Abstract
Background
It remains unclear how vaccine doses and combinations of vaccination and infection affect the magnitude and quality of immune responses, particularly against novel SARS-CoV-2 variants in subjects with immune-related disorders, such as people with multiple sclerosis (pwMS). Several studies have evaluated the duration of anti-SARS-CoV-2 immune protection in healthy individuals; however clinical data suggest an attenuated short-term humoral response to SARS-CoV-2 vaccines in pwMS receiving disease-modifying therapies (DMTs).
Methods
In this prospective study, we evaluated the humoral response to the third (3rd) BNT162b2 vaccine (booster) dose in a monocentric cohort of pwMS undergoing eight different DMTs, all without previous SARS-CoV-2 infection. Quantitative determination of SARS-CoV-2 IgG Spike titre was carried out by anti-SARS-CoV-2 S assay in 65 pwMS and 9 healthy controls, all without previous SARS-CoV-2 infection. Moreover, these measurements were also compared to their relative levels at 21 days (T1) and ∼6 months (T2) after the second (2nd) vaccination.
Results
We observed that the humoral response to the booster dose in Interferon β-1a-, Dimethyl fumarate- and Teriflunomide-treated pwMS is comparable to healthy controls, while increased in Cladribine-treated pwMS. Additionally, the 3rd dose elicits a seroconversion in the 100% of pwMS under Fingolimod and in the 65% of those under Ocrelizumab. Moreover, multivariate regression analysis showed that treatment with Interferon β-1a, Dimethyl fumarate and Cladribine positively associates with an increased humoral response.
Conclusions
Taken together this evidence strongly indicates the importance of the booster dose to enhance SARS-CoV-2-specific immunity especially in immunocompromised subjects, such as pwMS under DMTs.
Keywords: Severe acute respiratory syndrome coronavirus (SARS-CoV)-2, Coronavirus disease 2019 (COVID-19), BNT162b2 booster vaccine, Disease modifying therapies, Multiple sclerosis, Humoral response
Abbreviations: CLAD, Cladribine; COVID-19, Coronavirus disease 2019; DMF, Dimethyl fumarate; DMTs, Disease modifying therapies; EDSS, Expanded disability status scale; FTY, Fingolimod; GA, Glatiramer acetate; GMTR, Geometric mean titre ratio; HC, Healthy controls; IgG, Immunoglobulin G; IFN, Interferon β-1a; IQR, Interquartile range; MS, Multiple sclerosis; NAT, Natalizumab; OCRE, Ocrelizumab; TERI, Teriflunomide
1. Introduction
Mass vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been considered crucial to the containment of the coronavirus disease 2019 (COVID-19) pandemic. However, its success is challenged by breakthrough infection and disease in fully vaccinated individuals. Among the possible cause is the emergence of the different beta (B.1.351), delta (B.1.617.2) and omicron (B.1.1.529) SARS-CoV-2 variants that escape immunity leading to reduced effectiveness of the BNT162b2 vaccine (Pfizer-BioNTech) (Goldberg et al., 2021; Collie et al., 2022). Moreover, real-world data analysing the antibody kinetics after vaccination reported that breakthrough infection correlates with neutralizing antibody titre (Bergwerk et al., 2021). The BNT162b2 vaccine elicits high anti-spike IgG neutralizing response early after the second dose, while a significant decrease in antibody levels is observed from the peak (at 21 to 40 days) to 84 days after receipt of the second vaccine dose (Levin et al., 2021). In addition, lower antibody levels have been shown in elderly and in immunosuppressed subjects, thus suggesting that antibody titre in these individuals may decrease earlier than in other populations (Lusting et al., 2021). Multiple sclerosis (MS) subjects, especially those with older age, severe disabilities, progressive forms and comorbidities, have been shown to be at higher risk of serious illness or death secondarily to SARS-CoV-2 infection, even if this point is still debated (Sormani et al., 2021; Achiron et al., 2021; Naser Moghadasi et al., 2021). mRNA SARS-CoV-2 BNT162b2 vaccination has shown to be effective in people with multiple sclerosis (pwMS) undergoing different disease-modifying therapies (DMTs), with a humoral response comparable to healthy subjects, with the exception of those under Fingolimod and anti-CD20 therapies (Maniscalco et al., 2022; Sormani et al., 2021). However, the kinetics of the antibody response to the vaccine in pwMS is still not well defined and, due to the persistence of the SARS-CoV-2 pandemic, booster vaccinations have been considered necessary especially for those vulnerable groups. In light of the current discussion, several studies have evaluated the duration of anti-SARS-CoV-2 immune protection in healthy individuals to schedule the right booster vaccination program (Achiron et al., 2021; Andrews et al., 2022; Maniscalco et al., 2022). In this context, clinical data suggest an attenuated short-term SARS-CoV-2 humoral response and a faster decline in pwMS receiving some DMTs while increased antibody response and persistence overtime in others (Maniscalco et al., 2022; Tortorella et al., 2022; Capuano et al., 2022).
Here we present a prospective study aimed at assessing the humoral response to the 3rd BNT162b2 booster vaccination in a monocentric cohort of pwMS under eight different DMTs compared with healthy subjects, all without previous SARS-CoV-2 infection.
2. Materials and methods
2.1. Subjects and study design
This is a prospective monocentric study to evaluate the SARS-CoV-2 IgG Spike titre after BNT162b2 vaccine in pwMS undergoing vaccination at the Multiple Sclerosis centre of the Cardarelli Hospital (Naples, Italy) from March 2021 to January 2022. All human subjects were enrolled after obtaining informed consent. The study was approved by the Institutional Review Board of the Cardarelli Hospital. We enrolled 65 pwMS and 9 healthy controls (HC) receiving the third dose of BNT162b2 vaccine according to the recommendations of Italian Authority of Health, all without previous SARS-CoV-2 infection. pwMS were vaccinated according to specific timing; specifically, those treated with Interferon β1-a (IFN), Glatiramer acetate (GA), Teriflunomide (TERI), Dimethyl Fumarate (DMF), Fingolimod (FTY) and Natalizumab (NAT) were vaccinated without any interruption of immunomodulatory treatment, while Cladribine (CLAD)- and Ocrelizumab (OCRE)-treated pwMS were vaccinated at least 1 or 3 months respectively after the last therapeutic administration, according to the recommendations of Italian Authority of Health (Centonze et al., 2021). Blood samples were collected at 9:00AM into heparinized Vacutainers (BD Biosciences) and processed within the following 4 h. Demographic and clinical characteristics of the study cohort are shown in Table 1 . Inclusion criteria were: patients aged between 18 and 65 years, diagnosed with MS and treated with DMTs for at least 6 months. Exclusion criteria were: previous SARS-CoV-2 infection, any relapse and/or steroid use in the last 30 days before enrolment. HC were matched for age and sex and had no history of inflammation, endocrine or autoimmune disease. The ethnic distribution among the groups was comparable, with all participants being white.
Table 1.
Clinical and demographic characteristics of the study cohort.
| MS patients | Healthy Control | |
|---|---|---|
| Gender, n (%) | ||
| Male | 29 (44.6) | 4 (44.4) |
| Female | 36 (55.4) | 5 (55.6) |
| Age, years | ||
| Mean age (±SD) | 39.6 (±10.3) | 43.1 (±9.5) |
| Median age | 40 | 41 |
| IQR 25–75 | 14.5 | 11 |
| MS type, n (%) | ||
| RRMS | 63 (96.9) | |
| SPMS | 2 (3.1) | |
| EDSS, mean (range) | 1.9 (0 - 6.5) | |
| Disease duration, mean (±SD), years | 8.2 (±6.1) | |
| DMTs duration, mean (±SD), months | 47 (±40) | |
| DMTs, n (%) | ||
| Natalizumab | 13 (20.0) | |
| Dimethyl fumarate | 11 (16.9) | |
| Interferon β1-a | 10 (15.4) | |
| Fingolimod | 9 (13.8) | |
| Ocrelizumab | 8 (12.3) | |
| Cladribine | 6 (9.2) | |
| Teriflunomide | 5 (7.7) | |
| Glatiramer acetate | 3 (4.6) |
DMTs: Disease modifying therapy; EDSS: Expanded disability status scale; IQR: Interquartile range; MS: Multiple sclerosis; RRMS: Relapsing remitting multiple sclerosis; SD: Standard deviation;SPMS: Secondary progressive multiple sclerosis.
2.2. SARS-CoV-2 IgG Spike detection
Quantitative determination of antibodies to the SARS-CoV-2 Spike protein was carried out by Roche Elecsys® Anti-SARS-CoV-2 S assay (Roche Diagnostics International Ltd, Rotkreuz, Switzerland). The assay was performed using a recombinant protein representing the RBD of the S antigen leading to a double-antigen sandwich assay complex which favours detection of high affinity antibodies against SARS-CoV-2 (range between 0.4 to 250 U/mL), resulting in a sensitivity of 98.8% (95% CI: 98.1 – 99.3%). Samples above 250 U/mL were automatically diluted into the linear range of the assay with Diluent Universal (Roche Diagnostics, Rotkreuz, Switzerland). The analyzer automatically multiplies diluted results with the dilution factor. Positivity to the test was considered for values > 0.4 U/mL.
2.3. Ethics (Standard protocol approvals, registrations and patient consents)
The study was conducted according the Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki. Investigators obtained ethic committee approval for the study protocol and amendments by the local Ethic Committee of A.O.R.N. A. Cardarelli/Santobono-Pausilipon (protocol number 2821). All subjects gave written informed consent to participate to the study.
2.4. Statistical analysis
Descriptive analyses were presented as mean (± standard error of the mean), median and interquartile range (IQR). Categorial variables were described as frequency and percentage. A Shapiro-wilk test was performed to assess the normal distribution of data. In case of not-normal distribution appropriate non-parametric tests were performed (Wilcoxon test). The geometric mean titre ratio (GMTR) was calculated as the ratio between SARS-CoV-2 IgG titre at 21 days after the 3rd vaccine booster (T3) and at ∼6 months after the second dose (T2). p-value less than 0.05 indicated significance. A multilinear regression model was used to compare the antibody levels after booster dose across subjects treated with different DMTs after adjusting for age, sex, EDSS levels, disease and DMT duration. Data analyses were performed using Graphpad Prism (version 8).
3. Results
3.1. Study cohort
Data were collected from March 2021 to January 2022. In this prospective monocentric study, we excluded subjects previously infected with SARS-CoV-2. After evaluation, 65 pwMS were assessed for anti-Spike IgG levels 21 days after the 3rd SARS-CoV-2 BNT162b2 mRNA vaccine booster (T3) and compared to age and sex-matched HC. Moreover, these measurements were compared to their relative levels at 21 days (T1) and ∼6 months (T2) after the second (2nd) vaccination. The time lag between T2 and T3 was ∼3–4 months. The demographic and clinical characteristics are reported in Table 1. In the MS group, 29 were male (44.6%) and 36 female (55.4%) and the mean age was 39.6 ± 10.3 (mean ± SD) years. In the control group (HC), 4 subjects were males (44.4%) and 5 females (55.6%), with a mean age of 43.1 ± 9.5 (mean ± SD) years. In the MS cohort, 63 had relapsing-remitting (RR) (96.9%) and 2 secondary-progressive (SP) (3.1%) MS. Different types of DMTs were: NAT (n = 13; 20.0%), DMF (n = 11; 16.9%), IFN (n = 10; 15.4%), FTY (n = 9; 13.8%), OCRE (n = 8; 12.3%), CLAD (n = 6; 9.2%), TERI (n = 5; 7.7%), and GA (n = 3; 4.6%).
3.2. Third SARS-CoV-2-mRNA vaccine booster elicits different humoral response in pwMS undergoing distinct DMTs
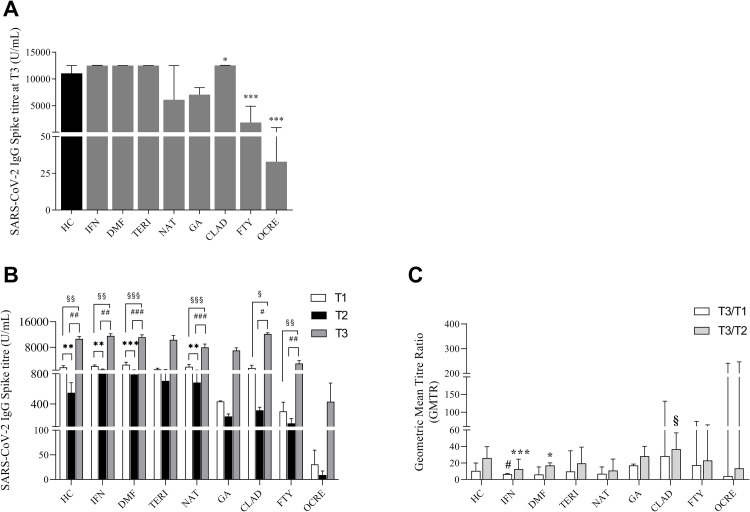
We evaluated SARS-CoV-2 IgG titre 21 days after the 3rd SARS-CoV-2 BNT162b2 mRNA vaccine dose (T3) in our monocentric cohort of pwMS undergoing different DMTs, compared to age and sex-matched HC. In total, 100% of HC and 97% of DMT-treated pwMS were seropositive after the 3rd vaccine booster; however, this is due to the lower percentage of seropositivity in OCRE-pwMS (65% of the subjects). We found that anti-Spike IgG levels in pwMS under treatment with IFN (median 12,500 (IQR: 11,604–12,550) U/mL), DMF (median 12,500 (IQR: 9054–12,501) U/mL) and TERI (median 12,500 (IQR: 6996–12,500) U/mL) were comparable to HC (median 11,048 (IQR: 8606–12,500) U/mL) (Fig. 1A and Table 2 ). On the contrary, humoral response at T3 was reduced in FTY- (median 1866 (IQR: 641.1–4914) U/mL) and almost undetectable in OCRE- (median 32.9 (IQR: 0.4–911) U/mL) treated pwMS, when compared to HC. A reduction was also observed in NAT- (median 6109 (IQR: 4738–12,500) U/mL) and GA- (median 7089 (IQR: 5442–8376) U/mL) treated pwMS, although this did not reach any statistical significance (Fig. 1A and Table 2). Strikingly, when compared to HC, we observed that CLAD-treated pwMS showed a significant increase of anti-Spike IgG levels at T3 (median 12,525 (IQR: 11,946–12,550) U/mL) (Fig. 1A and Table 2). Since pwMS of this monocentric cohort have been followed throughout the entire vaccination process, we performed an overall comparison of the kinetics of their specific humoral response to SARS-CoV-2 2nd and 3rd vaccine dose. More in detail, the serological response to SARS-CoV-2 vaccine was measured by detecting the anti-SARS-CoV-2 IgG level at 3 time-points: 21 days (T1) and ∼6 months (T2) after the 2nd vaccination, and 21 days (T3) after the 3rd vaccine booster. Fig. 1B summarizes the IgG levels at each time-point in vaccinated HC and pwMS under different DMTs. In HC, anti-Spike IgG levels after the 3rd vaccine booster increased 20-fold compared with the T2 (10,618 ± 737.7 vs 548.5 ± 133.2 (mean ± SEM) U/mL) and 6-fold compared with the T1 (10,618 ± 737.7 vs 1684 ± 527.8 (mean ± SEM) U/mL) levels (Fig. 1B). The same trend was also observed in pwMS under INF, DMF, TERI and NAT treatments. Specifically, in IFN-treated pwMS, the IgG levels at T3 increased 12-fold compared with the T2 (11,564 ± 660.1 vs 933.7 ± 173.5 (mean ± SEM) U/mL) and 5-fold compared with the T1 (11,564 ± 660.1 vs 2227 ± 377.9 (mean ± SEM) U/mL) levels (Fig. 1B). In pwMS under DMF treatment, anti-Spike IgG levels were 14-fold augmented at T3 (11,194 ± 690.8 (mean ± SEM) U/mL) compared to T2 (791.7 ± 115.2 (mean ± SEM) U/mL), and 4-fold compared to T1 (2491 ± 792.6 (mean ± SEM) U/mL) levels (Fig. 1B). This behavior was also observed in pwMS under TERI and NAT treatment, where anti-Spike IgG levels at T3 increased respectively 14-fold compared to T2 (10,298 ± 1371 (mean ± SEM) U/mL) vs 706.5 ± 311.4 (mean ± SEM) U/mL) and 10-fold compared to T1 (1061 ± 369.6 (mean ± SEM) U/mL) and 11-fold compared to T2 (7936 ± 1066 (mean ± SEM) U/mL) vs 683.5 ± 141.9 (mean ± SEM) U/mL) and 4-fold compared to T1 (1881 ± 731.1 (mean ± SEM) U/mL). Intriguingly, in GA-treated pwMS, the 3rd vaccine booster increased the anti-spike IgG levels 30-fold (6969 ± 849.1 (mean ± SEM) U/mL) compared with the levels at T2 (234.9 ± 34.48 (mean ± SEM) U/mL) and 16-fold compared to T1 (433 ± 8.4 (mean ± SEM) U/mL) (Fig. 1B). However, the differences observed in TERI- and GA-treated pwMS did not reach the statistical significance. Overall, the 3rd vaccine booster elicited the strongest humoral response in subjects under CLAD treatment either compared to the different DMT-treated pwMS or to HC. Indeed, the increase of IgG levels at T3 (12,156 ± 374.5 (mean ± SEM) U/mL) was 38-fold higher than those at T2 (316.7 ± 41.39 (mean ± SEM) U/mL) and 8-fold higher than T1 levels (1467 ± 896.7 (mean ± SEM) U/mL) (Fig. 1B). Conversely, anti-spike IgG levels after the 3rd vaccine booster were still low in FTY- when compared to HC and to the other groups of DMT-treated pwMS (Fig. 1A); nonetheless, anti-Spike IgG levels at T3 (2865 ± 928 (mean ± SEM) U/mL) increased near 20-times compared to T2 (143.7 ± 63.33 (mean ± SEM) U/mL) and 9-times compared to T1 levels (302.1 ± 122.3 (mean ± SEM) U/mL) (Fig. 1B). Furthermore, despite pwMS under OCRE treatment exhibited increased anti-spike IgG levels at T3, they were still the lowest and inadequate to provide a good and protective humoral response (431±246.2 (mean±SEM) U/mL). Finally, we calculated the geometric mean titre ratio (GMTR), evaluated as the ratio between the geometric mean titre at each time point in the different groups of DMT-treated pwMS, compared to HC. Specifically, we observed that the T3/T2 GMTR in IFN-treated pwMS was increased when compared to the relative T3/T1 value (median 12.64 (IQR: 8.73–24.75) vs (median 6.53 (IQR: 4.24–7.49)) (Fig. 1C). This trend was also maintained in DMF-treated pwMS (median 17.1 (IQR: 11.68–20.24) vs (median 6.12 (IQR: 3.94–15.31)). Moreover, the T3/T1 GMTR in IFN-treated pwMS was reduced when compared to HC (median 6.53 (IQR: 4.24–7.49) vs median 10.49 (IQR: 6.14–20.11)) (Fig. 1C) while CLAD-treated pwMS exhibited an increased T3/T2 GMTR respect to HC (median 36.85 (IQR: 32.3–56.58) vs median 26.12 (IQR: 15.08–39.73)). Finally, the results of the multivariate regression analysis reported in Table 3 suggested that the variables that positively associated to the SARS-CoV-2 IgG antibody levels at T3 were the DMTs used. More in detail, subjects under IFN, DMF and CLAD treatment showed significantly positive association with an increased humoral response among all the variables analysed (Table 3). Our data highlight how DMTs could differentially promote the protective SARS-CoV-2 humoral response to the 3rd vaccine booster in pwMS, notwithstanding the effect it could elicit with the 2nd dose.
Fig. 1.
(A) SARS-CoV-2 IgG Spike titre (median with interquartile range (IQR)) in MS subjects treated with different DMTs, 21 days (T3) after the third dose compared to healthy controls (HC). Mann U- Whitney two-tailed test vs HC subjects was performed and a p-value less than 0.05 was considered statistically significant. * p<0.05; ***p<0.001. (B) SARS-CoV-2 IgG Spike titre kinetic (mean ± SEM) of MS subjects treated with different DMTs at 21 days (T1), ∼6 months (T2) after the second vaccine dose and 21 days (T3) after the third dose compared to HC. Wilcoxon two-tailed test was performed to compare T1, T2 and T3 levels and a p-value less than 0.05 was considered statistically significant. *,§,#p<0.05; **,§§,##p<0.01; ***,§§§,###p<0.005. *T1 vs T2; §T1 vs T3; #T2 vs T3. (C) Geometric Mean Titre Ratio (GMTR) was obtained as the ratio between SARS-CoV-2 IgG Titre at 21 days after the third dose (T3) and at ∼6 months after the second (T2) dose of vaccine and between SARS-CoV-2 IgG Titre at 21 days after the third dose (T3) and 21 days after the second dose (T1) of vaccine. Data are reported as median with IQR. Mann U-Whitney two-tailed test vs HC and Wilcoxon two-tailed test were performed and a p-value less than 0.05 was considered statistically significant. *,§,#p<0.05; ***p<0.005;
*T3/T2 GMTR vs T3/T1 GMTR of pwMS; § T3/T2 GMTR of pwMS vs T3/T2 GMTR of HC; # T3/T1 GMTR of pwMS vs T3/T1 GMTR of HC.
Table 2.
SARS-CoV-2 IgG Spike titre in MS subjects treated with different DMTs 21 days after the third dose of vaccine (T3).
| DMTs | SARS-CoV-2 IgG spike titre (U/mL) | p-value |
|---|---|---|
| Healthy Controls | 11,048 (8606 – 12,500) | |
| Interferon β1-a | 12,500 (11,604 – 12,550) | 0.11 |
| Dimethyl fumarate | 12,500 (9054 – 12,501) | 0.27 |
| Teriflunomide | 12,500 (6996 – 12,500) | 0.99 |
| Natalizumab | 6109 (4738 – 12,500) | 0.17 |
| Glatiramer acetate | 7089 (5442 – 8376) | 0.06 |
| Cladribine | 12,525 (11,946 – 12,550) | 0.04 |
| Fingolimod | 1866 (641.1 – 4914) | 0.0007 |
| Ocrelizumab | 32.9 (0.4 – 911) | 0.0006 |
SARS-CoV-2 IgG Spike titre (median with IQR) in MS subjects treated with different DMTs, 21 days after the third vaccine dose (T3) compared to healthy controls (HC). Mann U-Whitney two-tailed test vs HC subjects was performed and a p-value less than 0.05 was considered statistically significant.
Table 3.
Multivariable analysis assessing factors associated to SARS-CoV-2 IgG antibody levels at 21 days after the third dose (T3).
| Variable | Β coefficient (SE) | CI 95% | p-value |
|---|---|---|---|
| Age | 82.2 (60.2) | −37.3 - 203.8 | 0.17 |
| Sex (male vs female) | 5121.7 (2697.8) | −299.6 - 10,543.2 | 0.06 |
| EDSS | −905.5 (466.3) | −1838 – 27.2 | 0.05 |
| Disease duration (years) | −48.8 (109.5) | −268 – 170.2 | 0.65 |
| DMTs duration (months) | −0.64 (16.3) | −33.4 - 32 | 0.96 |
| DMTs | |||
| Interferon β1-a | 5863.6 (2560) | 720.4 - 11,006.8 | 0.02 |
| Dimethyl Fumarate | 5714.2 (2560) | 571 – 10,857 | 0.03 |
| Teriflunomide | 2362.4 (1361.4) | −1874.8 - 6599.6 | 0.99 |
| Natalizumab | −105.3 (2606) | −5342 – 5132.3 | 0.96 |
| Glatiramer acetate | 2175 (3412.4) | −4682.6 - 9032.6) | 0.52 |
| Cladribine | 6324 (2697.8) | 902.5 - 11,745.4 | 0.02 |
| Fingolimod | −4076.5 (2643.2) | −9388.4 - 1235.3 | 0.12 |
| Ocrelizumab | −5341.6 (2786.2) | −11,030.8 - 167.6 | 0.06 |
Multivariable analysis was conducted to compare the SARS-CoV-2 IgG antibody levels at 21 days after the third dose (T3) adjusted for age, EDSS level, disease duration, DMT duration, sex and different DMTs. A p-value<0.05 was considered statistically significant.
CI: confidence interval; DMTs: Disease modifying therapy; EDSS: Expanded disability status scale; SE: Standard error.
4. Discussion
This study reports the anti-Spike IgG levels after the 3rd SARS-CoV-2 BNT162b2 mRNA vaccine booster, in a monocentric cohort of pwMS under eight different DMTs, all without previous SARS-CoV-2 infection. Our findings suggest that the SARS-CoV-2 specific humoral response in IFN-, DMF- and TERI-treated pwMS was comparable to HC, while significantly increased in CLAD-treated pwMS. Conversely, pwMS under NAT and GA treatment displayed a reduced humoral response to the booster dose, despite the impairment was not statistically significant probably due to small sample size. Furthermore, the anti-Spike IgG levels were significantly reduced in FTY- and OCRE-treated pwMS. The impairment observed in these two groups is in line with recent data from literature on the 3rd SARS-CoV-2 BNT162b2 mRNA vaccine booster (Brill et al., 2022; Madelon et al., 2022; Achtnichts et al., 2022; Achiron et al., 2021; Achiron et al., 2022) and with previous studies on the IgG titre after the 2nd SARS-CoV-2 BNT162b2 mRNA vaccine dose in pwMS under FTY and OCRE treatment (Sormani et al., 2021; Maniscalco et al., 2022; Achiron et al., 2021; Bigaut et al., 2021). Moreover, it has been shown that vaccinated COVID-19 convalescent OCRE-treated pwMS showed a humoral response comparable to healthy subjects, highlighting that SARS-CoV-2 IgG titre in OCRE-treated pwMS could be influenced by a previous SARS-CoV-2 infection (Habek et al., 2022).
It is worth noticing that despite the fact that CLAD-treated pwMS of our cohort failed to mount a good anti-SARS-CoV-2 humoral response to the 2nd SARS-CoV-2 vaccine dose, they fully recovered with the booster dose. Moreover, multivariate regression suggests that, among all the variables analysed, treatment with IFN, DMF and CLAD positively associated with a higher humoral response to the 3rd vaccine dose.
As unique feature of our study, we followed pwMS of our monocentric cohort throughout the entire vaccination course to obtain an overall comparison of the kinetics of the humoral response to both 2nd and 3rd SARS-CoV-2 vaccine doses, in eight different DMTs. Our results show that, when compared with the IgG levels at T2 (∼6 months after the 2nd dose), the booster dose induced a comparable increase of the anti-spike IgG levels in HC, IFN-, DMF- and TERI-treated pwMS (∼15-fold), while their levels were significantly augmented in pwMS under CLAD treatment (∼40-fold). In addition, CLAD-treated pwMS exhibited an increased T3/T2 GMTR respect to HC; this is particularly relevant since underlines the importance of the booster administration in this group of pwMS. We also found that the booster elicited a good rise in the humoral response also in GA- and OCRE-treated pwMS, despite still inadequate in this last group of subjects. Additionally, while detectable anti-spike IgG were found after the 2nd vaccination in the 89% FTY- and 60% of OCRE-treated pwMS subjects of our cohort (Maniscalco et al., 2022), the 3rd dose elicited a seroconversion in the 100% of pwMS under FTY and in the 65% of those under OCRE. Taken together this evidence strongly indicates the importance of the booster dose to enhance SARS-CoV-2 specific immunity, especially in those pwMS that did not develop an adequate humoral response after the first vaccination cycle. The added value of our study is that it depicts the SARS-CoV-2 humoral response in a monocentric cohort of pwMS that has been followed for the entire vaccination cycle (from the 1st to the booster dose). The limitation of our study is represented by the small sample size in some DMT groups; further studies are needed to confirm the effect of the booster dose on humoral response in a larger cohort of pwMS.
In agreement with another report from literature (Achtnichts et al., 2022) we could propose that, in some groups of DMT-treated pwMS, the humoral response may reach protective levels after the 3rd dose; as consequence, additional administrations may be a strategy to overcome the insufficient humoral immune response following the standard vaccination schedule. Several lines of research are proposing to administer a fourth vaccine dose six months after the 3rd vaccination in immunocompromised patients (Naaber et al., 2021). Also, our data further envisage novel strategies to shorten the time frame between the third and fourth vaccination in immunocompromised pwMS.
5. Conclusion
Our findings provide evidence that a third SARS-CoV-2 vaccine dose considerably enhances anti-Spike-specific antibody response especially in low-responder pwMS, highlighting the importance of an additional vaccine dose for those who may have achieved a weak and/or slow immune priming. Nevertheless, we highlight the urgent need to unveil the antibody titres necessary for protection against infection and/or severe disease, to implement serologic testing to maximize the benefit of additional vaccine doses in pwMS.
Funding
This work was supported by FISM 2018/R/4 from Fondazione Italiana Sclerosi Multipla and by Bando PRIN 2017 Prot. 2017K7FSYB from Ministry of Education, University and Research (MIUR) to VDR.
Declaration of Competing Interest
G.T. Maniscalco received personal compensations from Serono, Biogen, Novartis, Roche and TEVA for public speaking and advisory boards. The other authors have nothing to disclose.
Availability of data and material
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All the authors have reviewed the manuscript and have contributed to the work. Study concept and design: GTM, AL, VA, VDR; Acquisition of data: AL, ALF, EP, SS, MEDB, MN, AR, KL, RV, MB; Analysis and interpretation of data: GMT, ALF, AL, OM, DDG, VDR; Drafting of the manuscript: GTM, ALF, AL, VDR; Critical revision of the manuscript for important intellectual content: GTM, VA, VDR; Study supervision: GTM, VA, VDR; All authors read and approved the final manuscript.
Ethics approval
The study was conducted according the Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki. Investigators obtained ethic committee approval for the study protocol and amendments by the local Ethic Committee of A.O.R.N. A. Cardarelli/Santobono-Pausilipon (protocol number 2821).
Consent to participate
All subjects give written informed consent to participate to the study.
Consent for publication
Not applicable.
References
- Achiron A., et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., et al. Humoral SARS-COV-2 IgG decay within 6 months in COVID-19 healthy vaccinees: the need for a booster vaccine dose? Eur. J. Intern Med. 2021;94:105–107. doi: 10.1016/j.ejim.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J. Neurol. 2022;269(5):2286–2292. doi: 10.1007/s00415-022-11030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N., et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtnichts, L., et al., SARS-CoV-2 mRNA Vaccination in People with Multiple Sclerosis Treated with Fingolimod: protective Humoral Immune Responses May Develop after the Preferred Third Shot. Vaccines (Basel), 2022. 10(2). [DOI] [PMC free article] [PubMed]
- Bergwerk M., et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., et al. Impact of disease-modifying treatments on humoral response after COVID-19 vaccination: a mirror of the response after SARS-CoV-2 infection. Rev. Neurol. (Paris) 2021;177(10):1237–1240. doi: 10.1016/j.neurol.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L., et al. Severe Acute Respiratory Syndrome Coronavirus 2 Third Vaccine Immune Response in Multiple Sclerosis Patients Treated with Ocrelizumab. Ann. Neurol. 2022;91(6):796–800. doi: 10.1002/ana.26343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano R., et al. Six-month humoral response to mRNA SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab and fingolimod. Mult. Scler. Relat. Disord. 2022;60 doi: 10.1016/j.msard.2022.103724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D., et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J. Neurol. 2021;268(11):3961–3968. doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collie S., et al. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022;386(5):494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y., et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habek M., et al. Humoral and cellular immunity in convalescent and vaccinated COVID-19 people with multiple sclerosis: effects of disease modifying therapies. Mult. Scler. Relat. Disord. 2022;59 doi: 10.1016/j.msard.2022.103682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.G., et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig Y., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet. Respir. Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco G.T., et al. Interferon Beta-1a treatment promotes SARS-CoV-2 mRNA vaccine response in multiple sclerosis subjects. Mult. Scler. Relat. Disord. 2022;58 doi: 10.1016/j.msard.2021.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco G.T., et al. Long term persistence of SARS-CoV-2 humoral response in multiple sclerosis subjects. Mult. Scler. Relat. Disord. 2022;62 doi: 10.1016/j.msard.2022.103800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelon N., et al. Omicron-Specific Cytotoxic T-Cell Responses After a Third Dose of mRNA COVID-19 Vaccine Among Patients With Multiple Sclerosis Treated With Ocrelizumab. JAMA Neurol. 2022;79(4):399–404. doi: 10.1001/jamaneurol.2022.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaber P., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg. Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser Moghadasi A., et al. Can pulse steroid therapy increase the risk of infection by COVID-19 in patients with multiple sclerosis? Clin. Neurol. Neurosurg. 2021;203 doi: 10.1016/j.clineuro.2021.106563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella C., et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies. Neurology. 2022;98(5):e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.