Abstract
We developed immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assays (ELISAs) with four monovalent dengue virus antigens. We attempted to determine whether IgM responses in dengue virus infections are serotype specific or serotype cross-reactive. Serum samples from 14 confirmed dengue cases were examined. In these 14 cases, which consisted of 12 Japanese and 2 non-Japanese patients, infecting dengue virus serotypes were defined by reverse transcription-PCR. Thirteen of the 14 cases were IgM positive in ELISA. IgM responses were serotype cross-reactive in these 13 cases but were highest against infecting dengue virus serotype in 9 of the 13 cases. These results indicate that IgM responses are generally dengue serotype cross-reactive but that IgM levels are highest against the infecting serotype in most dengue cases.
Dengue is currently one of the most important arboviral diseases of humans. Dengue viruses, members of the family Flaviviridae, include four antigenically cross-reactive serotypes and are endemic in the tropical and subtropical countries of the world. The lack of effective mosquito control measures in areas where dengue is endemic maintains a high incidence of dengue. An increase in the number of people travelling by air results in a spread of dengue to most tropical and subtropical areas of the world.
Monitoring of dengue virus infections is an important component of assessing the disease risk to humans. Laboratory tests are necessary for confirmation of the diagnosis of dengue (8). Detection of the viral nucleic acids is an alternative to virus isolation and/or antigen detection. Reverse transcription-PCR (RT-PCR) is a sensitive technique for detection of dengue viral RNA in serum samples (7); however, dengue viral RNA is not usually detected in sera after defervescence (9). Enzyme-linked immunosorbent assay (ELISA) is a sensitive, specific, and rapid technique to detect virus-specific antibodies. In addition, ELISA requires less specialized equipment than PCR, making it a practical technique for use in laboratories.
Although detection of dengue immunoglobulin M (IgM) is widely used diagnostic method for dengue, it has not been determined whether IgM responses in dengue virus infections are dengue virus serotype specific or serotype cross-reactive. In this paper, we report the development of an IgM capture ELISA with monovalent dengue virus antigens. We applied the IgM capture ELISA to serum samples from confirmed dengue cases. We find that IgM responses are dengue virus serotype cross-reactive in most cases but that the highest responses are against the infecting serotype.
MATERIALS AND METHODS
Serum specimens.
Thirty-four acute- and convalescent-phase serum samples were obtained for laboratory diagnosis from 12 Japanese and 2 non-Japanese patients. The patients were infected with dengue viruses in areas where dengue is epidemic and became ill in Japan. Twenty-seven serum samples were also obtained from 17 suspected Japanese dengue cases.
Serum samples were collected from 33 healthy Japanese subjects who had never been to areas where dengue is epidemic, and these were used as anti-dengue virus IgM negative controls in the study. All 94 serum samples were first tested for the presence of anti-dengue virus IgM antibody by use of the commercially available Dengue Fever IgM Capture ELISA kit (MRL Diagnostics).
Anti-flavivirus IgG and preparation of the conjugate.
Pooled sera from Filipino dengue patients were collected, and IgG was purified by protein G affinity column chromatography (ImmunoPureG IgG purification kit; Pierce) according to the manufacturer's instruction. Purified IgG was conjugated with horseradish peroxidase (EZ-Link Plus Activated Peroxidase kit; Pierce) according to the manufacturer's instruction and used at a 1:500 dilution (1 μg of protein per ml).
ELISA antigens.
Four prototype dengue strains (type 1, Hawaii; type 2, New Guinea C; type 3, H87; and type 4, H241) were used as the antigens. Viruses were grown in the Aedes albopictus mosquito cell clone C6/36 (4). C6/36 cells were cultured in Dulbecco modified Eagle's medium supplemented with 10% fetal calf serum at 28°C. The cells were infected with dengue viruses at a multiplicity of infection higher than 1 PFU per cell and maintained at 28 or 32°C in 2% fetal calf serum–Dulbecco modified Eagle's medium for 5 to 6 days. Culture supernatant fluids were harvested and centrifuged at 10,000 × g for 30 min at 4°C to remove cell debris, and the resultant supernatants were used as the viral antigen in the assays. Control antigen was similarly prepared from uninfected C6/36 cells.
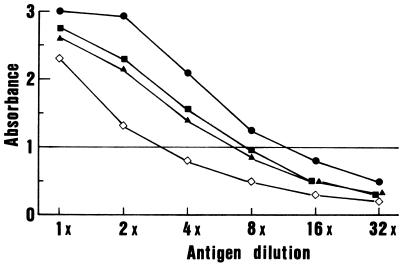
Antigen titration was carried out by an antibody sandwich ELISA using the human anti-flavivirus IgG and its peroxidase conjugate, in order to prepare the tetravalent and four monovalent dengue virus antigens (Fig. 1). Briefly, wells of the ELISA plate (Nunc) were coated with 0.1 ml of human anti-flavivirus IgG at a 1:500 dilution (1 μg of protein per ml) in 0.05 M carbonate buffer, pH 9.6. After 2.5 h of incubation at room temperature, the wells were washed with phosphate-buffered saline (PBS) (pH 7.4) containing 0.05% Tween 20 (PBS-Tween) and then reacted with serial dilutions of viral antigens in 0.1 ml per well for 1 h at room temperature. Captured antigens were detected by incubation for 1 h with 0.1 ml of the conjugated human anti-flavivirus IgG at a dilution of 1:500, and A492 readings were recorded. Based on antigen titration curves, each of the monovalent dengue virus antigens was adjusted to an A492 of 1.0. The tetravalent antigen was prepared by mixing equal volumes of each monovalent antigen at a 4-times-higher concentration.
FIG. 1.
Titration of dengue viral antigens by a sandwich ELISA. Prototype dengue virus type 1 (●)-, type 2 (⋄)-, type 3 (▪)-, and type 4 (▴)-infected C6/36 cell culture fluids were diluted with uninfected control culture fluid as the diluent.
Development of ELISA with monovalent and tetravalent antigens.
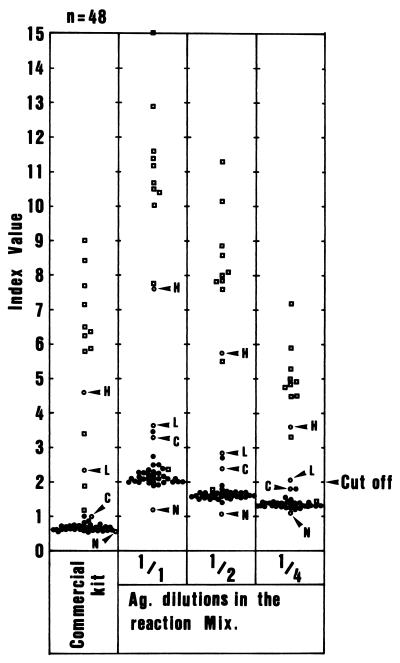
The tetravalent dengue virus antigen was used at dilutions of 1:1, 1:2, and 1:4 (Fig. 2). Serum samples obtained from 33 healthy Japanese adults were used as negative controls. Thirteen sera from 11 dengue patients and 4 reference sera which were provided with the commercial kit, two IgM-positive sera, one cutoff calibrator, and one IgM-negative serum were also tested in the assays.
FIG. 2.
Index values obtained in ELISA using tetravalent dengue virus antigen at various dilutions. The tetravalent dengue virus antigen (Ag.) was used at dilutions of 1:1, 1:2, and 1:4. Sera from 33 healthy Japanese adults (●) and 11 IgM-positive dengue patients (▫) and four reference sera given in the commercial kit (○) were used in the assay. H, L, C, and N, high IgM-positive, low IgM-positive, cutoff calibrator, and IgM-negative sera for the commercial ELISA kit, respectively.
The average (ςn − 1) and standard deviation (SD) (S2) using negative control sera were calculated for each antigen dilution. Average index values ± SDs of these negative control sera with antigen at 1:1, 1:2, and 1:4 dilutions were 2.14 ± 0.257, 1.61 ± 0.137, and 1.35 ± 0.087, respectively. Average index values + 3 SDs were 2.911, 2.021, and 1.611 for antigen dilutions of 1:1, 1:2, and 1:4, respectively. Based on these results, the tetravalent antigen was used at 1:4 dilution with the cutoff value of 2.0 in the ELISA.
ELISA.
IgM ELISA was performed according to the method reported by Bundo and Igarashi with modifications (1). Wells of the immunoplate were sensitized with 0.1 ml of the goat anti-human IgM (μ chain specific) antibody (Zymed) at a dilution of 1:500 (2 μg of protein per ml) in 0.05 M carbonate buffer, pH 9.6. After 2.5 h of incubation at room temperature, the wells were washed three times with PBS-Tween. One hundred microliters of the serum samples diluted at 1:101 in PBS plus 10% anti-Japanese encephalitis virus antibody-free calf serum (Wako Pure Chemical) was added to six wells and incubated for 1 h at room temperature. After washing with PBS-Tween, 0.1-ml portions of tetravalent antigen, each of the four monovalent antigens, and uninfected control antigens were added to each well and incubated for 1 h at room temperature. The plates were washed, and 1:500-diluted peroxidase-conjugated human anti-flavivirus IgG was added and incubated for 1 h at room temperature. After washing, an enzyme substrate, o-phenylenediamine 2HCl and H2O2 in 0.1 M citrate buffer (pH 5.0), was added and incubated for 30 min at room temperature. The stop reagent, 2.5 M H2SO4, was then added, and the resultant color change was quantified by A492 readings.
The index value was calculated by the formula A492 with the viral antigen/A492 with uninfected control antigen. We defined an index value of 2.0 or greater as positive. We always included negative and positive standard samples in each assay. The commercial ELISA kit (Dengue Fever IgM Capture ELISA kit) was purchased from MRL Diagnostics. The tests were carried out according to the manufacturer's instruction, and an index value of 1.0 or greater was considered positive.
RT-PCR.
Extraction of RNA was performed as previously reported (8). Fifty microliters of serum sample was mixed with 0.2 ml of Isogen-LS (Nippon Gene, Tokyo, Japan) for 10 s, and 0.04 ml of chloroform was added. The mixture was centrifuged for 10 min at 12,000 × g. One hundred fifty microliters of the aqueous phase was mixed with an equal volume of isopropanol for 10 s, and the mixture was kept at room temperature for 5 min. The mixture was centrifuged at 12,000 × g for 10 min. The RNA was washed once with 75% ethanol and resuspended in 0.05 ml of RNase-free water.
RT-PCR was performed according to the method reported by Morita et al., with a minor modification (7). RT and PCR were done sequentially in a single tube. The tubes were set in a oil bath type thermal cycler (Iwaki Co., Tokyo, Japan) and subjected to programmed incubation at 53°C for 10 min for reverse transcription, followed by 30 to 40 PCR cycles of amplification. PCR products were then subjected to agarose gel electrophoresis. Amplified DNA fragments were visualized by ethidium bromide staining. The primer sequences used to detect each serotype of dengue virus and the target sizes are listed in Table 1.
TABLE 1.
Nucleotide sequences of dengue virus primers
| Primera | Sequence | Gene | Product size (bp) |
|---|---|---|---|
| D1S | GGACTGCGTATGGAGTTTTG | E | 490 |
| D1C | ATGGGTTGTGGCCTAATCAT | NS1 | |
| D2S | GCATAGAGGCTAAGCTGACC | E | 263 |
| D2C | AAGGGGACTCACTCCACAAT | E | |
| D3S | GTGCTTACACAGCCCTATTT | E | 320 |
| D3C | TCCATTCTCCCAAGCGCCTG | NS1 | |
| D4S | CCATTATGGCTGTGTTGTTT | NS2a | 398 |
| D4C | CTTCATCCTGCTTCACTTCT | NS2b |
S, sense primer; C, complementary primer; D1, dengue virus type 1; D2, dengue virus type 2; D3, dengue virus type 3; D4, dengue virus type 4.
RESULTS
Comparison of the in-house ELISA with the commercial ELISA kit.
We attempted to determine dengue virus serotype specificity or cross-reactivity of IgM responses in dengue virus infections. Because ELISA using monovalent dengue virus antigen was not commercially available, we first developed ELISA with monovalent and tetravalent dengue virus antigens. The development of in-house dengue virus ELISA is described in Materials and Methods. We then compared the in-house ELISA with the commercial ELISA kit. Fifty-seven serum samples from confirmed and suspected dengue cases were tested by the in-house and the commercial ELISAs, and the results were compared (Table 2). When all of the results of the in-house ELISA with tetravalent and monovalent antigens are included, the results are consistent between our in-house ELISA and the commercial one, except for one sample (data not shown).
TABLE 2.
Comparison of the results obtained with the in-house ELISA and the commercial ELISA
| In-house ELISA result | No. of samples with the following commercial ELISA result:
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 43 | 0 | 43 |
| Negative | 1 | 13 | 14 |
| Total | 44 | 13 | |
Serodiagnosis of dengue by ELISA using monovalent dengue virus antigen.
We examined the serotype specificity or cross-reactivity of IgM antibody responses, using the in-house ELISA with the tetravalent dengue virus antigen and the antigens of each of four dengue virus serotypes. Thirty-four serum samples from 14 dengue patients were tested. Infecting dengue virus serotypes were determined by PCR for these 14 patients (cases 1 to 14) (Table 3).
TABLE 3.
Serodiagnosis of dengue by IgM ELISA
| Case no. | Days after illness | RT-PCR resulta | Commercial ELISA result (cutoff, <1.0) | Result with the following assay antigen in IgM ELISA (cutoff, <2.0)
|
||||
|---|---|---|---|---|---|---|---|---|
| Tetravalent | D1 | D2 | D3 | D4 | ||||
| 1 | 3 | D1 | 0.74 | 1.27 | 1.07 | 1.03 | 1.06 | 2.42 |
| 10 | 10.00 | 7.53 | 14.16 | 8.17 | 8.67 | 5.33 | ||
| 2 | 4 | D2 | 0.51 | 1.39 | 1.28 | 1.19 | 1.08 | 2.61 |
| 12 | 7.34 | 5.10 | 6.70 | 11.21 | 4.52 | 3.97 | ||
| 3 | 1 | D2 | 0.35 | 1.37 | 1.14 | 1.04 | 1.08 | 2.75 |
| 4 | 0.65 | 1.28 | 1.04 | 1.00 | 1.00 | 3.11 | ||
| 8 | 7.97 | 5.00 | 7.12 | 10.40 | 5.47 | 5.87 | ||
| 4 | 3 | D1 | 0.50 | 1.33 | 1.46 | 1.06 | 1.00 | 1.78 |
| 5 | 2.08 | 3.24 | 4.85 | 2.00 | 2.00 | 2.85 | ||
| 5 | 5 | D1 | 1.07 | 3.44 | 7.55 | 1.49 | 1.42 | 2.46 |
| 21 | 3.19 | 6.63 | 11.88 | 4.40 | 4.97 | 5.43 | ||
| 6 | 2 | D1 | 0.54 | 1.29 | 1.20 | 1.08 | 1.03 | 2.17 |
| 9 | 6.35 | 5.59 | 12.66 | 3.32 | 3.79 | 6.04 | ||
| 20 | 8.39 | 6.75 | 13.11 | 8.36 | 6.90 | 5.12 | ||
| 7 | 3 | D4 | 0.49 | 1.27 | 1.15 | 1.09 | 1.08 | 2.24 |
| 24 | 6.72 | 3.75 | 2.68 | 2.51 | 2.81 | 10.3 | ||
| 8 | 2 | D3 | 0.60 | 1.20 | 1.00 | 1.00 | 0.98 | 2.08 |
| 6 | 2.65 | 1.39 | 1.80 | 1.60 | 4.84 | 2.50 | ||
| 12 | 9.10 | 4.95 | 9.10 | 4.96 | 9.70 | 8.12 | ||
| 19 | 8.70 | 4.83 | 9.15 | 4.97 | 9.93 | 8.24 | ||
| 30 | 6.32 | 4.08 | 7.74 | 4.40 | 9.93 | 7.08 | ||
| 9 | 4 | D1 | 0.01 | 1.09 | 1.76 | 1.05 | 1.11 | 1.38 |
| 9 | 12.5 | 4.10 | 10.00 | 4.14 | 7.02 | 5.20 | ||
| 10 | 4 | D1 | 0.02 | 1.43 | 1.78 | 1.23 | 1.18 | 2.14 |
| 7 | 7.32 | 4.42 | 7.59 | 3.58 | 4.37 | 4.88 | ||
| 32 | 11.80 | 4.88 | 8.20 | 4.22 | 5.56 | 6.42 | ||
| 11 | 5 | D2 | 0.48 | 1.43 | 1.12 | 1.04 | 1.05 | 2.48 |
| 8 | 1.87 | 1.83 | 1.75 | 2.72 | 1.29 | 2.38 | ||
| 12 | 3 | D2 | 0.32 | 1.13 | 1.09 | 1.03 | 1.08 | 1.64 |
| 5 | 0.83 | 1.16 | 1.25 | 1.33 | 1.30 | 1.92 | ||
| 13 | 5 | D2 | 1.37 | 1.78 | 1.95 | 1.66 | 1.54 | 2.33 |
| 12 | 4.59 | 3.08 | 4.16 | 3.29 | 2.98 | 3.91 | ||
| 23 | 6.67 | 3.80 | 5.46 | 3.78 | 3.57 | 4.47 | ||
| 14 | 6 | D1 | 5.83 | 3.28 | 5.00 | 2.66 | 2.78 | 4.60 |
D1 to D4, dengue virus type 1 to 4, respectively.
Cases 1 to 14 included 12 Japanese cases and 2 non-Japanese cases (cases 13 and 14). Index values were greater than 2.0 by ELISA using tetravalent or monovalent antigens in 13 of the 14 cases. The day 8 serum sample from case 11, which was IgM negative in ELISA with tetravalent antigen, was positive in ELISA with dengue virus type 2 and type 4 antigens. Serum samples obtained from case 12 were determined to be IgM negative in the ELISA. These serum samples were obtained on days early in disease, and it is likely that IgM was not produced to a detectable level.
Index values were equal to or greater than 2 for all four dengue virus serotypes in 12 of the 13 IgM-positive cases. IgM levels when using the monovalent ELISA, however, were highest against the infecting dengue virus serotype in 9 of the 13 cases. These results suggest that IgM responses are dengue virus serotype cross-reactive but are highest against the infecting serotype in most cases.
DISCUSSION
We developed an IgM ELISA using tetravalent dengue virus antigen or antigens of each of four dengue virus serotypes. The sensitivity and specificity of our in-house ELISA were confirmed by comparison with a commercial one (Table 2). The ELISA was then applied to serum samples from 14 PCR-confirmed dengue cases (Table 3). The sensitivity of IgM ELISA in detecting dengue virus infection was again supported by the results that IgM responses were detected in 13 out of 14 confirmed cases. It is likely that serum samples from case 12 were negative for anti-dengue virus IgM because they were obtained on early days of the illness. We examined the serotype specificity and cross-reactivity of IgM antibody responses. In 9 of 12 cases, namely, 9 of 10 Japanese cases, IgM levels were higher against the infecting dengue virus serotype than against three other serotypes (Table 3), although IgM responses against the three other serotypes were also positive. The serotypes to which the highest IgM antibody responses were directed were consistent with the PCR-determined infecting dengue virus serotypes.
Since IgM ELISA was introduced for serodiagnosis of dengue (3), most of the ELISAs have utilized dengue virus antigens prepared from infected mouse brains or infected C6/36 cell culture fluids. We used culture fluids of dengue virus-infected C6/36 cells as assay antigens. It is important to develop culture systems which supply high titers of dengue viruses in the culture supernatants. Mohamed et al. (6) reported that the amount of dengue virus type 2 and type 3 viral antigens produced in C6/36 cell culture fluid increased when the incubation temperature was elevated from 28 to 32 or 37°C. Our study showed that dengue virus type 1, Hawaii strain, was produced to a higher titer at 32 or 37°C (data not shown). Similar attempts to produce higher titers of dengue virus types 2, 3, and 4 at 32 or 37°C were not successful.
The established IgM ELISA was useful for diagnosis of dengue. We already reported that RT-PCR was useful for diagnosis of dengue when serum samples were collected from patients with fever (9). On the other hand, the IgM ELISA tends to be positive after defervescence (9). Thus, application of both of IgM ELISA and RT-PCR increases the ability to diagnose dengue virus infections. In the present study, we examined serotype specificity and cross-reactivity of IgM responses, using serum samples from PCR-confirmed dengue cases. The serotype specificity of IgM responses in dengue patients has been variously stated (2, 3, 5). Burke reported that serotype-specific IgM responses corresponding to the isolated virus type were detected in all 16 patients with primary dengue virus infection tested (2, 5). Gubler reported that in dengue infection, monotypic IgM responses frequently are not correlated with the virus serotype isolated from a patients (3). We found that IgM responses were generally serotype cross-reactive but that IgM levels were highest against the infecting dengue virus serotype in most cases. Although more patient samples need to be tested to confirm our observation, the results provide interesting insights into human antibody responses to dengue viruses.
ACKNOWLEDGMENTS
We thank the doctors of 24 clinics and hospitals for providing us serum samples for diagnosis of dengue.
This work was supported by grants from the Research on Emerging and Re-emerging Infectious Diseases program, Ministry of Health and Welfare of Japan; from the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R & D Promotion and Product Review of Japan; and by Cooperative Research Grant 1999 (11-a-2) of the Department of Tropical Medicine, Nagasaki University. M. Nawa was supported by World Health Organization grant CDS/JPN/97/01.
REFERENCES
- 1.Bundo K, Igarashi A. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J Virol Methods. 1985;11:15–22. doi: 10.1016/0166-0934(85)90120-x. [DOI] [PubMed] [Google Scholar]
- 2.Burke D S. Rapid methods in the laboratory diagnosis of dengue virus infections. In: Pang T, Pathmananathan R, editors. Proceedings of the International Conference Dengue/Dengue Hemorrhagic Fever. Kuala Lumpur, Malasia: University of Malaya; 1983. pp. 72–84. [Google Scholar]
- 3.Gubler D J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to dengue and chikungunya viruses. J Gen Virol. 1978;40:531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- 5.Innis B L. Antibody responses to dengue virus infection. In: Gubler D J, Kuno G, editors. Dengue and dengue hemorrhagic fever. Wallingford, United Kingdom: CBA International; 1997. pp. 221–243. [Google Scholar]
- 6.Mohamed H, Castillo L D, Sinniah M, Igarashi A. Elevated incubation temperature enhanced antigen production of dengue type 2 and 3 viruses in the infected Aedes albopictus clone C6/36 cell culture. Trop Med. 1995;37:21–27. [Google Scholar]
- 7.Morita K, Tanaka M, Igarashi A. Rapid identification of dengue virus serotypes by using polymerase chain reaction. J Clin Microbiol. 1991;29:2107–2110. doi: 10.1128/jcm.29.10.2107-2110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, Takasaki T, Nawa M, Kurane I. Laboratory diagnosis of imported dengue cases. Jpn J Trop Med Hyg. 1999;27:75–77. [Google Scholar]
- 9.Yamada K, Nawa M, Takasaki T, Yabe S, Kurane I. Laboratory diagnosis of dengue virus infections by reverse transcriptase polymerase chain reaction (RT-PCR) and IgM-capture enzyme-linked immunosorbent assay (ELISA) Jpn J Infect Dis. 1999;52:150–155. [PubMed] [Google Scholar]