Introduction
The world is coping with the health, societal, and economic consequences of more than 2 years of the COVID-19 pandemic. Emerging diseases caused by new pathogens, or re-emerging infectious diseases, appear regularly and their frequency is increasing.1, 2 Several research papers3, 4, 5 have shown that the cost of preventing an infectious disease is much lower than the cost of managing one, especially at a global level. A change in health-care framework is needed to improve pandemic prevention, which will require a global understanding of disease emergence and an integrated One Health approach. The environmental, social, economic, ethical, and political factors that characterise a social ecosystem and influence the emergence of zoonoses should be considered to control these emergences.
COVID-19 has exposed the disconnection between governmental promotion of the One Health approach in international arenas (eg, political conferences) and the reality outside of government spaces—with the absence of an efficient, comprehensive One Health surveillance system that could have been in place from the start of the pandemic. As members of France's COVID-19 Scientific Council appointed in March, 2020, to support governmental efforts in managing the COVID-19 pandemic, we had to contend with this negative reality. Based on our experience, we propose an ambitious roadmap to prevent and mitigate future pandemic crises, including real-life implementation of intersectoral activities and processes. Our strategy will require a new, worldwide, One Health vision that includes ambitious national and international initiatives, and One Health education and training.
Lessons learnt from the pandemic
The pandemic is a One Health issue
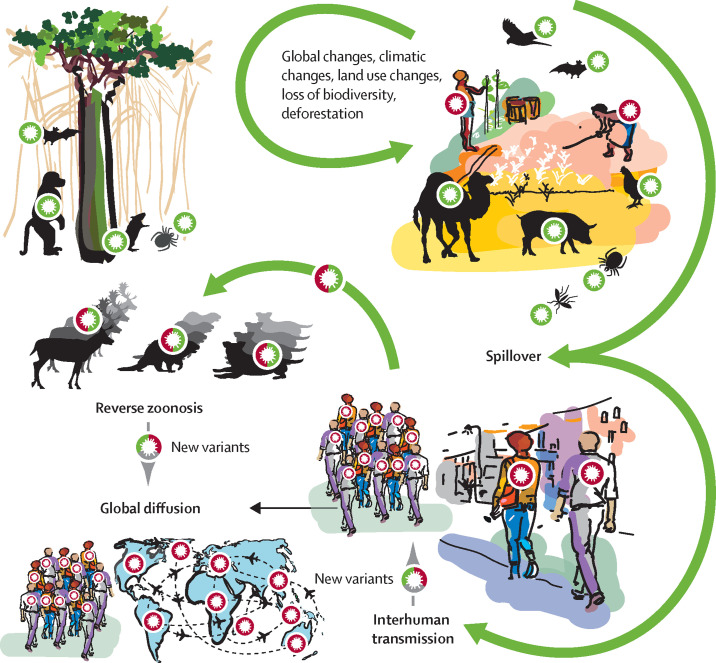
As with around 75% of listed emerging human diseases, COVID-19 is most probably a zoonosis caused by a coronavirus from an animal reservoir (figure ). Zoonotic coronaviruses can spread to humans, as observed in the past (OC43 in the 19th century, SARS-CoV in 2003, and MERS-CoV in 2011), and during the COVID-19 pandemic.6, 7 The absence of an efficient early warning system and early collaboration between stakeholders, and the knowledge gap regarding the overall process of SARS-CoV-2 adaptation to new hosts, had two consequences: unknown knowledge of the exact mechanisms or factors involved in virus emergence and adaptation steps, and speculation without scientific basis about the origin of the virus behind the pandemic. If crises similar to the COVID-19 pandemic are to be avoided in the future, these knowledge gaps about the emergence of infectious diseases need to be filled to allow the identification and rapid control of zoonotic risks before their introduction in humans. Optimum preparedness will depend on strategies and plans prepared jointly by scientists, decision makers, and all stakeholders involved in surveillance and early warning. The pandemic highlighted both the interdependence of human health, animal health, and environmental health and the need for an interdisciplinary vision to produce fundamental and comprehensive scientific and epidemiological knowledge. A strategic One Health8, 9 approach that fulfils this vision is only feasible with a comprehensive and multisectoral approach. However, despite some successful examples in Rwanda10 and Senegal, the vision for a One Health approach is still not shared by all, often poorly structured, and not operational.
Figure.
Transmission of zoonotic diseases
Stages involved in a pandemic crisis, from the initial circulation of pathogens within wild fauna and the natural environment, to the global dissemination after large-scale human-to-human transmission. Anthropological factors lead to changes in pathogen circulation and to increased contacts with new animal species, including domestic ones, which can induce spillover, with possible transmission to humans. The COVID-19 pandemic highlights the risk of reverse zoonoses, due to intensive circulation of SARS-CoV-2 in humans, transmission to new animal species, and circulation within some that have proven to be highly susceptible (hamsters, minks, and wild-tailed deer), with possible transmission of a mutated virus to humans. New variants can also emerge from circulation within humans.
An efficient One Health surveillance system is needed
The goal of the One Health strategy is to prevent and control the emergence, re-emergence, or dissemination of zoonotic pathogens;11, 12 it includes surveillance in animal reservoirs, deciphering factors facilitating emergence,13 and actions for disease control.
In an optimum One Health approach, because southeast Asian bat species harbour coronaviruses similar to SARS-CoV-2, coronavirus surveillance14 should have been triggered with regular biobanking from reservoirs, surveillance of transmission to potentially susceptible animals or to humans in contact with those animals, and an analysis of the environmental factors favouring transmission (ie, analysis of ecosystems and factors associated with carriage; figure). Action could then have been taken to control the risk of spillover or transmission to humans with reduced contact between animals and modified or resilient ecosystems reducing the risk of transmission. The cost of such a mechanism would have to be considered with the potential medium or long-term benefits arising from its use. In addition, the data generated, and the genetic resources collected would have to be shared and used beyond national, or even regional, public health strategies.
Control of zoonotic diseases requires an understanding of all factors that allow pathogens to cross the species barrier. In addition to modified ecosystems and virus molecular evolution that can trigger cross-species transmission, biological surveillance should also include how host responses influence the evolution of viruses, to help identify relevant evolutionary drivers responsible for changes that bring the virus to the point of transmissibility to humans.
Even 2 and a half years after the start of the pandemic, the COVID-19 emergence process remains unexplained. However, a comprehensive holistic One Health programme would have provided data on virus ecology, evolution, mechanisms involved in the acquisition of virulence factors (including furin or polybasic cleavage sites), and adaptive molecular traits. Surveillance would also have facilitated control and preventive measures, such as appropriate farming processes and prevention with restricted culling strategies.
An optimised surveillance system capable of providing early and robust data on a new pathogen will rely on three factors: (1) virus surveillance and detection in wildlife ecosystems; (2) identification of potential intermediate hosts (in wildlife and livestock) and factors favouring transmission (including adaptive processes); and (3) early detection of asymptomatic or symptomatic infections in exposed humans.
The risks of reverse transmissions between humans and animals
As with other zoonotic pathogens, SARS-CoV-2 is capable of reverse infection (reverse zoonosis), particularly in mustelid mammals,15 and in deer and hamsters.16, 17 As of September, 2022, the human SARS-CoV-2 virus has been detected in 25 animal species in 36 countries,18 and the figures are increasing. During reverse zoonosis, viruses might accumulate mutations leading to changes in different genes, with the potential acquisition of severity and (or) immunity escape factors. The omicron (B.1.1.529) variant is speculated to have possibly emerged after reverse zoonosis (figure).19 This understanding of potential evolutionary processes through reverse virus zoonosis and their consequences is part of the One Health strategy that needs to be addressed for each emergence (eg, monkeypox virus; appendix p 2).
Developing an ambitious roadmap for each step of a pandemic crisis
Going beyond past and current One Health projects and strategic plans
Much is being done to develop One Health approaches. More than just a concept, One Health is a strategy long used in research and surveillance in many zoonosis projects, mostly implemented in specific areas known to be places of emergence and re-emergence (eg, for Ebola virus, MERS-CoV, avian influenza virus, Nipah virus, vector diseases, and rabies), or regionally by setting up One Health surveillance networks (eg, One Health Indian Ocean, or Sega One Health). Some large multiregional projects (eg, Predict20) have implemented large-scale virus surveillance in the environment.
Beyond these research initiatives, true political awareness has emerged worldwide, with some symbolic declarations, including those from the Group of 7 (G7) Summit (June 4, 2021) and G20 (Sept 5–6, 2021) Summit health ministers. The COVID-19 pandemic has also prompted some institutional One Health strategies. A One Health High Level Expert Panel was created in May, 2021, with responsibility for gathering, analysing, passing on, and giving greater visibility to the scientific information available on the links between human, animal, and environmental health. The aim of the panel was to assist policy makers and international organisations in preventing and responding to future health crises. The panel published a new inclusive definition of One Health, encouraging intersectoral, but also interdisciplinary and multistakeholder approaches.21, 22 Several One Health operationalisation plans have also been developed, such as the One Health Joint Plan of Action developed by the Quadripartite Agreement, working together for the health of humans, animals, plants and the environment,23 and the One Health operational framework proposed by The World Bank.24
However, although declarations and action plans provided by each stakeholder in their own sector are a prerequisite for any change, they are not enough. The human health sector and agencies are well funded, but they are not experienced or equipped for the One Health concept to take an intersectoral approach. Conversely, this global understanding is more advanced in the ecology and animal health sectors, although One Health activities are poorly funded and mostly theoretical. These funding and commitment hurdles need to be addressed to increase our capacity to respond to epidemics and pandemics.
We consider that action plans should incorporate a solid long-term research programme rather than merely providing short-lived epidemiological data from a One Health surveillance system outsourced to high-income countries. Concrete and sustained surveillance and prevention actions are needed on all scales, together with education, training, and behavioural changes. These ambitious research projects need to be developed with all the stakeholders required for a One Health action plan to be taken into account, including local communities. Surveillance and prevention actions should be embedded in national plans with national stakeholders, not as external or international studies. Local empowerment is a prerequisite for programme implementation on a national and regional scale.
Targeted health prevention means developing resilient socioecological systems
This objective entails some knowledge prerequisites, notably an understanding of the associations between biodiversity, agriculture, food, and health; the concept of interdisciplinary data sharing; and the development of comprehensive indicators, including some from the human sciences. With such common and shared knowledge in advance of an emerging risk, economically viable and accepted socioecological systems can be jointly constructed with characteristics that are detrimental to long-term disease emergence, offering increased resilience to health crises.
Levers and monitoring systems could be deployed locally, with support from national and supranational bodies. Environmental intelligence sites (ie, sentinel sites) and operational monitoring of prevention initiatives (ie, living laboratories) can help to build socioecological systems that respect mandatory specifications for environmental and health management. This approach will require combined environmental and ecology research programmes, incorporating stakeholders responsible for the health of a territory, based on a predefined policy and identification of a risk for emerging diseases.
Preparedness and early detection should structure One Health action plans
A list of pathogens to be monitored should be jointly decided and updated regularly by all the stakeholders involved in public health surveillance. Corresponding indicators and innovative tools combining surveillance and alert should then be developed, and research programmes addressing the relevant emergence and transmission issues should be implemented. In the event of detection, these initiatives should be rapidly reinforced, with the surveillance upgraded to provide all relevant data for risk assessment and management.
This action plan can be facilitated by increased collaboration between the reference laboratories of health and agriculture ministries via joint funding or dual trusteeship. This joint effort would simplify the rapid mobilisation and early response of animal health and human health experts at the start of any health crisis. Reference laboratories with holistic expertise in real-life surveillance activities (eg, surveillance of emerging zoonotic infectious diseases or syndromic surveillance), such as the current World Organisation for Animal Health collaborative centres, could also be established. Embedded national or regional surveillance platforms could be set up with high-level diagnostic, sequencing, pathogen discovery, and surveillance capabilities.
Emergence surveillance gaps at the human health or animal health interface need to be identified, with a redefinition of monitoring and management responsibilities. For example, pathogens or diseases with a public health impact can circulate on livestock farms or in arthropod vectors without being the direct responsibility of a Ministry of Agriculture because they are not categorised, including swine flu virus infection, surveillance of H5N8 avian influenza virus in humans, or the circulation of Crimean–Congo haemorrhagic fever in animals.
A One Health inter-ministerial scientific platform, with governance at the highest political level in each country, could identify, analyse, and update major local risks involving emerging pathogens in the environment, in animals, or in humans, and could share that information with all stakeholders and develop control strategies.
Predefined One Health structures and task forces should drive crisis management and use all diagnostic and control capabilities
A rapid decision-making process with robust institutionalised support is essential to ensure that rapid responses agreed by different socioeconomic actors and political decision makers are implemented. Priority should be given to developing an operational alert–decision model, using a scientific basis and a multidisciplinary evaluation that includes the social sciences (eg, anthropology, sociology, health geography, and economics).
The early reporting of atypical and severe clinical presentations without any known causes (including imported diseases) requires hospital resources, ranging from infectious disease specialists, infection control specialists, and microbiologists, to resuscitation experts. In addition, rapid and innovative disease information reporting, with feedback on case monitoring to a large community, will ease the diagnostic process.
From the early days of an emergence, administrative and regulatory hurdles will have to be removed to mobilise all relevant laboratory resources, regardless of their affiliation (eg, veterinary laboratories), to allow diagnostics and research. Multisectoral laboratories will need to work together for sample handling and sharing of materials, results, and techniques.
Field collaborations developed between hospital, animal health, and wildlife or environmental professionals, and between institutional surveillance and research platforms, will include using common platforms for laboratory testing, metagenomics, sequencing, and all relevant diagnostic techniques.
Implementing these operational recommendations at each stage of a pandemic will require strong political will in each country to overcome the usual sectoral work, habits, and rules. Active local and international communities, together with civil society mobilisation, could thus become a game changer, as observed for climate change.
Inspiring new perspectives for a worldwide health vision
Improving surveillance and prevention by reinforcing both upstream and operational research
Research programmes should rely on multidisciplinary, multisectoral, and multiprofessional international projects, with a focus on novel approaches to environmental and ecological genomics (eg, open-minded detection of pathogens circulating in reservoirs and vectors and molecular identification of viruses with zoonotic potential),25, 26 modelling, artificial intelligence, and social science studies.
Such combined research approaches will accelerate control and prevention with the acquisition of basic knowledge in a coordinated manner, including on origins and adaptation mechanisms; strengthen sustained infrastructures and networks, allowing the coordinated collection of data for modelling and implementing a response strategy; and boost innovative public health strategies, or early development of innovative countermeasures (eg, diagnostics, vaccines, therapies, or behavioural recommendations), to mitigate the effect of emerging epidemic events.
International operational research initiatives, such as PREZODE,27 are thus needed to implement robust One Health programmes, surveillance programmes, and networks worldwide, with support from all the necessary stakeholders. Ambitious research projects targeting large-scale surveillance in the environment will need collaboration that focuses on bottom-up co-construction, local empowerment, and meaningful interactions between research and decision making. Projects will have to consider the Nagoya Protocol.28 Overcoming hurdles related to this protocol will require international collaboration and mutual trust and will have to be endorsed by policy makers.29, 30, 31
Developing a worldwide comprehensive and united vision of health
Diseases inherent to globalisation cannot be controlled on a single-country basis.32, 33 International institutions, including WHO, need to evolve to cope with the global challenges raised by global health and One Health. The effort needs to be focused on major emergence zones and be developed on a regional scale to have a global effect. Specific areas of Africa and southeast Asia are the epicentres of emerging and epidemic-prone infectious diseases. Recognised hotspots or evolving zones for emergence need to be identified, listed, and published on the basis of factors such as agricultural or human infringement into wildlife habitats, increasingly intensified livestock or poultry systems, live animal markets, poor biosecurity, and the emergence of megacities with poor hygiene and infection prevention management systems.
In addition, implementing One Health approaches with countries less advanced in health management will need to be supported by sustained twinning programmes (ie, collaborations between advanced, recognised laboratories and less advanced laboratories to improve their capacities) to improve diagnostics, sequencing, surveillance, and public health capacities (appendix p 2). On a regional scale, neighbouring countries share the same risks and the same socioeconomic and political constraints; regional One Health strategies34, 35 are needed to develop shared expertise, laboratories, and platforms, especially in countries with poor resources. The existing One Health regional health networks need to be further developed, with a focus on pandemic prevention and preparedness, which means solving the major issues related to animal diseases and natural disasters that affect food sustainability and security, and population resilience.
Regardless of the scale, a sustained political will and understanding of the One Health approach is necessary and should be modelled by leader countries with a long-term view, together with international organisations, and not only at the start of a crisis. A long-term plan means long-term financial guarantees provided by international communities from different mechanisms, including funding from The World Bank.
Ensuring adequate education and training for One Health
Implementing One Health and global health concepts will take time to produce operational effects. Training initiatives are needed to abolish boundaries between sectors.36
For all One Health professions (veterinarians, clinicians, pharmacists, biologists, and ecologists), the jointly constructed concept should be included in the curriculum of each discipline in its initial training. This common training should be widely available, based on teaching modules involving multidisciplinary expertise and combining public health; human and social sciences; the health of populations; territories and the environment; and research. Consistent education across One Health professions would subsequently improve collaboration between professionals.
One Health awareness campaigns and continuous training for decision makers, teachers, and members of civil society will lead to a population-based shared culture. Awareness education should be provided early in the school curriculum to support early behavioural changes, as observed for global warming. Specific training will develop cross-curricular skills needed to understand the One Health concept, such as complex thinking, systems thinking, and suitability for multidisciplinary collaboration capacity.
A change in health-care framework is needed
Health continues to be largely viewed through the restricted lens of human diseases. WHO clearly defines health as being “a state of complete physical, mental and social wellbeing and not merely the absence of disease or infirmity”. Environmental protection, animal health, and the sustainable health of a territory are not part of this definition.
For a holistic vision of health, we need to rethink how we approach the One Health concept by effectively integrating the environment into its implementation, thereby gaining an overall idea of the health of all living organisms in a given ecosystem.
The One Health concept goes beyond preventing health crises and is closely linked to a holistic vision of health and to the associations between health, environmental quality, climate, food and agriculture, and biodiversity. Studies in 2022,37, 38 and older, but nonetheless important studies,1, 39, 40, 41 have shown and reinforced the association between climate change, biodiversity crises, and emerging zoonotic diseases. One Health comprises the challenges grasped by all and shared by all societal objectives. The One Health objectives need to be addressed at a global level to fulfil the Sustainable Development Goals, together with global empowerment.
Declaration of interests
All authors are members of the French COVID-19 Scientific Council. J-FD is the President of the Council.
Acknowledgments
Acknowledgments
We thank all members of the French COVID-19 Scientific Council for their contribution to the advice on One Health published on Feb 8, 2022, on the French Ministry of Health website. We thank Patricia Doucet and Delphine Guard-Lavastre (CIRAD) for the figure illustration.
Contributors
All authors contributed to the writing of the manuscript (original draft, review, and editing). TL, DM, BL, and J-FD were involved in the conceptualisation and supervision. TL, DM, and BL designed the figure.
Supplementary Material
References
- 1.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marani M, Katul GG, Pan WK, Parolari AJ. Intensity and frequency of extreme novel epidemics. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2105482118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein AS, Ando AW, Loch-Temzelides T, et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci Adv. 2022;8 doi: 10.1126/sciadv.abl4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobson AP, Pimm SL, Hannah L, et al. Ecology and economics for pandemic prevention. Science. 2020;369:379–381. doi: 10.1126/science.abc3189. [DOI] [PubMed] [Google Scholar]
- 5.Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services . Intergovernmental Science Policy Platform on Biodiversity and Ecosystem Services; Bonn, Germany: 2020. Workshop report on biodiversity and pandemics. [Google Scholar]
- 6.Pekar JE, Magee A, Parker E, et al. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science. 2022;377:960–966. doi: 10.1126/science.abp8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worobey M, Levy JI, Serrano LM, et al. The Huanan seafood market in Wuhan was the early epicenter of the COVID-19 pandemic. Science. 2022;377:951–959. doi: 10.1126/science.abp8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osterhaus ADME, Vanlangendonck C, Barbeschi M, et al. Make science evolve into a One Health approach to improve health and security: a white paper. One Health Outlook. 2020;2:6. doi: 10.1186/s42522-019-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinsstag J, Schelling E, Waltner-Toews D, Whittaker MA, Tanner M. Théorie et pratique des approches intégrées de la santé. Éditions Quae; Versailles, France: 2020. One health, une seule santé. coordinateurs. [Google Scholar]
- 10.Nyatanyi T, Wilkes M, McDermott H, et al. Implementing One Health as an integrated approach to health in Rwanda. BMJ Glob Health. 2017;2 doi: 10.1136/bmjgh-2016-000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Garine-Wichatitsky M, Binot A, Morand S, et al. Will the COVID-19 crisis trigger a One Health coming-of-age? Lancet Planet Health. 2020;4:e377–e378. doi: 10.1016/S2542-5196(20)30179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs EP. The evolution of One Health: a decade of progress and challenges for the future. Vet Rec. 2014;174:85–91. doi: 10.1136/vr.g143. [DOI] [PubMed] [Google Scholar]
- 13.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaune D, Hul V, Karlsson EA, et al. A novel SARS-CoV-2 related coronavirus in bats from Cambodia. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goraichuk IV, Arefiev V, Stegniy BT, Gerilovych AP. Zoonotic and reverse zoonotic transmissibility of SARS-CoV-2. Virus Res. 2021;302 doi: 10.1016/j.virusres.2021.198473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickering B, Lung O, Maguire F, et al. Highly divergent white-tailed deer SARS-CoV-2 with potential deer-to-human transmission. bioRxiv. 2022 doi: 10.1101/2022.02.22.481551. published online May 24. (preprint). [DOI] [Google Scholar]
- 17.Yen HL, Sit THC, Brackman CJ, et al. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: a case study. Lancet. 2022;399:1070–1078. doi: 10.1016/S0140-6736(22)00326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Organisation for Animal Health SARS-CoV-2 in animals—situation report 13. 2022. https://www.woah.org/en/document/sars-cov-2-in-animals-situation-report-16/
- 19.Wei C, Shan KJ, Wang W, Zhang S, Huan Q, Qian W. Evidence for a mouse origin of the SARS-CoV-2 omicron variant. J Genet Genomics. 2021;48:1111–1121. doi: 10.1016/j.jgg.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PREDICT Consortium . One Health Institute, University of California, Davis; Davis, CA: 2020. Advancing global health security at the frontiers of disease emergence. [Google Scholar]
- 21.WHO Tripartite and UNEP support OHHLEP's definition of “One Health”. Joint tripartite (FAO, OIE, WHO) and UNEP statement. 2021. https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health
- 22.Adisasmito WB, Almuhairi S, Behravesh CB, et al. One Health: a new definition for a sustainable and healthy future. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Organisation for Animal Health One Health joint plan of action. Working together for the health of humans, animals, plants, and the environment. https://www.woah.org/app/uploads/2022/04/oh-joint-plan-of-action-summary.pdf
- 24.Berthe FCJ, Bouley T, Karesh WB, et al. World Bank Group; Washington, DC: 2018. Operational framework for strengthening human, animal, and environmental public health systems at their interface. [Google Scholar]
- 25.Mollentze N, Babayan SA, Streicker DG. Identifying and prioritizing potential human-infecting viruses from their genome sequences. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wardeh M, Baylis M, Blagrove MSC. Predicting mammalian hosts in which novel coronaviruses can be generated. Nat Commun. 2021;12:780. doi: 10.1038/s41467-021-21034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peyre M, Vourc'h G, Lefrançois T, Martin-Prevel Y, Soussana JF, Roche B. PREZODE: preventing zoonotic disease emergence. Lancet. 2021;397:792–793. doi: 10.1016/S0140-6736(21)00265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Secretariat of the Convention on Biological Diversity. United Nations Environmental Programme . Secretariat of the Convention on Biological Diversity; Montreal, QC: 2011. The Nagoya Protocol on access and benefit-sharing. [Google Scholar]
- 29.Lajaunie C, Morand S. Nagoya Protocol and infectious diseases: hindrance or opportunity? Front Public Health. 2020;8:238. doi: 10.3389/fpubh.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueni Katee S, Keambou Tiambo C. Discussing the drawbacks of the implementation of access and benefit sharing of the Nagoya Protocol following the COVID-19 pandemic. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.639581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho CW-L. Operationalizing “One Health” as “One Digital Health” through a global framework that emphasizes fair and equitable sharing of benefits from the use of artificial intelligence and related digital technologies. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.768977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in southeast Asia: regional challenges to control. Lancet. 2011;377:599–609. doi: 10.1016/S0140-6736(10)62004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nay O, Barré-Sinoussi F. Bridging the gap between science and policy in global health governance. Lancet Glob Health. 2022;10:e322–e323. doi: 10.1016/S2214-109X(21)00567-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakab Z, Selbie D, Squires N, Mustafa S, Saikat S. Building the evidence base for global health policy: the need to strengthen institutional networks, geographical representation, and global collaboration. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aarestrup FM, Bonten M, Koopmans M. Pandemics—One Health preparedness for the next. Lancet Reg Health Eur. 2021;9 doi: 10.1016/j.lanepe.2021.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montgomery M, Baitchman E. A call for One Health education [version 1] MedEdPublish. 2020;9:281. [Google Scholar]
- 37.Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607:555–562. doi: 10.1038/s41586-022-04788-w. [DOI] [PubMed] [Google Scholar]
- 38.Holmes EC. COVID-19-lessons for zoonotic disease. Science. 2022;375:1114–1115. doi: 10.1126/science.abn2222. [DOI] [PubMed] [Google Scholar]
- 39.Allen T, Murray KA, Zambrana-Torrelio C, et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun. 2017;8 doi: 10.1038/s41467-017-00923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 41.Karesh WB, Dobson A, Lloyd-Smith JO, et al. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.