Abstract
Coronavirus Disease 2019 (COVID-19) has significantly affected different physiological systems, with a potentially profound effect on athletic performance. However, to date, such an effect has been neither addressed nor investigated. Therefore, the aim of this study was to investigate fitness indicators, along with the respiratory and metabolic profile, in post-COVID-19 athletes. Forty male soccer players, were divided into two groups: non-hospitalized COVID-19 (n = 20, Age: [25.2 ± 4.1] years, Body Surface Area [BSA]: [1.9 ± 0.2] m2, body fat: 11.8% ± 3.4%) versus [vs] healthy (n = 20, Age: [25.1 ± 4.4] years, BSA: [2.0 ± 0.3] m2, body fat: 10.8% ± 4.5%). For each athlete, prior to cardiopulmonary exercise testing (CPET), body composition, spirometry, and lactate blood levels, were recorded. Differences between groups were assessed with the independent samples t-test (p < 0.05). Several differences were detected between the two groups: ventilation (: Resting: [14.7 ± 3.1] L·min−1 vs. [11.5 ± 2.6] L·min−1, p = 0.001; Maximal Effort: [137.1 ± 15.5] L·min−1 vs. [109.1 ± 18.4] L·min−1, p < 0.001), ratio VE/maximal voluntary ventilation (Resting: 7.9% ± 1.8% vs. 5.7% ± 1.7%, p < 0.001; Maximal Effort: 73.7% ± 10.8% vs. 63.1% ± 9.0%, p = 0.002), ratioVE/BSA (Resting: 7.9% ± 2.0% vs. 5.9% ± 1.4%, p = 0.001; Maximal Effort: 73.7% ± 11.1% vs. 66.2% ± 9.2%, p = 0.026), heart rate (Maximal Effort: [191.6 ± 7.8] bpm vs. [196.6 ± 8.6] bpm, p = 0.041), and lactate acid (Resting: [1.8 ± 0.8] mmol·L-1 vs. [0.9 ± 0.1] mmol·L-1, p < 0.001; Maximal Effort: [11.0 ± 1.6] mmol·L-1 vs. [9.8 ± 1.2] mmol·L-1, p = 0.009), during CPET. No significant differences were identified regarding maximal oxygen uptake ([55.7 ± 4.4] ml·min−1·kg−1 vs. [55.4 ± 4.6] ml·min−1·kg−1, p = 0.831). Our findings demonstrate a pattern of compromised respiratory function in post-COVID-19 athletes characterized by increased respiratory work at both rest and maximum effort as well as hyperventilation during exercise, which may explain the reported increased metabolic needs.
Keywords: Cardiopulmonary exercise testing, Infected with COVID-19, Respiratory work
Abbreviations
- COVID-19
Coronavirus Disease 2019
- CPET
cardiopulmonary exercise testing
- FEV1
forced expiratory volume in the 1st second
- HR
heart rate
- MVV
maximal voluntary ventilation
- PASC
post-acute coronavirus disease 2019syndrome
- PSQI
Pittsburg sleep quality index
- RER
respiratory exchange ratio
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- VE
ventilation
- VE/MVV
breathing reserve, ventilation/maximal voluntary ventilation ratio
- O2max
maximal oxygen uptake
- Δchest
chest circumference difference between maximal inhalation and exhalation
- ΔSpO2
difference values between resting and end of test in oxygen saturation measurement with pulse oximetry
- % predicted
percent of predicted values
- bpm
beats per minute
- cm
centimeters
- kg
kilograms
- kg·m-2
kilogram per square meter
- km·h−1
kilometers per hour
- L·min−1
liters per minute
- m2
square meter
- mmol·L-1
millimoles per liter
- μL
microliter
- ml·min−1·kg−1
milliliter per minute to kilograms
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has led to an increase in morbidity and mortality globally.1 After five pandemic waves, attention has shifted to the post-COVID-19 era, in which residual or nascent syndromes formulate the spectrum of long-COVID-19.2 Emerging data support the existence of systematic long-lasting symptoms in COVID-19 survivors, beyond the respiratory system, which is collectively described under the term of post-acute COVID-19 syndrome (PASC).3 PASC may manifest as breathlessness, impaired breathing, increased oxygen requirements, post-viral cough, cardiovascular muscular changes,4 sleep disorders, chronic fatigue, cognitive impairment,5 and sarcopenia.2,6 The multi-organ sequelae may extend up to 6 months post-COVID, making the prioritization of follow-up essential,7 regardless of comorbidity risk status and severity of illness. Along with PASC, a rather sedentary lifestyle prevailed due to local prohibiting legislation to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which worsened the disease burden.8 Recent studies have shown that even post-COVID-19 athletes, who have enhanced physical condition, as well as, no previous history of illness or comorbidities, may, face challenges upon their return to their training programs.9 In the absence of relevant data, the aim of this study was to investigate fitness indicators, along with their respiratory and metabolic profile, in post-COVID-19 athletes.
Materials and methods
Participants
Forty male professional soccer players from the Greek Super League 1 and 2 volunteered for this study and were divided into two groups: previously infected with SARS-CoV-2, but non-hospitalized (i.e. mild COVID-19) versus healthy ones (Table 1). All athletes previously infected with SARS-CoV-2 (Omicron variant), were without symptoms (e.g. chest pain, fever, runny nose, cough, sore throat, headaches, muscle pain, fatigue, etc.) and included in our study two days after polymerase chain reaction negative test. The total duration of virus positivity in athletes was (6.1 ± 1.1) days. Athletes were recruited between November 2021 to January 2022. For all athletes' inclusion criteria were: age ≥ 20 to ≤ 30 years, training age ≥ 6 years, without recent injury (> 12 months). Exclusion criteria were: the lack of medical history and recent athletes' transcripts (< 30 days),10 while for post-COVID-19 athletes were difference (Δ) value in blood oxygen saturation (SpO2) between resting and at the end of cardiopulmonary exercise testing (ΔSpO2 > 4%), hospitalization and self-reported symptoms (chest pain, fatigue and/or dyspnea).11 The study's protocol was approved by the Institutional Review Board/Ethics Committee of the University Hospital of Larissa, Greece (approval reference number: N◦ 13463). All athletes provided written informed consent, in accordance with the Helsinki declaration.
Table 1.
Athletes’ characteristics. Data are expressed as mean ± standard deviation (SD).
| Post-COVID-19 | Healthy | p value | ||
|---|---|---|---|---|
| Age | years | 25.2 ± 4.1 | 25.1 ± 4.4 | 0.971 |
| Body mass | kg | 75.0 ± 7.6 | 77.7 ± 7.4 | 0.257 |
| Body mass index | kg·m-2 | 23.2 ± 1.8 | 23.9 ± 1.3 | 0.147 |
| Body surface area | m2 | 1.9 ± 0.2 | 2.0 ± 0.3 | 0.333 |
| Body fat | % | 11.8 ± 3.4 | 10.8 ± 4.5 | 0.448 |
| Muscle mass | kg | 62.4 ± 4.3 | 64.1 ± 3.7 | 0.174 |
| Lean body mass | kg | 59.3 ± 4.2 | 60.6 ± 4.7 | 0.388 |
| Total body water | % | 63.0 ± 3.0 | 62.4 ± 3.4 | 0.574 |
| Δchest | cm | 7.0 ± 1.5 | 6.6 ± 1.5 | 0.301 |
| FEV1 | % predicted | 109.2 ± 4.5 | 111.7 ± 5.8 | 0.139 |
| PSQI | score | 6.1 ± 3.2 | 3.0 ± 1.7 | < 0.001 |
Abbreviations: % = percentage, % predicted = percent of predicted values, cm: centimeters, COVID-19 = Coronavirus Disease 2019, FEV1 = forced expiratory volume in the 1st second, kg·m-2 = kilogram per square meter, kg = kilograms, m2 = square meter, PSQI = Pittsburg sleep quality index, Δchest = chest circumference difference between maximal inhalation and exhalation.
Data collection
The study protocol initiated with the assessment of anthropometrical characteristics (i.e. body height (Seca 700, Seca Deutschland, Hamburg, Germany), chest circumference difference between maximal inhalation and exhalation (Δchest, Seca 201, Hamburg, Germany), body composition (Tanita MC-980, Tanita Europe BV, Amsterdam, The Netherlands) and calculated the percentage of body fat (from seven skinfold points measurement, Harpenden, Baty International Ltd, Burgess Hill, UK)12 and body surface area according to Mosteller's formula.13 All participants underwent standard spirometry and lung volume measurements in the sitting position using the MasterScreen-CPX pneumotachograph (VIASYS HealthCare, Germany). For each pulmonary function test, three maximal flow-volume loops were obtained to determine forced expiratory volume in the 1st second (FEV1) according to the American Thoracic Society/European Respiratory Society guidelines.14 Prior to the procedures, all athletes answered the Pittsburgh Sleep Quality Index (PSQI) questionnaire10,15 and it was recorded in their medical history. Cardiopulmonary exercise testing (CPET) was performed on a treadmill (h/p/Cosmos, Nussdorf-Traunstein, Germany). All participants initiated the CPET at a speed of 7 km·h−1. Thereafter the speed of the treadmill increased by 1 km·h−1 every minute until volitional exhaustion was reached. Following CPET, all participants engaged in a 3-min active recovery i.e. walking on the treadmill.
Prior to testing, 2-min familiarization sessions were provided for all participants; after the end of the maximal test (start of test with 7 km per min and increase 1 km per min until 18th km per min), they performed a 3-min walking (3 km·h−1) for recovery purposes. Analysis of breathing gases (Fitmate MED Cosmed, Italy) was used for all respiratory parameters while heart rate was recorded via Polar H10 (USA).10 Predicted values for oxygen uptake at peak (O2max) was calculated according to Wasserman et al.'s formula O2max (mL·min-1) = (Height [cm] – Age [years]) x 20 and maximum heart rate (HR) was calculated according the formula (HRmax [bpm] = 207 − 0.7 × Age [years]).16, 17, 18 Each trial was terminated when the participant reached symptom-limited maximum exercise, which was confirmed by the presence of respiratory exchange ratio (RER) > 1.10, HR ≥ 80% of predicted HRmax, and/or plateau of oxygen consumption with increasing workload.19 A sample of 0.5 μL of peripheral blood taken from the fingertip was drawn from each participant before, at the end and the 1st minute of recovery after the CPET for the evaluation of blood lactate levels. Blood lactate concentrations were evaluated with enzymatic amperometry detection method (Lactate Scout+, EKF diagnostic, Leipzig, Germany).
All sessions were performed at The Medical Project Center (Larissa, Greece), with the environmental temperature at (22.1 ± 1.1) °C and humidity at (32.6% ± 4.1%). The evaluation of patients was performed between 11:00 a.m. to 1:00 p.m.
Statistical analysis
Data are presented as mean ± standard deviation (SD) and percentage (%) where appropriate. Data normality was assessed via the Kolmogorov-Smirnov One Sample test. Independent Samples t-Test was used to assess differences between groups (post-COVID-19 versus healthy controls). For all tests, a p-value of < 0.05 was considered statistically significant. The IBM SPSS 21 statistical package (SPSS inc., Chicago, Illinois, USA) was used for all statistical analyses.
Results
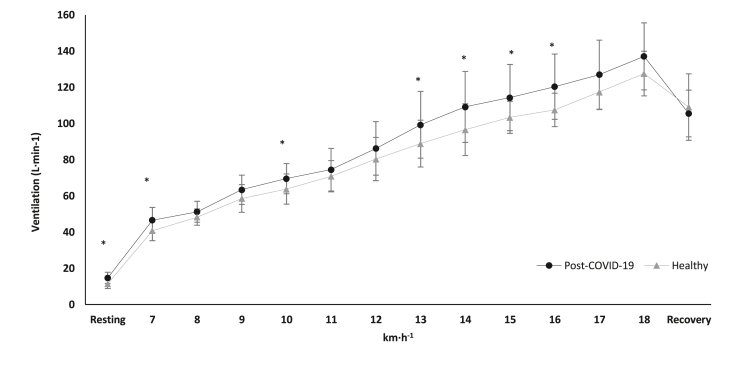
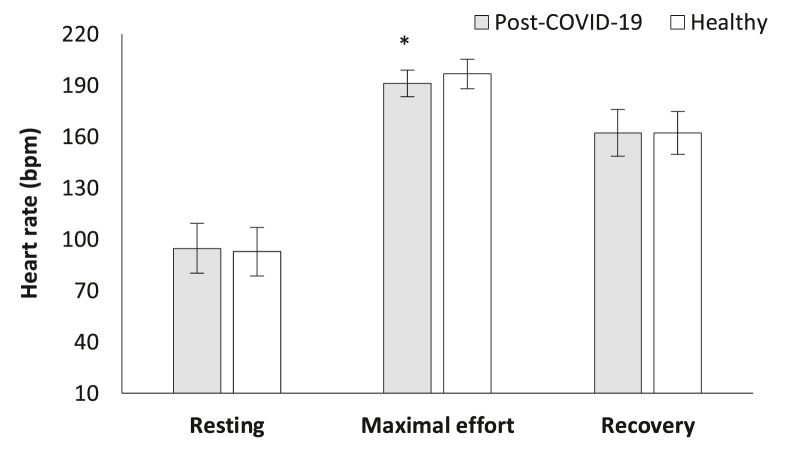
Table 1 presents athletes’ characteristics while the results of respiratory parameters during CPET are presented in Fig. 1, Fig. 2, Fig. 3. HR in the maximal effort was significantly different between the groups. COVID-19 athletes demonstrated significantly lower HR during maximal effort ([191.6 ± 7.8] bpm versus [196.6 ± 8.6] bpm, t[38] = −2.120, p = 0.041, Fig. 4) compared to the healthy group. However, mean arterial blood pressure did not reveal significant differences between groups during resting, maximal effort, or the 1st min of recovery (p > 0.05).
Fig. 1.
Ventilation alteration in cardiopulmonary exercise testing during resting phase, main test and recovery, between the groups. ∗p < 0.05.
Abbreviations: COVID-19 = Coronavirus Disease 2019, km·h−1 = kilometers per hour, L·min−1 = liters per minutes.
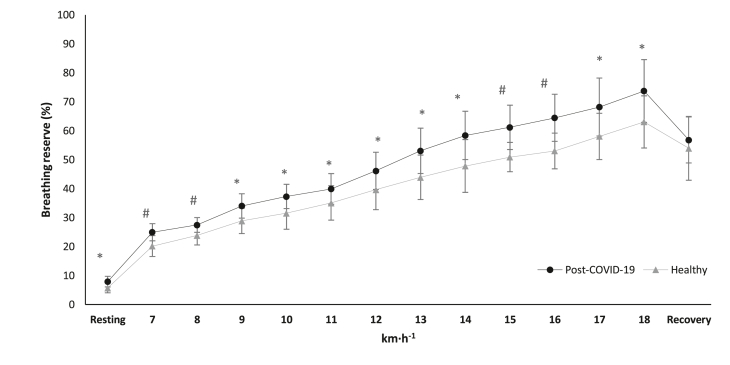
Fig. 2.
Breathing reserve alteration in cardiopulmonary exercise testing during resting phase, main test and recovery, between the groups. ∗p < 0.05, #p < 0.001.
Abbreviations: % = ventilation/maximal voluntary ventilation ratio, COVID-19 = Coronavirus Disease 2019, km·h−1 = kilometers per hour.
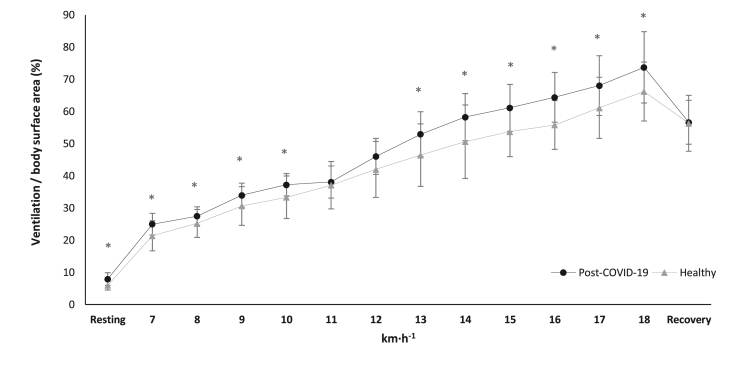
Fig. 3.
Ventilation to body surface area ratio alteration in cardiopulmonary exercise testing during resting phase, main test and recovery, between the groups. ∗p < 0.05.
Abbreviations: % = ventilation/body surface area ratio, COVID-19 = Coronavirus Disease 2019, km·h−1 = kilometers per hour.
Fig. 4.
Heart rate alteration in cardiopulmonary exercise testing during resting phase, maximal effort and recovery, between the groups. ∗p < 0.05.
Abbreviations: bpm = beats per minutes, COVID-19 = Coronavirus Disease 2019.
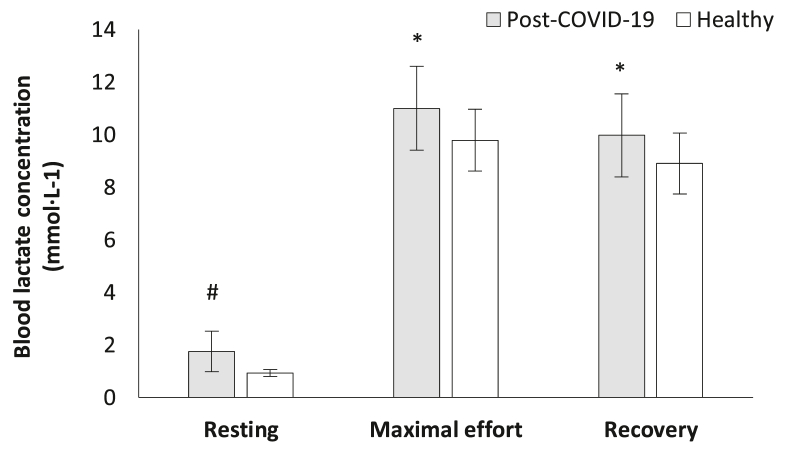
Blood lactate concentration was also significantly different between the groups. In specific, post-COVID-19 athletes showed higher values of blood lactate concentration in resting ([1.8 ± 0.8] mmol·L-1 versus [0.9 ± 0.1] mmol·L-1, t[38] = −4.695, p < 0.001, Fig. 5), during maximal effort ([11.0 ± 1.6] mmol·L-1 versus [9.8 ± 1.2] mmol·L-1, t[38] = 2.742, p = 0.009, Fig. 5) and the 1st min of recovery ([10.0 ± 1.6] mmol·L-1 versus [8.9 ± 1.2] mmol·L-1, t[38] = 2.441, p = 0.019, Fig. 5) compared to the healthy group.
Fig. 5.
Blood lactate concentration alteration during the cardiopulmonary exercise testing between the groups. #p < 0.001, ∗p < 0.05.
Abbreviations: COVID-19 = Coronavirus Disease 2019, mmol·L-1 = millimoles per liter.
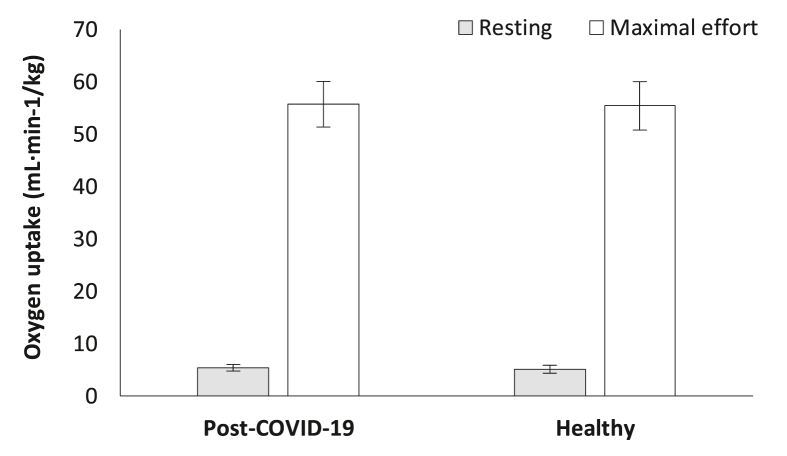
Oxygen uptake was not significantly different between groups in the resting condition (post-COVID-19: [5.4 ± 0.6] ml·min−1·kg−1 versus Healthy [5.1 ± 0.8] ml·min−1·kg−1, p > 0.05, Fig. 6): or maximal effort (post-COVID-19: [55.7 ± 4.4] ml·min−1·kg−1, 131.4% ± 9.3% of predicted versus Healthy: [55.4 ± 4.6] ml·min−1·kg−1, 131.8% ± 9.0% of predicted, p > 0.05, Fig. 6), between groups.
Fig. 6.
Oxygen uptake differentiation during cardiopulmonary exercise testing between the groups.
Abbreviations: COVID-19 = coronavirus disease 2019, ml·min−1·kg−1 = milliliter per minute to kilograms.
Sleep quality, as assessed by PSQI, revealed significant differences between the athlete groups with COVID-19 survivors demonstrating higher values compared to the healthy group (post-COVID-19: [6.1 ± 1.7] score versus Healthy: [3.0 ± 1.7] score, t[38] = 3.814, p < 0.001).
Oxygen saturation, self-assessed dyspnea, and leg fatigue also did not show significant differences between groups.
Discussion
To our knowledge, this is the first study to investigate differences in respiratory and metabolic parameters in post-COVID-19 athletes. Our study has indicated that, despite the non-significant differences in performance, it becomes evident that athletes surviving COVID-19 exhibit significant respiratory and musculature strain during exercise. Despite mild illness, these athletes display significant aerometric burdens, in order to achieve the same training performance, in contrast to non-infected ones. This may be due to the fact that COVID-19 transitions to long-COVID-19 regardless of the severity of the illness.20 Even in athletes, who are considered to have greater cardiorespiratory system capacity when compared to their age-matched sedentary controls, studies have reported a regression in the onset of the aerobic threshold, as well as lower O2max as a result of COVID-19 infection.21,22 In our study, despite the relatively equal O2max in both groups, increased respiratory work at both rest and maximum effort as well as hyperventilation during exercise, were documented. CPET was integrated with spirometry to offer a deeper understanding of lung function.
Moreover, blood lactate concentration was found significantly increased in post-COVID-19 athletes. In buffering potential hypoxia, hyperventilation is expected to occur at the expense of CO2. However, as relevant studies show, diffusing capacity of the lungs for carbon monoxide is impaired in post-COVID-19 patients.23 As the physiological process of gas exchange is hindered, hypoxia is perpetuated resulting in the recruitment of the anaerobic metabolism. The anaerobic energy pathways have higher rates of adenosine triphosphate production, but a smaller amount of total adenosine triphosphate production, compared to the aerobic ones.24 Anaerobic metabolism yields excessive levels of blood lactate concentration, disproportionately to pyruvate's (lactate/pyruvate ratio > 10).25 Therefore, we hypothesize that there exists a systematic deceleration in O2 utilization, amenable to impaired gas exchange capacity, secondary to SARS-CoV-2 infection.
Hypoxia depends on two elements: tissue-level oxidative metabolism and the supply of oxygen in the circulation (hyperventilation and tachycardia). As mentioned above, the shift to dominant-energy pathways via aerobic metabolism is delayed, while ventilatory response amplified metabolic demands and stress in post-infected athletes. However, the cardiovascular response did not yield similar differences between the two groups. One would expect that heart rate would be concomitantly increased, in line with ventilation. In a recent study, post-COVID-19 cases of previously hospitalized patients exhibited significantly increased heart rates during exercise assessment.11 Athletes with increased cardiac capacity, along with no previous history of cardiovascular pathology that could undermine ejection fraction (e.g. infection), may, indeed, require screening before returning to their prior training program. Małek et al.26 demonstrated that in a small proportion of professional post-COVID-19 athletes with mild or even asymptomatic infection, non-specific cardiac abnormalities could be identified by magnetic resonance imaging. Despite the overall low risk for cardiac involvement,27 engaging in competitive sports increases the risk of fatality and as such, guidelines for the safe return of athletes after COVID-19 infection have been published.28 It is worth noting that screening for such abnormalities should be streamlined with a personalized rehabilitation regimen for post-COVID-19 patients. Due to local restrictive legislation for COVID-19, medicine has shifted towards rehabilitation remotely supervised or even unsupervised.11,29
A consequence of the harmful effects of the SARS-CoV-2 infection, in conjunction with the aforementioned cardiorespiratory complications, is sleep disturbances. In the long-COVID-19 setting, athletes report higher scores in PSQI, suggesting the presence of persistent underlying sleep disorders. Sleep quality is essential for maximal performance, as it has been implicated in cognitive implications.30 Sleep-deprived athletes are prone to both injuries and affected perceptual ability with slower reaction times.10,31 It becomes obvious that long-COVID-19 manifests as a chronic burden, in which non-invasive means, like exercise, could be proved beneficial.32 Rehabilitation is advisable to extend to sleep hygiene intervention in the setting of holistic approaches.
Limitations, strengths, and context
Our study should be interpreted within the context of its potential limitations. The study included solely soccer athletes, whose sport combines the capability to perform in aerobic conditions for prolonged periods of time. The context and potential limitation here is that this conditioning provides a reserve against hypoxia and other noxious effects on stamina and oxygenation affected by COVID-19. The latter was reflected in our findings, indicating increased respiratory work at rest and maximum effort as well as hyperventilation. These effects would impact the performance of athletes in similar sports, and less so than others. Another caveat stemming from this is that other athletes from sports that require strength such as weightlifting would not be represented by our population. Another potential limitation is that omicron was associated with less severe respiratory illness, and thus cannot account for COVID-19 survivors infected with other variants. As a final potential limitation, the higher PSQI scores may indicate that sleep disturbances were an intermediate step in retracting from the athletes’ maximum capabilities, and thus may not be a specific effect of SARS-CoV-2.
Conclusion
In conclusion, a phenotype of post-COVID-19 implications was outlined in mild cases of previously infected athletes. The post-COVID-19 pattern was characterized by increased respiratory work at both rest and maximum effort as well as hyperventilation during exercise, which may have increased metabolic needs and mechanical stress. Such implications are not benign and require a carefully curated rehabilitation program, which could take into consideration principally the hindered oxygen supply, as well as the asymptomatic cases of myocarditis, which are gradually revealed in the post-COVID-19 era.
Submission statement
All authors have read and agree with manuscript content. In addition, as long as this manuscript is being reviewed for this journal, it will not be submitted elsewhere for review and publication.
Ethical approval statement
All participants provided informed consent, and the study's protocol was approved by the Institutional Review Board/Ethics Committee of the University Hospital of Larissa, Greece (approval reference number: N◦ 13463).
Authors’ contributions
VTS, IGF, GSM, KK and DK collected the data, VTS ran statistical analyses, VTS, KA, GDV, IGF and GSM drafted the manuscript and ZD, KIG and GB supervised the whole protocol. All authors reviewed the paper and agreed on the final form of submission.
Funding
The current research protocol was not financially supported.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank all the participants from the Medical Project, Prevention, Evaluation and Recovery Center, for volunteering in the current research protocol.
References
- 1.Gebru A.A., Birhanu T., Wendimu E., et al. Global burden of COVID-19: situational analyis and review. Hum Antibodies. 2021;29(2):139–148. doi: 10.3233/HAB-200420. [DOI] [PubMed] [Google Scholar]
- 2.Deer R.R., Rock M.A., Vasilevsky N., et al. Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stavrou V.T., Vavougios G.D., Boutlas S., et al. Physical fitness differences, amenable to hypoxia-driven and sarcopenia pathophysiology, between sleep apnea and COVID-19. Int J Environ Res Publ Health. 2022;19(2):669. doi: 10.3390/ijerph19020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vavougios G.D., Stavrou V.T., Papayianni E., et al. Investigating the prevalence of cognitive impairment in mild and moderate COVID-19 patients two months post-discharge: associations with physical fitness and respiratory function. Alzheimers Dement. 2021;17(suppl 6) doi: 10.1002/alz.057752. [DOI] [Google Scholar]
- 6.Greenhalgh T., Knight M., A'Court C., Buxton M., Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 7.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods J.A., Hutchinson N.T., Powers S.K., et al. The COVID-19 pandemic and physical activity. Sports Med Health Sci. 2020;2(2):55–64. doi: 10.1016/j.smhs.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hull J.H., Wootten M., Moghal M., et al. Clinical patterns, recovery time and prolonged impact of COVID-19 illness in international athletes: the UK experience. Br J Sports Med. 2022;56(1):4–11. doi: 10.1136/bjsports-2021-104392. [DOI] [PubMed] [Google Scholar]
- 10.Stavrou V.T., Astara K., Daniil Z., et al. The reciprocal association between fitness indicators and sleep quality in the context of recent sport injury. Int J Environ Res Publ Health. 2020;17(13):4810. doi: 10.3390/ijerph17134810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stavrou V.T., Tourlakopoulos K.N., Vavougios G.D., et al. Eight weeks unsupervised pulmonary rehabilitation in previously hospitalized of SARS-CoV-2 Infection. J Personalized Med. 2021;11(8):806. doi: 10.3390/jpm11080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos D.A., Dawson J.A., Matias C.N., et al. Reference values for body composition and anthropometric measurements in athletes. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosteller R.D. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 14.Miller M.R., Hankinson J., Brusasco V., et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 15.Stavrou V., Vavougios G.D., Bardaka F., Karetsi E., Daniil Z., Gourgoulianis K.I. The effect of exercise training on the quality of sleep in national-level adolescent finswimmers. Sports Med Open. 2019;5(1):34. doi: 10.1186/s40798-019-0207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dafoe W. Principles of exercise testing and interpretation. Can J Cardiol. 2007;23(4):274. [Google Scholar]
- 17.Stavrou V., Boutou A.K., Vavougios G.D., et al. The use of cardiopulmonary exercise testing in identifying the presence of obstructive sleep apnea syndrome in patients with compatible symptomatology. Respir Physiol Neurobiol. 2019;262:26–31. doi: 10.1016/j.resp.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka H., Monahan K.D., Seals D.R. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society; American College of Chest Physicians ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 20.Townsend L., Dowds J., O'Brien K., et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18(6):997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anastasio F., LA Macchia T., Rossi G., et al. Mid-term impact of mild-moderate COVID-19 on cardiorespiratory fitness in élite athletes. J Sports Med Phys Fit. 2022;62(10):1383–1390. doi: 10.23736/S0022-4707.21.13226-8. [DOI] [PubMed] [Google Scholar]
- 22.Vonbank K., Lehmann A., Bernitzky D., et al. Predictors of prolonged cardiopulmonary exercise impairment after COVID-19 Infection: a prospective observational study. Front Med. 2021;8 doi: 10.3389/fmed.2021.773788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuschillo S., Ambrosino P., Motta A., Maniscalco M. COVID-19 and diffusing capacity of the lungs for carbon monoxide: a clinical biomarker in postacute care settings. Biomarkers Med. 2021;15(8):537–539. doi: 10.2217/bmm-2021-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahlin K., Tonkonogi M., Söderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand. 1998;162(3):261–266. doi: 10.1046/j.1365-201X.1998.0298f.x. [DOI] [PubMed] [Google Scholar]
- 25.Alberti K.G. The biochemical consequences of hypoxia. J Clin Pathol Suppl. 1977;11:14–20. doi: 10.1136/jcp.s3-11.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Małek Ł.A., Marczak M., Miłosz-Wieczorek B., et al. Cardiac involvement in consecutive elite athletes recovered from COVID-19: a magnetic resonance study. J Magn Reson Imag. 2021;53(6):1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Hattum J.C., Spies J.L., Verwijs S.M., et al. Cardiac abnormalities in athletes after SARS-CoV-2 infection: a systematic review. BMJ Open Sport Exerc Med. 2021;7(4) doi: 10.1136/bmjsem-2021-001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene D.N., Wu A.H.B., Jaffe A.S. Return-to-play guidelines for athletes after COVID-19 infection. JAMA Cardiol. 2021;6(4):479. doi: 10.1001/jamacardio.2020.5348. [DOI] [PubMed] [Google Scholar]
- 29.Wang T.J., Chau B., Lui M., Lam G.T., Lin N., Humbert S. Physical medicine and rehabilitation and pulmonary rehabilitation for COVID-19. Am J Phys Med Rehabil. 2020;99(9):769–774. doi: 10.1097/PHM.0000000000001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astara K., Siachpazidou D., Vavougios G.D., et al. Sleep disordered breathing from preschool to early adult age and its neurocognitive complications: a preliminary report. Sleep Sci. 2021;14(Spec 2):140–149. doi: 10.5935/1984-0063.20200098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stavrou V.T., Astara K., Tourlakopoulos K.N., et al. Sleep quality's effect on vigilance and perceptual ability in adolescent and adult athletes. J Sports Med. 2021;2021 doi: 10.1155/2021/5585573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson E., Durstine J.L. Physical activity, exercise, and chronic diseases: a brief review. Sports Med Health Sci. 2019;1(1):3–10. doi: 10.1016/j.smhs.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]