Abstract
Disseminated tumor cells (DTCs) spread systemically yet distinct patterns of metastasis indicate a range of tissue susceptibility to metastatic colonization. Distinctions between permissive and suppressive tissues are still being elucidated at cellular and molecular levels. Although there is a growing appreciation for the role of the microenvironment in regulating metastatic success, we have a limited understanding of how diverse tissues regulate DTC dormancy, the state of reversible quiescence and subsequent awakening thought to contribute to delayed relapse. Several themes of microenvironmental regulation of dormancy are beginning to emerge, including vascular association, co-option of pre-existing niches, metabolic adaptation, and immune evasion, with tissue-specific nuances. Conversely, DTC awakening is often associated with injury or inflammation-induced activation of the stroma, promoting a proliferative environment with DTCs following suit. We review what is known about tissue-specific regulation of tumor dormancy on a tissue-by-tissue basis, profiling met major metastatic organs including the bone, lung, brain, liver, and lymph node. An aerial view of the barriers to metastatic growth may reveal common targets and dependencies to inform the therapeutic prevention of relapse.
Keywords: disseminated tumor cell dormancy, microenvironment, dormant niche, metastasis, quiescence
Introduction
In his writings on cancer, the Greek physician Celsus (25 BC-50 AD) remarked on patients who, “…attain to a ripe old age in spite of it. No one, however, except by time and experiment, can have the skill to distinguish a cacoethes which admits of being treated from a carcinoma which does not” [1]. Even today, with a plethora of advanced technology and medical knowledge, this statement rings true; a cancer diagnosis may no longer be a death sentence, but distinguishing between a cancer that requires vigilant management from one that will no longer pose a problem remains a distinct challenge.
In the millennia following Celsus, massive progress has been made in the areas of cancer prevention, prognosis, and treatment; yet metastasis continues to be a significant setback in patient health, with few treatments aimed at its specific nuances and presentation, A female patient who presents with a tumor in the breast has a 99% 5-year relative survival rate, which drops to 27% if she has distant metastases at that time (SEER database, 2010–2015, National Cancer Institute). The outlook worsens if the tumor is of the treatment-refractory triple-negative subtype, with a 5-year survival rate of 91% for localized disease, which drops to 11% with distant metastases. Analyses of matched primary and metastatic samples demonstrate that metastases share genomic alterations and may switch subtype when compared to the primary tumor [2–4]. The evolution of metastases correlates with time to detection, therapeutic selection, and tissue-specific alterations [5–10]. It would not be going too far to state that metastatic disease should be assessed independently from the tumor of origin.
Tumor dormancy is a piece of the metastatic puzzle yet to be fully understood. Although it has never been observed directly in humans that metastases arise from dormant DTCs, dissemination occurs early in tumor progression [11–14], and experimental models have demonstrated that single, quiescent DTCs initiate metastasis [15–19]. In models of breast cancer, DTCs can survive for long periods of time (at least 240 days for D2.0R mammary carcinoma cells [16]) as quiescent cells in organs like the lung and bone marrow; after isolation from the tissue, formerly quiescent DTCs including M4A4 and NM2C5 (clonally derived from the MDA-MB-435 breast cancer pleural effusion cell line) have been shown to resume proliferation and expand in culture, demonstrating that at least a portion of DTCs retain the capacity for proliferation [20–22]. These key data demonstrate convincingly that tumor cells capable of growth in idealized conditions (cell culture, the orthotopic site) are constrained at some secondary locations. The nature of these restrictive mechanisms may be cell-intrinsic, as acquisition of specific mutations may be required for colonizing a new landscape [13, 23]; extrinsic, via suppressive factors or lack of proliferative signals provided by the niche that limit DTC colonization; or a combination of the two, in which niche-derived signals differentially effect early versus late DTCs, reflecting genetic changes acquired by DTCs over time [24].
From a clinical perspective, the dormancy phase between treatment and metastatic emergence is a lull in disease progression that holds a measure of relief and a promise of reprieve. It may last a year, decades, or a lifetime. For the significant proportion of patients who experience delayed relapse, this is a golden window of opportunity to therapeutically intervene against future metastases. But first, we must capitalize on a wealth of historical observation and modern experimental data describing the biological and anatomical restrictions on tumor growth.
Rupert Willis formally introduced the concept of “tumor dormancy” in 1934 to account for seemingly inert tumor cells observed in autopsied tissues [25]. He was not the first to document these ectopic cells that seemed to contradict cancer’s defining quality of voracious and unbridled growth. The growing consensus among clinicians was that, upon careful examination only possible during autopsies, tumor cells could be found throughout most tissues and in far greater numbers than metastases [26]. Ernst Fuchs’ idea of tissue predisposition when describing uveal melanoma metastasis [27] and Stephen Paget’s seed and soil hypothesis [26] helped frame in broad terms that certain tissues are more amenable to metastasis than others depending on cancer type; yet, the observation that many tissues contain isolated tumor cells, hereafter referred to as disseminated tumor cells (DTCs), argued that colonization, rather than dissemination, is the limiting factor [26]. Twenty years after Willis, Geoffrey Hadfield noted in 1954 that, “The anatomical site of these minute quiescent growths must be significant, for in at least 50% of cases of malignant disease recurring after latent periods…was found in the hemopoietic bone marrow” [28]. This is one of many pieces of evidence that metastases display a tissue preference [29]. But what makes a tissue fertile or infertile soil? Can an infertile soil become more amenable to growth, either through changes to the soil or within the seed itself? And, in a therapeutic context, can a permissive soil be made more hostile to DTCs? These questions have significant impact on our understanding of tumor biology and how we approach this potent stage of disease.
Tumor dormancy: A parable of the microenvironment
When considering the suitability of tissue for metastatic colonization, an agricultural analogy beyond Paget’s “seed and soil” may be apt:
“‘A sower went out to sow his seed; and as he sowed, some fell on the path and was trampled on, and the birds of the air ate it up. Some f n on the rock; and as it grew up, it withered for lack of moisture. Some fell among thorns, and the thorns grew with it and choked it. Some fell into good soil, and when it grew, it produced a hundredfold.’” (Luke 8:5–8, NRSV)
Predators, insufficient nutrients, impediments to growth: these factors antagonize rampant DTC proliferation. DTCs are killed through direct elimination (immune editing) or fail to grow due to the absence of a hospitable niche and/or presence of anti-growth “morphostats” [30]. This review focuses on external tissue-specific factors and properties that impede metastasis through induction of cellular dormancy, defined as exiting the cell cycle into G0 phase (quiescence) or extremely slow cell division, and the conditions that break down these barriers to allow metastatic growth. Although each tissue is distinct, several themes emerge from an aerial view that provide insight into major factors that regulate DTC dormancy and its loss, providing clues for future studies.
1. Bone
1.1. Metastasis to the bone
“The evidence seems to me irresistible that in cancer of the breast the bones suffer in a special way…” –Stephen Paget, 1889 [26]
The bones are the third most common site of all solid tumor metastasis after liver and lung [31], reflected in the high rate of incidence of bone metastasis at diagnosis [32] and 10-year follow up [33]. A meta-analysis of SEER bone metastasis data found that 88.4% of metastatic prostate cancer patients have detectable bone metastases at diagnosis, followed by breast (53.71%), renal (38.65%), and lung adenocarcinoma (36.86%) [32]. Bone metastasis results in the painful destruction of hard bone and nerves, soft tissue inflammation, risk of fracture, and debilitation of the hematopoietic stem cell pool.
Tumor cell dissemination to the bone occurs frequently, as marrow aspirates reveal that 15.0–30.6% of breast cancer patients [34–37], and 57% of prostate cancer patients [38] who show no other evidence of disease harbor bone DTCs or micrometastases, and these correlate with worsened outcomes. The prevalence of DTCs in the bone has been attributed to wo factors: 1) the low blood pressure system of the sinusoidal beds, which aids in DTC extravasation [39]; and 2) DTC homing to the bone marrow via chemoattractant gradients including cXCL12 (SDF-1) via CXCR4 [40, 41]. The presence of DTCs in the bone predicts late relapse at that site and elsewhere [34, 35, 37, 42–44]; however, the discrepancy between high incidence of bone DTCs and relatively few detectable metastases in clinical observation and pre-clinical models [19] suggests growth-restrictive mechanisms in this niche.
1.2. The bone microenvironment
The bone is composed of hard, cancellous bone matrix surrounding an inner medullary cavity filled with vascularized fatty marrow. The inner surface of the hard bone, the endosteum, is a dynamic surface for cell interactions. It undergoes cycles of bone absorption and remodeling mediated by osteoclasts and osteoblasts, respectively, at punctuated sites across an otherwise homeostatic landscape [45, 46]. Arteries pass through the trabecular bone to the central cavity, emerging from the endosteum as arterioles. The endosteum therefore interfaces closely with arterioles, and associated cells including nestin promoter (Nes)+ neural/glia antigen 2 (NG2)+ αSMA+ mesenchymal stem cells (MSCs) [47], sympathetic neurons and associated non-myelinating Schwann cells [48], adipocytes, and bone-specific immune cells [49–51]. Arterioles extend into the central cavity where they transition into venous sinusoids which serve as the route of egress for mobilized bone marrow cells (Fig. 1). Sinusoidal vessels form a distinct vascular niche that includes immune cells and several lineages of mesenchymal-derived cells bearing overlapping markers: CXCL12-abundant reticular (CAR) cells [52, 53], leptin receptor (LepR)+ cells, Nes+ cells, and NG2+ cells, collectively referred to here as MSCs (reviewed extensively in [54]) (Fig. 1).
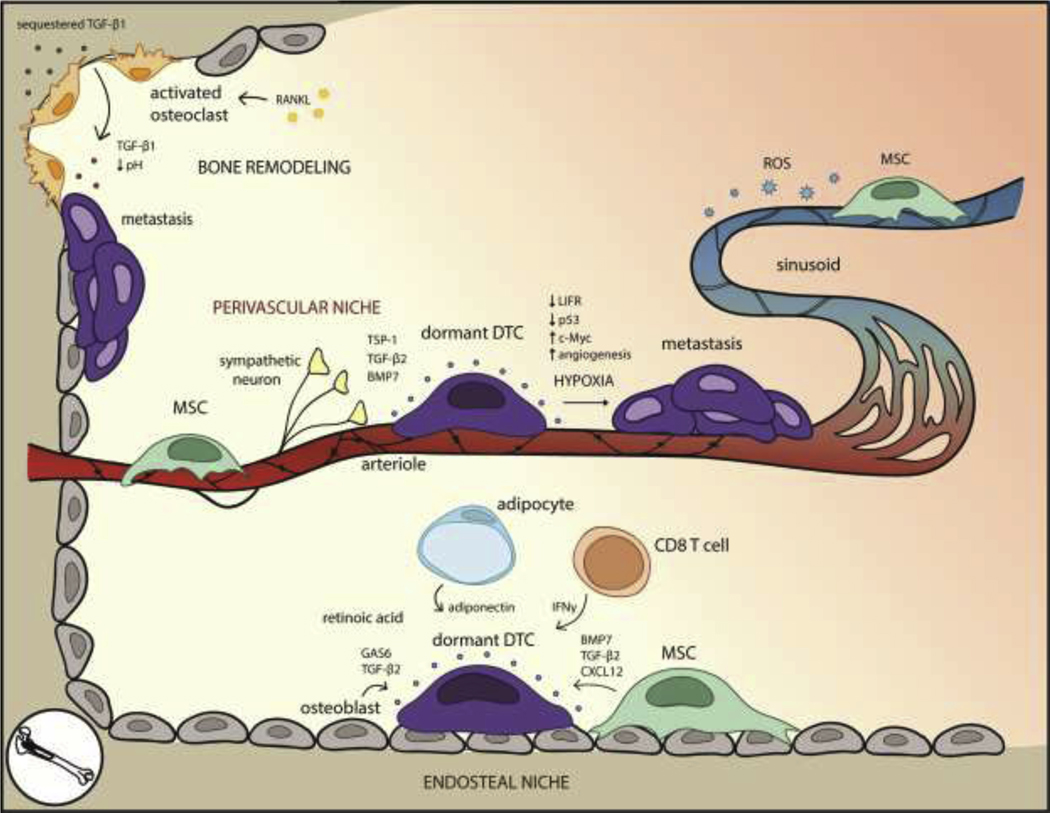
Figure 1. The bone microenvironment for dormant DTCs is well-defined due to established mechanisms that regulate HSC quiescence and bone resorption.
Within the endosteal niche, MSCs, osteoblasts, adipocytes, and CD8+ T cells secrete factors that preserve DTC quiescence. High levels of retinoic acid within the bone marrow induce cell cycle arrest. Awakening can occur as the result of environmental changes brought on by RANKL-activated osteoclasts and bone resorption. In the perivascular niche, arterioles and perivascular cells promote DTC quiescence through basement membrane factors such as TSP-1, although hypoxic regions near the vessels promote angiogenesis, which correlates with metastasis. The leakier sinusoids, by contrast, experience ROS on the basal side of the vessel, possibly inducing DNA damage and stress pathways associated with metastasis.
Another critical resident of the bone marrow is the hematopoietic stem cell (HSC), long-lived and reversibly quiescent stem cells that populate the blood lineages. The HSC niche is powerfully quiescent, and HSCs can be found in endosteal [55–59], arteriolar [60], and sinusoidal [59, 61, 62] compartments under the influence of osteoblastic cells, MSCs, neurons, and endothelia. The HSC capacity for reversible quiescence while maintaining stemness is remarkably similar to properties of dormant DTCs, and therefore many studies have investigated parallel mechanisms of regulation (reviewed in [63]).
1.3. Mechanisms of DTC dormancy in the bone
Animal models show that the endosteal niche [19, 40] and the perivascular niche [61, 64–66] harbor myeloma, prostate, and breast cancer DTCs among others, and identifying the specific interactions that enable and regulate tumor dormancy is the next critical step. There is substantial overlap between the endosteal niche and endothelial niche considering that the endosteum interfaces with arterioles (reportedly, 90% of osteoblasts are within 20 μm of a blood vessel [67]), but unique features of each niche drive distinct quiescence programs.
The endosteal niche
The endosteum is a primary niche for HSCs and can be co-opted by DTCs. Osteoblasts secrete pro-quiescence factors like osteopontin [68, 69] and growth arrest specific-6 (GAS6), which regulates cell division through binding specific TAM (Tyro3/Sk, Axl, Mer)-family receptor kinases: Tyro3 induces cell division while Axl promotes quiescence via ERK1/2 signaling, survival, and chemoresistance [70, 71]. Notably, slow-cycling prostate cancer cells [71] and quiescent multiple myeloma cells [19] in the bone express high levels of Axl. Prostate cancer DTCs that bind to osteoblasts [72] or osteoclasts [70] upregulate Axl, which is then stabilized by the hypoxic environment of the endosteal niche [73]. Despite its anti-proliferative effects, Axl cannot maintain long-term dormancy on its own, indicating that it collaborates with other quiescence drivers [74].
Osteoblasts also secrete transforming growth factor beta (TGF-β) family members, which suppress tumor progression at early stages through activation of cyclin-dependent kinase (CDK) inhibitors [75–77] (reviewed in the tum or dormancy context in [78]). TGF-β1 TGF-β1 is a key component of the vicious cycle of bone metastasis [79]; conversely, TGF-β2 limits DTC growth. Osteoblast-derived TGF-β2 induced quiescence in prostate cancer DTCs through TGF-β receptor RI (TβRI) and TβRIII [80], similar to its effect on head and neck squamous cell carcinoma DTCs through p38α/β, p27, and DEC2 upregulation and CDK4 downregulation [18]. Prostate cancer DTCs themselves express elevated levels of TGF-β2 mRNA [81], which can be induced by Axl-GAS6 binding [72]. Endosteal NG2+/Nestin+ MSCs produce TGF-β2 and BMP7, which promote breast and head and neck squamous cell carcinoma (HNSCC) DTC dormancy in bone [82]. Estrogen-receptor positive breast cancer patients with high levels of TGF-β2 and BMP7 in the bone marrow experience decreased risk of systemic recurrence and prolonged metastasis-free survival [82]. These studies collectively support the quiescence-promoting role of bone marrow TGF-β2 signaling and suggest therapeutic avenues to restrict metastatic growth.
In addition to TGF-β2, MSC-derived BMP7 promotes a quiescent epithelial phenotype for prostate cancer DTCs [83] through a Smad-independent pathway involving p38, p21, p27, and NDRG1 [84]. Intriguingly, MSC BMP7 can be induced by SPARC secreted by indolent prostate cancer DTCs, revealing that DTCs actively elicit support from their microenvironment [85]. MSCs also secrete CXCL12, which promotes quiescence of chronic myeloid leukemia stem cells [86], on top of its well-studied roles in bone marrow trafficking and HSC maintenance [87]. MSC-derived exosomes containing microRNAs like miRNA-23b [88] and miRNA-222/223 [89] induced quiescence and chemoresistance in breast cancer cells in culture and in vivo. The breadth of mechanisms through which MSCs interact with DTCs underscores their diverse functions as master regulators and maintainers of homeostasis within the bone microenvironment (Fig. 1).
Bidirectional interactions between DTCs and their niche have been identified in myeloma [19], breast [90], and prostate cancer [72, 85]. Breast cancer DTCs bias osteoblasts through direct contact to decrease secretions of IL-6, MMP3, and type I collagen [90], molecules shown to elicit proliferation [91, 92]. Direct co-culture of tumor-experienced osteoblasts and breast cancer cells results in increased p21 in the cancer cells, indicating that DTC-associated osteoblasts induce DTC cell cycle arrest through direct signaling and altered ECM deposition [90].
Acellular factors like oxygen tension also affect DTC behavior. Despite high vascularization throughout the bone marrow, hypoxic regions along the endosteum support HSC stemness by minimizing DNA damage from free radicals [93–95]. However, hypoxia may also promote escape from dormancy by inducing angiogenesis [66] and reposing leukemia inhibitory factor receptor (LIFR) in a hypoxia-induced factor (HIF)-independent manner [93]. LIF ligand is an IL-6-family cytokine that promotes proliferation in primary tumors [96] but inhibits DTC proliferation in the bone [93]. LIFR knockdown in different breast cancer cell lines correlated with decreased expression of quiescence and sternness genes including thrombospondin-1 (TSP-1), TGF-β2, and p53, as well as increased expression of proliferative genes c-Myc and pSrc, suggesting that LIFR is an environmentally-tuned gatekeeper for dormancy [93]. The seemingly contradictory role of hypoxia in the stem cell versus DTC niches reflects a nuanced balance between protection from free radical damage versus hypoxic stress.
The perivascular niche
In bone marrow, the perivascular niche refers to the microenvironment in and around arterioles concentrated at the endosteum and the sinusoids located in the central bone marrow cavity [97]. Arterioles are tight, VE-cadherinhi vessels supported by NG2+ MSCs, sympathetic nerves, and associated non-myelinating Schwann cells, whereas sinusoids are fenestrated, leaky vessels supported by CaR+ LepR+ MSCs. There is evidence that both niches support HSCs: quiescent HSCs depart from arterioles when activated by radiation injury [98] and in a mouse model of overactive HSCs [60]; in contrast, up to 85% of HSCs may be perisinusoidal [59, 61]. Differences in vessel permeability regulate HSC differentiation via ROS, with ROSlow arterioles maintaining HSC stemness and ROShigh sinusoids promoting HSC activation [99]. In the tumor context, spontaneously dormant DTCs preferentially reside in the perisinusoidal niche in the calvarium, whereas micrometastases are only detected in non-sinusoidal, non-perivascular regions [65]. Although these studies do not resolve the discrepancy between the arteriolar and sinusoidal niches, they provide strong evidence that the perivascular niche is critical for maintaining DTC dormancy.
Endothelia comprising blood vessels are the first residents of a tissue to interact with extravasating DTCs. Mouse models show that quiescent MDA-MB-231 breast cancer DTCs preferentially reside on the basal side of bone marrow vasculature [66] where they are exposed to quiescence factors including TSP-1, TGF-β2, and BMP7 [82, 84, 100]. The perivascular niche chemoprotects DTCs through integrin signaling, in a manner distinct from quiescence regulation [101]. Doxorubicin treatment selected for perivascular DTC survival, and knocking down integrins β1 or αvβ3 in tumor cells or their ligands (VCAM-1 or von Willebrand Factor, respectively) in an organotypic co-culture model of the bone marrow ablated the chemoprotective effect without altering proliferation. Inhibiting these integrins resulted in substantially fewer bone metastases (but was not totally preventative), providing a strategy to disrupt this protection [101].
Other bone marrow niche components that regulate tumor dormancy
Neural components of the bone marrow regulate HSC quiescence and may regulate DTCs in parallel, including pro-quiescent sympathetic nerve fibers and nonmyelinating Schwann cells [48, 60, 102]. Conversely, norepinephrine induced prostate DTC proliferation as well as downregulated the pro-quiescent factor GAS6 [103]. Chronic stress act’ vat's the sympathetic nervous system to induce RANKL signaling in osteoblasts, promoting breast cancer migration to the bone [104]. These studies highlight the neural response to stress that can, in turn, affect metastasis.
Adipocytes fill the spongy marrow of the central cavity, stratified into adipocyte-rich yellow marrow and adipocyte-poor red marrow. Red marrow is enriched in HSC activity, whereas yellow marrow suppresses hematopoiesis through pro-quiescent cytokines like adiponectin [105, 106], leading to the question of how adipocytes influence DTCs. Adipocyte-rich environments inhibited T-cell acute lymphoblastic leukemia (T-ALL) proliferation, resulting in slow-cycling, metabolically-altered, chemoresistant DTCs [107], and chemoprotected multiple myeloma cells via induction of anti-apoptotic autophagy [108]. Future studies should assess the effect of adipocytes on solid tumor DTCs.
Acellular environmental facers also play prominent roles in the bone marrow dormancy niche. Fibrillar fibronectin associated with urokinase plasminogen activator receptor suppresses p38 and de-represses ERK1/2 in the human squamous cell carcinoma HEp3 line [109]. The result is akin to simultaneously accelerating and lifting the breaks off dormant DTC growth. Environmental retinoic acid (RA) maintains HSC stemness [110], so it is no surprise that RA promotes DTC survival and cell cycle arrest through the orphan receptor NR2F1 and transcription factor NANOG [111]. This mechanism is not bone marrow-specific, as RA promotes quiescence in other tissues like the lung [111].
Immune regulation of bone dormancy
The bone marrow is a critical immune organ, organizing an immune cell network and coordinating B cell production [112]. DTC+ bone marrow from breast cancer patients is depleted in CD3+ T cells, yet enriched for tumor antigen-specific T cells [113]. The persistence of DTCs in such an environment suggests immune evasion, possibly through downregulation of MHCI [114] or regulatory T cell (Treg)-induced immune-suppression [115]. Interferon gamma (IFNγ) treatment may enhance DTC antigen presentation, boosting endogenous immune activity [116]. IFNγ also directly induced DTC dormancy through antiproliferative STAT1 signaling [117] and cyclin suppression [118], mirroring CD8+ T cell IFNγ induction of B cell leukemia cell cycle arrest [119]. IFN response genes were lost in proliferating bone-derived prostate cancer DTCs compared to those derived from the lung, and suppressing Type I IFN signals activated dormant RM1 prostate cancer DTCs to generate metastases in the bone [120]. Collectively, these data point to IFN signaling as an immune-derived mediator of dormancy in bone marrow. Perivascular MSCs alter the immune landscape for DTCs by inhibiting CD4+ and CD8+ T cell proliferation [121] and enhancing Treg and Th2 CD4+ T cell proliferation [122], generating an immunosuppressive environment. CD150hiTregs also play a non-immunological role in promoting HSC quiescence and engraftment via the production of extracellular adenosine, which protects DTCs from oxidative stress [123]; this protective role may be relevant to bone marrow DTCs as well.
1.4. Activation of quiescent DTCs in the bone
Activation of quiescent DTCs can be attributed to loss of the quiescent niche. Physiologic bone remodeling provides an illustrative example. The endosteum experiences local upheaval where bone is resorbed and remodeled. Osteoclast-driven resorption sites are rare, representing 1% of the endosteum, while osteoblast-driven remodeling of the tone occurs more frequently, across approximately 20% of the endosteum at a given time [45, 46, 124]. During remodeling, RANKL-activated osteoclasts secrete proteolytic enzymes that dissolve the bone matrix, releasing sequestered growth factors such as TGF-β1 (Fig. 1). These enzymes also cleave endosteal factors that maintain HSC and/or DTC quiescence including SCF, CXCL12, and osteopontin [125]. Decreasing oxygen levels and proton release result in an acidified, hypoxic environment [126] that can induce DTC proliferation directly or through angiogenesis [66, 93]. The capillary network directly above remodeling sites increases in density, enhancing trafficking of stromal cells and potentially DTCs as well as undergoing angiogenesis [124]. Endothelial sprouting induces proliferation of dormant MDA-MB-231 breast cancer DTCs through loss of TSP-1 and increased TGF-β1 and periostin (POSTN) in the niche [66]. This is supported by the observation that perivascular cells around remodeling endosteal capillaries increase proliferation [124], alluding to consequences for dormant perivascular DTCs.
The dynamic resorption environment directly activates quiescent myeloma DTCs [19], although the relatively low incidence of remodeling sites may explain the disparity between the frequency of bone DTCs and actual colonization and lesion formation. Activating osteoclasts led to fewer DTC niches and non-dividing myeloma DTCs, but curiously did not correlate with increased overall tumor burden [19]. A two-hit strategy of disturbing the quiescent niche and blocking the immune response would help parse out the source of DTC elimination. Increasing osteoclast activity and recruitment through enhanced VCAM-1 expression was also sufficient to induce dormant mammary DTC activation [127], supporting the role of osteoclasts in dormancy reversal.
The bone marrow is susceptible to changes due to fluctuating hormones during menopause, which is especially relevant for ER+ breast cancer DTCs. In a modified bone marrow microvascular niche model testing several breast cancer cell lines including MCF7, BT474, MDA-MB-361, and MDA-MB-231, loss of estrogen induced angiopoietin-2 expression by the bone marrow niche, antagonizing endothelial receptor Tie2 and TSP-1 signaling and leading to ER+ breast cancer cell growth [128]. Estrogen also negatively regulates IL-6 levels, which drive early breast cancer DTC progression [24]. During menopause, estrogen decreases and serum levels of IL-6 increase up to 10-fold [129–131], which may activate quiescent DTCs in postmenopausal women. Hormone replacement therapy counters rising IL-6 levels [129], potentially slowing DTC progression. Given the effects of estrogen loss, menopause may be a key point to anticipate relapse of residual bone marrow disease.
1.5. Therapeutic outlook
Adjuvant therapies against bone metastases have been successful particularly in breast (50% response) and prostate (70–80% response) cancer [132], but the treatment of dormant DTCs in bone is largely unaddressed. G-CSF mobilizes prostate cancer DTCs from supportive niches [133] and may chemosensitize DTCs when used prior to treatment; conversely, quiescence could be enforced by antagonizing bone resorption. Treatments such as the bisphosphonate zoledronic acid and anti-sclerostin antibodies counter bone resorption by killing osteoclasts [134, 135]; such an approach would preserve quiescent niches to maintain DTC dormancy. However, pharmacological inhibition of niche remodeling was behind the development of anti-RANKL therapies, targeting a pivotal signaling node associated with osteolytic bone degradation and metastasis [136]. Although anti-RANKL treatment benefitted lung cancer patients [137] and resulted in fewer fractures for postmenopausal breast cancer patients [138], it did not extend disease-free or bone metastasis-free survival in a large five-year breast cancer clinical trial [139]. Therefore, more specific approaches to target the DTC niche and deprive DTCs of pro-survival cues are urgently needed.
2. Lung
2.1. Metastasis to the lung
Breast and colorectal cancer have the highest incidence of metastasis to the lung. In a SEER meta-analysis, 36.4% of metastatic breast cancer patients [140] and 32.9% of metastatic colorectal patients [141] develop lung metastasis, which autopsies indicate could be as high as 71% of metastatic breast cancer patients [142]. Although only 3.6% of metastatic prostate cancer have pulmonary metastasis [143], hormone therapies may enhance this rate through transdifferentiation of the tumor into the androgen therapy resistant neuroendocrine subtype [144, 145], which is more likely to generate visceral metastases [146, 147].
Despite the frequency of lung metastasis, isolated lung metastases without a secondary metastatic site are rare across cancers [148, 149], suggesting that lung metastasis is driven by accessibility and permissiveness rather than site specificity. Nevertheless, the lungs are frequently studied in tumor dormancy, with ECM organization and immune influx as major contributors to dormancy regulation.
2.2. The lung microenvironment
Human lungs are comprised of five lobes, three on the right and two on the left side of the thoracic cavity. Each lobe contains branching units of alveoli that facilitate gas exchange with the adjacent capillary network. The small capillary diameter may slow circulating tumor cells to aid extravasation. The alveoli are lined by epithelial sheets line and surrounded by stroma including pericytes, fibroblasts, and mesenchymal stem cells (Fig. 2). These cells contribute to the epithelial and endothelial basement membrane of the lungs, provide contractile support, maintain the vasculature, and attract migrating immune cells [150]. The ECM of the lung is primarily composed of collagens, laminin, elastin, and glycosaminoglycans, which contribute to alveolar elasticity [151, 152]. Lung metastases tend to be concentrated in the basal and peripheral regions of the lobes due to their increased vascular density and, presumably, coincident frequency of DTC extravasation [153].
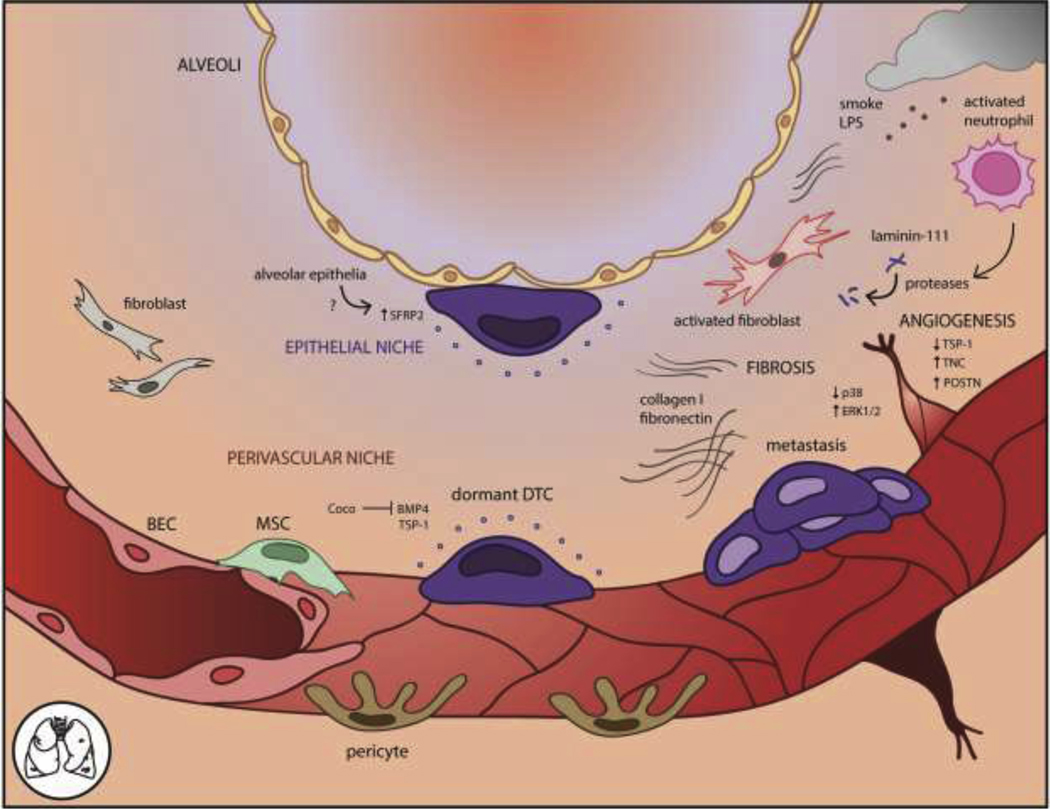
Figure 2. The lung microenvironment for dormant DTCs includes the perivascular and epithelial niches.
In the perivascular niche, deposited basement membrane components like TSP-1 induce cell cycle arrest. The lungs are rich in BMP signaling and BMP4 specifically promotes DTC quiescence, which is antagonized by the BMP inhibitor Coco. The epithelial niche induces SFRP2 in DTCs through an unknown factor. Environmental insults such as tobacco smoke or bacterial LPS elicit inflammation, leading to pro-metastatic angiogenesis, fibrosis, and ECM alterations such as cleavage of laminin-111 by activated neutrophil proteases. These changes are associated with metastatic outgrowth of dormant DTCs.
2.3. Mechanisms of DTC dormancy in the lung
The lungs are especially prone to metastasis, so it is of great interest to understand tumor progression at this site. Serially passaging breast cancer cells through the lungs generated a lung metastasis signature of 54 genes associated with relapse including chemokines, ECM remodeling proteins, cell adhesion, and transcriptions factors [154]. VCAM-1 was enriched on a lung-metastatic subline of MDA-MB-231 mammary tumor DTCs, enabling newly extravasated DTCs to survive by binding macrophage integrin α4 and activating the PI3K/Akt survival pathway [155]. VCAM-1 expression may be a selection force for pulmonary DTCs since the lungs are leukocyte-rich.
BMP signaling, implicated in bone dormancy [84], is even more active in the lung [156]. BMP4 produced by lung stroma suppressed 4T07 mammary tumor cell proliferation through inhibition of stem cell genes Nanog, Sox2, an Oct4 and induction of metastasis suppressors KIAA1199 and NDRG1 [156]. BMPs :s antagonized by the secreted protein Coco. Knocking down Coco imposed dormancy on highly metastatic 4T1 mammary tumor cells in the lung, but did not prevent metastases to brain, bone, or thyroid. Thus, BMP4 regulates lung-specific dormancy in opposition to Coco.
In lung, the perivascular niche is again implicated in promoting DTC dormancy through endothelial TSP-1, where it is also involved in vessel stability [157] and differentiation of alveolar epithelial cells 45e]. Spontaneous and experimental models of lung metastasis implicated endothelial TSP-1 as a quiescence factor for murine MDA-MB-231 and human T4–2 malignant breast epithelial cells [66].
The lung epithelia form a newly described DTC niche and stimulate D2.0R mammary carcinoma expression of SFRP2, which mediates integrin and survival pathways in a Src-dependent manner [159] (Fig. 2). SFRP2+ DTCs deposit fibrillar fibronectin and form cell protrusions to engage with the matrix, coinciding with increased lung metastasis. Although the epithelial signal that induces tumor cell SFRP2 was not identified, these results highlight integrin-ECM interactions as key mediators of lung DTC survival. Fibrillar fibronectin and rho kinase have also been tied to quiescence in a cell culture model of breast cancer, strengthening the association between matrix organization and dormancy [160].
Immune regulation of lung dormancy
Studies suggest that lung DTCs are constrained by the adaptive immune system [161, 162], and slow-cycling breast and lung carcinoma cells derived from patient tumors downregulate ligands for natural killer group 2 member D (NKG2D) receptors to evade NK cell detection [163]. CD8+ T cell depletion allowed emergence of previously undetectable DTCs in the lungs in fibrosarcoma [164], melanoma [162], and mammary carcinoma [165, 166] experimental and transgenic tumor models, while CD4+ T cell depletion only partially suppressed fibrosarcoma metastasis [164]. Intriguingly, suppression of melanoma and mammary carcinoma metastasis was tied to a cytostatic effect of CD8+ T cells [162, 165]. Metastatic outgrowth could be phenocopied by adoptive transfer of pro-tumorigenic MDSCs, which suppress CD8+ T cell activity [166]. While it is unclear whether CD8+ T cells directly suppress cell cycle engagement or select for non-proliferating cells through immune editing, these studies collectively demonstrate how adaptive immunity actively suppresses DTC awakening in the lung.
2.4. Activation of quiescent DTCs in the lung
As an air-tissue interface, the lungs are on the frontlines of environmental and pathological insult. Inflammation awakens dormant mammary carcinoma D2.0R lung DTCs by directly influencing the DTCs as well as modulating the stroma and immune milieu, indicating that therapeutically targeting inflammation may be particularly effective in this tissue [15].
The lung ECM is dominated by type I and II collagens, with additional type III collagen and elastin in the alveolar interstitium [151, 152]. The ECM organization becomes altered through environmental insult. Lung fibrosis dominated by collagen I (col-1) enabled integrin β1-mediated outgrowth of dormant D2.0R cells through phosphorylation of Src, FAK, myosin light chain kinase, and actin stress fiber formation [91, 167]. Fibrillar col-1 also promoted metastatic colonization through binding discoidin domain receptor 1 (DDR1) on the tumor cell membrane, subsequently activating PKC/JAK2/STAT3 signaling via syntenin 2 and tetraspanin TM4SF1 [92]. Fibrillar fibronectin led. to dormancy awakening of SCC HEp3 cells through independent but synergistic inhibition of cell cycle repressor p38 and de-repression of ERK1/2 [109]. While lung DTCs were not as dormant as those in the bone marrow, p38 was required for short-term SCC [109] and colon cancer dormancy in the lung [168]. Concordantly, MMP-2 degradation of a fibrillar fibronectin matrix led to outgrowth of several human breast cancer cell lines in culture [160], further pointing to the importance of ECM architecture to suppress DTC outgrowth.
As the mechanisms underlying the fibrotic awakening response are better understood, it is critical to identify the triggers of fibrosis. Chronic inflammation is a well-known inducer of systemic stress, which can lead to fibrosis. But recently, it was shown that even short, sustained inflammation from either bacterial lipopolysaccharide (LPS) or cigarette smoke can modulate a suppressive niche into a growth permissive one via the immune system. Brief exposure to LPS or cigarette smoke activated release of neutrophil extracellular traps (NETs) and proteolytic enzymes that cleave laminin-111, liberating a peptide that activates dormant D2.0R DTCs, and quiescence factor TSP-1, thus permitting DTC outgrowth [16, 169] (Fig. 2). Fibrosis may also be induced after surgery through lysyl oxidase (LOX)-mediated collagen crosslinking, which allows metastatic outgrowth of undetectable DTCs in lung [170]. Surgery plays a secondary role in activating T cell-dependent lung metastasis [15]. The effect could be abrogated with anti-inflammatory treatment, linking inflammation to adaptive immunity and tumor progression. This phenomenon appears to hold up in patients [171], offering an inexpensive intervention to counter inflammation and prevent DTC emergence.
Inflammation directly induces epithelial-to-mesenchymal (EMT) transition of dormant DTCs from quiescent, differentiated epithelia to an invasive, dedifferentiated mesenchymal phenotype. Different environmental stressors converge on an EMT response, imbuing dormant DTCs with metastatic capability. LPS induces EMT in a latent subline of mammary carcinoma D2A1 cells through Zeb1 expression, greatly enhancing metastasis in the lung [17]. Hypoxia also induces EMT of DTCs through upregulation of lysyl oxidase-like 2 (LOXL2) [172]. Canonically a crosslinking enzyme [173], LOXL2 initiates EMT and a stem cell-like phenotype in dormant mammary DTCs, resulting in lung metastasis [172].
Finally, inflammation has been linked to dormancy awakening through angiogenesis and concomitant loss of perivascular quiescence factors and gain of pro-metastatic factors like tenascin-C (TNC) and POSTN [66, 174]. Adult angiogenesis occurs during wound healing, hypoxia, or under oxidative stress, which is especially relevant to the lungs. Notably, TNC secreted by lung DTCs themselves promotes survival and invasiveness through Notch/Wnt signaling [175]. DTCs within the lung also instruct the stroma to produce POSTN, which further promotes metastasis [176]. Thus, under inflammatory conditions, dormant DTCs in the lung receive autocrine and paracrine growth cues, ultimately leading to metastatic awakening.
3. Brain
3.1. Metastasis to the brain
Brain metastases are detected in 8.5–20% of metastatic patients, most commonly in lung, renal, breast, melanoma, and colorectal cancer [177–180]. Autopsy studies indicate the incidence may be much higher, with up to 30% of breast cancer patients exhibiting metastatic lesions [180, 181]. The incidence of brain metastasis seems to be increasing due to more accurate imaging technologies and systemic therapies that extend patient survival, such as trastuzumab for HER-2+ breast cancer or taxanes for ovarian cancer [179, 182, 183] (although not all studies agree [177]). The increasing incidence of brain metastasis highlights a critical need to understand the niche(s) that enable DTC survival better so we can identify vulnerabilities and prevent metastasis.
Brain metastases tend to emerge at watershed areas between vascular beds [184]. These regions are supplied by the smallest arteriolar branches, suggesting that vessel diameter aids DTC extravasation. The cerebellum is an overrepresented target for breast and gastrointestinal metastases and the frontal lobe for melanoma metastases when normalized for lobe volume [185], suggesting different metastatic potentials between the different lobes. The distribution of DTCs in different mouse models varies (e.g., brain-tropic MDA-MB-231 breast cancer cells are enriched in the frontal and parietal cortex following intracardiac injection) [186], indicating additional species-specific differences that may necessitate new models to understand the differences observed in humans.
3.2. The brain microenvironment
The brain is a dense network of neurons, glia, and other stromal cells. The immune compartment consists of resident macrophages (microglia) as well as adaptive immune cells and natural killer cells at homeostasis and in disease states such as acute viral infection [187–194]. Fibrous ECM components like fibronectin and collagen are nearly absent from the adult brain, whereas proteoglycans fill the intercellular space and support synaptic contacts [195–197]. Endothelia bearing tight junctions, smooth muscle cells, pericytes, astrocyte endfeet, and neural synapses form a physical and molecular seal between solutes and the central nervous space called the blood-brain barrier (BBB) (Fig. 3). The basement membrane (BM) subtly changes between the vasculature and the outer side of the parenchyma (e.g., in laminin composition) [198–200]. Lymphatic vessels, only recently observed in the superficial dura mater, drain excess interstitial fluids but do not penetrate into the gray matter [201, 202].
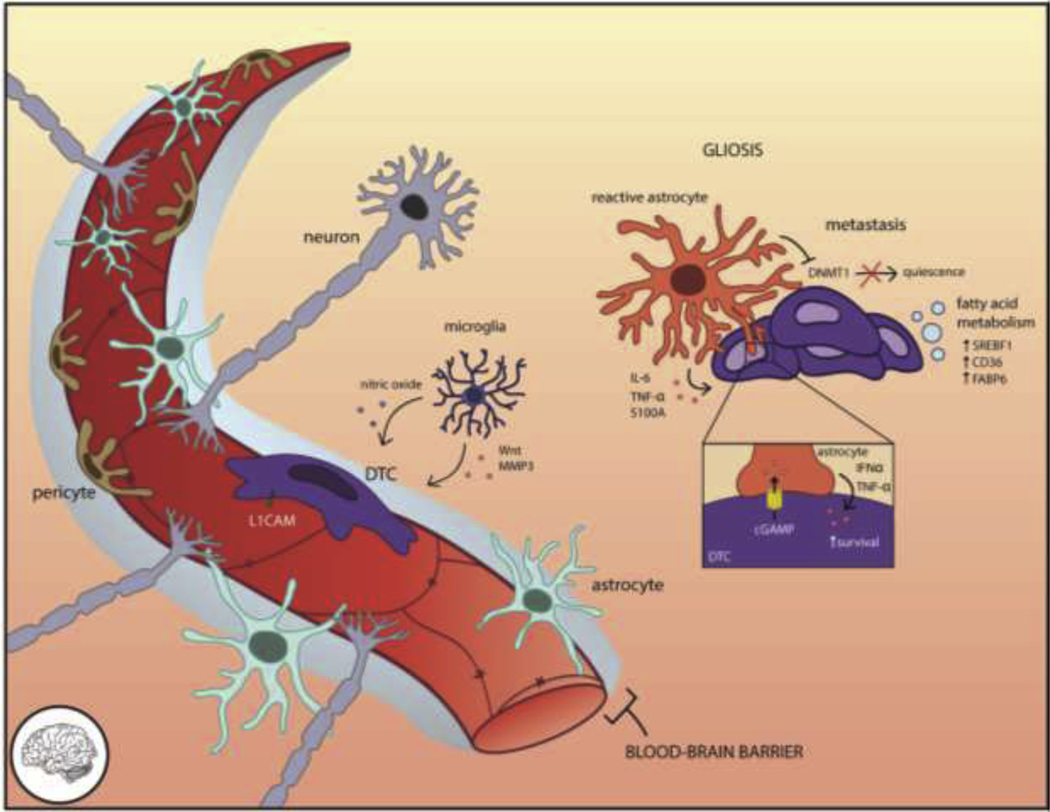
Figure 3. The brain microenvironment requires DTCs to adapt in order to survive.
Access to the brain parenchyma is restricted by the blood-brain barrier (BBB), a tightly reinforced layer including endothelia and concentric layers of supporting pericytes, astrocytes, and neurons. DTCs that manage to extravasate through the BBB rely on L1CAM-mediated spreading on the vasculature to trigger YAP-dependent survival. Astrocytes maintain the integrity of the vasculature and repel invasive cells through Fas ligand but undergo gliosis as an injury response. Reactive astrocytes secrete pro-metastatic cytokines such as IL-6, TNF-α, and S100a and exchange factors directly with DTCs through gap junctions, which elicits paracrine survival signaling (IFNα, TNF-α) back to the DTC. Microglia eliminate tumor cells through nitric oxide but also contribute pro-invasive Wnts and MMP3. The unique lipid composition of the brain necessitates DTCs adapt their fatty acid metabolism through SREBF1, CD36, or FABP6 for successful colonization.
3.3. Mechanisms of DTC dormancy and activation in the brain
Research on cellular dormancy of DTCs in the brain is not as well-established as in other organs. Few studies have attempted to model brain DTC dormancy, with notable exceptions [203, 204], but delayed relapse data [177], primary glioma research [205–207], and intravital imaging [208, 209] inform our understanding of the brain dormancy niche.
Tumor cell dissemination to the brain is not uncommon [210] but nevertheless is strenuous for DTCs [208, 209, 211]. Certain molecules are required to cross the BBB including the adhesion protein ST6GALNAC5 [212], proteolytic enzyme cathepsin-S [213], chemokine receptor CCR4 [214], and MMP9 to remodel the stroma [209]. Tumor-secreted exosomes containing cell migration-inducing and hyaluranon-binding protein (CEMIP) enhanced brain invasion and vascular interactions [215]. Fittingly, DTC extravasation to the brain takes longer than other tissues [216]. Once in the brain, the next major bottleneck for DTCs is overcoming microenvironmental barriers to growth. The emergence of brain metastasis only after chemotherapy and frequency of delayed relapse of a year or more after initial diagnosis (~28%, 66/232 cancer patients with brain metastasis) [177] suggests two interesting points: 1) that brain DTCs need time to overcome these barriers to growth, and 2) that DTCs are protected from chemotherapy.
Intravital imaging highlights the critical role of blood vessels in DTC survival. The endothelia are the first cell type that an extravasating DTC encounters, and failure to generate appropriate contacts with vessels leads to cell death for human melanoma, lung, and breast cancer DTCs [203, 208, 217]. Within this niche, some tumor cells (e.g., melanoma) are highly motile whereas others (lung carcinoma) remain static [208]. DTCs specifically engage in distinct ‘spreading’ over the vessels via the cell adhesion molecule L1 (L1CAM) (Fig 3.), which displaces vessel pericytes and promotes colonization through the mechanically-activated Yes-associated protein (YAP) and myocardin-related transcription factor (MTRF) [218]. VEGF-A blockade prevented PC14-PE6 lung carcinoma DTCs from progressing (ostensibly by stabilizing the vessels [66]) and pushed DTCs to adapt to different vessel interactions [208], highlighting a functional dependency on the vasculature. Brain endothelia provide chemoprotection to murine mammary tumor cells in culture through gap junction communication and secreted endothelin, resulting in 50% reduction of tumor cell death with taxol treatment [219]. When inflamed by tumor-secreted CEMIP+ exosomes, the endothelia commence vascular remodeling that promotes metastasis [215].
After emerging on the abluminal side of the vasculature, brain DTCs encounter glial and mural cells that support the vessels. Astrocytes are a significant cellular component of the brain parenchyme. At homeostasis, astrocytes support the integrity of the BBB through endfoot contact with the vessels and expression of Fas ligand, which repels infiltrating lymphocytes [220]. But in response to inflammation, metastasis, or glioma-secreted exosomes, astrocytes undergo reactive gliosis and become pro-metastatic [186, 209, 221–223]. Brain metastatic breast and lung carcinoma cells directly transfer cGAMP to astrocytes through gap junctions which activates astrocytes to secrete paracrine IFNα and TNF back to the tumor cells, promoting survival and chemoprotection through STAT1 and NF-κB pathways [224, 225]. Reactive astrocyte gap junctions also chemoprotect MDA-MB-231 breast and H226 lung cancer DTCs through endothelin-IL-6/IL-8 signaling [219], and sequester intracellular calcium from human melanoma cells [226]. Reactive astrocytes suppress DNA methyltransferase 1 (DNMT1) in human melanoma, breast, and lung DTCs through an unknown secreted factor, which activates pro-survival L1CAM and the anti-apoptotic molecular chaperone αB-crystallin (CRYAB) [204]. Astrocytes promote metastatic growth through secreted IL-6 and TNF-α [227] and S100A under systemic estrogen [228]. Strikingly, brain metastatic MLA-MB-231 breast cancer DTCs encouraged neural progenitor cell differentiation into astrocytes via BMP2, fine-tuning their niche into a permissive environment [229].
However, reactive astrocytes can also prevent metastasis through cytostatic functions. They produce plasminogen activator (pA), which liberates paracrine FasL to kill invading DTCs, and inhibit L1CAM preventing spreading and vascular co-option [217]. Brain-metastatic H2030 lung carcinoma and MDA-MB-231 breast carcinoma DTCs neutralize PA through serpin expression, which canonically protects neurons from elimination [217].
The chemical composition of the brain presents an additional barrier to DTC growth. The brain relies on specialized lipids instead of triacylglycerols to support neural activity [230], generating a unique lipid microenvironment to which brain DTCs must metabolically adapt. Using a panel of 50v human cancer cell lines across 21 different tumor types, two metabolic dependence have been uncovered: upregulation of SREBF1, a transcription factor that alters DTC fatty acid metabolism and enables proliferation of micrometastases, and engaging the fatty acid transporter CD36 and fatty acid-binding protein FABP6 to acquire lipids [231]. These adaptations demonstrate DTC plasticity to meet the unique demands of the brain microenvironment.
Immune regulation of brain dormancy
Brain DTC-immune cell interactions are similarly context-dependent. Microglia accumulate in or near metastases [60, 209, 232, 233]. They are canonically cytotoxic through secretion of nitric oxide [234] but assist human MCF7 breast and murine M410.4 mammary carcinoma DTC invasion through Wnt signaling [235] and MMP3 activation [236]. Contrary to most organs, inflammation reduced the pro-invasive effect of microglia, underscoring brain-specific protection from excessive inflammation [235]. Microglia-conditioned media upregulated quiescence and survival factors including TSP-1, BCL6, SMAD3, LIF, L1CAM [205] and GAS1 [206] in glioma organoid spheres, and it would be interesting to assess their effect on metastasizing cell types. Samples derived from human patients with breast or lung cancer metastasis to the brain revealed that NK cells actively survey for DTCs, which shed NKG2D ligands upon quiescence [237] and express them with re-entry into the cell cycle, resulting in elimination by NK cells [218].
As more barriers to growth in the brain are elucidated, we will gain a better understanding of the dormancy niche, and the diverse ways DTCs must adapt to co-opt the niche to survive (Fig 3). Future studies should consider the role of adaptive immunity [188, 238], which will be aided by advancing technologies and pre-clinical models of metals, en route to therapeutic approaches for this devastating aspect of metastasis.
4. Liver
4.1. Metastasis to the liver
The liver is an enzyme-rich organ that processes metabolites, synthesizes bile, and maintains blood glucose levels. It is the most common site of distant metastases, excluding lymph nodes [239, 240]. Across metastatic patients, liver metastases most frequently occur in pancreatic (85%), colorectal (78%), and esophageal cancers (52%), as well as breast (30%) and lung (16%) cancer [241]. Treatment option for liver metastasis are limited. Surgical resection is considered the only curative therapy [242, 243] and is standard of care for pancreatic and colorectal cancer metastasis. However, the benefits of surgery are modest and may induce liver injury, which in turn exacerbates metastasis [244–247]. The benefit of hepatic resection in other cancer types such as breast is promising [248], correlating with a 5-year survival rate of 22–41% [249, 250], yet only a subset of patients qualify for surgery [242]. Thus, better treatment options are crucially needed to manage metastatic liver disease.
Tumor dormancy in the liver is supported by autopsy and clinical samples [210, 251, 252] as well as experimental models [21, 252–255]. Mammary carcinoma DTCs persist as single cells in the liver for 11 weeks post-injection and reinitiate growth upon ex vivo isolation [21]. DTCs access the liver with relative ease through low-pressure, fenestrated sinusoids [256] and intravital imaging confirms that the liver is more prone to DTC extravasation than the lungs [257]. The liver also exhibits high retention rate of tumor cells: meta-analyses determined that the liver is the most frequent secondary site for metastases, and, conversely, primary liver cancer metastasizes the least compared to other primary tumor types [239, 240]. This paints a picture of the liver as a “sink” from which primary tumors or secondary metastases are less likely to spread. Although the steps of metastatic liver colonization have been described [258], investigating interactions between DTCs and the liver niche particularly during dormancy would illuminate the liver niche’s capacity for supporting dormancy.
4.2. The liver microenvironment
Macro- and micro-levels of organization foster liver cell specialization and function [259, 260]. Epithelial sheets of hepatocytes, the parenchymal cell that comprises the bulk of the liver mass and functions in metabolism, storage, and bile secretion, converge on central portal tracts made up of a triad of vessels: a portal vein, artery, and bile duct (Fig. 4). The portal triad is orthogonally connected to an efferent central vein via fenestrated sinusoids that run between the hepatocyte sheets. The sinusoids are comprised of liver sinusoidal endothelial cells (LSECs) and lack a typical organized basement membrane [261]. 70–80% of the liver’s blood supply passes through the low pressure portal vein and the remaining 20–30% comes from the portal artery [256, 262]. These blood supplies intermix as they flow across the sinusoids, another low-pressure system where Kupffer cells, the resident macrophage, reside. The subendothelial Space of Disse drains lymph and contains hepatic stellate cells (HSteCs) that store fat-soluble metabolites like retinoids and produce the majority of ECM fibers in a loose subendothelial basement membrane [263]. HSteCs also have contractile function and regulate sinusoidal blood flow through LSEC-generated nitric oxide and endothelin signaling [264].
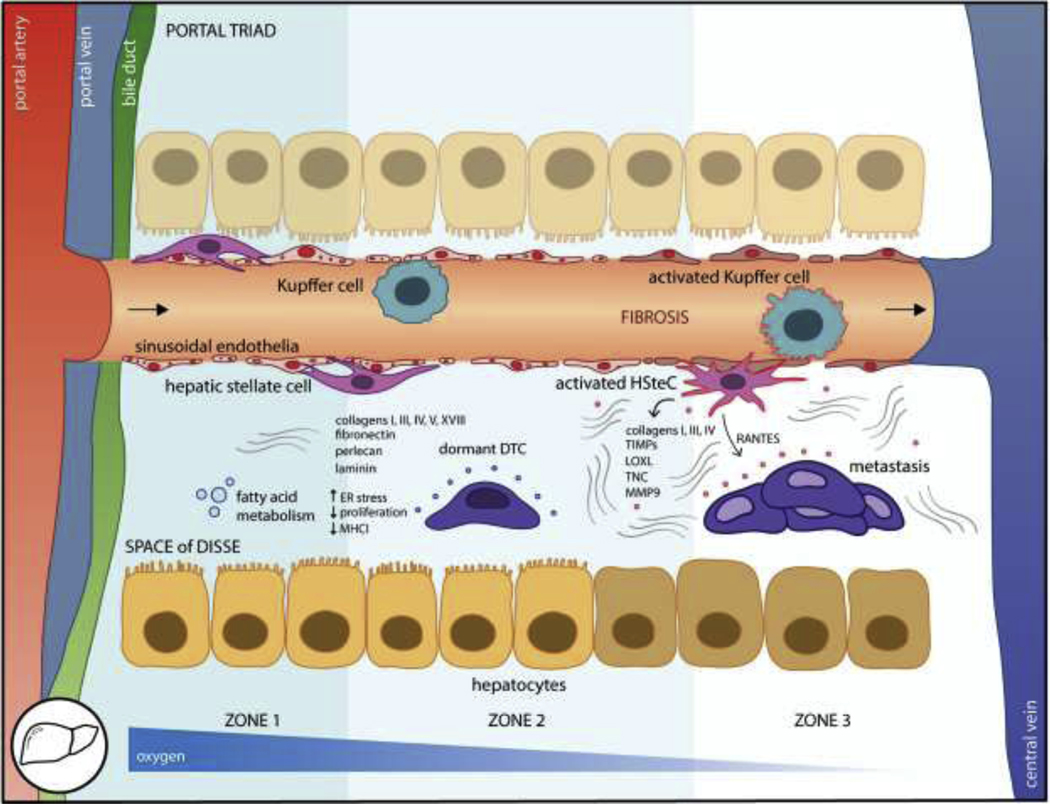
Figure 4. The liver is the most common major metastatic organ.
DTCs traffic from the portal triad vessels to the fenestrated sinusoids and into the subendothelial space of Disse where they encounter stromal cells including liver sinusoidal endothelial cells (LSEC), hepatic stellate cells (HSteC), Kupffer cells, and hepatocytes. Distinct zones occur along the portal triad to central vein axis following oxygen and functional gradients. The loosely organized fibers and proteoglycans basement membrane include collagens, fibronectin, perlecan, and laminin which impose quiescence. Metabolic adaptation slows DTC proliferation, although it also coincides with downregulation of MHCI and immune evasion. However, upon inflammation or injury, the liver become fibrotic which is associated with metastatic outgrowth. Activated HSteCs deposit vast quantities of ECM fibers and other pro-metastatic factors such as RANTES, endothelia undergo capillarization, and hepatocytes lose their microvilli. These changes to the liver microenvironment alter the niche to foster metastasis.
Single-cell analysis of the liver revealed stromal subpopulatioi.s and specializations across the hepatic zones [259], which radiate outward from the portal triad to the central vein following an oxygen and nutrient gradient [265] (Fig. 4). Zone 1, closest to the portal triad, experiences approximately twice the oxygen tension and, higher levels of ROS versus Zone 3, closest to the central vein [266]. Gluconeogenesis and ureagenesis occur in Zone 1, whereas Zone 3 is glycolytic and the site for glutamine synthesis [265]. ECM composition also changes between zones, with Zone 1 rich in type I and III collagens and Zone 3 in collagens IV and VI [267]. LSECs in Zone 3 undergo a greater fibrotic response to cirrhotic injury compared to Zone 1 LSECs, revealing differential vulnerability or activation thresholds across the zones [268]. How zonation impacts tumor dormancy is unknown.
4.3. Mechanisms of DTC dormancy in the liver
The liver imposes a strong quiescent phenotype on DTCs, as liver-derived mammary DTCs required two to three times as long to resume proliferation ex vivo compared to DTCs derived from the lung [253]. Patient autopsies revealed single tumor cells scattered throughout the liver that had not progressed to metastasis [210]. Additionally, primary hepatocellular carcinoma undergoes reversible quiescence and differentiation into normal stroma upon MYC inactivation [269]. Together, these studies indicate the liver microenvironment resists metastatic growth, but few studies have directly addressed the liver dormancy niche. Here, we examine known mechanisms of quiescence and potential dormancy niches to aid in this challenge.
Metabolism is a primary function of the liver and DTCs need to adapt accordingly in order to survive and grow. Inability to resolve endoplasmic reticulum (ER) stress in pancreatic ductal adenocarcinoma mM1DTLB DTCs led to quiescence and, critically, MHCI downregulation, which could be restored through pharmacological relief of ER stress or rescuing unfolded protein response machinery [252]. The immune system plays a key role in selecting for quiescent MHCI− DTCs: in T cell-depleted mice, quiescent DTCs reverted to an MHCI+ proliferative phenotype, whereas few DTCs or metastases could be detected in an immune-competent setting [252]. DTCs did not express NKG2D ligands required for NK recognition, so T cells are likely the dominant immune cell. Indeed, a ‘wave of apoptosis’ has been described in mouse models of hepatic mammary carcinoma metastasis as the immune system eliminates lesions [21]. Although immune recognition can be enhanced through relieving ER stress, this carries the risk of awakening quiescent DTCs. Alternatively, treatment with an ER stressor (tunicamycin) drove DTCs into quiescence, suggesting a strategy for maintaining dormancy if toxicities can be minimized [252]. Thus, adapting to the liver requires a trade-off between metabolic adaptation and immune evasion.
Although the preferential dormancy niche(s) for liver DTCs is unknown, the perisinusoidal space which DTCs encounter upon extravasation is rich in fibrillar collagens type III, and V, non-fibrillar collagens type IV and XVIII, as well as basement membrane components including fibronectin, perlecan, and laminins [267, 270, 271]. Type IV collagen promotes both survival and metastasis for liver DTCs [272, 273], and laminins and perlecan are noted quiescence promoters in other tissue sites [274, 275]. Ex vivo imaging confirms that vessel association is critical for extravasated DTC survival [257], but must be examined thoroughly in preclinical models.
Parenchymal hepatocytes induce mesenchymal-to-epithelial transition (MET) and quiescence in prostate cancer cells via p38 and ERK1/2 n a co-culture model [276]. Prostate and breast cancer models confirm that hepatocytes are critical for DTC quiescence and proliferation-independent chemoresistance [277–279]. Further, breast cancer DTCs adopt an intriguing hepatocyte-like ‘replacement’ organization in human patients [280], suggesting that communication with the parenchyma is beneficial for DTCs. Co-culture studies indicate that hepatocytes are the primary effector^ of tumor quiescence compared to NPCs [281]; however, growth on plastic activates HSteCs and phenocopies inflammation [282], which could confoundingly enhance tumor outgrow *h. Thus, it is likely that both hepatocytes and NPCs contribute to the dormant DTC phenotype in healthy, homeostatic tissue, which is lost upon stromal activation in response to injury.
Non-parenchyma cells (NPCs) including LSECs, Kupffer cells, and HSteCs support trafficking and quiescence of resident liver cells, and it is conceivable that these mechanisms impact DTCs. For example, LSECs secrete CXCL12, which attracts CXCR4+ DTCs [283], and nitric oxide, which promotes HSteC quiescence and contractility [284]. HSteCs promote vessel integrity through VEGF and nitric oxide [285], stabilizing a prospective quiescent niche.
4.4. Activation of quiescent DTCs in the liver
The quiescent hepatic microenvironment resists metastasis, which can be reversed upon stromal activation [286]. Two major sources of physiological disruption within the liver, fibrosis and regeneration after injury, are associated with activation [287–290] and may be key turning points in the dormancy timeline that promote awakening.
Liver fibrosis results from stromal cell activation following injury. It has numerous etiologies such as alcohol abuse, hepatitis, fatty liver disease, or surgery which converge on a state of inflammation [288, 291]. HSteCs are the primary effector of fibrosis, and activate from quiescent to proliferative hepatic myofibroblasts (HMFs), upregulate αSMA, lose their retinoid stores, and increase deposition of ECM fibers, especially collagens [292]. TGF-β1 [293, 294], PDGF [295], FGF [296], and ECM stiffness [297] contribute to the HSteC activation process, creating a pro-fibrotic feedback loop. The ECM stiffens as HSteCs deposit excessive collagen (predominantly collagens I, III, and IV [263, 298]), tissue inhibitor matrix metalloproteinase enzymes which antagonize MMPs to prevent matrix degradation, and LOX, which crosslinks collagen further [299, 300]. The liver sinusoids undergo capillarization [301] and hepatocytes lose their microvilli [302]. Proteins that are not normally a component of the healthy liver ECM appear, such as pro-metastatic TNC and MMP9 [303, 304].
Increased matrix stiffness [254, 305] and HMFs [286, 306] are highly associated with metastatic outgrowth, and potentially a source of awakening signals for dormant DTCs. HMFs secrete proliferative cytokines including RANTES [307] which may act on DTCs and inhibit T cell activity through B7-H1/PD-L1 ligation [308, 309]. In culture, MCF7 and MDA-MB-231 breast cancer cells treated with conditioned media from activated HSteCs exhibited increased proliferation when compared to conditioned media from quiescent HSteCs [306]. Thus, activated HSteCs promote DTC outgrowth and metastasis by altering the ECM, direct proliferative signaling, and immune suppression. Targeting the underlying causes of fibrosis [310–312] may be a viable strategy for maintain tumor dormancy [313]. A key aspect is restoring HSteC quiescence, which has been achieved through all-trans retinoic acid [286], vitamin D [314], and exposure to LSEC-generated nitric oxide [284]. It is also relevant to note that NK cells counter fibrosis by eliminating activated HSteCs with low MHCI expression [315, 316], and restrain activated hepatocyte proliferation in a IFN-γ-dependent manner [317]. It is possible that the anti-fibrotic role of NK cells helps sustain DTC quiescence, although a direct relationship between NK cells and DTC dormancy has yet to be established.
Liver regeneration is the controlled healing response to tissue injury such as surgical resection. Surgery is associated with relapse as a result of inflammation [15, 318, 319], but liver regeneration is unique from the trauma of surgery. During regeneration, the liver stroma undergo rapid yet controlled hyperplasia without incurring inflammation and actually suppress fibrosis [320, 321]. The endothelium may be at the heart of the balance between fibrosis and regeneration, as the angiocrine response to injury hinges on the dominance of CXCR7 versus CXCR4 signaling and the sphingosine-1-phosphate receptor [321, 322]. Depending on the balance of signals, activation is transduced from LSECs to HSteCs, cascading into either the fibrotic or regenerative response. When regeneration commences, hepatocytes and NPCs exit G0 phase and undergo mitosis under the regulation of cytokines including TNF-α, NF-κB [323], IL-6 [324], and growth factors [325, 326], while TGF-β and activin terminate stromal proliferation [327]. In this context, a bystander dormant DTC may be exposed to the rich proliferative signals in the regenerative microenvironment and enter the cell cycle. The role of liver regeneration in metastasis is well-studied [245, 290, 328], but the relationship between regeneration and awakening from dormancy has not been elucidated [255]. Since surgical resection is a standard treatment for liver metastasis for some cancers, it is critical to understand how fibrosis and regeneration impact dormant DTCs to avoid incidental DTC awakening.
In summary, the liver provides a dormancy niche for DTCs, which can be perturbed through fibrosis and injury. More research is needed to investigate hepatic regulators of quiescence and the interactions between DTCs and the stroma. While fibrosis activates the quiescent tissue, preventing inflammation or reversing it may help sustain DTC dormancy [313].
5. Lymph node (LN)
5.1. Metastasis to the LN
The lymph node (LN) is the most frequent site of metastases across tumor and tissue types [240]. Although LNs themselves are not vital organs, LN metastasis strongly associates with distant metastases and is a key prognostic factor in staging practices [329–334]. However, there is some controversy over their significance [335, 336]: Do LN DTCs contribute to distant metastases? Does the ability to disseminate to LNs reflect tumor aggression or the proximity and non-selective nature of LNs? How do the inevitable tumor-immune interactions within the LN influence tumor development at local and distant sites?
While we cannot track the cell of origin for a metastatic lesion within a human patient, experimental evidence has convincingly shown that LN DTCs ca n migrate to subsequent LNs [337] and seed metastases in distant tissues [334, 338–341], via blood or lymph [341–343]. Clinical evidence supports the notion that LN DTCs contribute to disease, especially over a longer duration of follow-up. The strongest example of this has been in breast cancer, where the MIRROR (Micrometastases and Isolated Tumor Cells: Relevant and Robust or Rubbish?) study, NSABP B-32 clinical trial, and meta-analyses have shown that isolated tumor cells in patient LNs independently predict worsened overall and disease-free survival over five to ten years post-diagnosis versus node-negative patients [344–349], and similar trends hold in colon cancer [350]. Further, this may be a conservative measurement given that 13–25% of histologically “node-negative” patients have occult metastases upon closer examination [346, 348, 351, 352]. Cumulative incidence curves comparing breast cancer patients stratified by nodal status reveal a latent phase in the isolated tumor cell (pN0(i+)) group that lasts approximately 36 months before the rate of adverse events rapidly increases [348, 353]. While it is not possible to assign a cause for this rapid switch base d on these data, it aligns with the paradigm of tumor dormancy in which single or small clusters of tumor cells reside in a quiescent niche within a tissue in a state of cell cycle arrest until stimulated to divide [354].
Late recurrence in LNs more than 10 years after diagnosis has also been documented for melanoma [355, 356] and gastric cancer [357], supporting the notion that LNs may harbor dormant DTCs for long periods of time. Establishing the role of the LN microenvironment in tumor dormancy is crucial for anticipating late relapse and guiding treatment strategies aimed at preventing distant relapse.
5.2. The LN microenvironment
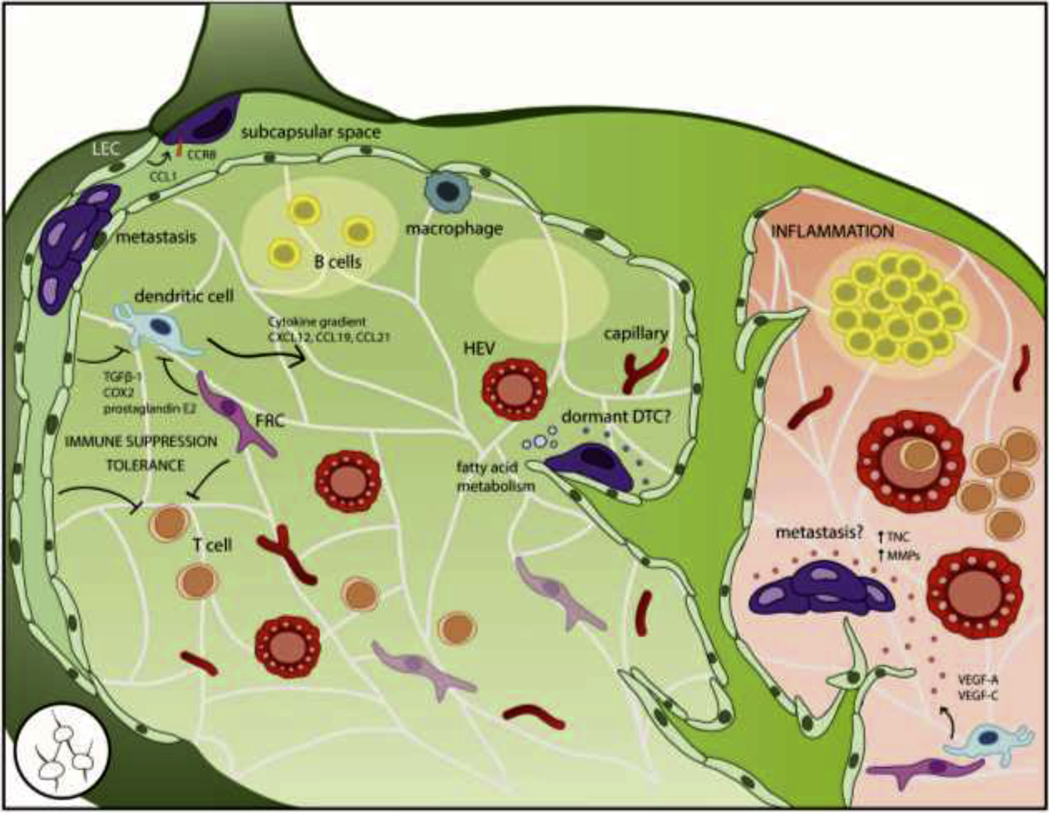
The lymphatic system is a network of one-way, leaky, fenestrated vessels that collect the interstitial fluid lost in peripheral capillary beds and return it to circulation through the thoracic duct to maintain blood pressure. This fluid, called lymph, carries proteins, cell debris, and immune cells, as well as pathogens and disseminating tumor cells. Intermittent nodules along the lymphatics serve as ‘field headquarters’ for peripheral immunity, allowing lymph to be surveyed by resident antigen-presenting cells (APCs) and adaptive immune cells in a centralized hub. To facilitate antigen sampling and presentation, LNs are highly compartmentalized, including four major subsets of stromal cells: lymphatic endothelial cells (LEC), blood endothelial cells (BEC), fibroblastic reticular cells (FRC), and contractile pericytes previously referred to as CD31− podoplanin− ‘double negative’ cells that are not as well-characterized [358, 359] (Fig 5.).
Figure 5. The lymph node (LN) is the most common site of all metastases but its role as a dormancy niche is unknown.
The LN is highly compartmentalized to support its primary function in peripheral immunity. In addition to resident and migrating immune cells, the LN stroma includes lymphatic endothelial cells (LECs) that form the outer capsule and draining sinusoids, blood endothelial cells (BECs) that form capillaries and specialized high endothelial venules (HEVs), and fibroblastic reticular cells (FRCs) that produce an ECM conduit network to facilitate antigen sampling. DTCs frequently access the LN by lymphatic or hematogenous routes, but must adapt to the LN microenvironment through metabolic pathways and co-opting signals that guide immune cells into the LN parenchyma such as CCL1-CCR8 signaling at the afferent lymphatic vessel and cytokine gradients of CXCL12, CCL19, and CCL21; otherwise, they risk remaining confined to the subcapsular space. LN DTCs may benefit from immune suppressive and tolerogenic functions of FRCs and LECs. However, the stromal components that contribute to a dormancy niche have not been formally described. During inflammation, the LN activates into a proliferative environment including angiogenesis and lymphangiogenesis; these changes to the stroma could conceivably induce outgrowth of dormant DTCs, but must be more fully investigated.
FRCs are the main parenchymal cells that provide structural and functional support to the LN resident cells. They produce and ensheath the conduit network, a series of ECM tubules that facilitates antigen sampling and serves as a scaffold for migrating leukocytes. The tubule core contains collagen I, IV, and XIV and proteoglycans (e.g., decorin, biglycan, and fibromodulin), surrounded by an outer layer of fibrillin, collagen VI, TNC and laminin-332 [359–361]. FRCs also control T cell migration [362] and egress [363], and regulate LN expansion though interactions with active dendritic cells (DCs) [364].
LN LEC form the outer capsule and internal sinuses. When lymph enters via the afferent vessel, it flows into the subcapsular space (SCS) which is separated ii.m the parenchyma by a layer of LEC. Lymph-borne particles are separated by size: small molecules less than 70 kDa or 5.5 nm in diameter can enter the conduit network and flow into the parenchyma, whereas larger particles are confined to the SCS to prevent possible pathogens from accessing the blood [365]. DCs sample small molecules within the conduit system [360, 366] and specialized subcapsular macrophages [367] and DCs [368] sample larger molecules in the SCS.
The outer cortex contains multiple lymphoid lobules consisting of B cells, follicular DCs, and supporting stroma. Lymphatic sinuses interdigitate between the lobules, reaching down to the medulla near the base of the node, which collects lymph from the sinuses into the efferent vessel. T cells reside and activate in the inner paracortex region. Throughout the parenchyma, LN BEC form two distinct vessels: arteries that enter at the hilar base and branch out into smaller capillaries to provide nutrients, and high endothelial venules (HEVs) that enable lymphocyte trafficking through adhesion molecules such as L-selectin and the absence of tight junctions [369]. These subcompartments form very distinct niches, though their respective effect on DTC phenotype is currently unknown.
5.3. Mechanisms of DTC Dormancy in the LN
The LN is a site of carefully coordinated migration and localization, and chemokines help set up the appropriate cues and compartments for immune function. Tumor cells co-opt these signals to enhance LN invasion. Initially, DTCs access the LN through intra- or peritumoral lymphatic vessels [370], as well as the aforementioned blood circulation [338, 339, 341]. Migrating cells entering via the afferent lymphatics express CCR8 which binds to LEC CCL1 to increase motility and facilitate entry into the LN [371]. These signals can be co-opted by DTCs and are required for LN access [372]. Inhibiting CCR8 caused melanoma DTCs to accumulate at the junction of the afferent vessel and LN capsule, conferring a gatekeeping function on CCL1-CCR8 signaling. Migrating DCs and T cells rely on gradients of CCL19, CCL21, and CXCL12 from FRCs and HEVs to transverse the LEC subcapsular floor and migrate into the parenchyma [373–375]. Breast, melanoma, and prostate cancer DTCs also express the cognate receptors CCR7 and CXCR4 [376–378], and inhibition of CXCR4 prevented breast cancer metastasis to the LN [376], suggesting DTCs follow the same cytokines gradients (Fig 5.).
A LN niche for dormant tumor cells has not been defined, but clues can be derived from patient and preclinical models. DTCs in human and animal models tend to concentrate in the SCS, which follows from afferent lymphatic entry [353, 379–385]. For breast cancer, metastases are isolated to the SCS in 47% of involved sentinel LNs, whereas only 3% contain metastatic deposits solely in the parenchyma [385]; for melanoma, 26% of sentinel LN metastasis is subcapsular, with no association found between location of the sentinel LN and microanatomic location of the metastatic deposit [381]. However, microanatomic location of the metastatic deposit predicts non-sentinel LN involvement and malignant outcome. Patients with metastases confined to the superficial SCS of the sentinel LN had an extremely low (statistically zero) probability of further LN involvement [381], indicating that the SCS is cap able of fostering growing metastases but stymies true tissue invasion. Egress through the LN blood vessels or efferent lymphatics would require DTCs to cross the subcapsular floor LECs and transverse the parenchyma, which is possible given that human breast cancer cells can perforate LEC monolayers through the metabolite 12S-HETE [386]. However, SCS containment underscores the notion that not all microanatomic regions of the LN are equally poised to promote metastasis.
Taking a clue from other tissues [66], the LN endothelia may contribute to the dormancy niche. While they come from a common endothelial progenitor [387], LN BEC and LEC are distinct in function and interactions with tumor cells. In one of the few studies addressing LN ECs and DTCs directly, human BEC suppressed MDA-MB-231 breast tumor growth in culture and when co-injected into mice, whereas LEC promoted tumor growth due to secreted EGF and PDGF-BB [388]. Further, LN LEC were conditioned by mammary tumor IL-6 to secrete CCL5 and promote angiogenesis, leading to enhanced LN metastasis [389]. Whether LN BEC suppression of tumor growth is due to a deficit in metastasis-promoting factors or the production of quiescence factors has yet to be determined. Further study would shed light into the functional distinction between the endothelial compartments with respect to tumor dormancy.
To that end, recent single-cell transcriptome profiling of FRC [390], LN BEC (capillaries and HEVs) [391], and LN LEC [392–394] has provided a wealth of insight into specializations and subpopulations of these cells, which may be relevant for tumor dormancy studies. For example, subcapsular ceiling LEC express the machinery to take up modified low-density lipoproteins (LDL) [392], a conserved function with the gut lymphatics [395]. LDL accumulation can disrupt macrophage function, a possible justification for scavenging LDLs from an immune organ [396], but its impact on LN metastasis and dormancy is not well-understood. LN melanoma DTCs upregulated lipid metabolism pathways to oxidize fatty acid species, and functional inhibition of fatty acid oxidation suppressed LN but not lung metastasis, indicating a unique adaptation for LN metastasis [397]. The effect of fatty acid oxidation inhibition on the immune compartment was not assessed but could hypothetically activate macrophages to eliminate LN metastases. This study opens the door for examining the role of dietary fats in providing fuel for LN DTC proliferation and determining whether LEC LDL scavenging has a protective effect.
5.4. Activation of quiescent DTCs in the LN
While the definition of the LN as a dormancy niche is still under scrutiny, two mechanisms of DTC awakening can be anticipated: the immune suppressive role of LN stromal cells and the dramatic changes that occur with inflammation. Although LN stroma maintain immune function [398], many studies highlight an immunoregulatory role for FRC and LEC [399–401]. Although this role is critical to mitigate autoimmunity, it may hinder defenses against tumor colonization. CD8+ T cells actively constrain LN metastasis [402], but FRC and LEC attenuate T cell effector function through prostaglandins [400], TGF-β1 [400], cyclooxygenase-2 [399], and nitric oxide [403] (Fig. 5).
FRC and LEC engage in direct and cross-presentation of self-antigens, which, in the absence of costimulation, elicits T cell exhaustion through PD-L1 [401, 404–408]. LN LEC cross-presentation of soluble tumor antigens selects against tumor-specific T cells, altering the immune repertoire and leading to enhanced LN metastasis of B16-F10 melanoma cells [409]. LN BEC do not seem to have the same immune-suppressive profile. The consequences of stromal tolerogenesis should be analyzed with respect to both the T cell repertoire and selection of immune-privileged tumor clones in order to clarify its systemic effects.
Inflammation alters the stable environment of DTCs, and has specifically been linked to emergence of D2.0R and latent D2A1 DTCs from dormancy through changes to the microenvironment at other sites [16, 17]. The LN is no exception; in fact, it is an exemplary organ for the study of inflammation-induced stromal changes. Upon recognition of a pathogen, the LN expands up to five-fold over several days with concomitant stromal proliferation and ECM remodeling to accommodate immune influx [410, 411]. After the inflammation is resolved, the LN undergoes shrinkage to report homeostasis. Since stromal stability plays such a prominent role in maintaining DTC quiescence in other organs, the dynamically responsive LN environment may disturb quiescent DTCs and induce awakening.
The initial phase of inflammation is regulated by DCs, and later expansion phases are maintained by B and T cells [412–414]. Upon inflammation, activated DC CLEC-2 binds FRC PDPN which relaxes the FRC network, allowing the LN to expand [364, 415] and resident cells to proliferate [416]. conduit network becomes leaky as FRCs loosen their attachments, exposing ECM components to the parenchyma. FRC expression of ECM components shifts, resulting in downregulation of netrin-1, which has been linked to quiescence factor TSP-1, and upregulation of metastatic niche components such as TNC and MMPs [359, 417]. DCs and FRCs coordinate secretion of VEGF-A and VEGF-C to induce BEC and LEC proliferation and angiogenesis and lymphangiogenesis [359, 412, 418–420]. As detailed in Sections 1.3 and 2.3, sprouting angiogenesis is associated with loss of the quiescent vascular niche and emergence of activated DTCs in lungs and bone marrow [66], and it remains to be seen whether inflammation-induced endothelial remodeling has a similar effect on LN DTCs. If so, then any kind of inflammatory insult may have drastic ramifications for activating dormant DTCs which could help explain the frequency of LN metastasis. Conversely, treating inflammation through non-steroidal anti-inflammatory drugs may be a viable method to stabilize the stromal architecture and reduce metastatic outgrowth. One might speculate that imposing stromal stability could restrict dissemination from the LN to other sites.
Conclusion
Tumor dormancy is a silent stage of cancer progression that continues to pose a clinical threat. Through a combination of extrinsic forces and ability to adapt, a portion of DTCs co-opt, educate, and evade their way to long-term survival. We are only beginning to appreciate how the unique physiology, architecture, and resident cells of each tissue contribute to the dormancy niche, but it is biologically and clinically compelling to identify common themes and distinct mechanisms of dormancy and awakening.
Vascular association is crucial across all organs discussed in this review. As the first microenvironment encountered by extravasating DTCs, the vasculature pr vides sustaining nutrients and potent pro-quiescence factors. A second theme is co-option of pre-existing niches, such as those that regulate HSCs in the bone marrow, and signaling cascades, such as those that regulate inter- and intra-lymph node trafficking of lymphocytes. Third, DTCs must adapt metabolically, as evidenced in the brain, liver, and lymph node, where fatty acid oxidation is necessary for DTC proliferation. Fourth, the immune system must be evaded or silenced, which DTCs accomplish through downregulation of recognition molecules (e.g., MHCI, NGK2D ligands) or benefitting from immunosuppressive environments. And finally, emergence from dormancy is tied to inflammation across tissues as a result of environmental insult, disease, and surgery, highlighting a common set of condition, that may predict delayed relapse in the clinic. Angiogenesis in response to injury exposes perivascular DTCs to a host of proliferative signals and concurrent loss of quiescence cues. Activated stromal cells release pro-inflammatory cytokines, initiate ECM remodeling, and lose their stabilizing functions. One silver lining may be the utility of treatments that resolve inflammation and restore stromal homeostasis, such as anti-inflammatory drugs and retinoic acid.
We have described known and prospective niches for dormant DTCs in several metastatic tissues, but many unknowns remain, not the least of which is exploring non-metastatic tissues to gain insight into mechanisms of dormancy [421]. As the field pursues these unknowns with rigor, we must deepen our understanding of the complex relationship between tumor cell, niche, and the thorns and rocks which challenge the metastatic process.
Acknowledgements
The authors wish to thank Dr. Jinxiang Dai for critical reading of the manuscript. ARL is supported by a fellowship from the National Cancer Institute (NCI; F99CA234840-02). Related work in the Ghajar Laboratory is funded by the Department of Defense Breast Cancer Research Program (W841XWH-15-1-0201, W81XWH-19-1-0071, W81XWH-19-1-0617, and W81XWH-20-1-0229) and the NCI (1R01CA252874-01; 1R01CA249528-01A1, and U54CA193461-01).
Footnotes
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers dthat apply to the journal pertain.
References
- 1.Spencer W, Celsus de Medicina. The American Journal of the Medical Sciences, 1936. 191(3): p. 424. [Google Scholar]
- 2.Hulsbergen AFC, et al. , Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro Oncol, 2020. 22(8): p. 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priedigkeit N, et al. , Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases. JAMA Oncol, 2017. 3(5): p. 666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lluch A, et al. , Dynamic clonal remodelling in breast cancer metastases is associated with subtype conversion. European Journal of Cancer, 2019. 120: p. 54–64. [DOI] [PubMed] [Google Scholar]
- 5.Nikbakht H, et al. , Latency and interval therapy affect the evolution in metastatic colorectal cancer. Scientific Reports, 2020. 10(1): p. 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labrecque MP, et al. , Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J Clin Invest, 2019. 129(10): p. 4492–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, et al. , Substantial interindividual and limited interindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med, 2016. 22(4): p. 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yates LR, et al. , Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell, 2017. 32(2): p. 169–184.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valastyan S.and Weinberg RA, Tumor metastasis: molecular insights and evolving paradigms. Cell, 2011. 147(2): p. 275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H, et al. , Genetic and clonal dissection of osteosarcoma progression and lung metastasis. Int J Cancer, 2018. 143(5): p. 1134–1142. [DOI] [PubMed] [Google Scholar]
- 11.Schardt JA, et al. , Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell, 2005. 8(3): p. 227–39. [DOI] [PubMed] [Google Scholar]
- 12.Hüsemann Y, et al. , Systemic spread is an early step in breast cancer. Cancer Cell, 2008. 13(1): p. 58–68. [DOI] [PubMed] [Google Scholar]
- 13.Hosseini H, et al. , Early dissemination seeds metastasis in breast cancer. Nature, 2016. 540(7634): p. 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harper KL, et al. , Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature, 2016. 540(7634): p. 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krall JA, et al. , The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Science Translational Medicine, 2018. 10(436): p. eaan3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albrengues J, et al. , Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science, 2018. 361(6409): p. eaao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Cock JM, et al. , Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Research, 2016: p. canres.0608.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bragado P, et al. , TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat Cell Biol, 2013. 15(11): p. 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson MA, et al. , Osteoclasts control reactivation of dormant myeloma cells by remodeling the endosteal niche. Nat Commun, 2015. 6: p. 8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodison S, et al. , Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clin Cancer Res, 2003. 9(10): p. 3808–3814. [PubMed] [Google Scholar]
- 21.Naumov GN, et al. , Persistence of Solitary Mammary Carcinoma Cells in a Secondary Site. Cancer Research, 2002. 62(7): p. 2162. [PubMed] [Google Scholar]
- 22.Suzuki M, et al. , Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. American Journal of Pathology, 2006. 169: p. 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner-Klein M, et al. , Genetic alterations driving metastatic colony formation are acquired outside of the primary tumour in melanoma. Nat Commun, 2018. 9(1): p. 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner-Klein M, et al. , Interleukin-6 trans-signaling is a candidate mechanism to drive progression of human DCCs during clinical latency. Nat Commun, 2020. 11(1): p. 4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis RA, The spread of tumours in the human body. 1934, London: J. & A. Churchill Ltd. [Google Scholar]
- 26.Paget S, The distribution of secondary growths in cancer of the breast. The Lancet, 1889. 133: p. 571–573. [PubMed] [Google Scholar]
- 27.Fuchs E, Das Sarkom des Uvealtractus. Archiv für Ophthalmologie, 1882. XII(2): p. 233. [Google Scholar]
- 28.Hadfield G, The dormant cancer cell. British Medical Journal, 1954. 2(4888): p. 607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q, et al. , Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget, 2017. 8(17): p. 27990–27996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter JD, Morphostats: a missing concept in cancer biology. Cancer Epidemiol Biomarkers Prev, 2001. 10(3): p. 161–70. [PubMed] [Google Scholar]
- 31.Disibio G.and French S, Metastatic patterns of cancers: Results from a large autopsy study. Arch Pathol Lab Med, 2008. 132(6): p. 931–939. [DOI] [PubMed] [Google Scholar]
- 32.Huang J-F, et al. , Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Annals of translational medicine, 2020. 8(7): p. 482–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez RK, et al. , Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC cancer, 2018. 18(1): p. 44–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartkopf AD, et al. , Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients - results from a large single-centre analysis. Eur J Cancer, 2014. 50(15): p. 2550–9. [DOI] [PubMed] [Google Scholar]
- 35.Tjensvoll K, et al. , Persistent tumor cells in bone marrow of non-metastatic breast cancer patients after primary surgery are associated with inferior outcome. BMC Cancer, 2012. 12: p. 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janni W, et al. , Persistence of Disseminated Tumor Cells in the Bone Marrow of Breast Cancer Patients Predicts Increased Risk for Relapse—A European Pooled Analysis. Clinical Cancer Research, 2011. 17(9): p. 2967. [DOI] [PubMed] [Google Scholar]
- 37.Braun S, et al. , A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med, 2005. 353(8): p. 793–802. [DOI] [PubMed] [Google Scholar]
- 38.Morgan TM, et al. , Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res, 2009. 15(2): p. 677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastro AM, Gay CV, and Welch DR, The skeleton as a unique environment for breast cancer cells. Clinical & Experimental Metastasis, 2003. 20(3): p. 275–284. [DOI] [PubMed] [Google Scholar]
- 40.Wang N, et al. , Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis: evidence from in vivo models. J Bone Miner Res, 2014. 29(12): p. 2688–96. [DOI] [PubMed] [Google Scholar]
- 41.Kucia M, et al. , Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells, 2005. 23(7): p. 879–94. [DOI] [PubMed] [Google Scholar]
- 42.Tjensvoll K, et al. , Detection of disseminated tumor cells in bone marrow predict late recurrences in operable breast cancer patients. BMC Cancer, 2019. 19(1): p. 1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schindlbeck C, et al. , Comparison of circulating tumor cells (CTC) in peripheral blood and disseminated tumor cells in the bone marrow (DTC-BM) of breast cancer patients. Journal of Cancer Research and Clinical Oncology, 2013. 139(6): p. 1055–1062. [DOI] [PubMed] [Google Scholar]
- 44.Hartkopf AD, et al. , Disseminated tumor cells from the bone marrow of patients with nonmetastatic primary breast cancer are predictive of locoregional relapse. Annals of Oncology, 2015. 26(6): p. 1155–1160. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi K, Croucher PI, and Compston JE, Comparison between the lengths of individual osteoid seams and resorption cavities in human iliac crest cancellous bone. Bone Miner, 1993. 23(1): p. 27–33. [DOI] [PubMed] [Google Scholar]
- 46.Andersen TL, et al. , A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol, 2009. 174(1): p. 239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Méndez-Ferrer S, et al. , Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature, 2010. 466(7308): p. 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamazaki S, et al. , Nonmyelinating Schwann Cells Maintain Hematopoietic Stem Cell Hibernation in the Bone Marrow Niche. Cell, 2011. 147(5): p. 1146–1158. [DOI] [PubMed] [Google Scholar]
- 49.Bruns I, et al. , Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med, 2014. 20(11): p. 1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao M, et al. , Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med, 2014. 20(11): p. 1321–6. [DOI] [PubMed] [Google Scholar]
- 51.Chang MK, et al. , Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol, 2008. 181(2): p. 1232–44. [DOI] [PubMed] [Google Scholar]
- 52.Sugiyama T, et al. , Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity, 2006. 25(6): p. 977–88. [DOI] [PubMed] [Google Scholar]
- 53.Omatsu Y, et al. , The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity, 2010. 33(3): p. 387–99. [DOI] [PubMed] [Google Scholar]
- 54.Asada N, Takeishi S, and Frenette PS, Complexity of bone marrow hematopoietic stem cell niche. International Journal of Hematology, 2017. 106(1): p. 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, et al. , Identification of the haematopoietic stem cell niche and control of the niche size. Nature, 2003. 425(6960): p. 836–41. [DOI] [PubMed] [Google Scholar]
- 56.Calvi LM, et al. , Osteoblastic cells regulate the haematopoietic stem cell niche. Nature, 2003. 425(6960): p. 841–6. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson SK, Johnston HM, and Coverdale JA, Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood, 2001. 97(8): p. 2293–9. [DOI] [PubMed] [Google Scholar]
- 58.Croucher PI, McDonald MM, and Martin TJ, Bone metastasis: the importance of the neighbourhood. Nature Reviews Cancer, 2016. 16(6): p. 373–386. [DOI] [PubMed] [Google Scholar]
- 59.Kiel MJ, et al. , SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell, 2005. 121(7): p. 1109–21. [DOI] [PubMed] [Google Scholar]
- 60.Kunisaki Y, et al. , Arteriolar niches maintain haematopoietic stem cell quiescence. Nature, 2013. 502(7473): p. 637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acar M, et al. , Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature, 2015. 526(7571): p. 126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butler JM, et al. , Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell, 2010. 6(3): p. 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cackowski FC and Taichman RS, Parallels between hematopoietic stem cell and prostate cancer disseminated tumor cell regulation. Bone, 2019. 119: p. 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sipkins DA, et al. , In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature, 2005. 435(7044): p. 969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price TT, et al. , Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Science Translational Medicine, 2016. 8(340): p. 340ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghajar C, et al. , The perivascular niche regulates breast tumour dormancy. Nat Cell Biol, 2013. 15: p. 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lo Celso C, et al. , Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature, 2009. 457(7225): p. 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bowers M, et al. , Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood, 2015. 125(17): p. 2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyerinas B, et al. , Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood, 2013. 121(24): p. 4821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shiozawa Y, et al. , GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia, 2010. 12(2): p. 116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taichman RS, et al. , GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One, 2013. 8(4): p. e61873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yumoto K, et al. , Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow. Sci Rep, 2016. 6: p. 36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mishra A, et al. , Hypoxia stabilizes GAS6/Axl signaling in metastatic prostate cancer. Mol Cancer Res, 2012. 10(6): p. 703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Axelrod HD, et al. , AXL Is a Putative Tumor Suppressor and Dormancy Regulator in Prostate Cancer. Mol Cancer Res, 2019. 17(2): p. 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Datto MB, et al. , Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA, 1995. 92(12): p. 5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hannon GJ and Beach D, p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature, 1994. 371(6494): p. 257–61. [DOI] [PubMed] [Google Scholar]
- 77.Polyak K, et al. , p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev, 1994. 8(1): p. 9–22. [DOI] [PubMed] [Google Scholar]
- 78.Prunier C, et al. , TGF-β Family Signaling Pathways in Cellular Dormancy. Trends Cancer, 2019. 5(1): p. 66–78. [DOI] [PubMed] [Google Scholar]
- 79.Yin JJ, et al. , TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest, 1999. 103(2): p. 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu-Lee L-Y, et al. , Osteoblast-Secreted Factors Mediate Dormancy of Metastatic Prostate Cancer in the Bone via Activation of the TGFβRIII–p38MAPK–pS249/T252RB Pathway. Cancer Research, 2018. 78(11): p. 2911–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bragado P, et al. , TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat Cell Biol, 2013. 15(11): p. 1351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nobre AR, et al. , NG2+/Nestin+ mesenchymal stem cells dictate DTC dormancy in the bone marrow through TGFβ2. bioRxiv, 2020: p. 2020.10.22.349514. [Google Scholar]
- 83.Buijs JT, et al. , BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol, 2007. 171(3): p. 1047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kobayashi A, et al. , Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med, 2011. 208(13): p. 2641–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma S, et al. , Secreted Protein Acidic and Rich in Cysteine (SPARC) Mediates Metastatic Dormancy of Prostate Cancer in Bone. J Biol Chem, 2016. 291(37): p. 19351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agarwal P, et al. , Mesenchymal Niche-Specific Expression of Cxcl12 Controls Quiescence of Treatment-Resistant Leukemia Stem Cells. Cell Stem Cell, 2019. 24(5): p. 769–784.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ding L.and Morrison SJ, Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature, 2013. 495(7440): p. 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ono M, et al. , Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signaling, 2014. 7(332): p. ra63. [DOI] [PubMed] [Google Scholar]
- 89.Bliss SA, et al. , Mesenchymal Stem Cell-Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res, 2016. 76(19): p. 5832–5844. [DOI] [PubMed] [Google Scholar]
- 90.Kolb AD, et al. , Osteoblasts are "educated" by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment. Breast Cancer Res, 2019. 21(1): p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barkan D, et al. , Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res, 2010. 70(14): p. 5706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao H, et al. , Multi-organ Site Metastatic Reactivation Mediated by Non-canonical Discoidin Domain Receptor 1 Signaling. Cell, 2016. 166(1): p. 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson RW, et al. , Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol, 2016. 18(10): p. 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parmar K, et al. , Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA, 2007. 104(13): p. 5431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chow DC, et al. , Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J, 2001. 81(2): p. 685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yue X, Wu L, and Hu W, The regulation of leukemia inhibitory factor. Cancer Cell Microenviron, 2015. 2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kusumbe AP, Vascular niches for disseminated tumour cells in bone. Journal of Bone Oncology, 2016. 5(3): p. 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie Y, et al. , Detection of functional haematopoietic stem cell niche using real-time imaging. Nature, 2009. 457(7225): p. 97–101. [DOI] [PubMed] [Google Scholar]
- 99.Itkin T, et al. , Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature, 2016. 532(7599): p. 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mirochnik Y, Kwiatek A, and Volpert OV, Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr Drug Targets, 2008. 9(10): p. 851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carlson P, et al. , Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat Cell Biol, 2019. 21(2): p. 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katayama Y, et al. , Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell, 2006. 124(2): p. 407–21. [DOI] [PubMed] [Google Scholar]
- 103.Decker AM, et al. , Sympathetic Signaling Reactivates Quiescent Disseminated Prostate Cancer Cells in the Bone Marrow. Molecular cancer research : MCR, 2017. 15(12): p. 1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Campbell JP, et al. , Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol, 2012. 10(7): p. e1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naveiras O, et al. , Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature, 2009. 460(7252): p. 259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DiMascio L, et al. , Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol, 2007. 178(6): p. 3511–20. [DOI] [PubMed] [Google Scholar]
- 107.Cahu X, et al. , Bone marrow sites differently imprint dormancy and chemoresistance to T-cell acute lymphoblastic leukemia. Blood Advances, 2017. 1(20): p. 1760–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Z, et al. , Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget, 2015. 6(33): p. 34329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguirre-Ghiso JA, et al. , Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell, 2001. 12(4): p. 863–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghiaur G, et al. , Regulation of human hematopoietic stem cell self-renewal by the microenvironmenťs control of retinoic acid signaling. Proc Natl Acad Sci USA, 2013. 110(40): p. 16121–16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sosa MS, et al. , NR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmes. Nature Communications, 2015. 6: p. 6170–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao E, et al. , Bone marrow and the control of immunity. Cell Mol Immunol, 2012. 9(1): p. 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feuerer M, et al. , Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer, 2001. 92(1): p. 96–105. [PubMed] [Google Scholar]
- 114.Pantel K, et al. , Frequent down-regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer Res, 1991. 51(17): p. 4712–5. [PubMed] [Google Scholar]
- 115.Fujisaki J, et al. , In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature, 2011. 474(7350): p. 216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Angell T, et al. , MHC Class I Loss Is a Frequent Mechanism of Immune Escape in Papillary Thyroid Cancer That Is Reversed by Interferon and Selumetinib Treatment In Vitro. Clinical cancer research : an official journal of the American Association for Cancer Research, 2014. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bromberg JF, et al. , Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci USA, 1996. 93(15): p. 7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kortylewski M, et al. , Interferon-gamma-mediated growth regulation of melanoma cells: involvement of STAT1-dependent and STAT1-independent signals. J Invest Dermatol, 2004. 122(2): p. 414–22. [DOI] [PubMed] [Google Scholar]
- 119.Farrar JD, et al. , Cancer Dormancy. VII. A Regulatory Role for CD8+ T Cells and IFN-γ in Establishing and Maintaining the Tumor-Dormant State. J Immunol, 1999. 162(5): p. 2842. [PubMed] [Google Scholar]
- 120.Owen KL, et al. , Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Rep, 2020. 21(6): p. e50162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramasamy R, et al. , The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol, 2008. 251(2): p. 131–6. [DOI] [PubMed] [Google Scholar]
- 122.Patel SA, et al. , Mesenchymal Stem Cells Protect Breast Cancer Cells through Regulatory T Cells: Role of Mesenchymal Stem Cell-Derived TGF-β. The Journal of Immunology, 2010. 184(10): p. 5885. [DOI] [PubMed] [Google Scholar]
- 123.Hirata Y, et al. , CD150(high) Bone Marrow Tregs Maintain Hematopoietic Stem Cell Quiescence and Immune Privilege via Adenosine. Cell Stem Cell, 2018. 22(3): p. 445–453.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kristensen HB, et al. , Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J Bone Miner Res, 2013. 28(3): p. 574–85. [DOI] [PubMed] [Google Scholar]
- 125.Kollet O, et al. , Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med, 2006. 12(6): p. 657–64. [DOI] [PubMed] [Google Scholar]
- 126.Arnett TR, et al. , Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol, 2003. 196(1): p. 2–8. [DOI] [PubMed] [Google Scholar]
- 127.Lu X, et al. , VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell, 2011. 20(6): p. 701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han HH, et al. , Angiopoietin-2 promotes ER+ breast cancer cell survival in bone marrow niche. Endocrine-Related Cancer, 2016. 23(8): p. 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abrahamsen B, et al. , Cytokines and bone loss in a 5-year longitudinal study—hormone replacement therapy suppresses serum soluble interleukin-6 receptor and increases interleukin-1-receptor antagonist: the Danish Osteoporosis Prevention Study. J Bone Miner Res, 2000. 15(8): p. 1545–54. [DOI] [PubMed] [Google Scholar]
- 130.Girasole G, et al. , Oestrogens prevent the increase of human serum soluble interleukin-6 receptor induced by ovariectomy in vivo and decrease its release in human osteoblastic cells in vitro. Clin Endocrinol (Oxf), 1999. 51(6): p. 801–7. [DOI] [PubMed] [Google Scholar]
- 131.Giuliani N, et al. , Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol, 2001. 36(3): p. 547–57. [DOI] [PubMed] [Google Scholar]
- 132.Ashford R, et al. , The modern surgical and non-surgical management of appendicular skeletal metastases. Orthopaedics and Trauma, 2012. 26(3): p. 184–199. [Google Scholar]
- 133.Shiozawa Y, et al. , Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest, 2011. 121(4): p. 1298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McDonald MM, et al. , Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood, 2017. 129(26): p. 3452–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corey E, et al. , Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clin Cancer Res, 2003. 9(1): p. 295–306. [PubMed] [Google Scholar]
- 136.Jones DH, et al. , Regulation of cancer cell migration and bone metastasis by RANKL. Nature, 2006. 440(7084): p. 692–6. [DOI] [PubMed] [Google Scholar]
- 137.Scagliotti GV, et al. , Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol, 2012. 7(12): p. 1823–1829. [DOI] [PubMed] [Google Scholar]
- 138.Gnant M, et al. , Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. The Lancet, 2015. 386(9992): p. 433–443. [DOI] [PubMed] [Google Scholar]
- 139.Coleman R, et al. , Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. The Lancet Oncology, 2020. 21(1): p. 60–72. [DOI] [PubMed] [Google Scholar]
- 140.Xiao W, et al. , Risk factors and survival outcomes in patients with breast cancer and lung metastasis: a population-based study. Cancer medicine, 2018. 7(3): p. 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li J, et al. , Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). Journal of Hematology & Oncology, 2019. 12(1): p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee YT, Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol, 1983. 23(3): p. 175–80. [DOI] [PubMed] [Google Scholar]
- 143.Fabozzi SJ, Schellhammer PF, and el-Mahdi AM, Pulmonary metastases from prostate cancer. Cancer, 1995. 75(11): p. 2706–9. [DOI] [PubMed] [Google Scholar]
- 144.Dang Q, et al. , Anti-androgen enzalutamide enhances prostate cancer neuroendocrine (NE) differentiation via altering the infiltrated mast cells → androgen receptor (AR) → miRNA32 signals. Molecular Oncology, 2015. 9(7): p. 1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dong B, et al. , Influence of abiraterone acetate on neuroendocrine differentiation in chemotherapy-naive metastatic castration-resistant prostate cancer. The Prostate, 2017. 77(13): p. 1373–1380. [DOI] [PubMed] [Google Scholar]
- 146.Conteduca V, et al. , Clinical features of neuroendocrine prostate cancer. Eur J Cancer, 2019. 121: p. 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aggarwal R, et al. , Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol, 2018. 36(24): p. 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saitoh H, et al. , Metastatic patterns of prostatic cancer. Correlation between sites and number of organs involved. Cancer, 1984. 54(12): p. 3078–84. [DOI] [PubMed] [Google Scholar]
- 149.Diaz-Canton EA, et al. , Clinical course of breast cancer patients with metastases confined to the lungs treated with chemotherapy. The University of Texas M.D. Anderson Cancer Center experience and review of the literature. Ann Oncol, 1998. 9(4): p. 413–8. [DOI] [PubMed] [Google Scholar]
- 150.Barron L, Gharib SA, and Duffield JS, Lung Pericytes and Resident Fibroblasts: Busy Multitaskers. The American Journal of Pathology, 2016. 186(10): p. 2519–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Suki B, Stamenović D, and Hubmayr R, Lung parenchymal mechanics. Compr Physiol, 2011. 1(3): p. 1317–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parker AL and Cox TR, The Role of the ECM in Lung Cancer Dormancy and Outgrowth. Frontiers in Oncology, 2020. 10(1766). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stella GM, et al. , Lung-Seeking Metastases. Cancers, 2019. 11(7): p. 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Minn AJ, et al. , Genes that mediate breast cancer metastasis to lung. Nature, 2005. 436(7050): p. 518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen Q, Zhang XHF, and Massagué J, Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer cell, 2011. 20(4): p. 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gao H, et al. , The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell, 2012. 150(4): p. 764–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bornstein P, Thrombospondins function as regulators of angiogenesis. J Cell Commun Signal, 2009. 3(3–4): p. 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee JH, et al. , Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell, 2014. 156(3): p. 440–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Montagner M, et al. , Crosstalk with lung epithelial cells regulates Sfrp2-mediated latency in breast cancer dissemination. Nature Cell Biology, 2020. 22(3): p. 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barney LE, et al. , Tumor cell–organized fibronectin maintenance of a dormant breast cancer population. Science Advances, 2020. 6(11): p. eaaz4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goddard ET, et al. , Dormant tumour cells, their niches and the influence of immunity. Nat Cell Biol, 2018. 20(11): p. 1240–1249. [DOI] [PubMed] [Google Scholar]
- 162.Eyles J, et al. , Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. Journal of Clinical Investigation, 2010. 120(6): p. 2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Malladi S, et al. , Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell, 2016. 165(1): p. 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Romero I, et al. , T lymphocytes restrain spontaneous metastases in permanent dormancy. Cancer Res, 2014. 74(7): p. 1958–68. [DOI] [PubMed] [Google Scholar]
- 165.Aqbi HF, et al. , Local and distant tumor dormancy during early stage breast cancer are associated with the predominance of infiltrating T effector subsets. Breast Cancer Res, 2020. 22(1): p. 116–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Piranlioglu R, et al. , Primary tumor-induced immunity eradicates disseminated tumor cells in syngeneic mouse model. Nature Communications, 2019. 10(1): p. 1430–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barkan D, et al. , Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer research, 2008. 68(15): p. 6241–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Urosevic J, et al. , Colon cancer cells colonize the lung from established liver metastases through p38 MAPK signalling and PTHLH. Nat Cell Biol, 2014. 16(7): p. 685–94. [DOI] [PubMed] [Google Scholar]
- 169.El Rayes T, et al. , Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA, 2015. 112(52): p. 16000–16005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rachman-Tzemah C, et al. , Blocking Surgically Induced Lysyl Oxidase Activity Reduces the Risk of Lung Metastases. Cell Reports, 2017. 19(4): p. 774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Demicheli R, et al. , The effects of surgery on tumor growth: a century of investigations. Ann Oncol, 2008. 19(11): p. 1821–8. [DOI] [PubMed] [Google Scholar]
- 172.Weidenfeld K, et al. , Dormant tumor cells expressing LOXL2 acquire a stem-like phenotype mediating their transition to proliferative growth. Oncotarget, 2016. 7(44): p. 71362–71377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu L.and Zhu Y, The function and mechanisms of action of LOXL2 in cancer (Review). Int J Mol Med, 2015. 36(5): p. 1200–1204. [DOI] [PubMed] [Google Scholar]
- 174.Calvo A, et al. , Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene, 2008. 27(40): p. 5373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oskarsson T, et al. , Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nature Medicine, 2011. 17(7): p. 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Malanchi I, et al. , Interactions between cancer stem cells and their niche govern metastatic colonization. Nature, 2012. 481(7379): p. 85–89. [DOI] [PubMed] [Google Scholar]
- 177.Schouten LJ, et al. , Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer, 2002. 94(10): p. 2698–705. [DOI] [PubMed] [Google Scholar]
- 178.Barnholtz-Sloan JS, et al. , Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol, 2004. 22(14): p. 2865–72. [DOI] [PubMed] [Google Scholar]
- 179.Smedby KE, et al. , Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer, 2009. 101(11): p. 1919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gavrilovic IT and Posner JB, Brain metastases: epidemiology and pathophysiology. Journal of Neuro-Oncology, 2005. 75(1): p. 5–14. [DOI] [PubMed] [Google Scholar]
- 181.Stemmler HJ and Heinemann V, Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: a treatment challenge. Oncologist, 2008. 13(7): p. 739–50. [DOI] [PubMed] [Google Scholar]
- 182.Kolomainen DF, et al. , Epithelial ovarian cancer metastasizing to the brain: a late manifestation of the disease with an increasing incidence. J Clin Oncol, 2002. 20(4): p. 982–6. [DOI] [PubMed] [Google Scholar]
- 183.Clayton AJ, et al. , Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer, 2004. 91(4): p. 639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Delattre JY, et al. , Distribution of brain metastases. Arch Neurol, 1988. 45(7): p. 741–4. [DOI] [PubMed] [Google Scholar]
- 185.Schroeder T, et al. , Mapping distribution of brain metastases: does the primary tumor matter? J Neurooncol, 2020. 147(1): p. 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fitzgerald DP, et al. , Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis, 2008. 25(7): p. 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Poli A, et al. , NK Cells in Central Nervous System Disorders. J Immunol, 2013. 190(11): p. 5355. [DOI] [PubMed] [Google Scholar]
- 188.Smolders J, et al. , Tissue-resident memory T cells populate the human brain. Nature Communications, 2018. 9(1): p. 4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Steinbach K, et al. , Brain-resident memory T cells generated early in life predispose to autoimmune disease in mice. Science Translational Medicine, 2019. 11(498): p. eaav5519. [DOI] [PubMed] [Google Scholar]
- 190.Sampson JH, et al. , Brain immunology and immunotherapy in brain tumours. Nature Reviews Cancer, 2020. 20(1): p. 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Domingues PH, et al. , Immunophenotypic Identification and Characterization of Tumor Cells and Infiltrating Cell Populations in Meningiomas. The American Journal of Pathology, 2012. 181(5): p. 1749–1761. [DOI] [PubMed] [Google Scholar]
- 192.Alsharifi M, et al. , NK cell-mediated immunopathology during an acute viral infection of the CNS. European Journal of Immunology, 2006. 36(4): p. 887–896. [DOI] [PubMed] [Google Scholar]
- 193.Wakim LM, et al. , The Molecular Signature of Tissue Resident Memory CD8 T Cells Isolated from the Brain. J Immunol, 2012. 189(7): p. 3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Smolders J, et al. , Characteristics of differentiated CD8+ and CD4+ T cells present in the human brain. Acta Neuropathologica, 2013. 126(4): p. 525–535. [DOI] [PubMed] [Google Scholar]
- 195.Celio MR, et al. , Perineuronal nets: past and present. Trends Neurosci, 1998. 21(12): p. 510–5. [DOI] [PubMed] [Google Scholar]
- 196.Dityatev A.and Schachner M, Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci, 2003. 4(6): p. 456–68. [DOI] [PubMed] [Google Scholar]
- 197.Matthews RT, et al. , Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci, 2002. 22(17): p. 7536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu C, et al. , Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med, 2009. 15(5): p. 519–27. [DOI] [PubMed] [Google Scholar]
- 199.Daneman R.and Prat A, The blood-brain barrier. Cold Spring Harb Perspect Biol, 2015. 7(1): p. a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Engelhardt B.and Sorokin L, The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Seminars in Immunopathology, 2009. 31(4): p. 497–511. [DOI] [PubMed] [Google Scholar]
- 201.Louveau A, et al. , Structural and functional features of central nervous system lymphatic vessels. Nature, 2015. 523(7560): p. 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Aspelund A, et al. , A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med, 2015. 212(7): p. 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Izraely S, et al. , The metastatic microenvironment: Brain-residing melanoma metastasis and dormant micrometastasis. International Journal of Cancer, 2012. 131(5): p. 1071–1082. [DOI] [PubMed] [Google Scholar]
- 204.Hirata E, et al. , The Brain Microenvironment Induces DNMT1 Suppression and Indolence of Metastatic Cancer Cells. iScience, 2020. 23(9): p. 101480–101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sarkar S, et al. , Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat Neurosci, 2014. 17(1): p. 46–55. [DOI] [PubMed] [Google Scholar]
- 206.Sarkar S, et al. , Microglia induces Gas1 expression in human brain tumor-initiating cells to reduce tumorigenecity. Sci Rep, 2018. 8(1): p. 15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sarkar S, et al. , ADAM-9 is a novel mediator of tenascin-C-stimulated invasiveness of brain tumor-initiating cells. Neuro Oncol, 2015. 17(8): p. 1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kienast Y, et al. , Real-time imaging reveals the single steps of brain metastasis formation. Nat Med, 2010. 16(1): p. 116–22. [DOI] [PubMed] [Google Scholar]
- 209.Lorger M.and Felding-Habermann B, Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol, 2010. 176(6): p. 2958–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Noltenius C.and Noltenius H, Dormant tumor cells in liver and brain: An autopsy study on metastasizing tumors. Pathology - Research and Practice, 1985. 179(4): p. 504–511. [DOI] [PubMed] [Google Scholar]
- 211.Heyn C, et al. , In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med, 2006. 56(5): p. 1001–10. [DOI] [PubMed] [Google Scholar]
- 212.Bos PD, et al. , Genes that mediate breast cancer metastasis to the brain. Nature, 2009. 459(7249): p. 1005–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sevenich L, et al. , Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol, 2014. 16(9): p. 876–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Klein A, et al. , The metastatic microenvironment: Brain-derived soluble factors alter the malignant phenotype of cutaneous and brain-metastasizing melanoma cells. International Journal of Cancer, 2012. 131(11): p. 2509–2518. [DOI] [PubMed] [Google Scholar]
- 215.Rodrigues G, et al. , Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nature Cell Biology, 2019. 21(11): p. 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Paku S, et al. , Organ-specificity of the extravasation process: an ultrastructural study. Clin Exp Metastasis, 2000. 18(6): p. 481–92. [DOI] [PubMed] [Google Scholar]
- 217.Valiente M, et al. , Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell, 2014. 156(5): p. 1002–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Er EE, et al. , Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nature Cell Biology, 2018. 20(8): p. 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim SW, et al. , Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro Oncol, 2014. 16(12): p. 1585–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang X, et al. , Astrocytic Fas ligand expression is required to induce T-cell apoptosis and recovery from experimental autoimmune encephalomyelitis. Eur J Immunol, 2013. 43(1): p. 115–24. [DOI] [PubMed] [Google Scholar]
- 221.Wasilewski D, et al. , Reactive Astrocytes in Brain Metastasis. Front Oncol, 2017. 7: p. 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schwartz H, et al. , Incipient Melanoma Brain Metastases Instigate Astrogliosis and Neuroinflammation. Cancer Research, 2016. 76(15): p. 4359. [DOI] [PubMed] [Google Scholar]
- 223.Yu T, et al. , Delivery of MGMT mRNA to glioma cells by reactive astrocyte-derived exosomes confers a temozolomide resistance phenotype. Cancer Lett, 2018. 433: p. 210–220. [DOI] [PubMed] [Google Scholar]
- 224.Chen Q, et al. , Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature, 2016. 533(7604): p. 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kim SJ, et al. , Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia, 2011. 13(3): p. 286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lin Q, et al. , Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia, 2010. 12(9): p. 748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Seike T, et al. , Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis, 2011. 28(1): p. 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sartorius CA, et al. , Estrogen promotes the brain metastatic colonization of triple negative breast cancer cells via an astrocyte-mediated paracrine mechanism. Oncogene, 2016. 35(22): p. 2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Neman J, et al. , Co-evolution of breast-to-brain metastasis and neural progenitor cells. Clin Exp Metastasis, 2013. 30(6): p. 753–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Piomelli D, Astarita G, and Rapaka R, A neuroscientisťs guide to lipidomics. Nat Rev Neurosci, 2007. 8(10): p. 743–54. [DOI] [PubMed] [Google Scholar]
- 231.Jin X, et al. , A metastasis map of human cancer cell lines. Nature, 2020. 588(7837): p. 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.He BP, et al. , Differential reactions of microglia to brain metastasis of lung cancer. Mol Med, 2006. 12(7–8): p. 161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Berghoff AS, et al. , Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin Exp Metastasis, 2013. 30(1): p. 69–81. [DOI] [PubMed] [Google Scholar]
- 234.Brantley EC, et al. , Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl Oncol, 2010. 3(6): p. 380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pukrop T, et al. , Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia, 2010. 58(12): p. 1477–89. [DOI] [PubMed] [Google Scholar]
- 236.Qiao S, et al. , Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. J Neuroinflammation, 2019. 16(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Flüh C, et al. , Dormancy and NKG2D system in brain metastases: Analysis of immunogenicity. Int J Mol Med, 2020. 45(2): p. 298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gemechu JM and Bentivoglio M, T Cell Recruitment in the Brain during Normal Aging. Front Cell Neurosci, 2012. 6: p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Budczies J, et al. , The landscape of metastatic progression patterns across major human cancers. Oncotarget, 2015. 6(1): p. 570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.diSibio G.and French SW, Metastatic Patterns of Cancers: Results From a Large Autopsy Study. Archives of Pathology & Laboratory Medicine, 2008. 132(6): p. 931–939. [DOI] [PubMed] [Google Scholar]
- 241.Hess KR, et al. , Metastatic patterns in adenocarcinoma. Cancer, 2006. 106(7): p. 1624–33. [DOI] [PubMed] [Google Scholar]
- 242.Kokudo N, et al. , Surgery for multiple hepatic colorectal metastases. J Hepatobiliary Pancreat Surg, 2004. 11(2): p. 84–91. [DOI] [PubMed] [Google Scholar]
- 243.Rose DM, et al. , Surgical Resection for Metastatic Melanoma to the Liver: The John Wayne Cancer Institute and Sydney Melanoma Unit Experience. Archives of Surgery, 2001. 136(8): p. 950–955. [DOI] [PubMed] [Google Scholar]
- 244.Chow FC-L and Chok KS-H, Colorectal liver metastases: An update on multidisciplinary approach. World journal of hepatology, 2019. 11(2): p. 150–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Paschos KA and Bird NC, Liver Regeneration and its Impact on Post-hepatectomy Metastatic Tumour Recurrence. Anticancer Research, 2010. 30(6): p. 2161–2170. [PubMed] [Google Scholar]
- 246.Jones RP, et al. , Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br J Surg, 2012. 99(4): p. 477–86. [DOI] [PubMed] [Google Scholar]
- 247.Gleisner AL, et al. , Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer, 2007. 110(11): p. 2484–92. [DOI] [PubMed] [Google Scholar]
- 248.Ruiz A, et al. , Surgical resection versus systemic therapy for breast cancer liver metastases: Results of a European case matched comparison. Eur J Cancer, 2018. 95: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 249.Selzner M, et al. , Liver metastases from breast cancer: Long-term survival after curative resection. Surgery, 2000. 127(4): p. 383–389. [DOI] [PubMed] [Google Scholar]
- 250.Adam R, et al. , Hepatic Resection for Noncolorectal Nonendocrine Liver Metastases: Analysis of 1452 Patients and Development of a Prognostic Model. Annals of Surgery, 2006. 244(4): p. 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ikawa K, et al. , Genetic detection of liver micrometastases that are undetectable histologically. J Surg Res, 2002. 106(1): p. 124–30. [DOI] [PubMed] [Google Scholar]
- 252.Pommier A, et al. , Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science, 2018. 360(6394). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Aqbi HF, et al. , Local and distant tumor dormancy during early stage breast cancer are associated with the predominance of infiltrating T effector subsets. Breast Cancer Research, 2020. 22(1): p. 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Clark AM, et al. , A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab on a chip, 2016. 17(1): p. 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Panis Y, et al. , Dormant liver metastases: an experimental study. BJS (British Journal of Surgery), 1992. 79(3): p. 221–223. [DOI] [PubMed] [Google Scholar]
- 256.Kan Z.and Madoff DC, Liver anatomy: microcirculation of the liver. Semin Intervent Radiol, 2008. 25(2): p. 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Martin MD, et al. , Rapid extravasation and establishment of breast cancer micrometastases in the liver microenvironment. Mol Cancer Res, 2010. 8(10): p. 1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Brodt P, Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin Cancer Res, 2016. 22(24): p. 5971–5982. [DOI] [PubMed] [Google Scholar]
- 259.Aizarani N, et al. , A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature, 2019. 572(7768): p. 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vekemans K.and Braet F, Structural and functional aspects of the liver and liver sinusoidal cells in relation to colon carcinoma metastasis. World Journal of Gastroenterology, 2005. 11(33): p. 5095–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wisse E, An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. Journal of Ultrastructure Research, 1970. 31(1): p. 125–150. [DOI] [PubMed] [Google Scholar]
- 262.Mathew RP and Venkatesh SK, Liver vascular anatomy: a refresher. Abdominal Radiology, 2018. 43(8): p. 1886–1895. [DOI] [PubMed] [Google Scholar]
- 263.Friedman SL, et al. , Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA, 1985. 82(24): p. 8681–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rockey DC, The Molecular Basis of Portal Hypertension. Trans Am Clin Climatol Assoc, 2017. 128: p. 330–345. [PMC free article] [PubMed] [Google Scholar]
- 265.Kietzmann T, Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biology, 2017. 11: p. 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jungermann K.and Kietzmann T, Role of oxygen in the zonation of carbohydrate metabolism and gene expression in liver. Kidney International, 1997. 51(2): p. 402–412. [DOI] [PubMed] [Google Scholar]
- 267.Martinez-Hernandez A.and Amenta PS, The extracellular matrix in hepatic regeneration. Faseb J, 1995. 9(14): p. 1401–10. [DOI] [PubMed] [Google Scholar]
- 268.Su T, et al. , Single-cell transcriptomics reveals zone-specific alterations of liver sinusoidal endothelial cells in cirrhosis. Cellular and Molecular Gastroenterology and Hepatology, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shachaf CM, et al. , MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature, 2004. 431(7012): p. 1112–7. [DOI] [PubMed] [Google Scholar]
- 270.Duarte S, et al. , Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biology, 2015. 44-46: p. 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rescan PY, et al. , Distribution and origin of the basement membrane component perlecan in rat liver and primary hepatocyte culture. The American Journal of Pathology, 1993. 142(1): p. 199–208. [PMC free article] [PubMed] [Google Scholar]
- 272.Burnier JV, et al. , Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene, 2011. 30(35): p. 3766–83. [DOI] [PubMed] [Google Scholar]
- 273.Vaniotis G, et al. , Collagen IV-conveyed signals can regulate chemokine production and promote liver metastasis. Oncogene, 2018. 37(28): p. 3790–3805. [DOI] [PubMed] [Google Scholar]
- 274.Albrengues J, et al. , Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science, 2018. 361(6409): p. eaao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Franses JW, et al. , Stromal Endothelial Cells Directly Influence Cancer Progression. Science Translational Medicine, 2011. 3(66): p. 66ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ma B.and Wells A, The mitogen-activated protein (MAP) kinases p38 and extracellular signal-regulated kinase (ERK) are involved in hepatocyte-mediated phenotypic switching in prostate cancer cells. J Biol Chem, 2014. 289(16): p. 11153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Chao Y, et al. , Hepatocyte induced re-expression of E-cadherin in breast and prostate cancer cells increases chemoresistance. Clin Exp Metastasis, 2012. 29(1): p. 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yates CC, et al. , Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer, 2007. 96(8): p. 1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ma B, et al. , Liver protects metastatic prostate cancer from induced death by activating E-cadherin signaling. Hepatology, 2016. 64(5): p. 1725–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Stessels F, et al. , Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer, 2004. 90(7): p. 1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Taylor DP, et al. , Hepatic nonparenchymal cells drive metastatic breast cancer outgrowth and partial epithelial to mesenchymal transition. Breast Cancer Research and Treatment, 2014. 144(3): p. 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.De Minicis S, et al. , Gene Expression Profiles During Hepatic Stellate Cell Activation in Culture and In Vivo. Gastroenterology, 2007. 132(5): p. 1937–1946. [DOI] [PubMed] [Google Scholar]
- 283.Wendel C, et al. , CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PLoS One, 2012. 7(1): p. e30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Deleve LD, Wang X, and Guo Y, Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology (Baltimore, Md.), 2008. 48(3): p. 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.DeLeve LD, et al. , Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2004. 287(4): p. G757–G763. [DOI] [PubMed] [Google Scholar]
- 286.Lenk L, et al. , The hepatic microenvironment essentially determines tumor cell dormancy and metastatic outgrowth of pancreatic ductal adenocarcinoma. Oncoimmunology, 2017. 7(1): p. e1368603-e1368603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kondo T, et al. , The impact of hepatic fibrosis on the incidence of liver metastasis from colorectal cancer. Br J Cancer, 2016. 115(1): p. 34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Miarka L, et al. , The Hepatic Microenvironment and TRAIL-R2 Impact Outgrowth of Liver Metastases in Pancreatic Cancer after Surgical Resection. Cancers (Basel), 2019. 11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hu X, et al. , Prediction of hepatic metastasis and relapse in colorectal cancers based on concordance analyses with liver fibrosis scores. Clin Transl Med, 2020. 9(1): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Harun N, et al. , Liver Regeneration Stimulates Tumor Metastases. Journal of Surgical Research, 2007. 138(2): p. 284–290. [DOI] [PubMed] [Google Scholar]
- 291.Bertolotti M, et al. , Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol, 2014. 20(39): p. 14185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tsuchida T.and Friedman SL, Mechanisms of hepatic stellate cell activation. Nature Reviews Gastroenterology & Hepatology, 2017. 14(7): p. 397–411. [DOI] [PubMed] [Google Scholar]
- 293.Gressner AM, et al. , Roles of TGF-beta in hepatic fibrosis. Front Biosci, 2002. 7: p. d793–807. [DOI] [PubMed] [Google Scholar]
- 294.Bissell DM, et al. , Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest, 1995. 96(1): p. 447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Pinzani M, et al. , Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest, 1989. 84(6): p. 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Schumacher JD and Guo GL, Regulation of Hepatic Stellate Cells and Fibrogenesis by Fibroblast Growth Factors. BioMed Research International, 2016. 2016: p. 8323747–8323747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Olsen AL, et al. , Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2011. 301(1): p. G110–G118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Rojkind M, Giambrone M-A, and Biempica L, Collagen Types in Normal and Cirrhotic Liver. Gastroenterology, 1979. 76(4): p. 710–719. [PubMed] [Google Scholar]
- 299.Murphy FR, et al. , Inhibition of Apoptosis of Activated Hepatic Stellate Cells by Tissue Inhibitor of Metalloproteinase-1 Is Mediated via Effects on Matrix Metalloproteinase Inhibition: IMPLICATIONS FOR REVERSIBILITY OF LIVER FIBROSIS. Journal of Biological Chemistry, 2002. 277(13): p. 11069–11076. [DOI] [PubMed] [Google Scholar]
- 300.Herbst H, et al. , Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol, 1997. 150(5): p. 1647–59. [PMC free article] [PubMed] [Google Scholar]
- 301.DeLeve LD, Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology (Baltimore, Md.), 2015. 61(5): p. 1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Friedman SL, The Cellular Basis of Hepatic Fibrosis -- Mechanisms and Treatment Strategies. New England Journal of Medicine, 1993. 328(25): p. 1828–1835. [DOI] [PubMed] [Google Scholar]
- 303.Kuriyama N, et al. , Tenascin-C: A novel mediator of hepatic ischemia and reperfusion injury. Hepatology, 2011. 54(6): p. 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Murakami T, et al. , Tenascin C in colorectal cancer stroma is a predictive marker for liver metastasis and is a potent target of miR-198 as identified by microRNA analysis. British Journal of Cancer, 2017. 117(9): p. 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Cox TR, et al. , LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer research, 2013. 73(6): p. 1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Khazali AS, Clark AM, and Wells A, Inflammatory cytokine IL-8/CXCL8 promotes tumour escape from hepatocyte-induced dormancy. British Journal of Cancer, 2018. 118(4): p. 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Schwabe RF, Bataller R, and Brenner DA, Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol, 2003. 285(5): p. G949–58. [DOI] [PubMed] [Google Scholar]
- 308.Chen CH, et al. , In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology, 2006. 44(5): p. 1171–81. [DOI] [PubMed] [Google Scholar]
- 309.Yu MC, et al. , Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology, 2004. 40(6): p. 1312–21. [DOI] [PubMed] [Google Scholar]
- 310.Hammel P, et al. , Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med, 2001. 344(6): p. 418–23. [DOI] [PubMed] [Google Scholar]
- 311.Poynard T, et al. , Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology, 2002. 122(5): p. 1303–1313. [DOI] [PubMed] [Google Scholar]
- 312.Nathwani R, et al. , A Review of Liver Fibrosis and Emerging Therapies. European Medical Journal, 2019. 4(4): p. 105–116. [Google Scholar]
- 313.Grzelak CA and Ghajar CM, Metastasis 'systems' biology: how are macro-environmental signals transmitted into microenvironmental cues for disseminated tumor cells? Curr Opin Cell Biol, 2017. 48: p. 79–86. [DOI] [PubMed] [Google Scholar]
- 314.Ding N, et al. , A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell, 2013. 153(3): p. 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Melhem A, et al. , Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. Journal of Hepatology, 2006. 45(1): p. 60–71. [DOI] [PubMed] [Google Scholar]
- 316.Radaeva S, et al. , Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology, 2006. 130(2): p. 435–52. [DOI] [PubMed] [Google Scholar]
- 317.Shen K, et al. , Activation of innate immunity (NK/IFN-gamma) in rat allogeneic liver transplantation: contribution to liver injury and suppression of hepatocyte proliferation. American journal of physiology. Gastrointestinal and liver physiology, 2008. 294(4): p. G1070–G1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Perego M, et al. , Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Science Translational Medicine, 2020. 12(572): p. eabb5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Chen Z, et al. , Surgical stress and cancer progression: the twisted tango. Molecular Cancer, 2019. 18(1): p. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Böhm F, et al. , Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med, 2010. 2(8): p. 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ding BS, et al. , HDL activation of endothelial sphingosine-1-phosphate receptor-1 (S1P(1)) promotes regeneration and suppresses fibrosis in the liver. JCI Insight, 2016. 1(21): p. e87058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ding BS, et al. , Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature, 2014. 505(7481): p. 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yang L, et al. , NF-κB activation in Kupffer cells after partial hepatectomy. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2005. 289(3): p. G530–G538. [DOI] [PubMed] [Google Scholar]
- 324.Selzner N, et al. , ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell–dependent release of TNF-α/IL-6 in mice. Gastroenterology, 2003. 124(3): p. 692–700. [DOI] [PubMed] [Google Scholar]
- 325.Steiling H, et al. , Fibroblast growth factor receptor signalling is crucial for liver homeostasis and regeneration. Oncogene, 2003. 22(28): p. 4380–8. [DOI] [PubMed] [Google Scholar]
- 326.Stolz DB, et al. , Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res, 1999. 59(16): p. 3954–60. [PubMed] [Google Scholar]
- 327.Oe S, et al. , Intact signaling by transforming growth factor beta is not required for termination of liver regeneration in mice. Hepatology, 2004. 40(5): p. 1098–105. [DOI] [PubMed] [Google Scholar]
- 328.Riddiough GE, et al. , Liver regeneration and liver metastasis. Seminars in Cancer Biology, 2020. [DOI] [PubMed] [Google Scholar]
- 329.Fisher B, et al. , Ten year follow-up results of patients with carcinoma of the breast in a cooperative clinical trial evaluating surgical adjuvant chemotherapy. Surg Gynecol Obstet, 1975. 140(4): p. 528–34. [PubMed] [Google Scholar]
- 330.Rosen PP, et al. , Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma: a study of 644 patients with median follow-up of 18 years. J Clin Oncol, 1989. 7(9): p. 1239–51. [DOI] [PubMed] [Google Scholar]
- 331.Krag DN, et al. , Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol, 2007. 8(10): p. 881–8. [DOI] [PubMed] [Google Scholar]
- 332.Kim JP, et al. , Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer, 1998. 1(2): p. 125–133. [DOI] [PubMed] [Google Scholar]
- 333.Datta K, et al. , Mechanism of lymph node metastasis in prostate cancer. Future Oncology, 2010. 6(5): p. 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Cho JK, et al. , Significance of lymph node metastasis in cancer dissemination of head and neck cancer. Transl Oncol, 2015. 8(2): p. 119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Giuliano AE, et al. , Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA, 2011. 306(4): p. 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ullah I, et al. , Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J Clin Invest, 2018. 128(4): p. 1355–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Fisher B.and Fisher ER, Transmigration of Lymph Nodes by Tumor Cells. Science, 1966. 152(3727): p. 1397. [DOI] [PubMed] [Google Scholar]
- 338.Brown M, et al. , Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science, 2018. 359(6382): p. 1408–1411. [DOI] [PubMed] [Google Scholar]
- 339.Pereira ER, et al. , Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science, 2018. 359(6382): p. 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Naxerova K, et al. , Origins of lymphatic and distant metastases in human colorectal cancer. Science, 2017. 357(6346): p. 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kodama T, Mori S, and Nose M, Tumor cell invasion from the marginal sinus into extranodal veins during early-stage lymph node metastasis can be a starting point for hematogenous metastasis. J Canc Met Treat, 2018. 4(56). [Google Scholar]
- 342.Fisher B.and Fisher ER, Significance of the interrelationship of the lymph and blood vascular systems in tumor cell dissemination. Prog Clin Cancer, 1970. 4: p. 84–96. [PubMed] [Google Scholar]
- 343.Coste A, et al. , Hematogenous Dissemination of Breast Cancer Cells From Lymph Nodes Is Mediated by Tumor MicroEnvironment of Metastasis Doorways. Front Oncol, 2020. 10: p. 571100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.de Boer M, et al. , Micrometastases or Isolated Tumor Cells and the Outcome of Breast Cancer. New England Journal of Medicine, 2009. 361(7): p. 653–663. [DOI] [PubMed] [Google Scholar]
- 345.Liikanen JS, et al. , Prognostic value of isolated tumour cells in sentinel lymph nodes in early-stage breast cancer: a prospective study. British Journal of Cancer, 2018. 118(11): p. 1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Weaver DL, et al. , Effect of Occult Metastases on Survival in Node-Negative Breast Cancer. New England Journal of Medicine, 2011. 364(5): p. 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Leidenius MH, et al. , Influence of isolated tumor cells in sentinel nodes on outcome in small, node-negative (pT1N0M0) breast cancer. Ann Surg Oncol, 2010. 17(1): p. 254–62. [DOI] [PubMed] [Google Scholar]
- 348.Querzoli P, et al. , Axillary Lymph Node Nanometastases Are Prognostic Factors for Disease-Free Survival and Metastatic Relapse in Breast Cancer Patients. Clinical Cancer Research, 2006. 12(22): p. 6696–6701. [DOI] [PubMed] [Google Scholar]
- 349.Andersson Y, et al. , Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Breast Cancer Research and Treatment, 2018. 171(2): p. 359–369. [DOI] [PubMed] [Google Scholar]
- 350.Faerden AE, et al. , Lymph node micrometastases and isolated tumor cells influence survival in stage I and II colon cancer. Dis Colon Rectum, 2011. 54(2): p. 200–6. [DOI] [PubMed] [Google Scholar]
- 351.Saphir O.and Amromin GD, Obscure axillary lymph-node metastasis in carcinoma of the breast. Cancer, 1948. 1(2): p. 238–241. [DOI] [PubMed] [Google Scholar]
- 352.Cummings MC, et al. , Occult axillary lymph node metastases in breast cancer do matter: results of 10-year survival analysis. Am J Surg Pathol, 2002. 26(10): p. 1286–95. [DOI] [PubMed] [Google Scholar]
- 353.Demicheli R, et al. , Microscopic tumor foci in axillary lymph nodes may reveal the recurrence dynamics of breast cancer. Cancer Communications, 2019. 39(1): p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ghajar CM, Metastasis prevention by targeting the dormant niche. Nature Reviews Cancer, 2015. 15(4): p. 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Osella-Abate S, et al. , Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer, 2015. 136(10): p. 2453–7. [DOI] [PubMed] [Google Scholar]
- 356.Scheri RP, et al. , Isolated Tumor Cells in the Sentinel Node Affect Long-Term Prognosis of Patients with Melanoma. Annals of Surgical Oncology, 2007. 14(10): p. 2861–2866. [DOI] [PubMed] [Google Scholar]
- 357.Yasuda T, et al. , Recurrence of Cutaneous and Lymph Node Metastases 12 Years after Radical Total Gastrectomy for Stage IIA Gastric Cancer. Intern Med, 2020. 59(11): p. 1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Willard-Mack CL, Normal structure, function, and histology of lymph nodes. Toxicol Pathol, 2006. 34(5): p. 409–24. [DOI] [PubMed] [Google Scholar]
- 359.Malhotra D, et al. , Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nature Immunology, 2012. 13(5): p. 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Sixt M, et al. , The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity, 2005. 22(1): p. 19–29. [DOI] [PubMed] [Google Scholar]
- 361.Kaldjian EP, et al. , Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. International Immunology, 2001. 13(10): p. 1243–1253. [DOI] [PubMed] [Google Scholar]
- 362.Bajénoff M, et al. , Stromal Cell Networks Regulate Lymphocyte Entry, Migration, and Territoriality in Lymph Nodes. Immunity, 2006. 25(6): p. 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Herzog BH, et al. , Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature, 2013. 502(7469): p. 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Astarita JL, et al. , The CLEC-2–podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nature Immunology, 2015. 16(1): p. 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Roozendaal R, Mebius RE, and Kraal G, The conduit system of the lymph node. Int Immunol, 2008. 20(12): p. 1483–7. [DOI] [PubMed] [Google Scholar]
- 366.Gretz JE, et al. , Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med, 2000. 192(10): p. 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Junt T, et al. , Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature, 2007. 450(7166): p. 110–114. [DOI] [PubMed] [Google Scholar]
- 368.Gerner Michael Y., P. Torabi-Parizi, and Ronald N. Germain, Strategically Localized Dendritic Cells Promote Rapid T Cell Responses to Lymph-Borne Particulate Antigens. Immunity, 2015. 42(1): p. 172–185. [DOI] [PubMed] [Google Scholar]
- 369.Ager A, High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function. Frontiers in Immunology, 2017. 8(45). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Skobe M, et al. , Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med, 2001. 7(2): p. 192–8. [DOI] [PubMed] [Google Scholar]
- 371.Qu C, et al. , Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med, 2004. 200(10): p. 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Das S, et al. , Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med, 2013. 210(8): p. 1509–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Gunn MD, et al. , Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med, 1999. 189(3): p. 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Luther SA, et al. , Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol, 2002. 169(1): p. 424–33. [DOI] [PubMed] [Google Scholar]
- 375.Cyster JG, Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annual Review of Immunology, 2004. 23(1): p. 127–159. [DOI] [PubMed] [Google Scholar]
- 376.Müller A, et al. , Involvement of chemokine receptors in breast cancer metastasis. Nature, 2001. 410(6824): p. 50–56. [DOI] [PubMed] [Google Scholar]
- 377.Maolake A, et al. , Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR7 upregulation. Cancer Science, 2018. 109(5): p. 1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Shields JD, et al. , Chemokine-mediated migration of melanoma cells towards lymphatics – a mechanism contributing to metastasis. Oncogene, 2007. 26(21): p. 2997–3005. [DOI] [PubMed] [Google Scholar]
- 379.Carr I, Lymphatic metastasis. Cancer Metastasis Rev, 1983. 2(3): p. 307–17. [DOI] [PubMed] [Google Scholar]
- 380.Hoshida T, et al. , Imaging Steps of Lymphatic Metastasis Reveals That Vascular Endothelial Growth Factor-C Increases Metastasis by Increasing Delivery of Cancer Cells to Lymph Nodes: Therapeutic Implications. Cancer Research, 2006. 66(16): p. 8065. [DOI] [PubMed] [Google Scholar]
- 381.Dewar DJ, et al. , The Microanatomic Location of Metastatic Melanoma in Sentinel Lymph Nodes Predicts Nonsentinel Lymph Node Involvement. Journal of Clinical Oncology, 2004. 22(16): p. 3345–3349. [DOI] [PubMed] [Google Scholar]
- 382.van Akkooi AC, et al. , High positive sentinel node identification rate by EORTC melanoma group protocol. Prognostic indicators of metastatic patterns after sentinel node biopsy in melanoma. Eur J Cancer, 2006. 42(3): p. 372–80. [DOI] [PubMed] [Google Scholar]
- 383.Atula T, et al. , Micrometastases and isolated tumour cells in sentinel lymph nodes in oral and oropharyngeal squamous cell carcinoma. European Journal of Surgical Oncology (EJSO), 2009. 35(5): p. 532–538. [DOI] [PubMed] [Google Scholar]
- 384.Sahin AA, Guray M, and Hunt KK, Identification and biologic significance of micrometastases in axillary lymph nodes in patients with invasive breast cancer. Arch Pathol Lab Med, 2009. 133(6): p. 869–78. [DOI] [PubMed] [Google Scholar]
- 385.van Deurzen CHM, et al. , The microanatomic location of metastatic breast cancer in sentinel lymph nodes predicts nonsentinel lymph node involvement. Annals of surgical oncology, 2008. 15(5): p. 1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Kerjaschki D, et al. , Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest, 2011. 121(5): p. 2000–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Oliver G.and Srinivasan RS, Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development, 2010. 137(3): p. 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Lee E, Pandey NB, and Popel AS, Lymphatic endothelial cells support tumor growth in breast cancer. Scientific Reports, 2014. 4(1): p. 5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Lee E, et al. , Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun, 2014. 5: p. 4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Rodda LB, et al. , Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity. Immunity, 2018. 48(5): p. 1014–1028.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Brulois K, et al. , A molecular map of murine lymph node blood vascular endothelium at single cell resolution. Nature Communications, 2020. 11(1): p. 3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Fujimoto N, et al. , Single-cell mapping reveals new markers and functions of lymphatic endothelial cells in lymph nodes. PLOS Biology, 2020. 18(4): p. e3000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Takeda A, et al. , Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity, 2019. 51(3): p. 561–572.e5. [DOI] [PubMed] [Google Scholar]
- 394.Xiang M, et al. , A Single-Cell Transcriptional Roadmap of the Mouse and Human Lymph Node Lymphatic Vasculature. Frontiers in Cardiovascular Medicine, 2020. 7(52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Cifarelli V.and Eichmann A, The Intestinal Lymphatic System: Functions and Metabolic Implications. Cellular and Molecular Gastroenterology and Hepatology, 2019. 7(3): p. 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Fessler MB and Parks JS, Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol, 2011. 187(4): p. 1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Lee C. k., et al. , Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science, 2019. 363(6427): p. 644–649. [DOI] [PubMed] [Google Scholar]
- 398.Mueller SN and Germain RN, Stromal cell contributions to the homeostasis and functionality of the immune system. Nature Reviews Immunology, 2009. 9(9): p. 618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Yu M, et al. , Fibroblastic reticular cells of the lymphoid tissues modulate T cell activation threshold during homeostasis via hyperactive cyclooxygenase-2/prostaglandin E2 axis. Scientific Reports, 2017. 7(1): p. 3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Christiansen AJ, et al. , Lymphatic endothelial cells attenuate inflammation via suppression of dendritic cell maturation. Oncotarget, 2016. 7(26): p. 39421–39435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Rouhani SJ, et al. , Regulation of T-cell Tolerance by Lymphatic Endothelial Cells. J Clin Cell Immunol, 2014. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Müller M, et al. , EblacZ tumor dormancy in bone marrow and lymph nodes: active control of proliferating tumor cells by CD8+ immune T cells. Cancer Res, 1998. 58(23): p. 5439–46. [PubMed] [Google Scholar]
- 403.Lukacs-Kornek V, et al. , Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol, 2011. 12(11): p. 1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Dubrot J, et al. , Lymph node stromal cells acquire peptide–MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J Exp Med, 2014. 211(6): p. 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Tewalt EF, et al. , Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood, 2012. 120(24): p. 4772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Rouhani SJ, et al. , Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nature Communications, 2015. 6(1): p. 6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Magnusson FC, et al. , Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology, 2008. 134(4): p. 1028–37. [DOI] [PubMed] [Google Scholar]
- 408.Hirosue S, et al. , Steady-State Antigen Scavenging, Cross-Presentation, and CD8+ T Cell Priming: A New Role for Lymphatic Endothelial Cells. J Immunol, 2014. 192(11): p. 5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Lund Amanda W., et al. , VEGF-C Promotes Immune Tolerance in B16 Melanomas and Cross-Presentation of Tumor Antigen by Lymph Node Lymphatics. Cell Reports, 2012. 1(3): p. 191–199. [DOI] [PubMed] [Google Scholar]
- 410.Fletcher AL, Acton SE, and Knoblich K, Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol, 2015. 15(6): p. 350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Dasoveanu DC, et al. , Regulation of Lymph Node Vascular-Stromal Compartment by Dendritic Cells. Trends in Immunology, 2016. 37(11): p. 764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Benahmed F, et al. , Multiple CD11c+ Cells Collaboratively Express IL-1β To Modulate Stromal Vascular Endothelial Growth Factor and Lymph Node Vascular–Stromal Growth. J Immunol, 2014. 192(9): p. 4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Chyou S, et al. , Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and/or B cells. J Immunol, 2011. 187(11): p. 5558–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Angeli V, et al. , B Cell-Driven Lymphangiogenesis in Inflamed Lymph Nodes Enhances Dendritic Cell Mobilization. Immunity, 2006. 24(2): p. P203–215. [DOI] [PubMed] [Google Scholar]
- 415.Acton SE, et al. , Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature, 2014. 514(7523): p. 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Yang C-Y, et al. , Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc Natl Acad Sci USA, 2014. 111(1): p. E109–E118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Martinez VG, et al. , Fibroblastic Reticular Cells Control Conduit Matrix Deposition during Lymph Node Expansion. Cell Reports, 2019. 29(9): p. 2810–2822.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Chyou S, et al. , Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol, 2008. 181(6): p. 3887–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Webster B, et al. , Regulation of lymph node vascular growth by dendritic cells. J Exp Med, 2006. 203(8): p. 1903–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Halin C, et al. , VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood, 2007. 110(9): p. 3158–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Crist SB and Ghajar CM, When a House Is Not a Home: A Survey of Antimetastatic Niches and Potential Mechanisms of Disseminated Tumor Cell Suppression. Annu Rev Pathol, 2020. [DOI] [PubMed] [Google Scholar]