Abstract
Evaluation of cytokine gene expression following in vitro stimulation is one means of examining the dysregulation of the immune system in human immunodeficiency virus (HIV) infection. We have assessed differences in the immune status of non-HIV-infected (HIV−) and HIV-infected (HIV+) individuals by evaluating the kinetics of the expression of cytokine genes. We compared detailed time courses of cytokine mRNA expression in HIV− and HIV+ peripheral blood mononuclear cells (PBMC) and found that there is a significant shift (P < 0.01) for all cytokines examined (interleukin 2 [IL-2], IL-6, IL-10, gamma interferon, and tumor necrosis factor alpha [TNF-α]) to an earlier time of mean peak mRNA expression by HIV+ PBMC (between 4 and 8 h) compared to HIV− PBMC (8 h) in response to either phytohemagglutinin (PHA) or anti-CD3 stimulation. Additional studies showed that although PHA-stimulated HIV+ PBMC showed decreased median IL-2, IL-4, and TNF-α mRNA levels, they typically demonstrated more rapid kinetics (increased mean 4-h/24-h cytokine mRNA ratios), with significant differences for IL-4 (P < 0.05) and TNF-α (P < 0.005), compared to HIV− PBMC. The use of fresh or frozen cells gave comparable cytokine mRNA data; however, the secretion of some cytokine proteins (IL-2 receptor, IL-10, and TNF-α) appeared to be reduced in HIV+ PBMC that had been frozen and thawed. Our studies demonstrate that the kinetics of cytokine gene expression can reveal additional dysregulation of the immune system in HIV infection, suggesting that PBMC of HIV-infected persons exist in an activated state in vivo that permits them to express cytokine genes more rapidly than a normal PBMC.
Infection with human immunodeficiency virus (HIV) is associated with signs of both immune deficiency and immune activation (9, 15, 16). The decline in the number and function of CD4+ T cells is one of the hallmarks of the infection. At the same time, there is ample evidence of immune hyperactivity in HIV-infected (HIV+) individuals, as indicated by numerous reports of increased levels in vivo, as well as increased spontaneous in vitro production of immunoglobulins, certain cytokines, and markers of immune activation (6, 9, 15, 16). It has been suggested that decreases in the in vitro production of some cytokines and increases in others may play a role in the progression of HIV disease (3, 4); however, this has not been universally observed (2, 7, 10, 14). There has been great interest, therefore, in examining cytokine activity in and by cells from HIV+ and non-HIV-infected (HIV−) subjects. There are many studies that document differences in the levels of cytokines secreted by and/or cytokine genes expressed in peripheral blood mononuclear cells (PBMC) from HIV− and HIV+ subjects (reviewed in references 9 and 16). While not a direct measure of cytokine production, semiquantitative determination of cytokine gene expression by the measurement of cytokine mRNA is generally considered an indicator of the potential of an individual's cells to ultimately secrete cytokine proteins.
Many cytokine mRNA studies have focused on the quantitative nature of the differences in cytokine gene expression, i.e., whether PBMC from HIV+ individuals had increased or decreased levels of cytokine mRNA compared to PBMC of HIV− individuals under the same experimental conditions in vitro. Such results are usually interpreted to suggest that the PBMC of HIV+ persons have an increased or decreased potential to express cytokine genes, which in turn may lead to differences in actual cytokine production in vivo. We have been interested in examining the kinetics of cytokine mRNA expression in response to in vitro stimuli, which may reflect the preexisting in vivo immune status of PBMC and their readiness to respond, rather than provide a quantitative measure of their overall potential to express cytokine genes.
Recently, we examined the kinetics of cytokine mRNA expression in PBMC from normal healthy subjects in response to various stimuli (8). We have extended those studies to compare the kinetics of cytokine mRNA expression in stimulated PBMC from HIV− and HIV+ subjects, and we show that PBMC from HIV+ subjects express mRNAs for several cytokines more rapidly than cells from HIV− subjects. In addition, we have examined the effects of using frozen HIV− and HIV+ PBMC for evaluating cytokine responses following in vitro stimulation.
MATERIALS AND METHODS
The cytokines and cytokine receptor examined were interleukin 2 (IL-2), IL-2-receptor (IL-2R), interleukin 4 (IL-4), interleukin 6 (IL-6), interleukin 10 (IL-10), gamma interferon (IFN-γ) and tumor necrosis factor-α (TNF-α).
Heparinized whole blood was obtained from known HIV− and HIV+ individuals. All HIV+ subjects were homosexual men currently enrolled in the Multicenter AIDS Cohort Study (MACS) at UCLA (5, 11). The two HIV+ individuals used for detailed kinetic analyses had absolute CD4 T-cell counts of 168 and 312 cells/mm3; the HIV+ group used for additional studies (n = 8) had a mean absolute CD4 cell count of 376/mm3, with a range from 213 to 476/mm3. HIV− subjects were healthy heterosexual controls or HIV-seronegative participants in the MACS. PBMC were purified from whole blood samples by density gradient centrifugation over 60% Percoll gradients and cultured in complete RPMI 1640 medium (100 U of penicillin per ml, 100 μg of streptomycin per ml, 0.3 mg of glutamine per ml) with 10% human AB serum.
For kinetic studies, 106 PBMC in a total volume of 0.5 ml were placed in 12- by 75-mm tubes, allowed to rest without stimulation for 1 h at 37°C, and then stimulated with phytohemagglutinin (PHA) (Sigma) (5 μg/ml), or anti-CD3 monoclonal antibody (NEN Research Products) (200 ng/ml), as previously described (8). PBMC pellets were collected at the indicated times (up to 72 h) poststimulation and stored at −70°C until RNA extraction. Culture supernatants (SN) were also collected at 24 and 72 h poststimulation and held at −20°C until assayed for cytokines or cytokine receptor.
RNA extraction and semiquantitative reverse transcription-PCR (RT-PCR) for cytokine gene expression were performed as previously described (7, 8), utilizing the primers shown in Table 1. Briefly, RNA was obtained by guanidinium isothiocyanate lysis and phenol-chloroform extraction, and 10 ng of total RNA was used for each RT-PCR. cDNA was synthesized using oligo(dT) priming and Moloney murine leukemia virus reverse transcriptase and then amplified using 0.2 μM 5′ and 3′ oligonucleotide cytokine or beta-actin primers, 2.5 μmol of AmpliTaq DNA polymerase (Perkin-Elmer Cetus), and a reaction mixture containing 10 mM Tris-HCl, 2.0 to 2.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphate (Perkin-Elmer Cetus), and 0.01 μM [α-32P]dATP. Reactions were amplified by 25 cycles (beta-actin) or 35 cycles (cytokines) of denaturation at 95°C, with annealing and extension at 60°C. PCR products were identified by autoradiography following acrylamide gel electrophoresis, the radioactive bands were cut from the dried gel, and activity was determined by liquid scintillation counting. The activity obtained (counts per minute) for each cytokine reaction product was normalized to reflect total RNA content by using the activity obtained for beta-actin in the same sample.
TABLE 1.
Primers for cytokine-specific RT-PCR
| Target gene | Primer source | Length (bp) | Primer sequence | Mg2+ (mM) |
|---|---|---|---|---|
| β-Actin | Clontech | 838 | 5′ ATCTGGCACCACACCTTCTACAATGAGCTGCG 3′ | 2.0 |
| 5′ CGTCATACTCCTGCTTGCTGATCCACATCTGC 3′ | ||||
| IL-2 | Perkin-Elmer | 229 | 5′ GAATGGAATTAATAATTACAAGAATCCC 3′ | 2.5 |
| 5′ TGTTTCAGATCCCTTTAGTTCCAG 3′ | ||||
| IL-4 | Clontech | 456 | 5′ ATGGGTCTCACCTCCCAACTGCT 3′ | 2.0 |
| 5′ CGAACACTTTGAATATTTCTCTCTCAT 3′ | ||||
| IL-6 | Synthetic Genetics | 190 | 5′ ATGTAGCCGCCCCACACAGA 3′ | 2.5 |
| 5′ CATCCATCTTTTTCAGCCAT 3′ | ||||
| IL-10 | Operon | 578 | 5′ CGTTAACCGCGGAAGGCATGCACAGCTCAGCACTGCTC 3′ | 2.0 |
| 5′ CCCGGGCTCGAGCCACCCTGATGTCTCAGTTTCGTATC 3′ | ||||
| IFN-γ | Synthetic Genetics | 520 | 5′ ATGAAATATACAAGTTATATCTTGCTGTT 3′ | 2.3 |
| 5′ GATGCTCTTCGACCTCGAAACAGCAT 3′ | ||||
| TNF-α | Perkin-Elmer | 325 | 5′ CAGAGGGAAGAGTTCCCCAG 3′ | 2.0 |
| 5′ CCTTGGTCTGGTAGGAGACG 3′ | ||||
| IL-2R | Clontech | 398 | 5′ GAATTTATCATTTCGTGGTGGGGCA 3′ | 2.5 |
| 5′ TCTTCTACTCTTCCTCTGTCTCCG 3′ |
Concentrations of soluble IL-2R (Endogen), IL-4 (Genzyme), IL-10 (Immunotech), and TNF-α (Innogenetics) in cell culture SN were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's protocol; IFN-γ (T Cell Diagnostics) was determined using a modified ELISA protocol (1).
For eight HIV− and eight HIV+ subjects included in the kinetic studies described above, parallel experiments were performed on aliquots of PBMC from the same blood draw that were frozen in 10% serum–10% dimethyl sulfoxide according to MACS protocols (12) and then thawed, stimulated, and cultured as described above. PBMC pellets and culture SN from frozen cells were collected at the same times, and tested in the same RT-PCR assays and cytokine ELISAs as the samples from fresh cells from the same subject.
The detailed kinetics of mRNA expression for each cytokine for each subject was represented by calculating the percentage of maximum mRNA expression obtained for a given cytokine at each time point over the 72-h time course. The mean percentage of maximum mRNA expression at each time point for each cytokine was determined for HIV− and HIV+ subjects and plotted as shown in Fig. 1 and 2. Statistical analysis of the kinetic plots were performed by calculating the time axis centroid for each plot, which represented the mean time of mRNA expression. These mean times were then compared simultaneously for all six cytokines and cytokine receptor between HIV− and HIV+ subjects using a mixed-effects linear model (13). Comparisons of mRNA values, mRNA ratios, and data for fresh versus frozen cells were performed using the nonparametric Wilcoxon rank sum test.
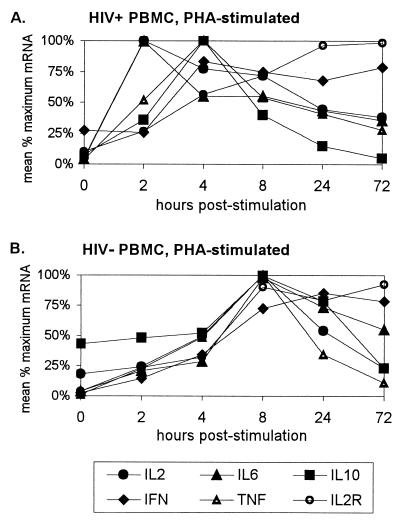
FIG. 1.
Kinetics of cytokine mRNA expression following PHA stimulation of PBMC. Each cytokine mRNA value from a subject was represented as a percentage of the maximum mRNA obtained for that cytokine over that subject's time course. (A) Mean percent maximum mRNA expression for two HIV+ subjects. (B) Mean percent maximum mRNA expression for two HIV− subjects.
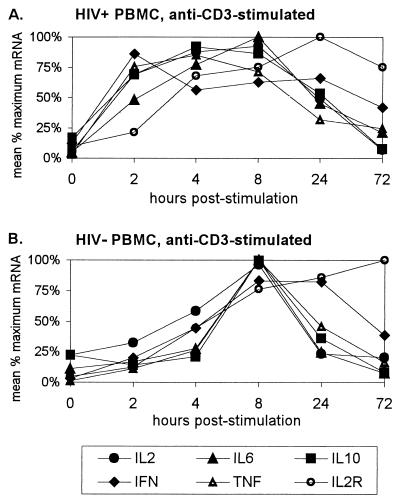
FIG. 2.
Kinetics of cytokine mRNA expression following stimulation of PBMC with anti-CD3 monoclonal antibody. (A) Mean percent maximum mRNA expression in the same HIV+ subjects as for Fig. 1A. (B) Mean percent maximum mRNA expression in the same HIV− subjects as for Fig. 1B.
RESULTS
Peak cytokine mRNA expression occurs earlier in stimulated HIV+ PBMC than in HIV− PBMC.
PBMC from HIV− and HIV+ subjects were stimulated with either PHA or anti-CD3 monoclonal antibody, and the expression of mRNAs for the cytokines IL-2, IL-6, IL-10, IFN-γ, and TNF-α and the cytokine receptor IL-2R was determined at multiple time points from 0 to 72 h poststimulation. The kinetics were expressed by representing each cytokine mRNA value from a subject as a percentage of the maximum mRNA obtained for that cytokine over the time course; i.e., among all the values obtained from a single subject for a given cytokine, the time point between 0 and 72 h that had the highest absolute mRNA result was set equal to 100%, and the results at all other time points for that particular cytokine were expressed as percentages of the highest value.
When the mean percentages of maximum mRNA values were plotted over time for HIV− and HIV+ PBMC simulated with PHA (Fig. 1), HIV+ cells showed a clear shift toward earlier peak mRNA expression for all five cytokines compared to HIV− cells. While HIV− PBMC showed peak mRNA expression for most cytokines at 8 h poststimulation (with IFN-γ as an exception at 24 h), HIV+ PBMC showed peak IL-2 and IL-6 mRNA expression at 2 h and peak IL-10, IFN-γ, and TNF-α expression at 4 h. Consistent with previous observations (8), the kinetics of IL-2R mRNA expression differed from those of the cytokines, exhibiting a more sustained time course that peaked at 72 h in both HIV+ and HIV− cells.
Plots of cytokine mRNA expression by HIV− and HIV+ PBMC stimulated with anti-CD3 antibody showed similar results (Fig. 2). HIV− cells showed distinct peaks of mRNA expression at 8 h for all five cytokines, while earlier times of peak expression were seen in HIV+ cells. Although a mean IL-2 and IL-6 mRNA values in HIV+ cells reached their maximum at 8 h, mRNA levels for both of these cytokines were near maximal levels at 4 h, suggesting that the peak may have occurred between 4 and 8 h. In the anti-CD3-stimulated cells, the shift toward earlier peak mRNA expression in HIV+ cells was also seen for IL-2R, which clearly peaked at 24 h, in comparison to 72 h in HIV− cells.
The shift toward earlier peak cytokine mRNA expression in HIV+ PBMC seen in the plots was evaluated by calculating a weighted mean time of mRNA expression for each cytokine time course for HIV− and HIV+ subjects. This value, called a time axis centroid, takes into account the overall shape of the kinetic curve rather than just the one time point or mode that has the maximum mRNA expression. When the mean times of mRNA expression for all cytokines and IL-2R following stimulation of HIV− and HIV+ PBMC with PHA were compared simultaneously, there was a significant shift to an earlier mean time of mRNA expression in HIV+ cells (P < 0.01). When the same type of comparison was made for anti-CD3-stimulated cells, HIV+ cells once again showed a highly significant shift to an earlier mean time of expression (P < 0.001). The increase in significance in the anti-CD3 comparison was most likely due to the more obvious shift seen in peak mRNA IL-2R expression. In both comparisons, the mean time of mRNA expression in HIV+ PBMC was between 4 and 8 h, compared to greater than or equal to 8 h in HIV− PBMC. When the same types of analyses were performed on cytokine data only (omitting IL-2R), a statistically significant shift to earlier mean mRNA expression in HIV+ PBMC was still seen in both PHA- and anti-CD3-stimulated cells (P < 0.01 and P < 0.001, respectively).
Stimulated PBMC from HIV+ subjects have decreased levels, but more rapid kinetics, of expression of cytokine mRNAs than HIV− PBMC.
In order to further explore the possibility of more rapid cytokine mRNA expression in HIV+ PBMC, additional mRNA data were obtained from a larger number of subjects. As it was not practical to attempt larger-scale studies with multiple stimuli, time points, and cytokine and cytokine receptor assays, a simpler experimental protocol was utilized. Since PHA stimulation demonstrated a clear shift in mean peak cytokine mRNAs in HIV+ PBMC to either 2 or 4 h poststimulation for all of the cytokines examined initially, PHA was chosen as the stimulus, with cell pellets collected at 4 and 24 h poststimulation. Cytokine mRNA levels were determined for IL-2, IL-4, TNF-α, and IFN-γ, and median levels were compared between HIV− and HIV+ PBMC (Table 2). The relative kinetics of cytokine mRNA expression were evaluated using the ratio of 4-h mRNA to 24-h mRNA (Table 3). A ratio of >1 indicates more mRNA expression at 4 h than at 24 h, and when comparing ratios, a higher ratio suggests an earlier peak of mRNA expression.
TABLE 2.
Cytokine mRNA levels in PHA-stimulated HIV− and HIV+ PBMCa
| Time (h) | PBMC group | cpmb
|
|||
|---|---|---|---|---|---|
| IL-2 | IL-4 | TNF-α | IFN-γ | ||
| 4 | HIV− | 13,554 | 1,032 | 12,064 | 1,029 |
| HIV+ | 5,098 | 654 | 5,302 | 2,862 | |
| 24 | HIV− | 12,589 | 2,862 | 9,099 | 3,509 |
| HIV+ | 5,875 | 618 | 2,706* | 7,376 | |
n = 8 HIV− subjects and 8 HIV+ subjects.
Median counts per minute obtained from RT-PCR for the indicated cytokine mRNA.
*, P < 0.05 for comparison of the HIV− and HIV+ groups.
TABLE 3.
Relative kinetics of cytokine mRNAs in PHA-stimulated HIV− and HIV+ PBMC
| PBMC group (n) | Mean 4-h/24-h ratiod (SEM)
|
|||
|---|---|---|---|---|
| IL-2 | IL-4 | IFN-γ | TNF-α | |
| HIV− (8) | 1.17 (0.17) | 0.62 (0.13) | 0.55 (0.20) | 1.03 (0.20) |
| HIV+ (8) | 1.48 (0.53) | 2.56* (1.13) | 1.17 (0.39) | 2.14** (0.33) |
Mean of the ratios obtained by dividing the 4-h mRNA result (counts per minute) by the 24-h mRNA result (counts per minute) for the indicated cytokine.
*, P < 0.05 for comparison of the HIV− and HIV+ groups.
**, P < 0.005 for comparison of the HIV− and HIV+ groups.
When the median mRNA values obtained for each cytokine in PBMC from HIV− and HIV+ subjects were compared, our data were consistent with other reports showing lower median IL-2, IL-4, and TNF-α expression and higher median IFN-γ expression in HIV+ cells than in cells from HIV− subjects (Table 2). However, the differences were not statistically significant except in the case of TNF-α mRNA at 24 h (P < 0.05). When the ratio of 4- to 24-h mRNA expression for each cytokine was examined for evidence of a shift to earlier expression in HIV+ cells, the mean ratio was higher in HIV+ than HIV− cells for every cytokine, suggesting an earlier peak of mRNA expression (Table 3). Due to the relatively small sample size, however, only the differences in ratios for IL-4 and TNF-α were statistically significant. Therefore, in spite of a reduced capacity to express most kinds of cytokine mRNAs in response to stimulation, an evaluation of relative kinetics suggests that cytokine mRNA responses in HIV+ PBMC tend to occur more rapidly, with an earlier peak time of cytokine mRNA response, than those in HIV− PBMC.
Cytokine responses by HIV+ and HIV− PBMC may differ in fresh and frozen cells.
Cytokine mRNA expression was measured in parallel cultures of PHA-stimulated fresh and “frozen” cells but showed no statistically significant differences within either the HIV− or HIV+ group (data not shown). Similarly, the relative kinetics of expression (4-h/24-h ratio) for each cytokine showed no significant differences between fresh and frozen cells in the HIV+ group, and only IL-2 ratios were slightly increased in fresh HIV− PBMC compared to frozen HIV+ PBMC (P < 0.05) (data not shown).
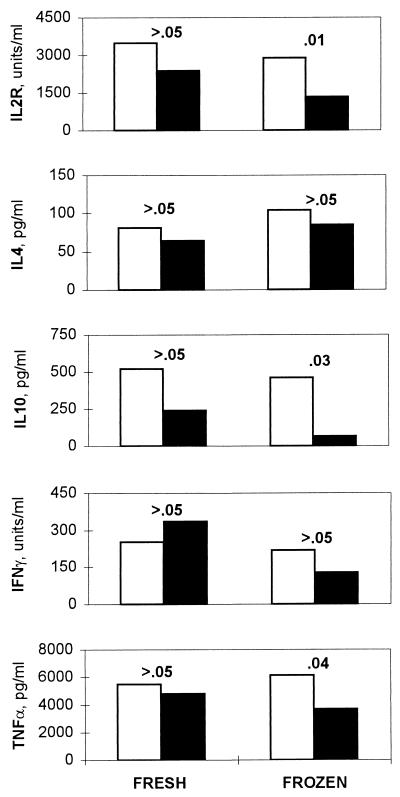
The effect of freezing and thawing on cytokine production was also evaluated with the same fresh and frozen PBMC cultures by measuring cytokine or cytokine receptor concentrations in culture SN collected 72 h poststimulation. Soluble IL-2R, which is secreted in response to IL-2 production, was measured as a surrogate for IL-2 (6). For each cytokine, mean levels in SN were compared between the HIV− and HIV+ groups, first using the data obtained from fresh cells and then again using the data from frozen cells (Fig. 3). There were no statistically significant differences in mean SN cytokine levels between the HIV− and HIV+ groups (P > 0.05) when fresh cells were used. However, when mean SN cytokine levels in the HIV− and HIV+ groups were compared using the data from cells that had been frozen and thawed before culture, IL-2R, IL-10, and TNF-α appeared to be significantly decreased in the HIV+ group compared to the HIV− group. While the changes in IFN-γ were not statistically significant, it is interesting that the HIV+ group had a higher mean IFN-γ level than the HIV− group in fresh cell SN but that the trend was reversed in frozen SN, with the HIV− group having the higher mean.
FIG. 3.
Cytokine production following PHA stimulation in 72-h culture supernatants. Open bars, mean cytokine concentrations in the HIV− group; filled bars, mean cytokine concentrations in the HIV+ group. The P values shown are for comparison of the HIV− versus HIV+ groups using data from either fresh or frozen PBMC.
DISCUSSION
Although the early descriptions of immune system changes in HIV infection and AIDS focused on the immunodeficiency associated with the loss of T-helper cells, there is now ample evidence of widespread immune dysregulation in HIV+ persons, leading to a somewhat paradoxical mix of both immune deficiency and immune hyperactivation. Studies of changes in cytokines in HIV+ persons are an excellent example of this paradox, as the circulating levels in serum of some cytokines, such as IL-2, are decreased, while those of others, such as the inflammatory cytokines IL-6 and TNF-α, are increased in association with HIV infection. While serum cytokine levels are a measure of in vivo cytokine activity, they reflect the equilibrium that has been established between cytokine production and uptake by cells expressing cytokine receptors and/or clearance from the circulation. Many investigators, therefore, have evaluated the ability of cells to secrete cytokines in vitro in response to stimulation with mitogens, as a means of determining changes in the potential or capacity of cells to produce cytokines. Yet another approach is to examine cytokine responses following in vitro stimulation at the level of cytokine gene expression, by determining levels of cytokine-specific mRNA. This approach examines the capacity of a cell to mount a cytokine response at its most basic level and lends itself not only to addressing the magnitude of a response but also to characterizing the time course over which the response occurs. We have been interested in examining the kinetics of cytokine expression by PBMC following in vitro stimulation, and we have previously described the cytokine mRNA kinetics seen in healthy, HIV− subjects in response to a number of mitogens commonly employed in the immunology laboratory (8). In the work reported here, we have extended those studies to compare the kinetics of cytokine mRNA expression in HIV+ and HIV− subjects, and we demonstrate that although the magnitude of most cytokine mRNA responses may be less than in PBMC from HIV+ subjects, these cells respond more rapidly to in vitro stimulation.
In detailed time courses following stimulation with either PHA or anti-CD3, two of the most commonly used laboratory mitogens, we observed a clear shift in mean peak mRNA expression for all cytokines examined to less than 8 h in HIV+ PBMC, compared to mean peaks at 8 or 24 h in HIV− PBMC (Fig. 1 and 2). Evidence of a more rapid response to stimulation by HIV+ PBMC was also seen when the relative kinetics of PHA-stimulated PBMC were examined in a larger group of subjects, where gene expression for IL-2, IL-4, TNF-α, and IFN-γ tended to occur more rapidly in the HIV+ group than in the HIV− group (Table 3). This was in spite of decreased levels of mRNA for all but IFN-γ (Table 2). Therefore, even though cells from HIV+ persons failed to express comparable levels of multiple cytokine-specific mRNAs following stimulation, they appeared to be able to respond more quickly to a stimulus. This observation suggests that cells within the HIV+ PBMC exist in a preactivated state in vivo that enables them to initiate this process more quickly. These cells should be viewed, therefore, as hyperresponsive to the initial cytokine-inducing signals but unable to sustain comparable levels of cytokine mRNA expression and production. The characterization of these cells as both hyperactive and yet ultimately immunodeficient reflects the immune dysregulation that characterizes HIV infection in the immune system as a whole. From the perspective of the immunology laboratory, it is important to recognize that the more rapid peak of cytokine gene expression seen in HIV+ cells should be taken into account when designing studies comparing cytokine mRNA responses between HIV− and HIV+ subjects.
All of the HIV+ subjects in this work were participants in the MACS, a study of the natural history of AIDS in which participants have been providing blood samples every 3 to 6 months since the mid-1980s (5, 11). While the kinetic studies described here were performed on PBMC isolated from fresh blood samples, the MACS and other cohort studies like it have repositories of frozen PBMC that provide unique opportunities for study design. We were interested to see if frozen and thawed PBMC would give results comparable to those obtained with fresh cells in studies of cytokine responses following in vitro stimulation, and so we performed parallel mRNA and cell culture SN assays. While the use of fresh versus frozen cells did not alter the comparisons of cytokine mRNA between the HIV− and HIV+ groups, three out of five cytokines examined (IL-2R, IL-10, and TNF-α) showed effects of freezing on cytokine protein production in culture SN. For each of these cytokines, there was no statistically significant difference between concentrations seen in SN from the HIV− and HIV+ groups when fresh cells were used, but there was a significant decrease in concentration for the HIV+ groups when frozen cells were used. This suggests that HIV+ PBMC may be more sensitive to freezing than HIV− PBMC and that the use of frozen cells might bias the data when comparing cytokine responses by HIV− and HIV+ cells. These observations indicate that although cryopreserved cells from the MACS appear to be suitable for other laboratory studies (12), the use of fresh cells may be preferable for evaluating cytokine responses in vitro.
ACKNOWLEDGMENTS
We gratefully acknowledge the men who participate in the MACS, who have made this and many other studies possible. We also thank Thang Nguyen and Najib Aziz for technical assistance and Pari Nishanian for scientific input and manuscript review.
This work was supported by grants from the National Institutes of Health (AI36086, AI35040, TW00003, and T32AI07388).
REFERENCES
- 1.Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey J L. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diagn Lab Immunol. 1999;6:89–95. doi: 10.1128/cdli.6.1.89-95.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breen E C, Salazar-Gonzalez J F, Shen L P, Kolberg J A, Urdea M, Martínez-Maza O, Fahey J L. Circulating CD8 T cells show increased interferon-gamma (IFNγ) mRNA expression in HIV infection. Cell Immunol. 1997;178:91–98. doi: 10.1006/cimm.1997.1115. [DOI] [PubMed] [Google Scholar]
- 3.Clerici M, Shearer G M. A TH1 → TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 4.Clerici M, Shearer G M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 5.Detels R, Phair J P, Saah A J, Rinaldo C R, Muñoz A, Kaslow R A, Seminara D, Schrager L, Vermund S. Recent scientific contributions to understanding HIV/AIDS from the Multicenter AIDS Cohort Study. J Epidemiol (Japan) 1992;2:S11–S19. [Google Scholar]
- 6.Fahey J L. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998;5:597–603. doi: 10.1128/cdli.5.5.597-603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan J, Bass H Z, Fahey J L. Elevated IFNγ and decreased IL-2 gene expression are associated with HIV infection. J Immunol. 1993;151:5031–5040. [PubMed] [Google Scholar]
- 8.Fan J, Nishanian P, Breen E C, McDonald M, Fahey J L. Cytokine gene expression in normal human lymphocytes in response to stimulation. Clin Diagn Lab Immunol. 1998;5:335–340. doi: 10.1128/cdli.5.3.335-340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 10.Graziosi C, Pantaleo G, Gantt K R, Fortin J P, Demarest J F, Cohen O J, Sekaly R P, Fauci A S. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 11.Kaslow R A, Ostrow D G, Detels R, Phair J P, Polk B F, Rinaldo C R. The multicenter AIDS cohort study: rationale, organization and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 12.Kleeberger C A, Lyles R H, Margolick J B, Rinaldo C R, Phair J P, Giorgi J V. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immunol. 1999;6:14–19. doi: 10.1128/cdli.6.1.14-19.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Littell R C, Milliken G A, Stroup W W, Wolfinger R D. SAS system for mixed models. Cary, N.C: SAS Institute Inc.; 1996. [Google Scholar]
- 14.Maggi E, Mazzetti M, Ravina A, Annunziato F, de Carli M, Piccinni M P, Manetti R, Carbonari M, Pesce A M, del Prete G, Romagnani S. Ability of HIV to promote a Th1 to Th0 shift and to replicate preferentially in Th2 and Th0 cells. Science. 1994;265:244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 15.Pantaleo G, Fauci A S. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;113:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 16.Poli G. Cytokines and the human immunodeficiency virus: from bench to bedside. Eur J Clin Invest. 1999;29:723–732. doi: 10.1046/j.1365-2362.1999.00525.x. [DOI] [PubMed] [Google Scholar]