Abstract
The epidemiology of COVID-19 has dramatically changed since the beginning of the pandemic as we witnessed significant changes in transmissibility and pathogenicity with the emergence of SARS-CoV-2 variants of concern (VoCs). Here I present and comment on working hypotheses about the evolutionary dynamics of the emergence of VoCs.
Keywords: SARS-CoV-2, COVID-19, pathogenicity, transmission, immune escape, variants of concern
Main text
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) VoCs is a phenomenon with unprecedented characteristics. A SARS-CoV-2 VoC is a viral strain with a combination of ‘transmissibility, severity and/or immunity that is likely to have an impact on the epidemiological situation’ (www.ecdc.europa.eu/en/covid-19/variants-concern). Although antigenic evolution resulting in escape from past infection immunity is a very common feature of virus epidemics, we have never witnessed such dramatic phenotypic switches on transmission and pathogenicity in such a short time. I note that, apart from the immune response, human-dependent factors of pathogenicity include genetics and demographics (e.g., age structure of the population), which, however, are not expected to significantly change in the timescale of the pandemic; thus, I will not analyse them further.
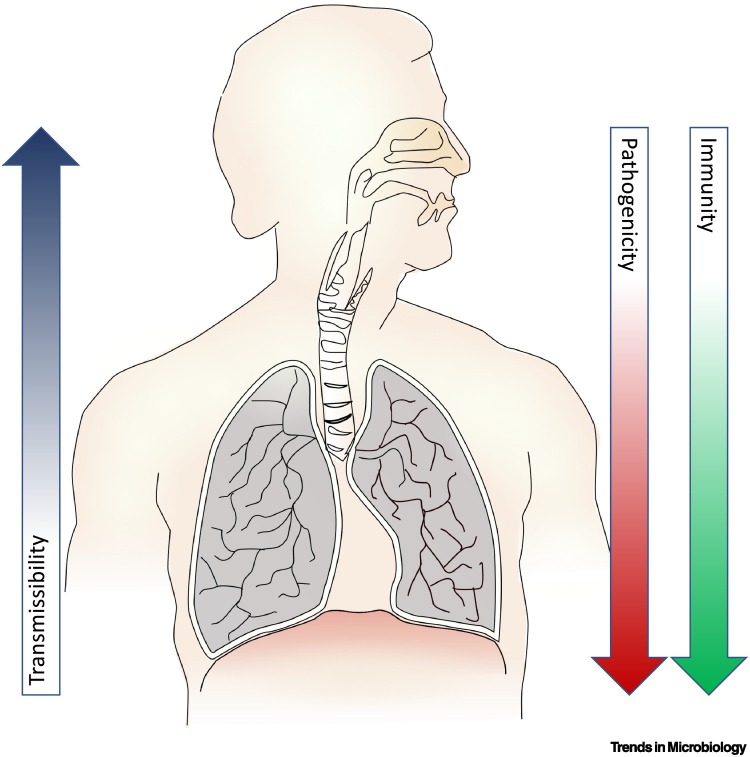
The dependency of the three traits of transmissibility, pathogenicity, and immune escape is a complex one (Figure 1 ). An increase of transmission might also mean an increase in pathogenicity if the underlying mechanism that increases transmission results in a more pathogenic feature [1]. For example, an increase in viral load would result in more infectious respiratory secretions and thus would be more easily transmitted, but it might also mean an increase in pathogenicity because it could be more likely that the payload would reach the lungs of the newly infected person. However, if transmission is led by better adaptation to the upper respiratory tract, this would also mean a decrease of pathogenicity because the likelihood of infecting the lungs would be reduced [2]. The latter might also mean more chances for immune escape because the immune surveillance of the upper respiratory tract seems to be less stringent [3]. For example, it is not uncommon to see asymptomatic colonisation of the upper respiratory tract with microbes highly virulent in the lower respiratory tract (e.g., Streptococcus pneumoniae [4]). The biological mechanism that results in a change of one specific feature could also have an effect on other traits.
Figure 1.
Relationship of transmissibility, pathogenicity, and immunity with the anatomical proliferation of the virus, which is dependent on the receptor and coreceptor tropism.
An interesting working hypothesis is that the distribution of potential receptors and coreceptors on the human respiratory tract has been shaped by the deep-in-time evolutionary pressure of respiratory tract viruses in a way that a virus proliferating efficiently on the upper respiratory tract is unlikely to proliferate efficiently on the lower respiratory tract.
As of August 2022, more than 12 million SARS-CoV-2 genome records are available (www.gisaid.org), making SARS-CoV-2 the most massively sequenced human virus. However, our understanding of how VoCs emerged, such as Alpha with higher transmissibility and higher pathogenicity than cocirculating strains [5], Delta with higher transmissibility and higher pathogenicity than Alpha [6], and Omicron with higher transmissibility and lower pathogenicity than Delta [7,8], remains unresolved.
Transmission from humans to animals, such as the white-tailed deer [9], has been suggested as a potential VoC-generating route. Circulation of SARS-CoV-2 in animal populations in theory may result in distinctly adapted viruses with significantly modified properties. It is unclear if further adaptation of human-adapted SARS-CoV-2 in animals is likely to result in optimised adaptation for humans, and because there has not been substantial evidence in support of a VoC coming from animals, I will not further discuss this hypothesis here.
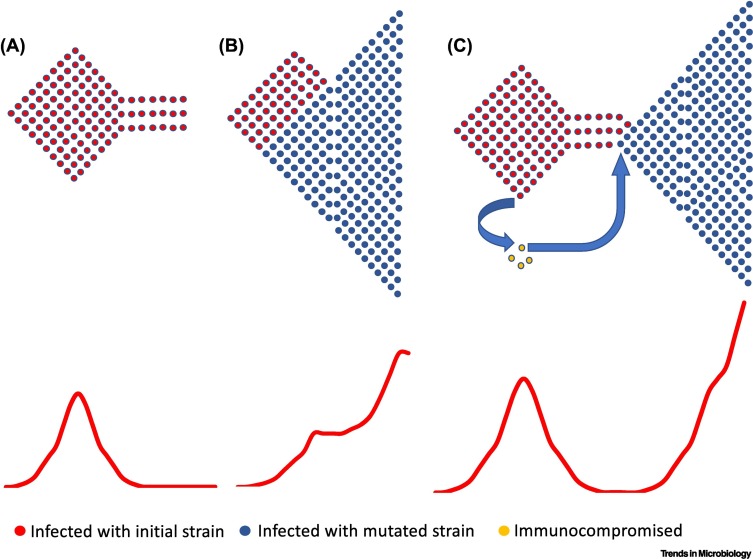
The most likely hypothesis for the emergence of a novel VoC is the chronic infection of SARS-CoV-2 in immunosuppressed individuals (CII) [10., 11., 12.] (Figure 2C); the alternative hypothesis is the gradual accumulation of mutations in multiple hosts (GMM) (Figure 2B).
Figure 2.
Schematic of hypothetical epidemic waves.
(A) An epidemic resurgence without generation of a highly transmissible variant subsides into a period of low transmission until the next resurgence (likely dependent on waning immunity of the population). (B) The emergence of a highly transmissible variant after the gradual accumulation of mutations that results in a second ‘wave-on-a-wave’ resurgence. (C) The emergence of a highly transmissible variant that has evolved within an immunocompromised patient. The emergence of the new variant wave requires introduction of the variant into the general population.
In any case, the probability of generating a new variant increases with the number of infected persons as the mutation repertoire of the epidemic increases with the population size of the viruses. However, one major difference between GMM and CII is that, for GMM, the virus mutation repertoire and thus the probability of generating a highly mutated virus is analogous to the total size of the infected population, whereas for CII, the same probability is a function of the size of the CII population but, most important, the duration of the chronic infection.
Apart from the probability of generating a single highly mutated virus, the CII hypothesis is a significant departure from GMM. First, it changes the evolutionary framework of VoCs. The GMM hypothesis suggests little if any selective pressure on pathogenicity because the virus has the opportunity to spread before the development of pneumonia and respiratory distress; thus, pathogenicity is unlikely to reduce the fitness of the virus. However, the CII hypothesis provides a significant limitation for the development of pathogenicity because the immunocompromised host is weaker than the average host, but also the time needed to accumulate mutations in the immunocompromised host requires tolerable pathogenicity for the prolonged period of infection. This cannot preclude a tolerable increase in pathogenicity, though, and indeed we have witnessed the emergence of the more pathogenic Alpha and Delta variants. However, it suggests that eventually, in the long term, less virulent strains are more likely to emerge and prevail through this mechanism because the scenario favours less pathogenic VoCs. Thus, in time, a trend toward lower pathogenicity might be expected. These long-term expected dynamics on pathogenicity might explain why the more pathogenic Alpha and Delta variants did not provide successful subvariants, whereas the less pathogenic Omicron variant has provided at least four successful subvariants as of August 2022. Crucially, it is the only VoC that has successfully ‘survived’ for almost 1 year with no signs for emergence of a VoC from an unrelated lineage. In any case, higher pathogenicity blips (i.e., subvariants with tolerable increase of pathogenicity) cannot be excluded if this scenario is eventually proved to be correct.
Second, the CII hypothesis changes the population dynamics for the emergence of VoCs compared with the GMM hypothesis. Under the CII hypothesis, the virus evolves independently from the epidemic spread and is reintroduced as a novel variant at some time point (Figure 2C), whereas under the GMM hypothesis, the virus coevolves with the epidemic spread (Figure 2B). At first glance, under the CII hypothesis, the evolution of a VoC seems to be independent from the viral spread in the rest of the population, whereas for the GMM, the emergence of a VoC is directly dependent on the level of the viral spread. A more careful look, however, reveals an interesting dependency between the epidemic spread and the emergence of VoCs under the CII hypothesis. For novel variants to be established in a population, where other variants have already established an epidemic, they need to have the opportunity to spread within that same population where cross-immunity from past infection can be an obstacle. Simple epidemiological models that consider the effect of cross-immunity [13] show that the likelihood of success of a novel variant is dependent on the level and prevalence of cross-immunity against circulating variants. For SARS-CoV-2 we know that the level of immunity and cross-immunity is dependent on the recency of the previous infection, making less likely a novel infection within the first weeks of recovery [14]. These realisations suggest that novel VoCs are more likely to emerge, because of a jump from a chronically infected immunosuppressed host, during lower (postpeak) activity of cocirculating variants than during the peaks of the epidemic waves. However, the GMM hypothesis suggests that the generation of a VoC is a function of the number of active infections; thus, they are more likely to emerge close to the peak of an epidemic wave, at which time they will gradually displace the less fit variants and become dominant.
The emergence of novel VoCs (see the emergence of Omicron in South Africa, Delta in India, and Gamma in Brazil) seems to have occurred during lower (postpeak) activity periods of viral spread, suggesting that the CII hypothesis is more likely than the GMM hypothesis to be true. This does not preclude the possibility that both mechanisms might operate at different time points.
If immunosuppressed patients are more likely to be the source of novel VoCs, it will be of utmost importance to monitor such patients closely with molecular testing and, if infected, treat them until prolonged eradication of the virus is achieved. In weaker health systems, however, monitoring of immunosuppressed individuals poses a significant challenge, especially in areas with high prevalence of HIV infection. For example, the 90-90-90 target (90% diagnosed, 90% on treatment, 90% virologically suppressed) to end the HIV pandemic will be of utmost importance to reduce the likelihood of coronavirus disease 2019 (COVID-19) resurgences. In any case, if the CII hypothesis is true for the emergence of VoCs, the importance of health equity around the world is once more underpinned.
Declaration of interests
No interests are declared.
References
- 1.May R.M., Anderson R.M. Epidemiology and genetics in the coevolution of parasites and hosts. Proc. R. Soc. Lond. B Biol. Sci. 1983;219:281–313. doi: 10.1098/rspb.1983.0075. [DOI] [PubMed] [Google Scholar]
- 2.Medina R.A., García-Sastre A. Influenza A viruses: new research developments. Nat. Rev. Microbiol. 2011;9:590–603. doi: 10.1038/nrmicro2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel M.M., et al. Immune-mediated attenuation of influenza illness after infection: opportunities and challenges. Lancet Microbe. 2021;2:e715–e725. doi: 10.1016/S2666-5247(21)00180-4. [DOI] [PubMed] [Google Scholar]
- 4.Weiser J.N., et al. Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol. 2018;16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Challen R., et al. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheikh A., et al. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maslo C., et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. 2022;327:583–584. doi: 10.1001/jama.2021.24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolter N., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hale V.L., et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602:481. doi: 10.1038/s41586-021-04353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallapaty S. Where did Omicron come from? Three key theories. Nature. 2022;602:26–28. doi: 10.1038/d41586-022-00215-2. [DOI] [PubMed] [Google Scholar]
- 11.Callaway E. How months-long COVID infections could seed dangerous new variants. Nature. 2022;606:452–455. doi: 10.1038/d41586-022-01613-2. [DOI] [PubMed] [Google Scholar]
- 12.Ghafari M., et al. Investigating the evolutionary origins of the first three SARS-CoV-2 variants of concern. Front. Virol. 2022;2 [Google Scholar]
- 13.May R.M., et al. Infectious disease dynamics: what characterizes a successful invader? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001;356:901–910. doi: 10.1098/rstb.2001.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg Y., et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N. Engl. J. Med. 2022;386:2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]