Abstract
Severe SARS-CoV-2 infection causes systemic inflammation, cytokine storm, and hypercytokinemia due to activation of the release of pro-inflammatory cytokines that have been associated with case-fatality rate. The immune overreaction and cytokine storm in the infection caused by SARS-CoV-2 may be linked to NLRP3 inflammasome activation which has supreme importance in human innate immune response mainly against viral infections. In SARS-CoV-2 infection, NLRP3 inflammasome activation results in the stimulation and synthesis of natural killer cells (NKs), NFκB, and interferon-gamma (INF-γ), while inhibiting IL-33 expression. Various efforts have identified selective inhibitors of NLRP3 inflammasome. To achieve this, studies are exploring the screening of natural compounds and/or repurposing of clinical drugs to identify potential NLRP3 inhibitors. NLRP3 inflammasome inhibitors are expected to suppress exaggerated immune reaction and cytokine storm-induced-organ damage in SARS-CoV-2 infection. Therefore, NLRP3 inflammasome inhibitors could mitigate the immune-overreaction and hypercytokinemia in Covid-19 infection.
Keywords: Covid-19, NLRP3 inflammasomes, SARS-CoV-2, Therapeutic inhibitors
Introduction
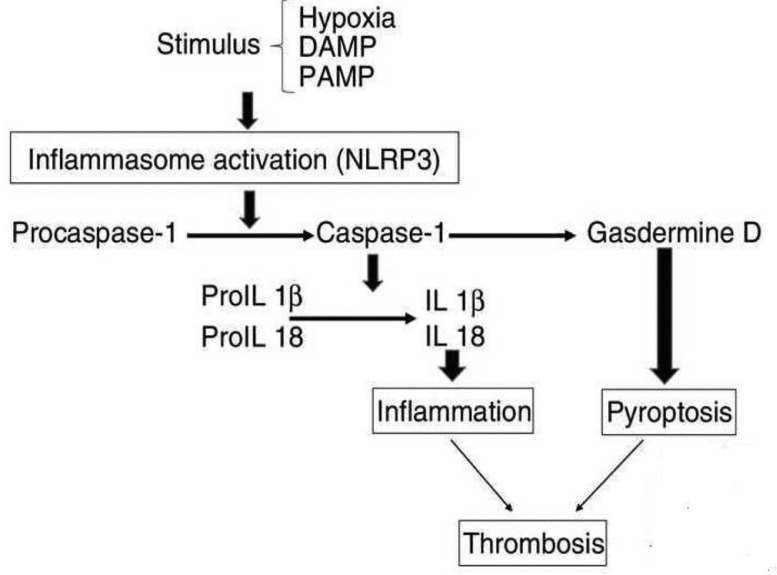
The inflammasomes are multi-protein of innate immunity responsible for the regulation of pro-inflammatory responses [1]. Inflammasomes enhances proteolytic cleavage and release the proinflammatory cytokines (IL-18, IL-1β) and gasdermin-D which are N-terminal fragment responsible for the induction of cytokine release and pyroptosis [2]. In turn, the inflammasomes are activated through cytosolic pattern recognition receptors (PRRs) that are stimulated by pathogen-associated molecular patterns (PAMPs) from microbial pathogen and damage-associated molecular patterns (DAMPs) from host cell damage. The PRRs comprise leucine-rich receptors (NLRs) and nucleotide-binding domains [3,4]. Activation of inflammasomes receptors activates caspase-1 for proteolytic cleavage of immature pro-inflammatory cytokines. Some inflammasomes are activated independently of the caspase-1 pathway, by bacterial lipopolysaccharide through caspase-11 leading to pyroptosis [5]. In brief, activation of NLRP3 inflammasomes by hypoxia, DAMPs, and PAMPs leads to proteolytic conversion to produce caspase-1 from pro-caspase-1 which activates the conversion of pro-IL1β and pro-IL-18 to IL-1β and IL-18, respectively that together induce inflammation. Likewise, caspase-1 activates Gasdermine D leading to pyroptosis. Both pyroptosis and inflammation increase the risk of thrombosis and other coagulopathy (Fig. 1 ) [6]. These inflammasomes are named conical inflammasomes like NLR1, NLR2, NLR3, and NLR4 [7]. NLR1 is found in the neurons, while NLR2 and NLR3 are found in the microglia. NLR1 is activated by bacterial toxins and inhibited by Bcl-2 [8]. NLR3 is the largest one among other NLRs, and it is regulated by PAMPs and DAMPs [9]. NLR3 is also activated by cholesterol crystals and monosodium urate, thus explaining the role of NLRP3 inflammasome in the origin and development of atherosclerosis and gout [10,11]. NLRP3 inflammasome is inhibited by dapansutrile and diarylsulfonylurea MCC-950. NLR4 inflammasome is activated by palmitate and inhibited by cyclic adenosine monophosphate (cAMP) [12].
Fig. 1.
Consequence of NLRP3 activation (adopted from Paramo et al. [7]).
During acute infection, PRRs recognize PAMPs and DAMPs, either through toll-like receptors (TLRs) in the membrane or by nod-like receptors (NLRs) within the cytoplasm that activate NLR3 inflammasome in the macrophages [13]. IL-1β and IL-18 are released after NLRP3 inflammasome activation leading to stimulation of natural killer cells (NKs), NFκB, and interferon-gamma (INF-γ) secretion with inhibition of IL-33 [14]. Activation of NLRP3 inflammasome is regulated by a priming progression that upregulates NLRP3 genes in response to DAMPs and PAMPs through purine sensing receptors [15]. DAMPs and PAMPs activate PRRs like TLRs and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) with subsequent stimulation of the NFκB pathway [16]. This priming contributes to macrophage activation and increases the expression of the IL1β gene with post-translation modification of NLRP3 inflammasome through modulation of the activation of cell membrane ion channels, lysosome disruption, and mitochondrial dysfunction [17]. In addition, activation of NLRP3 inflammasome induces T cells pyroptosis via gasdermin D (GSDMD) dependent-activation of caspase 1, 4, 5. Also, GSDMD provokes IL-1β and IL-18 release [18].
Covid-19 and NLRP3 inflammasome
Covid-19 also known as Coronavirus disease has become a global challenge and is caused by a virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The SARS-CoV-2 is a single-strand RNA virus with similar features as SARS-CoV-1 and MERS-CoV-1 (Middle East respiratory syndrome-coronavirus 1) [19]. The focal targets of the virus in the human host are the angiotensin-converting enzyme 2 (ACE2) receptors that are highly expressed in the lung epithelial cells, proximal renal tubules, brain, and heart. The infection of this virus-induced an acute host immune response, cytokine storm, and inflammatory reaction which leads to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [20].
The clinical manifestation among Covid-19 patients revealed that around 85% had asymptomatic to mild cases, while severe and critical cases were about 10% and 5%, respectively. Severe SARS-CoV-2 infection causes ALI and ARDS with systemic inflammation, cytokine storm, and hypercytokinemia due to activation of the release of pro-inflammatory cytokines IL-1β and IL-6) that are associated with case-fatality rate [21,22]. Wen et al. [23] illustrated that IL-1β producing monocytes are increased with reduction in T cells in the early recovery phase in severe Covid-19 patients suggesting immune-dysregulations. The immune overreaction and cytokine storm during the infection may be due to activation of NLRP3 inflammasome which has supreme importance in human innate immune response mainly against viral infections [24].
It has been reported that SARS-CoV activates NLRP3 INFs via 3a protein in lipopolysaccharide primed macrophages with subsequent release of IL-1β [25]. Sun et al. [26] reported that down-regulation of ACE2 during SARS-CoV infection with an elevation of angiotensin II (AngII) might cause AngII-dependent NLRP3 inflammasome activation. In addition, the activated NLRP3 inflammasome drive AngII to cause proliferation of vascular smooth muscle cells and vascular remodeling [27]. Moreover, plasma and bronchoalveolar fluid of patients with MERS-CoV-1 and SARS-CoV infections have higher IL-1β concentrations which correlated with the development of ALI, ARDS, and poor clinical outcomes [28]. Similarly, a high IL-1β level is associated with ALI in influenza infection [29]. Therefore, IL-1β receptor antagonists may attenuate respiratory viral infection induced-ALI since NLRP3 inflammasome and IL-1β are involved in the pathogenesis of viral complications [30].
The interaction between the ACE2 receptor and SARS-CoV-2 leads to direct activation of NLRP3 inflammasome or indirectly through DAMPs and PAMPs from injured and apoptotic type II alveolar cells that activate lung macrophages [31]. Besides, SARS-CoV-2 activate lung macrophage to release IL-1β and TNF-α that organize a feedback loop for NLRP3 inflammasome activation and immune cell recruitments through the generation of DAMPs and PAMPs [32].
Up to date, SARS-CoV-2 infection may induce local pulmonary inflammatory microenvironment by inducing TNF-α and IL-1β, release that mutually participate into pulmonary vascular endothelial injury and development of pulmonary edema [33]. TNF-α and IL-1β activate the release of IL-6 from the NLRP3 inflammasome which disrupts the alveolar-capillary unit, with subsequent respiratory failure and systemic inflammatory storm. Cell membrane TNF-α in Covid-19 patients activates TLR4 which increases the sensitivity of the NLRP3 inflammasome [34].
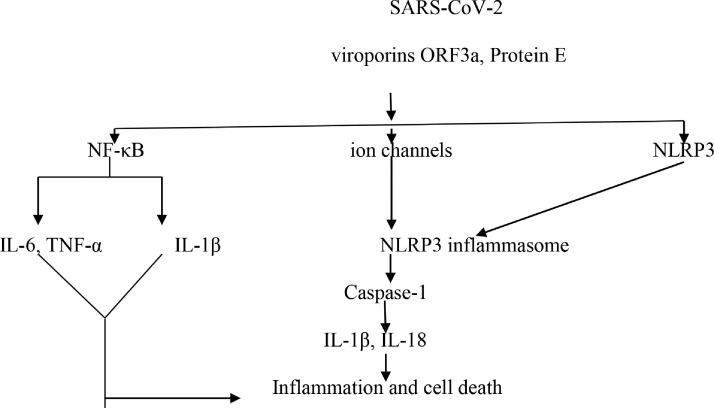
Genomic analysis of SARS-CoV illustrated that ion channel proteins like E protein, open reading frame 3a (ORF3a), and ORF8a required for virulence and replication, act as NLRP3 inflammasome agonist for the release of IL-1β [35]. These ion channel proteins are also found in SARS-CoV-2 and participate in the induction of cellular organelle stress, production of free radicals, and oxidative stress via NF-κB and caspase-1 activation. ORF8a is involved in SARS-CoV-2 pathogenesis and virulence through suppression of interferon from virally-infected cells [36]. It has been shown, that memeantine and gliclazide are potent SARS-CoV-2 E protein inhibitors (Fig. 2 ) [37]. Moreover, genetic variation in the host NLRP3 inflammasome may affect the binding with the viral protein of SARS-CoV-2. [38].
Fig. 2.
SARS-Co viruses and inflammasomes.
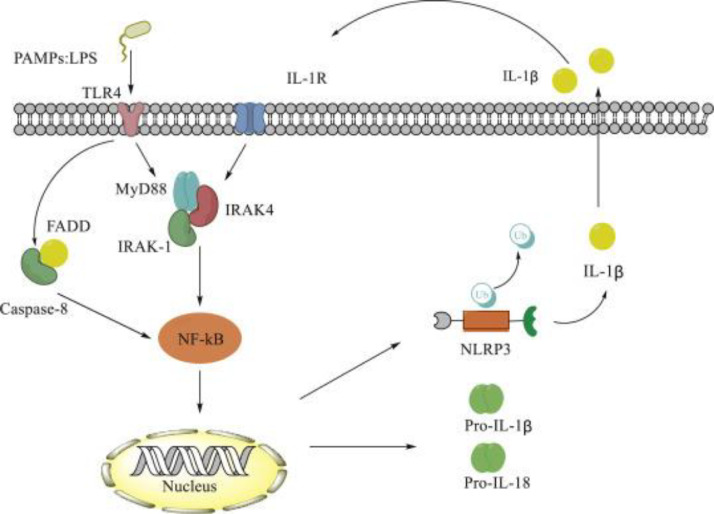
During SARS-CoV-2 infections, NLRP3 inflammasome has been noted to have a potential interaction myeloid differentiation primary response 88 (MYD88). Activated TLR4 and high IL-1β levels stimulate NF-κB through cytoplasmic MYD88 or through caspase-8, which led to IL-1β synthesis and the stimulation of NLRP3 inflammasome (Fig. 3 ) [39].
Fig. 3.
Role of MYD88 in activating NF-κB (adopted from Zhang et al. [39]).
During the recovery phase, NLRP3 inflammasome cytokines are decreased with the compensatory immunosuppressive phase which is characterized by IL-10 elevation and polarization of anti-inflammatory macrophage (M2). In this phase fibroblasts and platelets are recruited and deposited in the lung extracellular matrix with fibrosis and collagen formation, a hallmark of ARDS [40].
NLRP3 inflammasome inhibitors in SARS-CoV-2 infection
Aberrant hyperactivation of NLRP3 inflammasome during SARS-CoV-1, MERS-CoV, SARS-CoV-2, and other respiratory viral infections is associated with ALI and ARDS development due to pro-inflammatory cytokines release and cytokine storm development [41].
Several studies showed that the inhibitors of NLRP3 inflammasome either naturally or by the repurposing of clinically approved drugs. Juliana et al. [42] recently reported that both a natural product parthenolide, and synthetic Bay 11-7082 are potent inhibitors of NLRP3 inflammasome ATPase, independent of NF-κB signaling. Similarly, oridonin which is the active ingredient of Rabdosia rubescens has an anti-inflammatory effect by inhibiting cysteine 279 of the NLRP3 inflammasome, thus making it an effective agent against NLRP3-driven inflammatory disorders. [43].
In addition, mefenamic acid and flufenamic acid which are members of the non-steroidal anti-inflammatory drugs (NSAID), are non-selective inhibitors of cyclooxygenase enzyme, and they also suppress the activity of NLRP3 inflammasome via inhibition of membrane volume anion chloride channel [44]. Recently, mefenamic acid was observed as an effective therapeutic option against SARS-CoV-2 infection-associated hyperinflammation by inhibiting NLRP3 inflammasome with reduction of viral entry through inhibition of transmembrane protease serine 2 (TMPRSS2) [45]. The animal model study by Zhou et al. [46] demonstrated that a low dose of aspirin inhibits endothelial injury through suppression of the synthesis and activation of NLRP3 inflammasome. Furthermore, in a multi-center cohort study, aspirin was independently linked with a low risk of admission into intensive care unit, mechanical ventilation, and mortality in patients with Covid-19 pneumonia [47]. Moreover, indomethacin attenuated acute pancreatitis in mice through inhibition of NLRP3 inflammasome [48]. Thus, indomethacin might be effective for mild Covid-19 by its anti-inflammatory and antiviral activities, while abating the progression of cytokine storm through modulation of the activity of NLRP3 inflammasome [49,50].
However, omega-3 fatty acids have anti-inflammatory activities through inhibition of NLRP3 inflammasome and subsequent reduction in the release of IL-1β and caspase-1 activation. The omega-3 fatty acids are mediated by the down-streaming of the scaffold protein β-arrestin-2 in mice [51]. Weil et al. [52], illustrated that the beneficial effect of omega-3 fatty acids against SARS-CoV-2 infection-induced-inflammatory changes is mediated through a reduction in the activity of NLRP3 inflammasome. However, prolonged uses of omega-3 fatty acids makes the cell membrane vulnerable and susceptible to ROS and other free radicals that may increase the risk of paradoxical oxidative stress, which is a component in the origin and development of SARS-CoV-2 infection [53].
Glyburide is an oral hypoglycemic drug from sulfonylurea group and it has been widely used in managing type 2 diabetes mellitus (T2DM) [54]. Glyburide inhibits the activation of NLRP3 inflammasome and IL-1β release in RNA virus-infected cells [55].
Besides, metformin a first-line drug in the management of T2DM acts through activation of AMP-activated protein kinase (AMPK) and blocking of mitochondrial complex I [56]. Metformin inhibits NLRP3 inflammasome through AMPK activation and autophagy with mTOR pathway inhibition in dilated cardiomyopathy. Also, metformin blocks caspase-1 and GSDMD-N that correlate with the NLRP3 inflammasome activation [57]. Different studies reported the beneficial effect of metformin therapy against SARS-CoV-2 infection by inhibiting viral replication, ACE2 phosphorylation dependent-inhibition of viral entry, and amelioration of associated cytokine activation [58,59].
In addition, pioglitazone, a peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonist ameliorated diabetic nephropathy through inhibition of NLRP3 inflammasome [60]. Pioglitazone and other PPAR-γ agonists may have a potential role in the management of Covid-19-associated cytokine storm through inhibition of pro-inflammatory cytokines synthesis, NF-κB signaling, and NLRP3 inflammasome [61].
Furthermore, a NF-κB signaling inhibitor tranilast is an effective anti-inflammatory drug used in the management of asthma. Tranilast prevents the assembly and activation of NLRP3 inflammasome as well as inhibition of the release of pro-inflammatory cytokines [62]. A review study reveals that tranilast may reduce Covid-19 severity during clinical trials [63].
Colchicine which is an alkaloid derivative drug has a marked anti-inflammatory effect by inhibiting NLRP3 inflammasome, neutrophil recruitments, and adhesion molecules. Colchicine is widely used in the management of acute gout, familial meditation fever, pericarditis, and other inflammatory disorders [64]. In Covid-19, high neutrophil recruitment is correlated with disease severity; thereby colchicine may reduce Covid-19 severity through inhibition of NLRP3 inflammasome, neutrophil recruitments, adhesion molecules, and release of pro-inflammatory cytokines [65].
Polyphenolic resveratrol indirectly inhibits NLRP3 inflammasome, through suppression of mitochondrial damage and induction of autophagy [66]. Thus, resveratrol may serve as adjuvant therapy in severe Covid-19 patients via mitigation of NLRP3 inflammasome induced inflammation and augmentation of cell autophagy [67]. In addition, resveratrol upregulates the expression of ACE2 receptors with significant inhibition of pro-inflammatory cytokines [68].
Lipid-lowering drugs may affect NLRP3 inflammasome activity and decrease complications related to inflammatory disorders regardless of lipid profile. Parsamanesh et al. [69] found that statins have anti-inflammatory and immunomodulatory effects through regulation of the activity of NLRP3 inflammasome. Statins regulate the molecular platform of lysosomal function, ATP signaling, cathepsin-B, and K+ ion efflux that contribute to the NLRP3 inflammasome activation. Notably, in vitro studies revealed that statins inhibit NLRP3 inflammasome activity due to atherogenic stimuli through blocking of pregnane x receptors (PXR) [70]. However, separate studies reported that statins therapy is linked with NLRP3 inflammasome activation, caspase-1, IL-1β release that collectively contribute to the induction of T2DM [71]. Koushki et al. [72] demonstrated that statins may exert a stimulatory or inhibitory effect on the NLRP3 inflammasome depending on their chemical structure and pharmacokinetic profile. Lipophilic statins like atorvastatin and simvastatin exert more effect on the TLR4/MYD88/NF-κB signaling and NLRP3 inflammasome compared with hydrophilic statins like rousovastatin. Therefore, all statins inhibit the activity of NLRP3 inflammasome except simvastatin which might have a stimulatory effect on NLRP3 INFs.
Furthermore, a systematic review on statins usage in Covid-19 patients showed that drug use is correlated with reduced death rate and severe cases in Covid-19 patients by 30%. Therefore, statins therapy is suggested to be an effective therapy against moderate-severe Covid-19 [73].
Therefore, NLRP3 inflammasome inhibitors play an essential role in the mitigation of immune-overaction and hypercytokinemia in Covid-19 (Table 1 ).
Table 1.
the relevance of NLRP3 inflammasome inhibitors in Covid-19
| NLRP3 inhibitors | The mechanisms | References | Relevance in Covid-19 | Refs. |
|---|---|---|---|---|
| Natural products | ||||
| Parthenolide | Inhibits NLRP3 inflammasome ATPase | 42 | Cytokine storm inhibition | 43 |
| Oridonin | Inhibits NLRP3 inflammasome cysteine 279 | 43 | Cytokine storm inhibition | 43 |
| Resveratrol | Inhibits NLRP3 inflammasome | 66 | Inhibition of autophagy, cytokine storm, ACE2 upregulation | 67 |
| NSAIDs | ||||
| Mefenamic acid | Inhibits NLRP3 inflammasome via membrane volume anion chloride channel suppression | 44 | Reduction of viral entry through inhibition of transmembrane protease serine 2 (TMPRSS2) | 45 |
| Aspirin | Inhibits NLRP3 inflammasome | 46 | Reduction in patient's mortality rate with Covid-19 pneumonia | 47 |
| Indomethacin | Inhibits NLRP3 inflammasome | 48 | Anti-inflammatory and antiviral activities | 49,50 |
| Anti-diabetic drugs | ||||
| Glyburide | Inhibition of NLRP3 inflammasome | 55 | Anti-inflammatory and antiviral activities with upregulation of ACE2 | 58,59 |
| Metformin | Inhibition of NLRP3 inflammasome, block caspase-1 and GSDMD-N | 57 | Inhibition of cytokine storm and upregulation of ACE2 | 61 |
| Lipid-lowering drugs | ||||
| Pioglitazone | Inhibition of NLRP3 inflammasome | 60 | Anti-inflammatory effects | 52 |
| Omega-3 fatty acids | Inhibition of NLRP3 inflammasome through down-streaming of scaffold protein β-arrestin-2 | 51 | Reduced death in Covid-19 patients by 30%. | |
| Statins | Inhibits NLRP3 inflammasome, Regulation of molecular platform of ATP signaling, cathepsin-B, lysosomal function, blocking of pregnane x receptors (PXR). | 69-72 | Inhibits pro-inflammatory cytokines release so as to reduce Covid-19 severity | 73 |
| Others | ||||
| Tranilast | Prevents the assembly and activation of NLRP3 inflammasome | 62 | Inhibits pro-inflammatory cytokines release so as to reduce | 63 |
| Colchicine | Inhibits NLRP3 inflammasome, neutrophil recruitments and adhesion molecules | 64 | Covid-19 severity | 65 |
Ethics approval and consent to participate
Not required.
Consent for publication
not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Funding
None.
CRediT authorship contribution statement
Gaber El-Saber Batiha: Conceptualization. Ali I. Al-Gareeb: Conceptualization. Damilare Rotimi: Conceptualization, Resources, Data curation, Formal analysis. Oluyomi Stephen Adeyemi: Conceptualization, Writing – original draft. Hayder M. Al-kuraishy: Conceptualization.
Declaration of Competing Interest
None.
Acknowledgments
None.
Editor: DR B Gyampoh
References
- 1.De Zoete M.R., Palm N.W., Zhu S., Flavell R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014;6(12) doi: 10.1101/cshperspect.a016287. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Man S.M., Karki R., Kanneganti T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017;277(1):61–75. doi: 10.1111/imr.12534. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh J.E., Lee H.K. Pattern recognition receptors and autophagy. Front. Immunol. 2014;5:300. doi: 10.3389/fimmu.2014.00300. Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratsimandresy R.A., Dorfleutner A., Stehlik C. An update on PYRIN domain-containing pattern recognition receptors: from immunity to pathology. Front. Immunol. 2013;4:440. doi: 10.3389/fimmu.2013.00440. Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vande Walle L., Lamkanfi M. Inflammasomes: caspase-1-activating platforms with critical roles in host defense. Front. Microbiol. 2011;2:3. doi: 10.3389/fmicb.2011.00003. Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Páramo JA. Inflammatory response in relation to COVID-19 and other prothrombotic phenotypes. Reumatol. Clín. 2020 doi: 10.1016/j.reumae.2020.06.007. (English Edition)Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greaney A.J., Leppla S.H., Moayeri M. Bacterial exotoxins and the inflammasome. Front. Immunol. 2015;6:570. doi: 10.3389/fimmu.2015.00570. Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Almeida E., Frantz S.R., Cesar P., Tarragô A.M., de Amorim Xabregas L., Garcia N.P., Costa A.G., de Paula E.V., Malheiro A. Frequency of interleukins IL1ß/IL18 and inflammasome NLRP1/NLRP3 polymorphisms in sickle cell anemia patients and their association with severity score. Curr. Mol. Med. 2019;19(10):776–783. doi: 10.2174/1566524019666190826143749. Dec 1. [DOI] [PubMed] [Google Scholar]
- 9.Jin C., Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J. Clin. Immunol. 2010;30(5):628–631. doi: 10.1007/s10875-010-9440-3. Sep 1. [DOI] [PubMed] [Google Scholar]
- 10.Rock K.L., Kataoka H., Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 2013;9(1):13. doi: 10.1038/nrrheum.2012.143. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldrighi M., Mallat Z., Li X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis. 2017;267:127–138. doi: 10.1016/j.atherosclerosis.2017.10.027. Dec 1. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Fernández A., Skouras D.B., Dinarello C.A. López-Vales R. Olt1177 (dapansutrile), a selective nlrp3 inflammasome inhibitor, ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front. Immunol. 2019;10:2578. doi: 10.3389/fimmu.2019.02578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paroli A.F., Gonzalez P.V., Díaz-Luján C., Onofrio L.I., Arocena A., Cano R.C., Carrera-Silva E.A., Gea S. NLRP3 inflammasome and caspase-1/11 pathway orchestrate different outcomes in the host protection against Trypanosoma cruzi acute infection. Front. Immunol. 2018;9:913. doi: 10.3389/fimmu.2018.00913. May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigue-Gervais I.G., Doiron K., Champagne C., Mayes L., Leiva-Torres G.A., Vanié P., Douglas T., Vidal S.M., Alnemri E.S., Saleh M. The mitochondrial protease HtrA2 restricts the NLRP3 and AIM2 inflammasomes. Sci. Rep. 2018;8(1):1. doi: 10.1038/s41598-018-26603-1. May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath G.L., Schrum J.E., De Nardo C.M., Latz E. Intracellular sensing of microbes and danger signals by the inflammasomes. Immunol. Rev. 2011;243(1):119–135. doi: 10.1111/j.1600-065X.2011.01050.x. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Z., Zhuang Q., Ye X., Ning M., Wu S., Lu L., Wan X. Adiponectin inhibits NLRP3 inflammasome activation in nonalcoholic steatohepatitis via AMPK-JNK/ErK1/2-NFκB/ROS signaling pathways. Front. Med. 2020;7:705. doi: 10.3389/fmed.2020.546445. Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cecil J.D., O'Brien-Simpson N.M., Lenzo J.C., Holden J.A., Singleton W., Perez-Gonzalez A., Mansell A., Reynolds E.C. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front. Immunol. 2017;8:1017. doi: 10.3389/fimmu.2017.01017. Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malireddi R.K., Kesavardhana S., Kanneganti T.D. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis) Front. Cell Infect. Microbiol. 2019;9:406. doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Kuraishy H.M., Al-Gareeb A.I., Al-Niemi M.S., Al-Buhadily A.K., Al-Harchan N.A., Lugnier C. COVID-19 and phosphodiesterase enzyme type 5 inhibitors. J. Microsc. Ultrastruct. 2020;8(4):141. doi: 10.4103/JMAU.JMAU_63_20. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Kuraishy H.M., Hussien N.R., Al-Naimi M.S., Al-Buhadily A.K., Al-Gareeb A.I., Lungnier C. Renin–Angiotensin system and fibrinolytic pathway in COVID-19: one-way skepticism. Biomed. Biotechnol. Res. J. (BBRJ) 2020;4(5):33. doi: 10.4103/bbrj.bbrj_105_20. Aug 1. [DOI] [Google Scholar]
- 21.Al-kuraishy H.M., Al-Maiahy T.J., Al-Gareeb A.I., Musa R.A., Ali Z.H. COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery. Asian Pac. J. Reprod. 2020;9(3):156. doi: 10.4103/2305-0500.282984. May 1. [DOI] [Google Scholar]
- 22.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. May 12. [DOI] [PubMed] [Google Scholar]
- 23.Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., Liu X., Xie L., Li J., Ye J., Dong L. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6(1):1–8. doi: 10.1038/s41421-020-0168-9. May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heneka M.T. Inflammasome activation and innate immunity in Alzheimer's disease. Brain Pathol. 2017;27(2):220–222. doi: 10.1111/bpa.12483. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H.J., Ren X.S., Xiong X.Q., Chen Y.Z., Zhao M.X., Wang J.J., Zhou Y.B., Han Y., Chen Q., Li Y.H., Kang Y.M. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death. Dis. 2017;8(10):e3074. doi: 10.1038/cddis.2017.470. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Tawfiq J.A., Assiri A., Memish ZA. Middle East respiratory syndrome novel corona (MERS-CoV) infection. Saudi Med. J. 2013;34(10):991–994. [PubMed] [Google Scholar]
- 29.Chiaretti A., Pulitanò S., Barone G., Ferrara P., Romano V., Capozzi D., Riccardi R. IL-1β and IL-6 upregulation in children with H1N1 influenza virus infection. Mediators Inflamm. 2013:2013. doi: 10.1155/2013/495848. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei J., Vermillion M.S., Jia B., Xie H., Xie L., McLane M.W., Sheffield J.S., Pekosz A., Brown A., Klein S.L., Burd I. IL-1 receptor antagonist therapy mitigates placental dysfunction and perinatal injury following Zika virus infection. JCI Insight. 2019;4(7) doi: 10.1172/jci.insight.122678. Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigues T.S., de Sá K.S., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R., Veras F.P. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021;218(3) doi: 10.1084/jem.20201707. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020:1–6. doi: 10.1007/s12250-020-00207-4. Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallo O., Locatello L.G., Mazzoni A., Novelli L., Annunziato F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal. Immunol. 2020:1–2. doi: 10.1038/s41385-020-00359-2. Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipworth B., Chan R., Lipworth S., Kuo CR. Weathering the cytokine storm in susceptible patients with severe SARS-CoV-2 infection. J. Allergy Clin. Immunol. Pract. 2020;8(6):1798–1801. doi: 10.1016/j.jaip.2020.04.014. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouayad A. Innate immune evasion by SARS-CoV-2: Comparison with SARS-CoV. Rev. Med. Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2135. Nov. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A., Prasoon P., Kumari C., Pareek V., Faiq M.A., Narayan R.K., Kulandhasamy M., Kant K. SARS-CoV-2-specific virulence factors in COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26615. Sep 22. [DOI] [PubMed] [Google Scholar]
- 37.Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ungerbäck J., Belenki D., Jawad ul-Hassan A., Fredrikson M., Fransén K., Elander N., Verma D., Söderkvist P. Genetic variation and alterations of genes involved in NFκB/TNFAIP3-and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis. 2012;33(11):2126–2134. doi: 10.1093/carcin/bgs256. Nov 1. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X., Xu A., Lv J., Zhang Q., Ran Y., Wei C., Wu J. Development of small molecule inhibitors targeting NLRP3 inflammasome pathway for inflammatory diseases. Eur. J. Med. Chem. 2020;185 doi: 10.1016/j.ejmech.2019.111822. Jan 1. [DOI] [PubMed] [Google Scholar]
- 40.Yang J.R., Deng D.T., Wu N., Yang B., Li H.J., Pan X.B. Persistent viral RNA positivity during the recovery period of a patient with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25940. Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao C., Zhao W. NLRP3 inflammasome-a key player in antiviral responses. Front. Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juliana C., Fernandes-Alnemri T., Wu J., Datta P., Solorzano L., Yu J.W., Meng R., Quong A.A., Latz E., Scott C.P., Alnemri E.S. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010;285(13):9792–9802. doi: 10.1074/jbc.M109.082305. Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majnooni M.B., Fakhri S., Shokoohinia Y., Kiyani N., Stage K., Mohammadi P., Gravandi M.M., Farzaei M.H., Echeverría J. Phytochemicals: potential therapeutic interventions against coronavirus-associated lung injury. Front. Pharmacol. 2020;11:1744. doi: 10.3389/fphar.2020.588467. Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels M.J., Rivers-Auty J., Schilling T., Spencer N.G., Watremez W., Fasolino V., Booth S.J., White C.S., Baldwin A.G., Freeman S., Wong R. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer's disease in rodent models. Nat. Commun. 2016;7(1):1. doi: 10.1038/ncomms12504. Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pareek R.P. Use of mefenamic acid as a supportive treatment of COVID-19: a repurposing drug. Int. J. Sci. Res. 2020 doi: 10.21275/SR20530150407. (IJSR) ISSN: 2319-7064. [DOI] [Google Scholar]
- 46.Zhou X., Wu Y., Ye L., Wang Y., Zhang K., Wang L., Huang Y., Wang L., Xian S., Zhang Y., Chen Y. Aspirin alleviates endothelial gap junction dysfunction through inhibition of NLRP3 inflammasome activation in LPS-induced vascular injury. Acta Pharm. Sin. B. 2019;9(4):711–723. doi: 10.1016/j.apsb.2019.02.008. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chow J.H., Khanna A.K., Kethireddy S., Yamane D., Levine A., Jackson A.M., McCurdy M.T., Tabatabai A., Kumar G., Park P., Benjenk I. Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19. Anesth. Analg. 2020 doi: 10.1213/ANE.0000000000005292. Nov 16. [DOI] [PubMed] [Google Scholar]
- 48.Lu G., Pan Y., Kayoumu A., Zhang L., Yin T., Tong Z., Li B., Xiao W., Ding Y., Li W. Indomethacin inhabits the NLRP3 inflammasome pathway and protects severe acute pancreatitis in mice. Biochem. Biophys. Res. Commun. 2017;493(1):827–832. doi: 10.1016/j.bbrc.2017.08.060. Nov 4. [DOI] [PubMed] [Google Scholar]
- 49.Kanakaraj A. Low dose indomethacin in the outpatient treatment of COVID-19 in kidney transplant recipients—a case series. Open Access Library J. 2020;7(10):1. doi: 10.4236/oalib.1106860. [DOI] [Google Scholar]
- 50.Ilie P.C., Stefanescu S., Veronese N. A paradigm shift in the role of NSAIDs in COVID-19: new pathological mechanisms and potential treatment targets. Atena J. Public Health. 2020;2:6. Sep 20. [Google Scholar]
- 51.Yan Y., Jiang W., Spinetti T., Tardivel A., Castillo R., Bourquin C., Guarda G., Tian Z., Tschopp J., Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38(6):1154–1163. doi: 10.1016/j.immuni.2013.05.015. Jun 27. [DOI] [PubMed] [Google Scholar]
- 52.Weill P., Plissonneau C., Legrand P., Rioux V., Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–280. doi: 10.1016/j.biochi.2020.09.003. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogero M.M., Leão M.D., Santana T.M., de M.B., Pimentel M.V., Carlini G.C., da Silveira T.F., Gonçalves R.C., Castro IA. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radical Biol. Med. 2020 doi: 10.1016/j.freeradbiomed.2020.07.005. Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hussien N.R., Al-Naimi M.S., Rasheed H.A., Al-Kuraishy H.M., Al-Gareeb A.I. Sulfonylurea and neuroprotection: the bright side of the moon. J. Adv. Pharm. Technol. Res. 2018;9(4):120–123. doi: 10.4103/japtr.JAPTR_317_18. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X., Qu C., Jia J., Zhan Y. NLRP3 inflammasome inhibitor glyburide expedites diabetic-induced impaired fracture healing. Immunobiology. 2019;224(6):786–791. doi: 10.1016/j.imbio.2019.08.008. Nov 1. [DOI] [PubMed] [Google Scholar]
- 56.Al-Kuraishy H.M., Sami O.M., Hussain N.R., Al-Gareeb A.I. Metformin and/or vildagliptin mitigate type II diabetes mellitus induced-oxidative stress: the intriguing effect. J. Adv. Pharm. Technol. Res. 2020;11(3):142. doi: 10.4103/japtr.JAPTR_18_20. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang F., Qin Y., Wang Y., Meng S., Xian H., Che H., Lv J., Li Y., Yu Y., Bai Y., Wang L. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int. J. Biol. Sci. 2019;15(5):1010. doi: 10.7150/ijbs.29680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheen AJ. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab. 2020;46(6):423–426. doi: 10.1016/j.diabet.2020.07.006. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.C. Bramante, N. Ingraham, T. Murray, S. Marmor, S. Hoversten, J. Gronski, C. McNeil, R. Feng, G. Guzman, N. Abdelwahab, S. King. Observational study of metformin and risk of mortality in patients hospitalized with Covid-19. medRxiv. 2020 Jan 1. doi: 10.1101/2020.06.19.20135095. [DOI] [PMC free article] [PubMed]
- 60.Wang Y., Yu B., Wang L., Yang M., Xia Z., Wei W., Zhang F., Yuan X. Pioglitazone ameliorates glomerular NLRP3 inflammasome activation in apolipoprotein E knockout mice with diabetes mellitus. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181248. Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carboni E., Carta A.R., Carboni E. Can pioglitazone be potentially useful therapeutically in treating patients with covid-19? Med. Hypotheses. 2020 doi: 10.1016/j.mehy.2020.109776. Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Y., Jiang H., Chen Y., Wang X., Yang Y., Tao J., Deng X., Liang G., Zhang H., Jiang W., Zhou R. Tranilast directly targets NLRP 3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018;10(4):e8689. doi: 10.15252/emmm.201708689. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol. Sci. 2020 doi: 10.1016/j.tips.2020.03.006. Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., Pinto F.J., Ibrahim R., Gamra H., Kiwan G.S., Berry C. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. Dec 26. [DOI] [PubMed] [Google Scholar]
- 65.Parra-Medina R., Sarmiento-Monroy J.C., Rojas-Villarraga A., Garavito E., Montealegre-Gómez G., Gómez-López A. Colchicine as a possible therapeutic option in COVID-19 infection. Clin. Rheumatol. 2020;39(8):2485–2486. doi: 10.1007/s10067-020-05247-5. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang L., Zhang L., Kang K., Fei D., Gong R., Cao Y., Pan S., Zhao M., Zhao M. Resveratrol ameliorates LPS-induced acute lung injury via NLRP3 inflammasome modulation. Biomed. Pharmacother. 2016;84:130–138. doi: 10.1016/j.biopha.2016.09.020. Dec 1. [DOI] [PubMed] [Google Scholar]
- 67.I. Mittra, R. de Souza, R. Bhadade, T. Madke, P.D. Shankpal, M. Joshi, B. Qayyumi, A. Bhattacharya, V. Gota, S. Gupta, P. Chaturvedi. Resveratrol and copper for treatment of severe COVID-19: an observational study (RESCU 002). medRxiv. 2020 Jan 1. doi: 10.1101/2020.07.21.20151423. [DOI]
- 68.Hoang T. An approach of fatty acids and resveratrol in the prevention of COVID-19 severity. Phytother. Res. 2020 doi: 10.1002/ptr.6956. Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parsamanesh N., Moossavi M., Bahrami A., Fereidouni M., Barreto G., Sahebkar A. NLRP3 inflammasome as a treatment target in atherosclerosis: a focus on statin therapy. Int. Immunopharmacol. 2019;73:146–155. doi: 10.1016/j.intimp.2019.05.006. Aug 1. [DOI] [PubMed] [Google Scholar]
- 70.Wang S., Xie X., Lei T., Zhang K., Lai B., Zhang Z., Guan Y., Mao G., Xiao L., Wang N. Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFα in vascular endothelial cells through a PXR-dependent mechanism. Mol. Pharmacol. 2017;92(3):256–264. doi: 10.1124/mol.116.108100. Sep 1. [DOI] [PubMed] [Google Scholar]
- 71.Henriksbo B.D., Schertzer J.D. Is immunity a mechanism contributing to statin-induced diabetes? Adipocyte. 2015;4(4):232–238. doi: 10.1080/21623945.2015.1024394. Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koushki K., Shahbaz S.K., Mashayekhi K., Sadeghi M., Zayeri Z.D., Taba M.Y., Banach M., Al-Rasadi K., Johnston T.P., Sahebkar A. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin. Rev. Allergy Immunol. 2020 doi: 10.1007/s12016-020-08791-9. May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kow C.S., Hasan S.S. Meta-analysis of effect of statins in patients with COVID-19. Am. J. Cardiol. 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.