Abstract
Purpose of Review
Atopic dermatitis (AD) and allergic asthma are complex disorders with significant public health burden. This review provides an overview of the recent developments on Mas-related G protein-coupled receptor-X2 (MRGPRX2; mouse counterpart MrgprB2) as a potential candidate to target neuro-immune interaction in AD and allergic asthma.
Recent Findings
Domestic allergens directly activate sensory neurons to release substance P (SP), which induces mast cell degranulation via MrgprB2 and drives type 2 skin inflammation in AD. MRGPRX2 expression is upregulated in human lung mast cells and serum of asthmatic patients. Both SP and hemokinin-1 (HK-1 generated from macrophages, bronchial cells, and mast cells) cause degranulation of human mast cells via MRGPRX2.
Summary
MrgprB2 contributes to mast cell-nerve interaction in the pathogenesis of AD. Furthermore, asthma severity is associated with increased MRGPRX2 expression in mast cells. Thus, MRGPRX2 could serve as a novel target for modulating AD and asthma.
Keywords: Mast cell, Mas-related G protein-coupled receptor X2/B2, Atopic dermatitis, Allergic asthma, Itch, Neuro-immune interaction
Introduction
Allergic diseases, such as allergic rhinitis, asthma, food allergy, and eczema, are common in all age groups in the United States with a high burden in pediatric populations. Allergies are the 6th leading cause of chronic illness in the U.S. affecting over 50 million Americans with an annual cost of $18 billion [1, 2]. Growing adverse impact of allergic diseases on public health highlights the need for novel therapeutic development. Intense research in the field has increased our understanding of disease pathogenesis, and the critical role of mast cells in the manifestation of allergic diseases has become evident.
Mast cells are unique tissue-resident immune cells strategically located in tissues that have direct interface with the external environment such as the skin and mucosal surfaces (e.g., respiratory tract, oral/gastrointestinal tract) [3–5]. They are well-characterized central effectors of T-helper-2 type (Th2)-dominated immune response [6–8]. Upon activation via crosslinking of high affinity immunoglobulin E (IgE) receptor (FcεRI) on mast cells with allergen, they release a wide range of pre-stored inflammatory mediators (histamine, serotonin, protease), followed by de novo synthesis of mediators (prostaglandins and leukotrienes) and a variety of cytokines such as interleukin (IL)-4, IL-5, IL-9, and IL-13 [9–12]. These inflammatory mediators are the drivers of the pathology observed in allergic inflammatory diseases including atopic dermatitis (AD) and allergic asthma [5, 13–16].
While Th2 immune dysregulation has been comprehensively studied in AD and allergic asthma, the pathophysiology of these diseases are complex and multifactorial [17]. A growing body of evidence suggests that neuro-immune interaction is a key modulator in the manifestation of pathological outcomes in AD and allergic asthma [18, 19]. Mast cells are located in close proximity to peripheral nerve endings in most tissues and act as the first responder to sensory nerve activation [20, 21]. Thus, it is not surprising that the crosstalk between mast cells and neurons has been implicated in many pathologies including neurogenic inflammation and itch [22••, 23••]. While extensive studies have focused on IgE-FcεRI-mediated mast cell activation in allergic diseases, recent studies demonstrated the existence of an IgE-independent pathway, which is mediated via a novel G protein-coupled receptor (GPCR) known as Mas-related GPCR-X2 (MRGPRX2; mouse counterpart MrgprB2). MRGPRX2 is a class A GPCR, and thus shares the common structural feature containing seven transmembrane (TM) α-helices with other GPCRs [24]. Ligand binding to the extracellular region of GPCRs leads to the conformational changes in the intracellular region, exposing an intracellular pocket that can effectively engage the binding of G proteins and other intracellular signaling proteins, and initiating a downstream signaling cascade [25]. The function of GPCRs is regulated by a process known as desensitization (uncoupling of G proteins from the receptor), which requires phosphorylation of agonist-occupied receptor at Ser/Thr residues in the carboxyl-terminus by GPCR kinases and the recruitment of adaptor proteins β-arrestins [26].
MRGPRX2 is expressed predominantly in mast cells and is activated by a broad range of cationic ligands including many Food and Drug Administration (FDA)-approved drugs, host defense peptides (HDPs), and neuropeptides [27–30]. Endogenous neuropeptides such as substance P (SP) and hemokinin-1 (HK-1), which are implicated in the pathogenesis of AD and allergic asthma, were historically known to activate the GPCR, neurokinin-1 receptor (NK-1R) [31–33]. However, the recent realization that SP and HK-1 activate human mast cells via MRGPRX2, independently of NK-1R [22••, 34••], raises the interesting possibility that the previously described effects of these neuropeptides on AD and allergic asthma are mediated via MRGPRX2.
The main objective of this article is to review the recent literature on the function of MRGPRX2/B2 as the novel receptor for neuropeptides, SP and HK-1, as opposed to NK-1R. We will provide an overview on the roles of MRGPRX2/B2 in the context of neuro-immune interaction in allergic diseases with primary focus on AD and allergic asthma. We will further discuss the prospects and unmet needs in targeting MRGPRX2 as an option for the modulation of AD and allergic asthma.
Discovery of the Novel Mast Cell Receptor, MRGPRX2
Based on their location and mediator contents, mast cells exhibit distinct heterogeneity. Human mast cells are classified into 2 subtypes based on the protease content of their secretory granules and their tissue localization. Thus, mast cells that contain both tryptase and chymase are known as MCTC and are found predominantly in connective tissues such as the skin [35–37]. By contrast, mast cells that contain only tryptase in their granules are known as MCT and are present in mucosal surfaces such as the gut and the lung. In rodents, connective tissue mast cells resemble MCTC and mucosal mast cells resemble MCT. While both subtypes express FcεRI, MRGPRX2 is predominantly expressed in MCTC [35]. The expression profile of NK-1R in different mast cell subtypes has not been studied extensively. Earlier studies showed that mouse bone marrow-derived mast cells (BMMCs) cultured in stem cell factor (SCF) and IL-4, considered to be a connective tissue phenotype, express NK-1R [38], but human intestinal mast cells, considered to be of the mucosal type, do not constitutively express NK-1R [39]. Later, Sumpter et al. [40] reported that BMMCs express low levels of NK-1R which is significantly increased upon FcεRI activation. However, Fujisawa et al. [41] reported that human skin mast cells that do not express NK-1R respond to SP for degranulation via MRGPRX2. Additionally, Manorak et al. [34••] showed that a human mast cell line (LAD2 cells), which express both NK-1R and MRGPRX2, respond to SP and HK-1 for degranulation solely via MRGPRX2. This interesting finding warrants studies targeting MRGPRX2-SP/HK-1 axis.
After the identification of MRGPRX2 as an IgE-independent pathway for mast cell activation by Tatemoto et al. [42] in 2006, our lab was the first to show that HDPs activate mast cells via this receptor [27, 28]. At that time, it was believed that this receptor was expressed only in primates [43]. However, a landmark study by McNeil et al. [29] led to the discovery of the mouse counterpart (MrgprB2), which opened up a new era for mast cell research in the context of its effect on pseudoallergic drug reactions. More recently, MrgprB2 has been shown to be involved in experimental neuro-immune interactions with regard to the regulation of neurogenic inflammation, pain, itch, and AD in mice [22••, 23••]. In humans, the pathological manifestations of chronic idiopathic urticaria is associated with MRGPRX2 activation [41, 44, 45••]. Additionally, its potential role in modulating allergic asthma has increasingly been recognized [34, 46••].
SP Elicits Inflammatory Responses via MRGPRX2 and MrgprB2
Besides close anatomical localization, mast cells and sensory neurons demonstrate bidirectional communications. Mast cell-derived mediators, such as histamine, leukotrienes, and tryptase, interact with their specific receptors on sensory nerve endings, resulting in the release of neuropeptides [47–49]. For example, tryptase activates proteinase-activated receptor-2 (PAR-2) on sensory afferent neurons, which promotes the secretion of pre-stored neuropeptide SP, a member of the tachykinin family of neurokinins [47, 50•, 51]. In turn, SP released from nerve endings evokes further mast cell degranulation, resulting in the release of a wide array of proinflammatory cytokines and chemokines, and subsequently leading to the recruitment of innate immune cells including neutrophils, monocytes, and macrophages [38, 52, 53]. Immune cell recruitment further amplifies local inflammatory responses and facilitates peripheral nerve sensitization, which are critical characteristics of neurogenic inflammation. Activation of mast cells by SP has been implicated in the pathogenesis of a number of neuroinflammatory conditions such as neurogenic inflammation [22••], chronic urticaria [41], and AD [45••]. In addition, SP expression is also upregulated in asthmatic lungs when compared to healthy controls [54].
Initial studies showed that SP induced Ca2+ mobilization in HEK-293 cells ectopically expressing NK-1R [55]. Neurogenic inflammation and pain caused by SP were then postulated to be mediated via NK-1R [56–58]. Based on these studies, several antagonists of NK-1R were developed as potential therapies for inflammatory pain, AD, and asthma [59–61]. Although these NK-1R antagonists were effective at reducing airway hyperresponsiveness, inflammation, and pain in animal models, they failed to demonstrate significant anti-inflammatory and analgesic effects in several human clinical studies [62, 63]. Interestingly, Azimi et al. [64] showed that conventional NK-1R antagonists have an off-target effect on the mouse MrgprB2 but not human MRGPRX2. Hence, this failure of NK-1R antagonists in humans suggests the possibility that the inflammatory effect of SP may be mediated via alternative mechanisms, presumably through MRGPRX2.
Emerging evidence suggests that MrgprB2 regulates SP-mediated neurogenic inflammation in mice [22••]. Using post-operative incision and complete Freund’s adjuvant (CFA) pain models, Green et al. [22••] demonstrated that activation of MrgprB2 by SP is required for inflammatory and thermal hyperalgesia, pro-inflammatory cytokine and chemokine production, and innate immune cell recruitment. Intriguingly, these responses are unaffected in NK-1R-deficient (NK-1R−/−) mice. Furthermore, SP-stimulated release of inflammatory cytokines and chemokines in LAD2 cells are reduced in MRGPRX2-silenced cells and not modulated by an NK-1R antagonist. These findings suggest that both SP-induced immune cell recruitment and cytokine release are mediated by MRGPRX2, independent of the canonical SP receptor NK-1R [22••]. These discoveries that SP elicits inflammatory responses via MRGPRX2/B2 have therefore challenged our previous understanding on SP/NK-1R axis.
Novel Role of MRGPRX2/B2 in Atopic Dermatitis
Atopic dermatitis (AD), also known as atopic eczema, is a chronic inflammatory skin condition that is clinically characterized by recurrent eczematous skin lesions with intense pruritus and type 2 immunity-associated hypersensitivity to common domestic allergens such as house dust mites (HDMs) [65]. It affects approximately 15–20% of children and 1–3% of adults worldwide, with an increasing prevalence, especially in urbanized and industrialized areas [66]. AD is frequently associated with other atopic diseases including allergic asthma, allergic rhinitis, and food allergy [65, 67]. Additionally, AD causes a profound impairment of quality of life for patients and their families. Individuals with AD, particularly those with moderate-to-severe disease, often suffer from chronic/relapsing itch and pain, as well as psychosocial morbidities, such as anxiety, depression, and suicidal ideation [68–70]. Despite significant public health burden associated with AD, the pathogenesis of chronic itch and inflammation in AD is not completely understood, and therapeutic options are limited. Thus, there is an urgent need to better understand the mechanisms underlying the pathophysiology of AD to develop novel therapeutic and preventative strategies.
Although the etiology of AD is not fully understood, skin barrier dysfunction, microbial dysbiosis, and aberrant Th2-immune dysregulation are implicated as the primary pathological drivers [71, 72]. Skin barrier defects can stimulate keratinocytes to release thymic stromal lymphoprotein (TSLP) and other cytokines that promote Th2-type immune response [72, 73]. They also allow the penetration of irritants/allergens and colonization of Staphylococcus aureus (S. aureus), which promote further inflammation, thus leading to a vicious cycle [74]. S. aureus is frequently isolated from the skin of AD patients and is found in 70–100% of lesional skin when compared to 5–40% of healthy control skin [75, 76]. S. aureus-derived exotoxins with superantigen (SAg) activity, such as staphylococcal enterotoxins B (SEB), contribute significantly to dermal T cell infiltration and disease severity [74, 77]. Patients whose skin is colonized with SEB-secreting S. aureus have more accumulation of skin-infiltrating T cells and a higher severity of AD than those colonized with non-toxigenic strains [77].
Mast cells have long been implicated in the pathogenesis of AD [15, 78]. Although mast cells serve as a functional homeostatic regulatory unit to facilitate neuro-immune interaction [50•, 79], their inappropriate activation and peripheral nerve sensitization account for inflammatory and pruritoceptive pathways in AD. Lesional skin of AD patients is hyper-innervated with SP-positive nerve fibers, enhanced mast cell-nerve fiber contacts, and increased numbers of activated mast cells when compared to non-lesional skin [80], suggesting an important role of mast cell-nerve interaction in the pathogenesis of AD. Moreover, plasma level of SP is elevated in AD patients with a positive correlation to disease severity [81, 82].
The gene encoding SP precursor, Tac1, is highly expressed in the transient receptor potential cation channel subfamily V number 1 (TRPV1+) nociceptors [83]. Recently, Serhan et al. [45••] showed that Tac1 expressed in the neuronal compartment of the skin is required for the development of experimental allergic skin inflammation in mice. Application of Dermatophagoides farinae (D. farinae) extracts and SEB on the dorsal skin has been widely used and shown to induce AD-like skin lesions in mice with histological and immunological characteristics similar to those of human AD [84, 85]. Indeed, treatment of wild-type mice with D. farinae and SEB induced a systemic D. farinae-specific Th2 response and developed macroscopic skin lesion with increased epidermal thickness. However, Tac1−/− mice exhibited substantially reduced skin lesion development and histological abnormalities [45••]. As SP is shown to function through MrgprB2 and MRGPRX2 to regulate neurogenic inflammation, itch sensation, and chronic urticaria [22••, 41], neuro-immune interaction in AD is likely to be mediated via this receptor.
Using intravital imaging approach, a new method to visualize the activation of sensory neurons and mast cells simultaneously in live mice, Serhan et al. [45••] demonstrated that the majority of TRPV1+ neuron endings are found in close proximity and/or form physical contacts with skin mast cells, which express MrgprB2. They further showed that D. farinae directly activates TRPV1+Tac1+ neurons to release SP, which induces degranulation in mast cells via MrgprB2 (Fig. 1a). Unlike wild-type mice, MrgprB2mut/mut mice showed substantial reductions in skin lesions, skin barrier disruption, and immune cell recruitment [45••]. Taken together, this study suggests that upon allergen exposure, TRPV1+Tac1+ neurons release SP, which induces degranulation in mast cells via MrgprB2 to promote the development of AD-like allergic skin inflammation (Fig. 1a). These findings identify MrgprB2 as a key receptor that facilitates communication between skin mast cells and TRPV1+ nociceptors. Whether MRGPRX2 in human functions via a similar mechanism has yet to be determined.
Fig. 1.
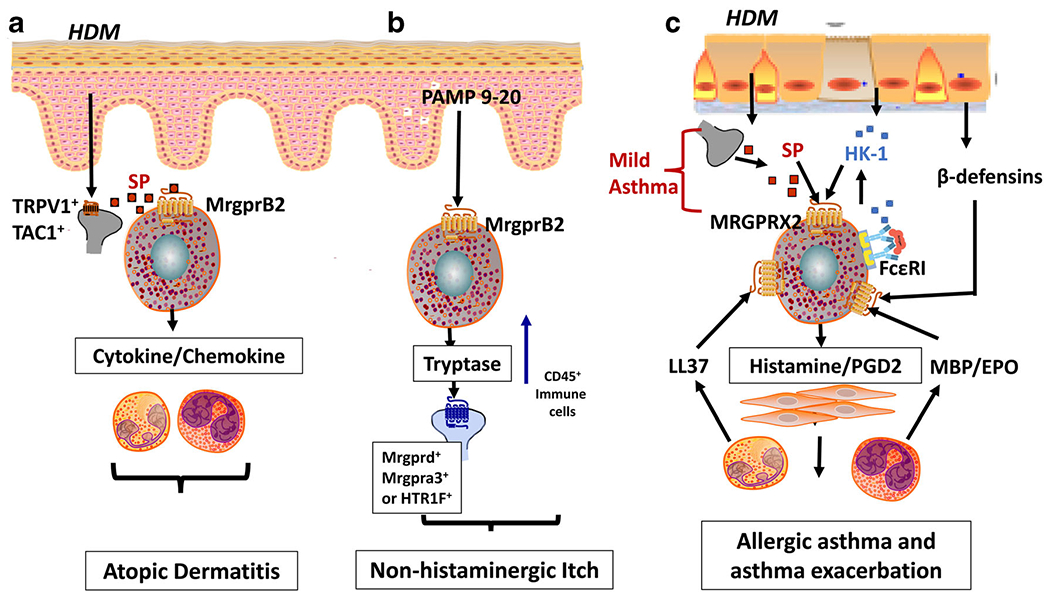
MRGPRX2-mediated mast cell-nociceptor interaction in atopic dermatitis, itch, and allergic asthma. a Common domestic allergens, house dust mites (HDMs), directly activate TRPV1+Tac1+ sensory neurons to release neuropeptide substance P (SP), which induces mast cell degranulation and cytokine/chemokine production via MrgprB2 that drives the development of inflammation in atopic dermatitis. b Pro-adrenomedullin peptide 9-20 (PAMP 9-20) released from keratinocytes activates MrgprB2 resulting in the release of tryptase. Compared to classical IgE-FcεRI, MrgprB2 recruits CD45+ immune cells and excites distinct itch-sensory neuron populations expressing either Mrgprd, Mrgpra3, or HTR1F to induce non-histaminergic itch. c HDM exposure activates sensory nerves to produce SP, which activates MRGPRX2 in lungs to induce degranulation resulting in mild asthma. Hemokinin-1 (HK-1) produced from lung macrophages, bronchial cells and mast cells, and ligands generated from eosinophils (major basic protein (MBP) and eosinophil peroxidase (EPO)), neutrophils (cathelicidin LL-37), and epithelial cells (β-defensins) further activates mast cells via MRGPRX2 to induce degranulation and release inflammatory mediators (histamine, prostaglandin 2 (PGD2) to amplify the airway hyperresponsiveness resulting in allergic asthma exacerbation
MRGPRX2 in Non-histaminergic Itch
IgE-FcεRI-mediated mast cell activation and histamine release have been demonstrated to be crucial for the development of itch. However, antihistamines are largely ineffective for the treatment of chronic itch sensation including AD-associated itch [86]. This phenomenon is not completely understood and it likely reflects the involvement of an additional pathway. Dysregulation of mast cell-nerve interaction has been implicated in itch sensation [87•, 88–90]. Itch is a classic hallmark for AD and is exacerbated by pruritogens, inflammatory mediators, and activation of sensory nerves [87•]. Recently, MrgprB2 has been demonstrated to regulate non-histaminergic itch in mice. Compared to classical IgE-FcεRI-mediated activation, MrgprB2-mediated mast cell activation induces differential mediator release and distinct pattern of activated itch-sensory neurons [23••]. For example, MrgprB2 activation induced by pro-adrenomedullin peptide 9–20 (PAMP 9–20) results in the release of tryptase B predominantly with low levels of monoamines, histamine and serotonin, and excites distinct itch-sensory neuron populations expressing either Mrgprd, Mrgpra3, or HTR1F to induce itch (Fig. 1b). By contrast, FcεRI-mediated activation is associated with greater release of histamine and serotonin, and mainly triggers histamine-sensitive neurons. Not surprisingly, while histamine receptor antagonists substantially reduce itch induced by anti-IgE, they have insignificant effects on MrgprB2 agonist-mediated itch [23••]. Thus, it is possible that AD-associated itch ineffective with antihistamine treatment may be MRGPRX2 mediated. However, this association is yet to be investigated. Of note, AD patients have increased risks of allergic contact dermatitis [70, 91], whose pathogenesis has been recently shown to be regulated via MRGPRX2/B2 [23••, 92, 93]. Therefore, targeting MRGPRX2 could be a promising therapeutic modality for the prevention and/or treatment of non-histaminergic itch.
Emerging Role of MRGPRX2 in Allergic Asthma
Allergic asthma is an upper airway inflammatory disease caused by a complex interaction between structural and immune cells (airway smooth muscles, T cells, and leukocytes) [94–96]. Similar to AD pathogenesis, mast cells are widely recognized as a major player in allergic asthma by mediating effector function in Th2-immune response driven inflammation [94–96]. Mast cell-released histamine causes airway smooth muscle contraction, mucus hypersecretion, and plasma extravasation within the airway wall, which consequently results in airway narrowing [97–99]. Mast cell-derived cysteinyl leukotrienes are some of the most potent bronchoconstrictors known to cause asthmatic airway obstruction as well as increase mucus production [100, 101•].
There are a number of similarities between AD and allergic asthma. For example, sensory nerve fibers and mast cells are present in both skin [21] and lungs [102], which are continuously exposed to environmental allergens such as HDMs [103, 104]. Given the important roles of mast cells in Th2 immune response, it is possible that these diseases share a common mechanism for their initiation involving mast cell-nociceptor interaction. The level of SP (MRGPRX2 agonist) is elevated in lungs, bronchoalveolar lavage, and sputum obtained from asthmatic patients compared to normal controls [105, 106]. Furthermore, mast cells obtained from bronchoalveolar lavage (from patients at routine bronchoscopy) are activated by SP [107]. Although it was generally accepted that normal human lung mast cells are of the MCT type and do not express MRGPRX2 [41, 108], it now appears that ~20% of normal human lung mast cells express MRGPRX2 [109••]. Thus, it is possible that, similar to AD pathogenesis (Fig. 1a), upon allergen challenge, SP is released from sensory nerves and activates MRGPRX2-expressing lung mast cells to cause bronchoconstriction and Th2-mediated immune response, resulting in mild allergic asthma (Fig. 1c). Moreover, in individuals with severe asthma, airway submucosa and lung epithelium are dominated by MCTC type mast cells rather than MCT [97, 110, 111]. Manorak et al. [34••] demonstrated that the number of MRGPRX2 positive mast cells in lungs of the patients who died with asthma is significantly enhanced compared to patients who died with unrelated cause.
Neuropeptide HK-1, an important mediator implicated in the bronchoconstriction in asthma, belongs to the same family of neurokinins as SP [112]. Using transfected HEK-293 cells, Morteau et al. [55] showed that HK-1 is a full agonist for NK-1R and induces Ca2+ mobilization with similar binding affinity as SP. Unlike SP, HK-1 is not produced by sensory nerves but is released by lung macrophages, human bronchial cells, and FcεRI-activated mast cells [40, 112, 113]. Interestingly, FcεRI activation of murine mast cells results in enhanced production of HK-1, which further activates mast cells via NK-1R to provide adjuvancy in the development of chronic airway inflammation in vivo [40]. However, Manorak et al. [34••] showed that HK-1 induces degranulation in rat basophilic leukemic (RBL-2H3) cells stably expressing MRGPRX2 and a human mast cell line (LAD2) endogenously expressing MRGPRX2. This effect was not inhibited by an NK-1R antagonist, but knockdown of MRGPRX2 in LAD2 cells resulted in substantial reduction of HK-1-induced degranulation [34••]. These findings suggest that in addition to SP, HK-1 also activates human mast cell via MRGPRX2 and the potential adjuvancy in the chronic airway inflammation is likely mediated via MRGPRX2, not NK-1R. Thus, it is possible that allergen-induced mast cell/FcεRI activation, which is involved in asthma pathogenesis, produces HK-1 which may further activate MRGPRX2 and produce inflammatory mediators such as histamine and PGD2 to amplify the airway hyperresponsiveness, resulting in more severe asthma (Fig. 1c). These findings suggest that allergic asthma severity could be linked with MRGPRX2 upregulation.
In addition to mast cells, granulocytes such as eosinophils and neutrophils also play a role in asthma pathogenesis. Upon activation, eosinophils release cytoplasmic proteins such as eosinophil-derived major basic protein (MBP) and eosinophil peroxidase (EPO) [114–116]. Lung neutrophils are also shown to secrete cathelicidin LL-37 in asthmatic patients [27, 44]. Moreover, respiratory epithelium is shown to produce β-defensins in response to rhinoviral infection [117, 118] Interestingly, LL-37, MBP, EPO, and β-defensins all activate human mast cells via MRGPRX2 [27, 28, 41]. Since MRGPRX2 is upregulated in asthmatic lungs, it is likely that HK-1 released from bronchial cells and activated mast cells as well as LL-37, MBP, EPO, and β-defensins released from granulocytes and epithelial cells further amplify mast cell-mediated responses in allergic asthma through the activation of this receptor (Fig. 1c). These possibilities thus introduce MRGPRX2 both in the early events in allergic asthma as well as its exacerbation.
MRGPRX2 Mutations and Their Prospective Clinical Relevance in AD and Asthma
Molecular remodeling and mutagenesis studies have identified putative ligand binding pocket and G protein-coupling region for MRGPRX2. It has been shown that negatively charged residues E164 and D184 of MRGPRX2 are important for binding several cationic ligands, including opioids and SP [119••, 120, 121] (Fig. 2a, b). Structural and computational studies have identified residues in TM domains that are highly conserved among class A GPCRs and are likely to participate in G protein coupling [122]. These predicted residues for MRGPRX2 are V123, I225, and Y279 [123••]. Furthermore, for receptor phosphorylation, the carboxyl terminus of MRGPRX2 contains five Ser/Thr residues (Fig. 2a, b).
Fig. 2.
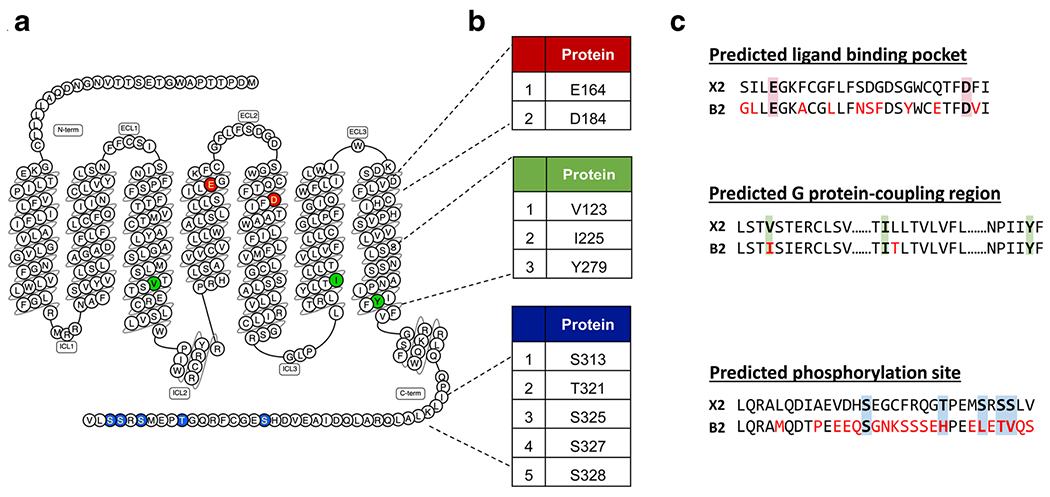
Predicted ligand binding, G protein coupling, and phosphorylation regions of MRGPRX2 with sequence comparisons to its mouse ortholog MrgprB2. a Snake diagram of secondary structure of MRGPRX2 with solid background denoting residues responsible for ligand binding (red), G protein coupling (green), and phosphorylation (blue) of MRGPRX2. b Amino acid numbering for each MRGPRX2 residues. c Site-specific pairwise sequence alignment of human MRGPRX2 (top) with mouse MrgprB2 (bottom) at predicted ligand binding (red background), G protein-coupling (green background), and phosphorylation regions (blue background). Red font color indicates different residues in MrgprB2 when compared to MRGPRX2
Utilizing structural information from crystal structures of other class A GPCRs and naturally occurring MRGPRX2 missense variants (Fig. 3a, b), Alkanfari et al. [ 119••] identified 4 naturally occurring MRGPRX2 missense variants (G165E, D184H, W243R, and H259Y) that failed to respond to SP and HK-1 [119••]. Additionally, Chompunud Na Ayudhya et al. [123••] recently identified potentially G protein-coupling-deficient variants with loss-of-function phenotype (V123F, R138C, R141C, and V282M) and phosphorylation-defective variants with gain-of-function phenotype (S325L and L329Q) for SP-induced mast cell activation and degranulation [123••] (Fig. 3a, b). It is possible that individuals with loss-of-function MRGPRX2 variants may be resistant, while those who harbor gain-of-function variants may be more susceptible in the context of both SP and HK-1-mediated inflammatory response, including AD and allergic asthma. However, these possibilities are yet to be explored.
Fig. 3.
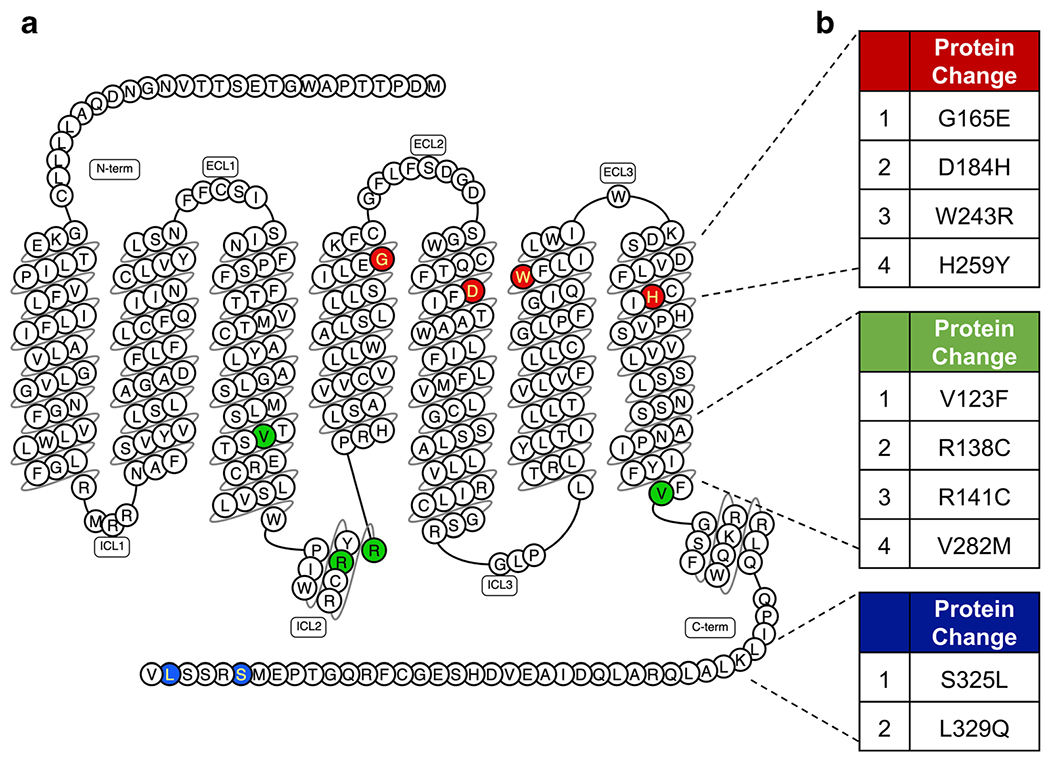
Naturally occurring missense variants of MRGPRX2. a Snake diagram of secondary structure of MRGPRX2 with solid background denoting the gain- and loss-of-function naturally occurring missense MRGPRX2 variants. b Amino acid change for each MRGPRX2 variant
Therapeutic Prospects and Unmet Needs in MRGPRX2 Studies
Based on the recent evidence of MrgprB2’s vital functional roles in AD pathogenesis [45••], neurogenic inflammation and itch [22••, 23••], and its prospective role in allergic asthma [34••, 124], it is reasonable to speculate that MRGPRX2-targeted antagonists could be an attractive therapeutic intervention for AD and allergic asthma. However, although MrgprB2mut/mut mice have been extensively utilized to study the function of MRGPRX2 in vivo, MrgprB2 markedly differs from MRGPRX2 for agonist affinity. For example, SP activates MRGPRX2 with EC50 of 152 nM but MrgprB2 requires EC50 of 54 μM [29, 120]. Of note, the overall sequence similarity between these two receptors is only about 53% [120]. Site-specific pairwise sequence alignment shows distinct dissimilarity in ligand binding and phosphorylation sites, while G protein coupling region seems to be conserved (Fig. 2c). Azimi et al. [64] reported that antagonists which block MrgprB2 do not inhibit MRGPRX2 activation. Furthermore, Ogasawara et al. [125] recently reported novel MRGPRX2 antagonists that inhibited SP-mediated degranulation in human mast cells but had no inhibitory cross reactivity against MrgprB2. These species-specific dissimilarity in amino acids sequence may attribute to the striking differences in agonist/antagonist affinity between two receptors. This restricts the testing of antagonists for an in vivo translation and highlights the need for development of better animal models.
Development of humanized mice have started to gain attention in allergy research [126–128]. Recently, Mencarelli et al. [129•] reported the generation of humanized mice expressing MRGPRX2 by hydrodynamic injection of plasmids expressing human GM-CSF and IL-3 into immunodeficient mice, NOD-scid IL2Rγ−/− (NSG) strain. Even though these mice were enriched with human mast cells, not all mast cells expressed MRGPRX2. Another major disadvantage of this model is the inability of host microenvironment to fully support human hematopoiesis. One of the prospective ways to address the limitations would be to develop humanized mice by genetically deleting MrgprB2 and replacing with MRGPRX2. This approach, however, has not been reported yet.
Conclusion
Recent studies have implicated MRGPRX2/B2 as a novel potential receptor in mast cell-mediated neuro-immune interaction associated with Th2-mediated allergic diseases, AD, and allergic asthma (Fig. 1a, c) [34••, 45••, 46••]. Emerging evidence that MRGPRX2 contributes to SP-mediated neurogenic inflammation [22••] and non-histaminergic itch [23••] strongly supports the notion that MRGPRX2 could serve as a potential key player in AD-associated inflammation and itch (Fig. 1a, b). Moreover, in addition to the neuropeptides SP and HK-1, cationic peptides released from eosinophils, neutrophils, and epithelial cells activate human mast cells via MRGPRX2 (Fig. 1c). Thus, MRGPRX2 appears to be a missing link for understanding of the pathogenesis of AD and allergic asthma, and developing a specific antagonist for this receptor could provide a novel approach for the treatment of these and other allergic diseases.
Abbreviation
- AD
Atopic dermatitis
- SP
Substance P
- HK-1
Hemokinin-1
- NK-1R
Neurokinin-1 receptor
- MRGPRX2/B2
Mas-related G protein-coupled receptor-X2/B2
- FcεRI
High-affinity immunoglobulin receptor
- HDM
House dust mite
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.National Center for Health Statistics. FastStats - Allergies and Hay Fever [Internet], 2019. Available from: https://www.cdc.gov/nchs/fastats/allergies.htm. Accessed 8 May 2020.
- 2.American College of Allergy, Asthma & Immunology. Allergy Facts | ACAAI Public Website [Internet]. 2015. Available from: https://acaai.org/news/facts-statistics/allergies. Accessed 10 May 2020.
- 3.da Silva EZM, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014;62(10):698–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immnnol. 2010;40(7):1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–92. [DOI] [PubMed] [Google Scholar]
- 7.Smarr CB, Bryce PJ, Miller SD. Antigen-specific tolerance in immunotherapy of Th2-associated allergic diseases. Crit Rev Immunol. 2013;33(5):389–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–79. [DOI] [PubMed] [Google Scholar]
- 9.Galli SJ, Gordon JR, Wershil BK. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3(6):865–72. [DOI] [PubMed] [Google Scholar]
- 10.Galli SJ, Gordon JR, Wershil BK. Mast cell cytokines in allergy and inflammation. Agents Actions Suppl. 1993;43:209–20. [DOI] [PubMed] [Google Scholar]
- 11.Echtenacher B, Männel DN, Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381(6577):75–7. [DOI] [PubMed] [Google Scholar]
- 12.Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999; 11(1):53–9. [DOI] [PubMed] [Google Scholar]
- 13.Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy. 2008;38(1):4–18. [DOI] [PubMed] [Google Scholar]
- 14.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131(6):1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21(6):666–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuerwegh AJ, De Clerck LS, De Schutter L, Bridts CH, Verbruggen A, Stevens WJ. Flow cytometric detection of type 1 (IL-2, IFN-gamma) and type 2 (IL-4, IL-5) cytokines in T-helper and T-suppressor/cytotoxic cells in rheumatoid arthritis, allergic asthma and atopic dermatitis. Cytokine. 1999;11(10):783–8. [DOI] [PubMed] [Google Scholar]
- 17.Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the nomenclature review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–6. [DOI] [PubMed] [Google Scholar]
- 18.Voisin T, Bouvier A, Chiu IM. Neuro-immune interactions in allergic diseases: novel targets for therapeutics. Int Immunol. 2017;29(6):247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabata H, Artis D. Neuro-immune crosstalk and allergic inflammation. J Clin Invest. 2019;129(4):1475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148(5):1002–1011.e4. [DOI] [PubMed] [Google Scholar]
- 21.Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97(3):575–85. [DOI] [PubMed] [Google Scholar]
- 22.••.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A mast cell-specific receptor mediates neurogenic inflammation and Pain. Neuron. 2019;101(3):412–420.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated novel role of MrgprB2 in neurogenic inflammation and pain.
- 23.••.Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of mast-cell-expressed Mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity. 2019;50(5):1163–1171.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that MrgprB2 mediates non-histaminergic itch.
- 24.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494(7436):185–94. [DOI] [PubMed] [Google Scholar]
- 25.Gurevich VV, Gurevich EV. GPCR signaling regulation: the role of GRKs and Arrestins. Front Pharmacol. 2019;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–92. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian H, Gupta K, Guo Q, Price R, Ali H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells. J Biol Chem. 2011;286(52):44739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. β-Defensins activate human mast cells via Mas-related gene-X2 (MrgX2). J Immunol. 2013;191(1):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navinés-Ferrer A, Serrano-Candelas E, Lafuente A, Muñoz-Cano R, Martín M, Gastaminza G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci Rep. 2018;8(1):11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci. 2011;1217:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellucci F, Carini F, Catalani C, Cucchi P, Lecci A, Meini S, et al. Pharmacological profile of the novel mammalian tachykinin, hemokinin 1. Br J Pharmacol. 2002;135(1):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger A, Paige CJ. Hemokinin-1 has substance P-like function in U-251 MG astrocytoma cells: a pharmacological and functional study. J Neuroimmunol. 2005; 164(1–2):48–56. [DOI] [PubMed] [Google Scholar]
- 34.••.Manorak W, Idahosa C, Gupta K, Roy S, Panettieri R, Ali H. Upregulation of Mas-related G protein coupled receptor X2 in asthmatic lung mast cells and its activation by the novel neuropeptide hemokinin-1. Respir Res. 2018;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that MRGPRX2 is upregulated in asthmatic lungs and that HK-1 activates MRGPRX2 not NK-1R.
- 35.Varricchi G, Pecoraro A, Loffredo S, Poto R, Rivellese F, Genovese A, et al. Heterogeneity of human mast cells with respect to MRGPRX2 receptor expression and function. Front Cell Neurosci. 2019;13:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997;61(3):233–45. [DOI] [PubMed] [Google Scholar]
- 37.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37(1):25–33. [DOI] [PubMed] [Google Scholar]
- 38.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bischoff SC, Schwengberg S, Lorentz A, Manns MP, Bektas H, Sann H, et al. Substance P and other neuropeptides do not induce mediator release in isolated human intestinal mast cells. Neurogastroenterol Motil. 2004;16(2):185–93. [DOI] [PubMed] [Google Scholar]
- 40.Sumpter TL, Ho CH, Pleet AR, Tkacheva OA, Shufesky WJ, Rojas-Canales DM, et al. Autocrine hemokinin-1 functions as endogenous adjuvant for IgE-mediated mast cell inflammatory responses. J Allergy Clin Immunol. 2015;135(4):1019–1030.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujisawa D, Kashiwakura J-I, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134(3):622–633.e9. [DOI] [PubMed] [Google Scholar]
- 42.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349(4):1322–8. [DOI] [PubMed] [Google Scholar]
- 43.Burstein ES, Ott TR, Feddock M, Ma J-N, Fuhs S, Wong S, et al. Characterization of the Mas-related gene family: structural and functional conservation of human and rhesus MrgX receptors. Br J Pharmacol. 2006;147(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016;138(3):700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.••.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol. 2019;20(11):1435–43 [DOI] [PMC free article] [PubMed] [Google Scholar]; This important study implicated the novel role MrgprB2 as a facilitator of mast cell-nerve interaction in atopic dermatitis-like type 2 skin inflammation.
- 46.••.An J, Lee J-H, Won H-K, Kang Y, Song W-J, Kwon H-S, et al. Clinical significance of serum MRGPRX2 as a new biomarker in allergic asthma. Allergy. 2020;75(4):959–62 [DOI] [PubMed] [Google Scholar]; This study identified upregulation of serum MRGPRX2 as a new biomarker for allergic asthma.
- 47.Taylor-Clark TE, Nassenstein C, Undem BJ. Leukotriene D4 increases the excitability of capsaicin-sensitive nasal sensory nerves to electrical and chemical stimuli. Br J Pharmacol. 2008;154(6):1359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6(2):151–8. [DOI] [PubMed] [Google Scholar]
- 49.Shim W-S, Oh U Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.•.Forsythe P Mast cells in neuroimmune interactions. Trends Neurosci. 2019;42(1):43–55 [DOI] [PubMed] [Google Scholar]; Excellent review on the role of mast cells in neuroimmune interaction.
- 51.Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40(3):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73(22):4249–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201(2):167–80. [DOI] [PubMed] [Google Scholar]
- 54.Chu HW, Kraft M, Krause JE, Rex MD, Martin RJ. Substance P and its receptor neurokinin 1 expression in asthmatic airways. J Allergy Clin Immunol. 2000;106(4):713–22. [DOI] [PubMed] [Google Scholar]
- 55.Morteau O, Lu B, Gerard C, Gerard NP. Hemokinin 1 is a full agonist at the substance P receptor. Nat Immunol. 2001;2(12):1088. [DOI] [PubMed] [Google Scholar]
- 56.Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regul Pept. 1997;70(2–3):75–90. [DOI] [PubMed] [Google Scholar]
- 57.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94(1):265–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Recio S, Gascón P. Biological and pharmacological aspects of the NK1-receptor. Biomed Res Int. 2015;2015:495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pintér E, Pozsgai G, Hajna Z, Helyes Z, Szolcsányi J. Neuropeptide receptors as potential drug targets in the treatment of inflammatory conditions. Br J Clin Pharmacol. 2014;77(1):5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramalho R, Soares R, Couto N, Moreira A. Tachykinin receptors antagonism for asthma: a systematic review. BMC Pulm Med. 2011;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boot JD, de Haas S, Tarasevych S, Roy C, Wang L, Amin D, et al. Effect of an NK1/NK2 receptor antagonist on airway responses and inflammation to allergen in asthma. Am J Respir Crit Care Med. 2007;175(5):450–7. [DOI] [PubMed] [Google Scholar]
- 62.Borsook D, Upadhyay J, Klimas M, Schwarz AJ, Coimbra A, Baumgartner R, et al. Decision-making using fMRI in clinical drug development: revisiting NK-1 receptor antagonists for pain. Drug Discov Today. 2012;17(17–18):964–73. [DOI] [PubMed] [Google Scholar]
- 63.Lönndahl L, Holst M, Bradley M, Killasli H, Heilborn J, Hall MA, et al. Substance P antagonist aprepitant shows no additive effect compared with standardized topical treatment alone in patients with atopic dermatitis. Acta Derm Venereol. 2018;98(3):324–8. [DOI] [PubMed] [Google Scholar]
- 64.Azimi E, Reddy VB, Shade K-TC, Anthony RM, Talbot S, Pereira PJS, et al. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight. 2016;1(16):e89362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–22. [DOI] [PubMed] [Google Scholar]
- 66.Nutten S Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl. 1):8–16. [DOI] [PubMed] [Google Scholar]
- 67.Saunders SP, Moran T, Floudas A, Wurlod F, Kaszlikowska A, Salimi M, et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J Allergy Clin Immunol. 2016;137(2):482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35(3):283–9. [DOI] [PubMed] [Google Scholar]
- 69.Ring J, Zink A, Arents BWM, Seitz IA, Mensing U, Schielein MC, et al. Atopic eczema: burden of disease and individual suffering - results from a large EU study in adults. J Eur Acad Dermatol Venereol. 2019;33(7):1331–40. [DOI] [PubMed] [Google Scholar]
- 70.Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–51. [DOI] [PubMed] [Google Scholar]
- 71.Ahn K, Kim BE, Kim J, Leung DY. Recent advances in atopic dermatitis. Curr Opin Immunol. 2020;66:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Werfel T, Allam J-P, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138(2):336–49. [DOI] [PubMed] [Google Scholar]
- 73.Ong PY. New insights in the pathogenesis of atopic dermatitis. Pediatr Res. 2014;75(1–2):171–5. [DOI] [PubMed] [Google Scholar]
- 74.Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 2018;26(6):484–97. [DOI] [PubMed] [Google Scholar]
- 75.Park H-Y, Kim C-R, Huh I-S, Jung M-Y, Seo E-Y, Park J-H, et al. Staphylococcus aureus colonization in acute and chronic skin lesions of patients with atopic dermatitis. Ann Dermatol. 2013;25(4):410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Totté JEE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SGMA. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2016;175(4):687–95. [DOI] [PubMed] [Google Scholar]
- 77.Bunikowski R, Mielke ME, Skarabis H, Worm M, Anagnostopoulos I, Kolde G, et al. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol. 2000;105(4):814–9. [DOI] [PubMed] [Google Scholar]
- 78.Liu F-T, Goodarzi H, Chen H-Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41(3):298–310. [DOI] [PubMed] [Google Scholar]
- 79.Forsythe P, Bienenstock J. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chem Immunol Allergy. 2012;98:196–221. [DOI] [PubMed] [Google Scholar]
- 80.Järvikallio A, Harvima IT, Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res. 2003;295(1):2–7. [DOI] [PubMed] [Google Scholar]
- 81.Salomon J, Baran E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22(2):223–8. [DOI] [PubMed] [Google Scholar]
- 82.Toyoda M, Nakamura M, Makino T, Fuh H, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147(1):71–9. [DOI] [PubMed] [Google Scholar]
- 83.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–53. [DOI] [PubMed] [Google Scholar]
- 84.Kawakami Y, Yumoto K, Kawakami T. An improved mouse model of atopic dermatitis and suppression of skin lesions by an inhibitor of Tec family kinases. Allergol Int. 2007;56(4):403–9. [DOI] [PubMed] [Google Scholar]
- 85.Kawakami Y, Kawakami T. A mouse model of atopic dermatitis. Methods Mol Biol. 2015;1220:497–502. [DOI] [PubMed] [Google Scholar]
- 86.Yang T-LB, Kim BS. Pruritus in allergy and immunology. J Allergy Clin Immunol. 2019;144(2):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.•.Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev. 2018;282(1):168–87 [DOI] [PMC free article] [PubMed] [Google Scholar]; Informative review on the role of mast cell-nerve interaction in pain and itch.
- 88.Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yosipovitch G, Berger T, Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(2):239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uehara M, Sawai T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch Dermatol. 1989;125(3):366–8. [PubMed] [Google Scholar]
- 92.Zheng Y, Che D, Peng B, Hao Y, Zhang X, He L, et al. All-transretinoic acid activated mast cells via Mas-related G-protein-coupled receptor-X2 in retinoid dermatitis. Contact Dermatitis. 2019;81(3):184–93. [DOI] [PubMed] [Google Scholar]
- 93.Peng B, Che D, Hao Y, Zheng Y, Liu R, Qian Y, et al. Thimerosal induces skin pseudo-allergic reaction via Mas-related G-protein coupled receptor B2. J Dermatol Sci. 2019;95(3):99–106. [DOI] [PubMed] [Google Scholar]
- 94.Brightling CE, Bradding P. The re-emergence of the mast cell as a pivotal cell in asthma pathogenesis. Curr Allergy Asthma Rep. 2005;5(2):130–5. [DOI] [PubMed] [Google Scholar]
- 95.Page S, Ammit AJ, Black JL, Armour CL. Human mast cell and airway smooth muscle cell interactions: implications for asthma. Am J Phys Lung Cell Mol Phys. 2001;281(6):L1313–23. [DOI] [PubMed] [Google Scholar]
- 96.Robinson DS. The role of the mast cell in asthma: induction of airway hyperresponsiveness by interaction with smooth muscle? J Allergy Clin Immunol. 2004;114(1):58–65. [DOI] [PubMed] [Google Scholar]
- 97.Andersson CK, Bergqvist A, Mori M, Mauad T, Bjermer L, Erjefält JS. Mast cell-associated alveolar inflammation in patients with atopic uncontrolled asthma. J Allergy Clin Immunol. 2011;127(4):905–912.e1–7. [DOI] [PubMed] [Google Scholar]
- 98.Andersson C, Tufvesson E, Diamant Z, Bjermer L. Revisiting the role of the mast cell in asthma. Curr Opin Pulm Med. 2016;22(1):10–7. [DOI] [PubMed] [Google Scholar]
- 99.Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev. 2017;278(1):162–72. [DOI] [PubMed] [Google Scholar]
- 100.Hallstrand TS, Henderson WR. An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010;10(1 ):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.•.Méndez-Enríquez E, Hallgren J. Mast cells and their progenitors in allergic asthma. Front Immunol. 2019;10:821. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent overview of the role of mast cells in allergic asthma.
- 102.Undem BJ, Riccio MM, Weinreich D, Ellis JL, Myers AC. Neurophysiology of mast cell-nerve interactions in the airways. Int Arch Allergy Immunol. 1995;107(1–3):199–201. [DOI] [PubMed] [Google Scholar]
- 103.Caubet J-C, Eigenmann PA. Allergic triggers in atopic dermatitis. Immunol Allergy Clin N Am. 2010;30(3):289–307. [DOI] [PubMed] [Google Scholar]
- 104.Singh M, Hays A. Indoor and outdoor allergies. Prim Care. 2016;43(3):451–63. [DOI] [PubMed] [Google Scholar]
- 105.Tomaki M, Ichinose M, Miura M, Hirayama Y, Yamauchi H, Nakajima N, et al. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am J Respir Crit Care Med. 1995;151(3 Pt 1):613–7. [DOI] [PubMed] [Google Scholar]
- 106.Nieber K, Baumgarten CR, Rathsack R, Furkert J, Oehme P, Kunkel G. Substance P and β-endorphin-like immunoreactivity in lavage fluids of subjects with and without allergic asthma. J Allergy Clin Immunol. 1992;90(4, Part 1):646–52. [DOI] [PubMed] [Google Scholar]
- 107.Heaney LG, Cross LJ, Stanford CF, Ennis M. Substance P induces histamine release from human pulmonary mast cells. Clin Exp Allergy. 1995;25(2):179–86. [DOI] [PubMed] [Google Scholar]
- 108.Kajiwara N, Sasaki T, Bradding P, Cruse G, Sagara H, Ohmori K, et al. Activation of human mast cells through the plateletactivating factor receptor. J Allergy Clin Immunol. 2010;125(5):1137–1145.e6. [DOI] [PubMed] [Google Scholar]
- 109.••.Plum T, Wang X, Rettel M, Krijgsveld J, Feyerabend TB, Rodewald H-R. Human mast cell proteome reveals unique lineage, putative functions, and structural basis for cell ablation. Immunity. 2020;52(2):404–416.e5 [DOI] [PubMed] [Google Scholar]; Important study that showed MRGPRX2 is expressed in lung mast cells as opposed to previous observation that it was not.
- 110.Balzar S, Fajt ML, Comhair SAA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the severe asthma research program. Am J Respir Crit Care Med. 2011;183(3):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sverrild A, Bergqvist A, Baines KJ, Porsbjerg C, Andersson CK, Thomsen SF, et al. Airway responsiveness to mannitol in asthma is associated with chymase-positive mast cells and eosinophilic airway inflammation. Clin Exp Allergy. 2016;46(2):288–97. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol. 2000;1(5):392–7. [DOI] [PubMed] [Google Scholar]
- 113.Grassin-Delyle S, Naline E, Buenestado A, Risse P-A, Sage E, Advenier C, et al. Expression and function of human hemokinin-1 in human and Guinea pig airways. Respir Res. 2010;11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Amin K, Janson C, Bystrom J. Role of eosinophil granulocytes in allergic airway inflammation endotypes. Scand J Immunol. 2016;84(2):75–85. [DOI] [PubMed] [Google Scholar]
- 115.Doran E, Cai F, Holweg CTJ, Wong K, Brumm J, Arron JR. Interleukin-13 in asthma and other eosinophilic disorders. Front Med (Lausanne). 2017;4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yasukawa A, Hosoki K, Toda M, Miyake Y, Matsushima Y, Matsumoto T, et al. Eosinophils promote epithelial to mesenchymal transition of bronchial epithelial cells. PLoS One. 2013;8(5):e64281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duits LA, Nibbering PH, van Strijen E, Vos JB, Mannesse-Lazeroms SPG, van Sterkenburg MAJA, et al. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol. 2003;38(1):59–64. [DOI] [PubMed] [Google Scholar]
- 118.Proud D, Sanders SP, Wiehler S. Human rhinovirus infection induces airway epithelial cell production of human beta-defensin 2 both in vitro and in vivo. J Immunol. 2004;172(7):4637–45. [DOI] [PubMed] [Google Scholar]
- 119.••.Alkanfari I, Gupta K, Jahan T, Ali H. Naturally occurring missense MRGPRX2 variants display loss of function phenotype for mast cell degranulation in response to substance P, hemokinin-1, human β-defensin-3, and icatibant. J Immunol. 2018;201(2):343–9 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified naturally occurring MRGPRX2 variants with a loss of function phenotype in response to SP and HK-1 induced mast cell degranulation.
- 120.Lansu K, Karpiak J, Liu J, Huang X-P, McCorvy JD, Kroeze WK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 2017;13(5):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reddy VB, Graham TA, Azimi E, Lerner EA. A single amino acid in MRGPRX2 necessary for binding and activation by pruritogens. J Allergy Clin Immunol. 2017;140(6):1726–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Venkatakrishnan AJ, Deupi X, Lebon G, Heydenreich FM, Flock T, Miljus T, et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature. 2016;536(7617):484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.••.Chompunud Na Ayudhya C, Roy S, Alkanfari I, Ganguly A, Ali H. Identification of gain and loss of function missense variants in MRGPRX2’s transmembrane and intracellular domains for mast cell activation by substance P. Int J Mol Sci. 2019;20(21):5247. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified both gain and loss of function variants of MRGPRX2 for SP induced mast cell activation with prospective direct clinical relevance.
- 124.Ali H Mas-related G protein coupled receptor-X2: a potential new target for modulating mast cell-mediated allergic and inflammatory diseases. J Immunobiol. 2016;1(4):115. [PMC free article] [PubMed] [Google Scholar]
- 125.Ogasawara H, Furuno M, Edamura K, Noguchi M. Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells. J Leukoc Biol. 2019;106(5):1069–77. [DOI] [PubMed] [Google Scholar]
- 126.Burton OT, Stranks AJ, Tamayo JM, Koleoglou KJ, Schwartz LB, Oettgen HC. A humanized mouse model of anaphylactic peanut allergy. J Allergy Clin Immunol. 2017;139(1):314–322.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bryce PJ, Falahati R, Kenney LL, Leung J, Bebbington C, Tomasevic N, et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J Allergy Clin Immunol. 2016;138(3):769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ito R, Maruoka S, Gon Y, Katano I, Takahashi T, Ito M, et al. Recent advances in allergy research using humanized mice. Int J Mol Sci. 2019;20(11):2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.•.Mencarelli A, Gunawan M, Yong KSM, Bist P, Tan WWS, Tan SY, et al. A humanized mouse model to study mast cells mediated cutaneous adverse drug reactions. J Leukoc Biol. 2020;107(5):797–807 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study established a humanized mice model expressing MRGPRX2 to address species-specific limitation in in vivo studies.


