2C). Following the decoding of the study, sera from 2-month-old infants were retested by ELISA and SBA, and the lack of agreement between the two test methods was confirmed. Additionally, Western blot analysis did not demonstrate the presence of IgG or IgM against bacterial cell components for those sera with SBA titers where no detectable meningococcal group C-specific IgG antibodies were detected by ELISA (data not shown).
FIG. 2.
(A) Relationship between serogroup-specific IgG concentration and serum bactericidal activity for infant subjects (n = 45) approximately 2 months of age (sera obtained prior to immunization). (B) Infant subjects following two immunizations (6 months of age). Filled triangles, immunized subjects (n = 30); open triangles, age-matched control group (n = 15). (C) Infant subjects following three immunizations (7 months of age) (n = 30) versus an age-matched control group (n = 15). Boxed symbols represent the indicated number of individual data points.
Analysis of sera from toddler subjects.
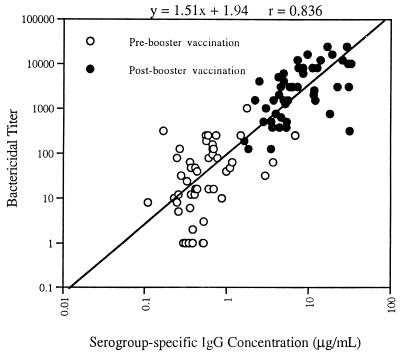
Toddlers who had previously received the MnCC as an infant immunization series at 2, 4, and 6 months of age were administered a booster dose of MnCC at 12 to 15 months of age. Tests using the pre- and postbooster sera (n = 48 pairs) showed that the relationship between serum bactericidal activity and serogroup-specific IgG concentration was linear (Fig. 3).
FIG. 3.
Correlation between N. meningitidis serogroup-specific IgG concentration and serum bactericidal titer. Sera were collected prior to and approximately 30 days after administration of a fourth immunization dose (booster dose given at 12 to 15 months of age) to toddlers, following completion of a primary immunization series as infants (immunized at 2, 4, and 6 months of age). Forty-eight pre- and postimmunization sera were analyzed.
Intralaboratory SBA reproducibility.
The reproducibility of the SBA over the course of several years indicated that more than 83% of the human serum control values for the assays performed were within a single twofold dilution of the established median value for that serum control (n = 156). Furthermore, 98.7% of the human serum control values were within 2 twofold dilutions of the median value. Although one would not expect a bioassay to demonstrate as fine a differentiation as an ELISA, the SBA reproducibility is in close agreement with that established through multilaboratory comparisons (24).
Intralaboratory serogroup C ELISA reproducibility.
The reproducibility of the ELISA method over the time period in which this study was conducted was evaluated by using three human sera derived from healthy adults immunized with quadrivalent polysaccharide vaccine. In each ELISA run, duplicate titrations of the reference serum (CDC1992) and one of three different human serum quality control (QC) specimens were performed on each ELISA plate. The mean antibody concentration of each QC serum for the duration of this study was calculated from individual daily runs consisting of 5 to 10 ELISA plates. The serogroup C ELISA was shown to be reproducible by demonstrating low coefficients of variation (CV) for the repeated testing of these control sera. The observed CV were 24.91, 17.24, and 19.36% for QC specimens having low (5.54-μg/ml), middle (16.99-μg/ml), and high (63.60-μg/ml) anti-MnC IgG concentrations (n = 153), respectively. The calculated slope for the regression line describing optical density values versus dilution for the QC specimens and the reference standard indicates that the assay yields reliable parallelism and reproducibility. Similar slopes, with a CV of 4.54%, were also obtained for toddler and infant test sera (n = 153). These results indicate that intralaboratory assay variation was low over a period of 2 years. Medium-binding ELISA plates were used in these studies and resulted in good assay specificity, with a mean inhibition of ≥90% by specific antigen and ≤10% inhibition by other polysaccharide or protein antigens (data not shown).
DISCUSSION
Titers assigned by SBA provided the original link between a serologic test method and efficacy against invasive meningococcal group C disease (9–13, 19, 27). While the original SBA method has evolved through efforts at interlaboratory standardization, it remains a valuable test of functional activity. However, bactericidal activity may be antibody dependent, reflecting antibodies to other extracellular components as well as to the group C capsule, or such activity may be antibody independent. Furthermore, IgM antibodies not quantitated by the IgG ELISA may contribute to SBA activity. It has previously been demonstrated that approximately 50% of neonates have bactericidal antibodies, resulting from maternal transfer at birth (9, 10). These antibodies rapidly diminish, reaching their lowest level between 6 and 12 months of age, coincident with the increased susceptibility to disease.
While the SBA provides a link to protection, alternative test methods such as the ELISA can quantitate more directly the humoral antibody response to the vaccine for clinical trial evaluations. The correlation of the ELISA to the SBA results may be poor with specimens not dominated with meningococcal group C capsule-specific antibodies, as shown here with sera from 2-month-old infants. The specificity of the bactericidal activity in the absence of significant meningococcal group C IgG antibodies in several of these infant sera has not been elucidated. Neonates and young infants do have age-dependent variation in acute-phase proteins in the serum, such as mannan-binding proteins, which may explain non-antibody-mediated bactericidal activity (1, 26). While maternal antibody and non-antibody-mediated factors may have a detrimental effect on the correlation of ELISA IgG and SBA at 2 months of age, control serum samples from infants aged 6 and 7 months show better correlation with our methods.
The data shown herein describe the MnC ELISA as performed by Wyeth-Lederle Vaccines. Most significant is the decreased amount of endotoxin present in the meningococcal group C antigen preparation (101 endotoxin units [EU]/mg) compared to the lot used in previously published studies (1,243 EU/mg) (14, 21, 25; G. Campagne et al., Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 192, 1997). Key to this strong relationship is the use of an appropriate qualified antigen in the ELISA, as well as other ELISA components (e.g., pyrogen-free buffers for antigen coating), for quantification of IgG. Previously described poor correlation of IgG ELISA results to SBA titers in other laboratories using similar methodology may be related to differences in antigen preparations, ELISA components or SBA reagents, or test sera with different characteristics than those used in these studies (14, 25). While this study does not address directly the issue of endotoxin, it should be pointed out that human sera contain antibodies which bind to endotoxin, presumably acquired through environmental exposure to multiple pathogens (16, 29). Other contaminants in the antigen preparations or assay buffer systems also may affect the relationship between the ELISA and SBA methods under some test conditions. In our hands, the selection of ELISA plates is an important component of the quality assurance for the ELISA method. In our experience, the use of a high-binding plate such as the Immulon II plates used in other laboratories (14, 21, 25; Campagne et al., 37th ICAAC) may augment the binding of nonpolysaccharide contaminants present in some polysaccharide antigen preparations. Medium-binding ELISA plates were used in these studies and resulted in good assay specificity, with a mean inhibition of ≥90% by specific antigen and ≤10% inhibition by other polysaccharide or protein antigens (data not shown).
A good correlation between the SBA and ELISA is observed for all sera tested before and after immunization with MnCPs or MnCC, with the exception of 2-month-old preimmunization sera. Two-month-old infant sera are not typical of unimmunized sera from older infants or toddlers or adults (1, 26). Presumably, this reflects the unique status of these sera with transferred maternal antibodies and other neonate-specific increases in certain classes of serum proteins. The good correlation for all other sera tested suggests that the ELISA is useful for routine testing of specimens from clinical trials. Because these correlations measure two different assay outcomes, an evaluation of closeness of fit or equivalence is not appropriate. However, a predictive IgG concentration associated with a particular threshold SBA titer can be developed within each laboratory for the test methods used, as the relationship will vary by complement source, subject age and immunization status, population, sample timing, vaccine formulation, sensitivity and specificity of test methods, antibody avidity and affinity, and other factors (1, 2, 4–6, 23, 25, 28, 31, 32).
Development of the relationship between IgG concentrations and SBA for evaluating clinical trial specimens is also dependent on demonstration of consistent assay performance. Both the MnC ELISA and the SBA showed consistent performance over the 2-year time frame when these sera were tested.
The analyses presented here not only characterize two different antibody quantitation methods but also show their relationship with a new conjugate vaccine for N. meningitidis group C. In adults, toddlers, and infants, this vaccine elicited high levels of IgG serogroup C-specific antibody, which are associated with functional activity. The data show a strong relationship between specific MnC IgG ELISA results with our method and an MnC serum bactericidal assay using baby rabbit complement. The ELISA can be used with adult, toddler, and infant sera to show the level of IgG serogroup C-specific antibody associated with functional activity, given appropriate age control for a study. Evaluations such as those we describe here enable establishment of the relationship of IgG antibody to functional activity for sera obtained from clinical vaccine studies.
REFERENCES
- 1.Aittoniemi J, Meittinen A, Laippala P, Isoauri E, Viikari J, Ruuska T, Soppi E. Age-dependent variation in the serum concentration of mannan-binding protein. Acta Paediatr. 1996;85:906–909. doi: 10.1111/j.1651-2227.1996.tb14182.x. [DOI] [PubMed] [Google Scholar]
- 2.Alper C A, Abramson N, Johnston R B, Jandl J H, Rosen F S. Increased susceptibility to infection associated with abnormalities of complement-mediated functions and of the third component of complement (C3) N Engl J Med. 1970;282:349–354. doi: 10.1056/nejm197002122820701. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommended childhood immunization schedule—United States, 1998. Morb Mortal Wkly Rep. 1998;47:8–12. [PubMed] [Google Scholar]
- 4.Densen P. Complement deficiencies and meningococcal disease. Clin Exp Immunol. 1991;86(Suppl. 1):57–62. doi: 10.1111/j.1365-2249.1991.tb06209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Densen P, Weiler J M, Griffiss J M, Hoffmann L G. Familial properdin deficiency and fatal meningococcemia: correction of the bactericidal defect by vaccination. N Engl J Med. 1987;316:922–926. doi: 10.1056/NEJM198704093161506. [DOI] [PubMed] [Google Scholar]
- 6.Ellison R T, Kohler P F, Curd J G, Judson F N, Reller L B. Prevalence of congenital or acquired complement deficiency in patients with sporadic meningococcal disease. N Engl J Med. 1983;308:913–916. doi: 10.1056/NEJM198304213081601. [DOI] [PubMed] [Google Scholar]
- 7.Fairley C K, White J M, Begg N T. Fast-tracking meningococcal vaccination. Lancet. 1994;344:1164–1165. [PubMed] [Google Scholar]
- 8.Gheesling L L, Carlone G M, Pais L B, Holder P F, Maslanka S E, Plikaytis B D, Achtman M, Densen P, Frasch C E, Käyhty H, Mays J P, Nencioni L, Peeters C, Phipps D C, Poolman J T, Rosenqvist E, Siber G R, Thiesen B, Tai J, Thompson C M, Vella P P, Wenger J D. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J Clin Microbiol. 1994;32:1475–1482. doi: 10.1128/jcm.32.6.1475-1482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotschlich E C, Liu T Y, Artenstein M S. Human immunity to the meningococcus. III. Preparations and immunochemical properties of the Group A, Group B, and Group C meningococcal polysaccharides. J Exp Med. 1969;129:1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotschlich E C, Goldschneider I, Artenstein M S. Human immunity to the meningococcus. IV. Immunogenicity of Group A and Group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotschlich E C, Liu T Y, Artenstein M S. Human immunity to the meningococcus. V. The effect of immunization with meningococcal Group C polysaccharide on the carrier state. J Exp Med. 1969;129:1385–1395. doi: 10.1084/jem.129.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granoff D M, Maslanka S E, Carlone G M, Plikaytis B D, Santos G F, Mokatrin A, Raff H V. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol. 1998;5:479–485. doi: 10.1128/cdli.5.4.479-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holder P K, Maslanka S E, Pais L B, Dykes J, Plikaytis B D, Carlone G M. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin Diagn Lab Immunol. 1995;2:132–137. doi: 10.1128/cdli.2.2.132-137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins C, Chart H. Antibodies to lipopolysaccharides of Escherichia coli in serogroups 05 and 0165 in healthy adults. Lancet. 1999;354:649. doi: 10.1016/s0140-6736(99)02214-x. [DOI] [PubMed] [Google Scholar]
- 17.Jones D. Epidemiology of meningococcal disease in Europe and the USA. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley and Sons; 1995. pp. 147–158. [Google Scholar]
- 18.Jones D M, Kaczmarski E B. Meningococcal infections in England and Wales: 1994. Communicable Dis Rep. 1995;5:R125–R129. [PubMed] [Google Scholar]
- 19.Kayhty H, Karanko V, Peltola H, Sarna S, Makela P H. Serum antibodies to capsular polysaccharide vaccine of group A Neisseria meningitidis followed for three years in infants and children. J Infect Dis. 1980;142:861–867. doi: 10.1093/infdis/142.6.861. [DOI] [PubMed] [Google Scholar]
- 20.Kuzma J W. Basic statistics for the health sciences. 2nd ed. 1992. pp. 199–212. . Mayfield Publishing Co., Mountain View, Calif. [Google Scholar]
- 21.Lieberman J M, Chiu S C, Wong V K, Partridge S, Chang S, Chiu C, Gheesling L L, Carlone G M, Ward J I. Safety and immunogenicity of a serogroups A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. JAMA. 1996;275:1499–1503. [PubMed] [Google Scholar]
- 22.Lim D, Gewurz A, Lint T F, Ghaze M, Sepheri B, Gewurz H. Absence of the sixth component of complement in a patient with repeated episodes of meningococcal meningitis. Pediatrics. 1976;89:42–47. doi: 10.1016/s0022-3476(76)80924-9. [DOI] [PubMed] [Google Scholar]
- 23.Lucas A H, Granoff D M. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type b polysaccharide-protein conjugates. J Immunol. 1995;106:4195–4202. [PubMed] [Google Scholar]
- 24.Maslanka S E, Gheesling L L, Libutti D E, Donaldson K B J, Harakeh H S, Dykes J K, Arhin F F, Devi S J N, Frasch C E, Huang J C, Kriz-Kuzemenska P, Lemmon R D, Lorange M, Peeters C C A M, Quataert S A, Tai J Y, Carlone G M the Multilaboratory Study Group. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–167. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maslanka S E, Tappero J W, Plikaytis B D, Brumberg R S, Dykes J K, Gheesling L L, Donaldson K B J, Schuchat A, Pullman J, Jones M, Bushmaker J, Carlone G M. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect Immun. 1998;66:2453–2459. doi: 10.1128/iai.66.6.2453-2459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita M, Fujita T. Cleavage of the third component of complement (C3) by mannose-binding protein-associated serine protease (MASP) with subsequent complement activation. Immunobiology. 1995;194:443–448. doi: 10.1016/S0171-2985(11)80110-5. [DOI] [PubMed] [Google Scholar]
- 27.Peltola H, Makela P H, Kayhty H, Jousimes H, Herva E, Hallstrom K, Sivonen A, Renkonen O V, Pettay O, Karanko V, Ahvonen P, Sarna S. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977;297:686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- 28.Petersen B H, Graham J A, Brooks G F. Human deficiency of the eighth component of complement: the requirement of C8 for serum Neisseria gonorrhoeae bactericidal activity. J Clin Investig. 1976;57:283–290. doi: 10.1172/JCI108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pruslin F H, To S E, Winston R, Rodman T C. Caveats and suggestions for the ELISA. J Immunol Methods. 1991;137:27–35. doi: 10.1016/0022-1759(91)90390-2. [DOI] [PubMed] [Google Scholar]
- 30.Rennels M B, Edwards K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P R, Malinowski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 31.Ross S C, Rosenthal P J, Berberich H M, Densen P. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987;155:1266–1275. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- 32.Schlesinger Y, Granoff D M the Vaccine Study Group. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA. 1992;267:1489–1494. [PubMed] [Google Scholar]
- 33.World Health Organization. Requirements for meningococcal polysaccharide vaccine (requirements for biological substances no. 23) WHO Tech Rep Ser. 1976;594:72–73. [Google Scholar]