Abstract
Our aims were to 1) evaluate the capacity of hollow hydroxyapatite (HA) microspheres (212–250 μm) to serve as a delivery system for controlled release of BMP-2 in vitro and 2) examine relaxin as an enhancer of BMP-2 for bone regeneration. Hollow HA microspheres were converted from borate glass microspheres and characterized using X-ray diffraction, Fourier-transform infrared spectroscopy, scanning electron microscopy, and the Brunauer–Emmett–Teller method. The microspheres loaded with BMP-2 and relaxin were implanted for 6 weeks in Sprague Dawley rats with calvarial defects. BMP-2 alone in the range up to 1 μg per defect exhibited dose-dependent bone regeneration while relaxin alone in the range up to 0.25 μg per defect did not promote bone regeneration. When compared with BMP-2 alone (1 μg per defect), a 50% reduction in the BMP-2 dose was achieved with the addition of 0.05, 0.1, or 0.25 μg of relaxin per defect. These results show that loading HA microspheres with a combination of relaxin and BMP-2 can significantly reduce the BMP-2 dose required to regenerate an equivalent amount of bone.
Keywords: bone morphogenetic protein-2, bone regeneration, hydroxyapatite microsphere, protein growth factor, rat calvarial defect model, relaxin
1 |. INTRODUCTION
Approximately three million bone grafts are performed annually in the United States for treatment of large bone defects (Jahangir et al., 2008). Bone defects can result from trauma, malignancy, or congenital diseases and are a significant clinical problem. Trauma in the craniofacial region poses a great challenge to bone growth due to the location of highly important anatomic structures (Herford, 2017). Bone has a high capability to regenerate on its own with a three-stage repair process. These three stages are the early inflammatory stage, the repair stage, and the late remodeling stage (Kalfas, 2001). During the inflammatory stage a hematoma develops, allowing inflammatory cells and fibroblasts to enter and form granulation tissue (Kalfas, 2001). In the repair stage, fibroblasts form a stroma to support vascular growth (Kalfas, 2001). A collagen matrix is laid down and mineralized, leading to the formation of a soft callus (Kalfas, 2001). The callus eventually ossifies and forms a bridge of bone over the fracture (Kalfas, 2001). The remodeling stage is the longest and results in the bone being restored to its original shape and mechanical strength (Kalfas, 2001).
When it is impractical to allow bone to naturally heal on its own, bone grafts can be used to treat bone defects. Bone grafts can have osteoconductive and/or osteoinductive properties. Osteoconduction is the process in which the graft allows osteogenic cells to have a biological role, while osteoinduction involves the molecules, such as growth factors, that influence the fate of osteogenic cells (Gomes & Fernandes, 2011). Autografts are considered the gold standard for treatment because they have osteoinductive effects and do not cause an adverse immune response in the patient. However, donor site limitations and morbidity remain obstacles for using them (Liu et al., 2012). Allografts lack the risk of host site morbidity, but are costly and risk a negative host immune response (Jahangir et al., 2008). A variety of synthetic bone grafts are being developed to circumvent limitations of both autografts and allografts. These include bioceramics (e.g., hydroxyapatite [HA], beta-tricalcium phosphate [β-TCP], and biphasic calcium phosphate [BCP]), bioactive glass, and polymers (e.g., hyaluronic acid) (Jahangir et al., 2008). β-TCP is known for its high strength similar to cancellous bone but has a low replacement ratio (more β-TCP is resorbed than replaced with new bone) which has limited its clinical use (Szpalski, Barr, Wetterau, Saadeh, & Warren, 2010). BCPs are mixtures of β-TCP and HA and release calcium and phosphate ions into the defect as the scaffold is resorbed (Szpalski et al., 2010). Bioactive glass, such as 45S5 glass, reacts in the body to form a layer of hydroxyapatite and supports the regeneration of bone (Jahangir et al., 2008). Polymers, like hyaluronic acid, have the ability to combine with other materials to enhance their properties but natural polymers impose a risk for an immune response (Kisiel, 2013). Hyaluronic acid has also been used as a delivery method for the release of growth factors (Kisiel, 2013). Synthetic bone grafts can be made in large quantities, have reproducible quality, and typically do not have an adverse host response (Xiao, Fu, Rahaman, Liu, & Bal, 2013). Unfortunately, synthetic bone grafts are limited by their osteoconductive rather than osteoinductive capabilities because they lack the osteoprogenitor cells found in natural grafts (Giannoudis, Dinopoulos, & Tsiridis, 2005; Xiao et al., 2013). Since they do not produce significant bone regeneration compared to autografts, the addition of growth factors is needed.
Hydroxyapatite (HA) is an ideal biomaterial for synthetic bone grafts because it consists of the inorganic elements found in natural bone (Szpalski et al., 2010). Its chemical formula is Ca10(PO4)6(OH)2 and thus it is comprised mainly of calcium and phosphate ions. This makes HA highly biocompatible since it produces no toxicity or immune response (Giannoudis et al., 2005). HA has also been known to promote cell adhesion and growth when produced with adequate pore sizes, typically 100 μm (Szpalski et al., 2010; Tang et al., 2016). Another quality of HA is its ability to adsorb different types of chemical species on to its surface (Ginebra, Traykova, & Planell, 2006). For instance, HA microspheres have a high affinity to growth factors and can be used as a delivery vehicle (Fu, Rahaman, Brown, & Day, 2013). Delivery systems are typically composed of porous particles, granules, or scaffolds in which a bioactive protein is either adsorbed to the surfaces of the porous material, or encapsulated within the pores (Xiong et al., 2015). Hollow HA microspheres have a large surface area due to their mesoporous shell (Xiong et al., 2015). This allows for an enhanced capability to release growth factors because absorption can occur in the shell and within the hollow core (Fu et al., 2013). The mesoporous structure of the HA shell can also enhance cell adhesion (Cholas et al., 2016). Disadvantages of using HA include its low mechanical strength, limiting its use in load bearing bones, and its slow degradation rate (Szpalski et al., 2010; Zhou & Lee, 2011). However, the absorption and release of growth factors from HA microspheres makes them a model carrier.
Bone morphogenetic protein 2 (BMP-2), a member of the TGF-β superfamily, is a strong inducer of osteogenesis (Xiao, Xiang, & Shao, 2007). BMP-2 is naturally found at a concentration of 2 ng/g of bone and in serum at a concentration of about 90 pg/ml (Oryan, Alidadi, Moshiri, & Bigham-Sadegh, 2014; Park et al., 2008). BMP-2 is one of two BMP proteins currently used in clinical settings for orthopedic and dental applications (Oryan et al., 2014). Unfortunately, BMP-2 tends to be rapidly degraded by proteases (half-life of about 7 to 16 minutes) when injected directly into a defect site. Thus, a supraphysiological dose of the protein is typically required. This supraphysiological dose can cause some undesired side effects like swelling and an increased risk for cancer (more than three times the incident rate) (Carragee et al., 2013; Kisiel, 2013; Zhang et al., 2012). Because of this, it is preferable to have controlled release of BMP-2 in a localized environment since it will lower the required dose and reduce side-effects associated with extensive exposure. For instance, at similar doses it has been shown that controlled, long-term delivery of BMP-2 is more effective and safer compared to short-term delivery (Jeon et al., 2008). For controlled, localized delivery, a carrier is needed. Currently, the only carriers clinically approved for BMP-2 delivery are collagen scaffolds (Oryan et al., 2014). Unfortunately, these scaffolds tend to have high initial burst release and growth factors have a low affinity to them (Lee et al., 2013). HA microspheres have been shown to be a novel system for controlled drug release due to decreased initial burst and sustained release of BMP-2 (Fu, Rahaman, Day, & Brown, 2011; Luginbuehl, Meinel, Merkle, & Gander, 2004).
In additional to providing the controlled release of BMP-2 to reduce undesired effects, an enhancer to BMP-2 can be used to potentially decrease the required dose of BMP-2. Relaxin is an example of such an enhancer. Relaxin is a pleiotropic hormone of the insulin family (Bathgate, Ivell, Sanborn, Sherwood, & Summers, 2005). It is typically known as a pregnancy hormone, in which it has a role in promoting cervical softening to facilitate birth (Bathgate et al., 2005). Relaxin also plays a part in connective tissue metabolism, collagen turnover, angiogenesis, and tumor metastasis (Bathgate et al., 2013; Moon et al., 2014). Relaxin-2, the only relaxin protein found circulating in the blood, acts equally through the relaxin/insulin-like family peptide receptors (Rxfp) 1 and 2, although Rxfp2 is known to play a larger role in osteogenesis (Bathgate et al., 2005; Duarte, Kobayashi, Morita, Kawamoto, & Moriyama, 2016). These receptors have been recently found on bone cells, namely osteoblasts and notably on mouse calvarial tissue (Duarte, Kobayashi, Kawamoto, & Moriyama, 2014; Ferlin et al., 2008). While examining the effects of relaxin on mesenchymal stem cell differentiation into osteoblasts and bone formation, Moon et al. found that relaxin augmented BMP-2-induced osteogenesis (Moon et al., 2014). When determining the effects that relaxin played on C3H/10 T1/2 mouse embryonic fibroblast cells, it was found that relaxin was not an inducer of osteogenesis but an enhancer to BMP-2. It is believed that a combination of BMP-2 and relaxin may provide satisfactory bone regeneration, therefore leading to a reduction of the necessary BMP-2 dose.
Our previous studies have investigated bone regeneration using HA microspheres and two growth factors: transforming growth factor beta (TGF-β) and BMP-2. Loading the microspheres with 1 μg of BMP-2 per defect provided considerable bone regeneration at 6 weeks (Xiao et al., 2013). TGF-β was not as successful in inducing osteogenesis compared to BMP-2 and required a higher dosage (5 μg per defect) (Xiong et al., 2015). In a quick comparison, this dose of BMP-2 is lower than the dosage used by other types of calcium phosphates and other biomaterials (Xiao et al., 2013). However, continuing to lower this dose will contribute to a reduction in clinically relevant side effects.
The present study hypothesizes that the use of relaxin will lower the dose of BMP-2 required to achieve efficient bone regeneration. To test the hypothesis, closed HA microspheres were prepared by reacting glass microspheres in a phosphate buffer. The HA microspheres were characterized using scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier Transform Infrared (FTIR) spectroscopy, and the Brunauer–Emmett–Teller method. Specific surface area and carbon content were estimated. Release profiles of BMP-2, relaxin, and a combination of BMP-2 and relaxin were determined. A rat calvarial defect model was used because it is a standard technique for studying bone regeneration (Gomes & Fernandes, 2011; Szpalski et al., 2010). Bone regeneration in rat calvarial defects was analyzed using histomorphometric analysis.
2 |. MATERIALS AND METHODS
2.1 |. Preparation and characterization of HA microspheres
Hollow HA microspheres were prepared by reacting borate glass microspheres in an aqueous phosphate solution as described in a previous publication (Fu et al., 2011). Briefly, a glass with the composition 15% CaO, 11% Li2O, and 74% B2O3 (by weight) was prepared by melting reagent grade CaCO3, Li2CO3, and H3BO3 in a Pt crucible at 1200°C for 45 minutes and quenching the melt between cold stainless-steel plates. Particles of size 212–250 μm were obtained by grinding the glass in a hardened steel mortar and pestle, and sieving through 60 and 70 mesh sieves. The glass particles were allowed to fall down a vertical tube furnace at 1000°C to form spherical particles. The glass microspheres were reacted for 2 days in 0.02 M K2PO4 solution at 37°C at a starting pH of 9.0 to produce hollow HA microspheres. The converted microspheres were washed three times with distilled water, soaked in ethanol to remove residual water and dried for 12 hours at room temperature, followed by another 12 hours at 90°C.
Characterization of the hollow HA microspheres was performed using methods described previously (Fu et al., 2011). Briefly, the microstructure of the surface of the microspheres was examined using scanning electron microscopy (SEM; S4700; Hitachi, Tokyo, Japan). The specific surface area of the microspheres and pore size of the microsphere shell was measured using the Brunauer–Emmett–Teller method (Xiao et al., 2013). The carbon content was measured using a combustion technique at a commercial laboratory (LECO Corp., St Joseph, MI). X-ray diffraction (XRD) and Fourier-transform infrared (FTIR) spectroscopy were used to check the phase composition of the microspheres. XRD was performed using Cu Kα radiation (λ = 0.15406 nm) at a rate of 1.8°/min in the 2θ range 20–70°, while FTIR was performed in the wavenumber range of 400–4,000 cm−1.
2.2 |. Release profiles of BMP-2, relaxin, and a combination of BMP-2 and relaxin
The hollow HA microspheres were sterilized by soaking in anhydrous ethanol, followed by drying in an incubator at 120°C. Then the microspheres were loaded with BMP-2 (Human recombinant BMP-2; Shenandoah Inc., Warwick, PA). The BMP-2 was diluted in sterile water to a concentration of 10 μg/ml and 10 mg of HA microspheres were placed in the BMP-2 solution. A small vacuum was applied to remove any air trapped in the microspheres. The BMP-2 loaded microspheres were then allowed to dry at 4°C overnight. To measure the BMP-2 release profile, the microspheres were placed in a release buffer consisting of 400 μl of 50% fetal bovine serum (FBS) in phosphate buffered saline (PBS) and kept at 37°C with gentle agitation. After 1 hour, 1 day, 3 days, 5 days, 7 days, and 14 days, the entire release buffer was collected. The microspheres were then rinsed with additional 200 μl of release buffer and the rinse was collected and pooled with the initial 400 μl collection, making sure not to collect any microspheres in the process. Fresh buffer was then added to continue the experiment for the next time point. The amount of BMP-2 released into the buffer was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Peprotech, Rocky Hill, NJ). Absorbance was measured with a microplate reader. The concentration of the unknown samples was compared to a standard curve ran at the same time.
The release profile of relaxin was measured using a procedure similar to that described above for BMP-2. Human recombinant relaxin-2 was purchased from R&D systems (Minneapolis, MN) and diluted in sterile water to a concentration of 1 μg/ml. Ten milligrams of HA microspheres were placed in the relaxin solution. The remaining procedure was followed as detailed for BMP-2.
The combined release was conducted in a similar manner to the individual release profiles. Briefly, 10 mg of HA microspheres were placed in a solution containing both 10 μg/ml of BMP-2 and 1 μg/ml of relaxin. The remaining procedure was conducted exactly like the individual release profiles mentioned above.
2.3 |. Animals and surgery
All animal procedures were approved by the Missouri University of Science and Technology Institutional Animal Care and Use Committee (IACUC), in compliance with the NIH Guide for Care and Use of Laboratory Animals (Committee for the update of the guide for the care use of laboratory animals, 2011). Male Sprague Dawley rats of 3-months-old were purchased from Envigo (Indianapolis, IN) (weighing 350–400 g) and housed under a 12 h/12 h light/dark cycle. Rats were allowed to acclimate to diet, water, and housing for 14 days before experiments were conducted. All animal procedures follow our previous publications (Liu, Rahaman, Liu, Bal, & Bonewald, 2013; Xiao et al., 2013; Xiong et al., 2015).
The rats were anesthetized with 3–5% isoflurane in oxygen administered by inhalation or an intraperitoneal injection of ketamine (70 mg/kg) and xylazine (7 mg/kg). The surgical area was shaved, scrubbed with 70% ethanol, and draped with a sterile drape. Using aseptic technique, a cranial skin incision was made in an anterior-to-posterior direction. The subcutaneous tissue, musculature, and periosteum were dissected to expose the calvarium. Bilateral full-thickness defects 4 mm in diameter were created using a trephine burr and an electric drill. The site was constantly irrigated with sterile PBS to prevent overheating. The defects were filled with one type of 13 different implants consisting of closed hollow HA microspheres (10 mg per defect) loaded with varying amounts of relaxin and BMP-2 which are detailed in Table 1.
TABLE 1.
Experimental design of the amount of BMP-2 and relaxin loaded in the 13 groups of animals
| Group | N | BMP-2 (μg) | Relaxin (μg) | Description |
|---|---|---|---|---|
| 1 | 5 | 0 | 0 | As prepared microspheres |
| 2 | 8 | 0 | 0.05 | Relaxin alone |
| 3 | 7 | 0 | 0.1 | Relaxin alone |
| 4 | 8 | 0 | 0.25 | Relaxin alone |
| 5 | 6 | 0.25 | 0 | BMP-2 alone |
| 6 | 7 | 0.5 | 0 | BMP-2 alone |
| 7 | 5 | 1 | 0 | BMP-2 alone |
| 8 | 6 | 0.25 | 0.05 | 0.25 μg BMP-2 + Relaxin |
| 9 | 9 | 0.25 | 0.1 | 0.25 μg BMP-2 + Relaxin |
| 10 | 9 | 0.25 | 0.25 | 0.25 μg BMP-2 + Relaxin |
| 11 | 8 | 0.5 | 0.05 | 0.5 μg BMP-2 + Relaxin |
| 12 | 8 | 0.5 | 0.1 | 0.5 μg BMP-2 + Relaxin |
| 13 | 8 | 0.5 | 0.25 | 0.5 μg BMP-2 + Relaxin |
The animals were monitored daily for condition of the surgical wound, food intake, activity, and clinical signs of infection. After 6 weeks, the animals were sacrificed by CO2 inhalation. The calvarial defect sites and surrounding bone were harvested.
2.4 |. Histology and histomorphometric analysis
The calvarial samples were washed and fixed in a 10% formalin solution for 5 days. The fixed samples were cut transversely in half; half of each sample was embedded in paraffin and the other half was embedded in methyl methacrylate. The samples destined for paraffin embedding were decalcified for 10 days in 14% EDTA, dehydrated in ethanol, and embedded in paraffin using standard histological techniques. Sections of 5 μm were made from the samples and sections were stained with hematoxylin and eosin (H&E). The samples designated for methyl methacrylate embedding were dehydrated in ethanol and embedded in poly(methyl methacrylate) (PMMA). Sections were affixed to acrylic slides, ground down to 40 μm using a surface grinder (EXAKT 400CS, Norderstedt, Germany), and stained using the von Kossa method to observe mineralization (McGee-Russell, 1958).
Transmitted light images of the stained sections were taken with an Olympus BX 53 microscope connected to a CCD camera (DP70, Olympus, Japan) and were analyzed using the ImageJ software (National Institutes of Health). The percentage area of new bone formed was determined from the H&E stained samples. The total defect area was identified by measuring one edge of the old calvarial bone to the other edge including the implants and tissue within. The new bone within the defect area was outlined and expressed as a percentage of the total defect area.
2.5 |. Statistical analysis
Data sets are presented as the mean ± standard deviation (SD). Data were compiled in Microsoft Excel and statistical analysis was performed using Minitab 17. Student’s t-tests were used to determine statistical significance between groups. One-way analysis of variance (ANOVA) with Dunnett’s method pairwise comparison plots were used to determine and identify statistically significant values. Values were considered significant for p-values smaller than .05 (p < .05).
3 |. RESULTS
3.1 |. Characterization of HA microspheres
The hollow HA microspheres had an external diameter of 212–250 μm, a surface area of 168 ± 7 m2/g, and a mesoporous shell wall with an average pore size of 10–18 nm (Figure 1a,b). The total specific surface of the HA microspheres, determined by the Brunauer–Emmett–Teller method using nitrogen adsorption, was 0.425 cm2/g. The XRD patterns and FTIR spectra confirmed that the hollow microspheres were composed of nanometer-sized HA, as described previously (Fu et al., 2011; Xiao et al., 2013). The FTIR results also showed a weak resonance corresponding to a carbonate group, indicating that the microspheres were composed of a carbonate-substituted hydroxyapatite. The carbonate substitution (2.1% [w/w]) may be due to dissolved CO2 in the phosphate solution used in the glass conversion process.
FIGURE 1.
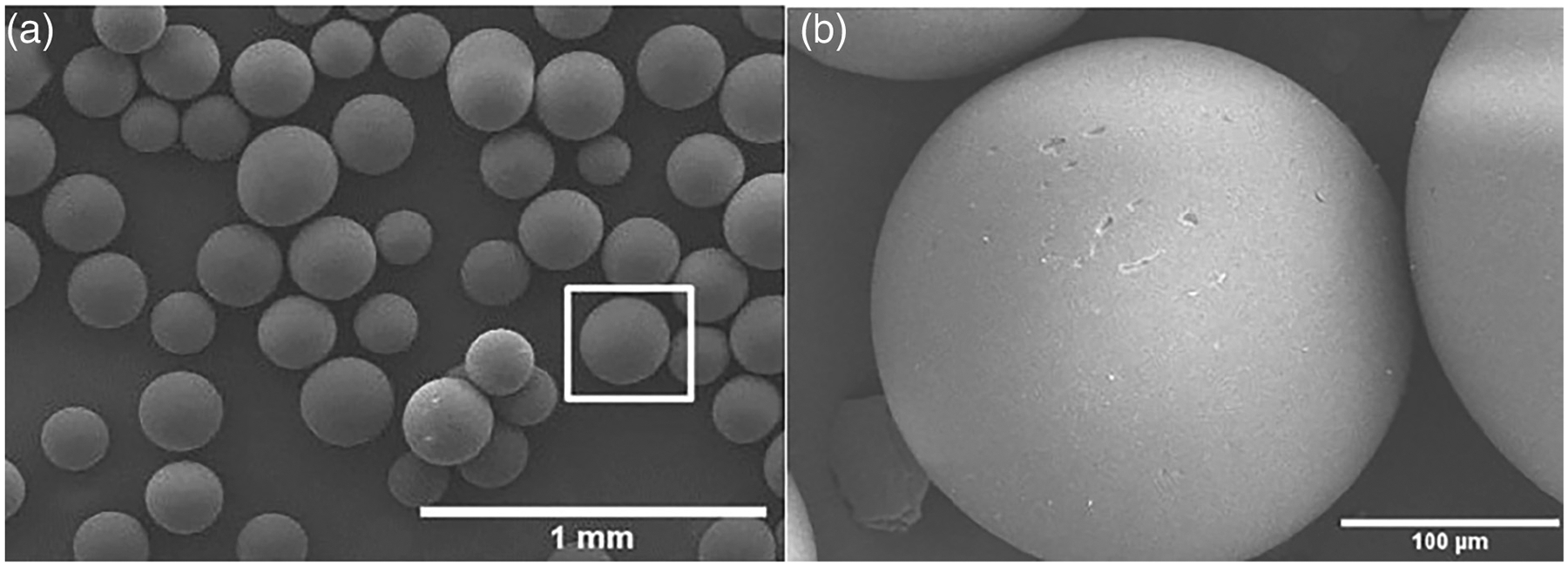
SEM images of as prepared HA microspheres. (a) low magnification and (b) high magnification from the box in (a)
3.2 |. Release profile
The cumulative amount of BMP-2 and relaxin released from the hollow HA microspheres into the release buffer (50% FBS in PBS) is shown as a function of time in Figure 2. A general trend was observed: when microspheres were loaded with both BMP-2 and relaxin, the release rates of BMP-2 and relaxin from the microspheres were faster than those of the proteins loaded alone. At day 14, the cumulative release rate of BMP-2 from the microspheres loaded with both BMP-2 and relaxin was 57% higher than that of BMP-2 from the microspheres loaded with BMP-2 alone (p < .05; n = 3). At day 14, the cumulative release rate of relaxin from the microsphere loaded both BMP-2 and relaxin was 86% higher than that of the microspheres loaded with relaxin alone (p < .05; n = 3).
FIGURE 2.
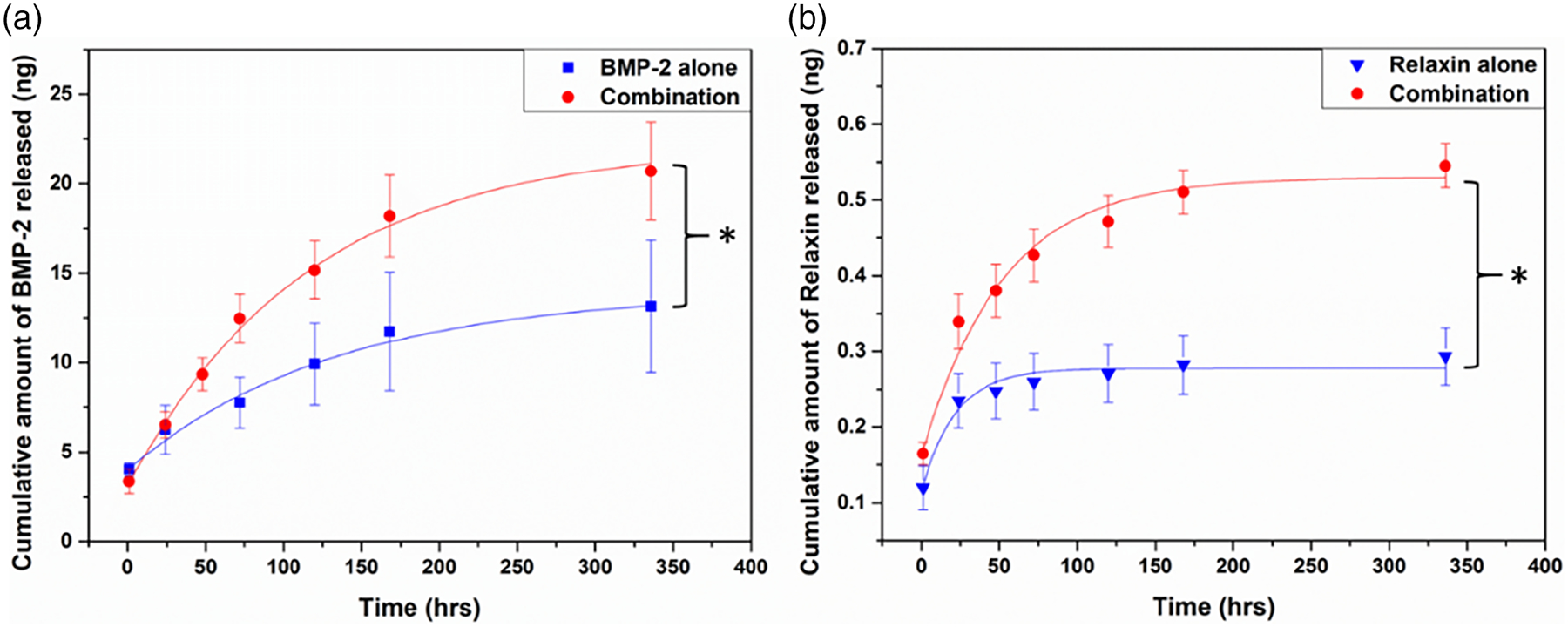
Release profiles of BMP-2 and relaxin from hollow HA microspheres. Cumulative amount of BMP-2 (a) and relaxin (b) released as a function of time. Results are expressed as the mean ± SD. (*p < .05; n = 3)
3.3 |. H&E stained sections
H&E stained sections of rat calvarial defects implanted with as-prepared HA microspheres (without relaxin or BMP-2) and microspheres loaded with different amounts of BMP-2 are shown in Figure 3. The as-prepared microspheres showed little new bone growth (4.0 ± 6.2%) which occurred along the edges of the host bone (Figure 3a). The defect area instead consisted of fibrous connective tissue. The addition of 0.25 μg of BMP-2 per defect did not significantly increase new bone formation when compared to the as-prepared microspheres (Figure 3b; 17.9 ± 8.4%; p = .129). In comparison, new bone formation increased considerably as the amount of BMP-2 was increased to 0.5 μg per defect (Figure 3c; 23.9 ± 12.4%; p = .006). The greatest new bone growth was seen when using microspheres loaded with 1 μg per defect (Figure 3d; 40.2 ± 6.3%; p < .001), with new bone bridging the defect within the 6-week implantation period.
FIGURE 3.
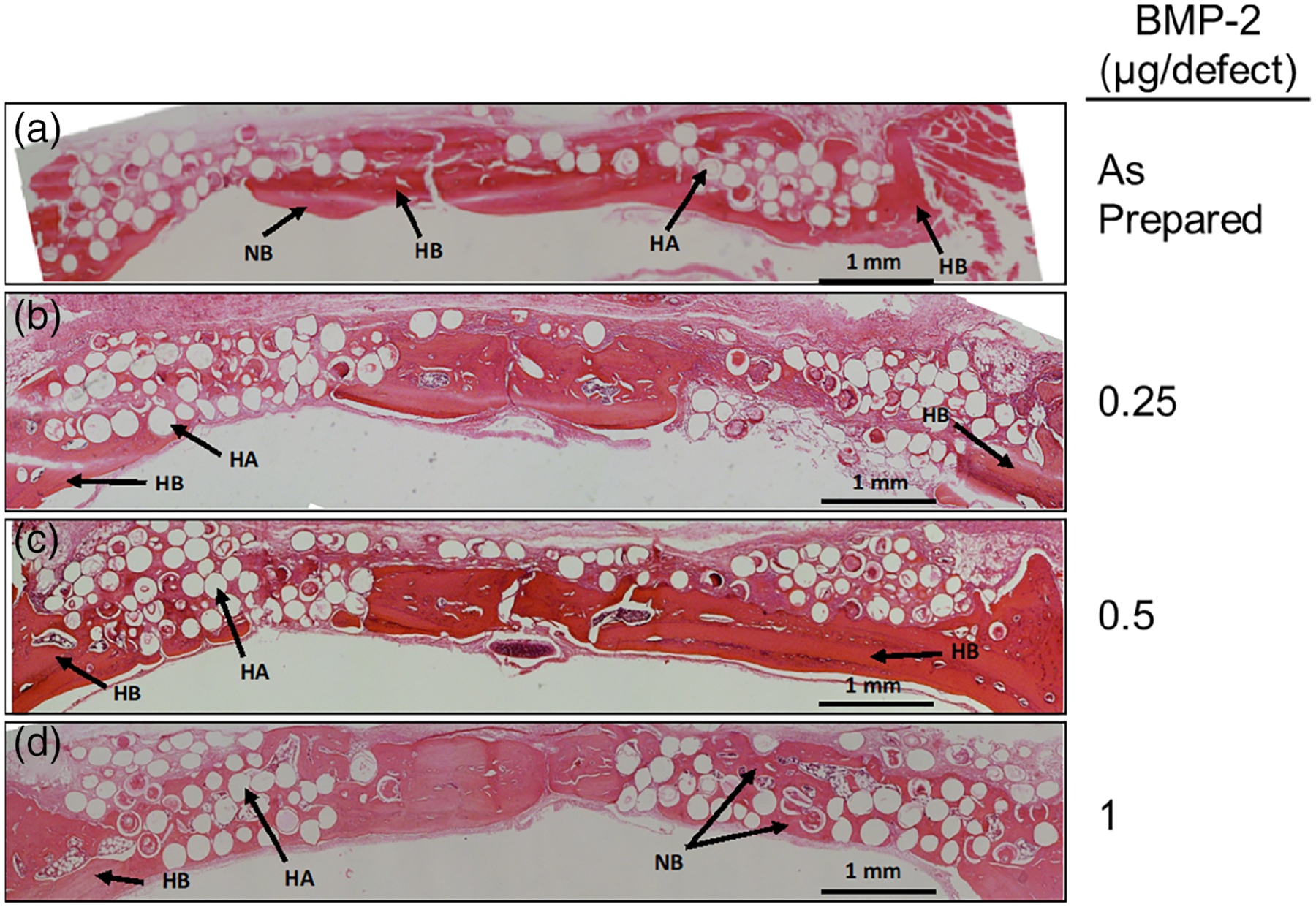
H&E stained sections of rat calvarial defects implanted with HA microspheres and various concentrations of BMP-2 alone for 6 weeks. HB, host bone; NB, new bone; HA, hydroxyapatite. Scale bar = 1 mm
H&E stained sections of rat calvarial defects implanted with HA microspheres loaded with different amounts of relaxin are shown in Figure 4. Little new bone growth was seen in defects loaded with 0.05, 0.1, and 0.25 μg of relaxin and growth was not significantly different from that in defects implanted with as-prepared HA microspheres (Figure 4b–c; p = .177, .541, and .401, respectively). Bone growth was only observed adjacent to host bone and from the dura mater, which is a source of osteogenic cells and growth factors.
FIGURE 4.
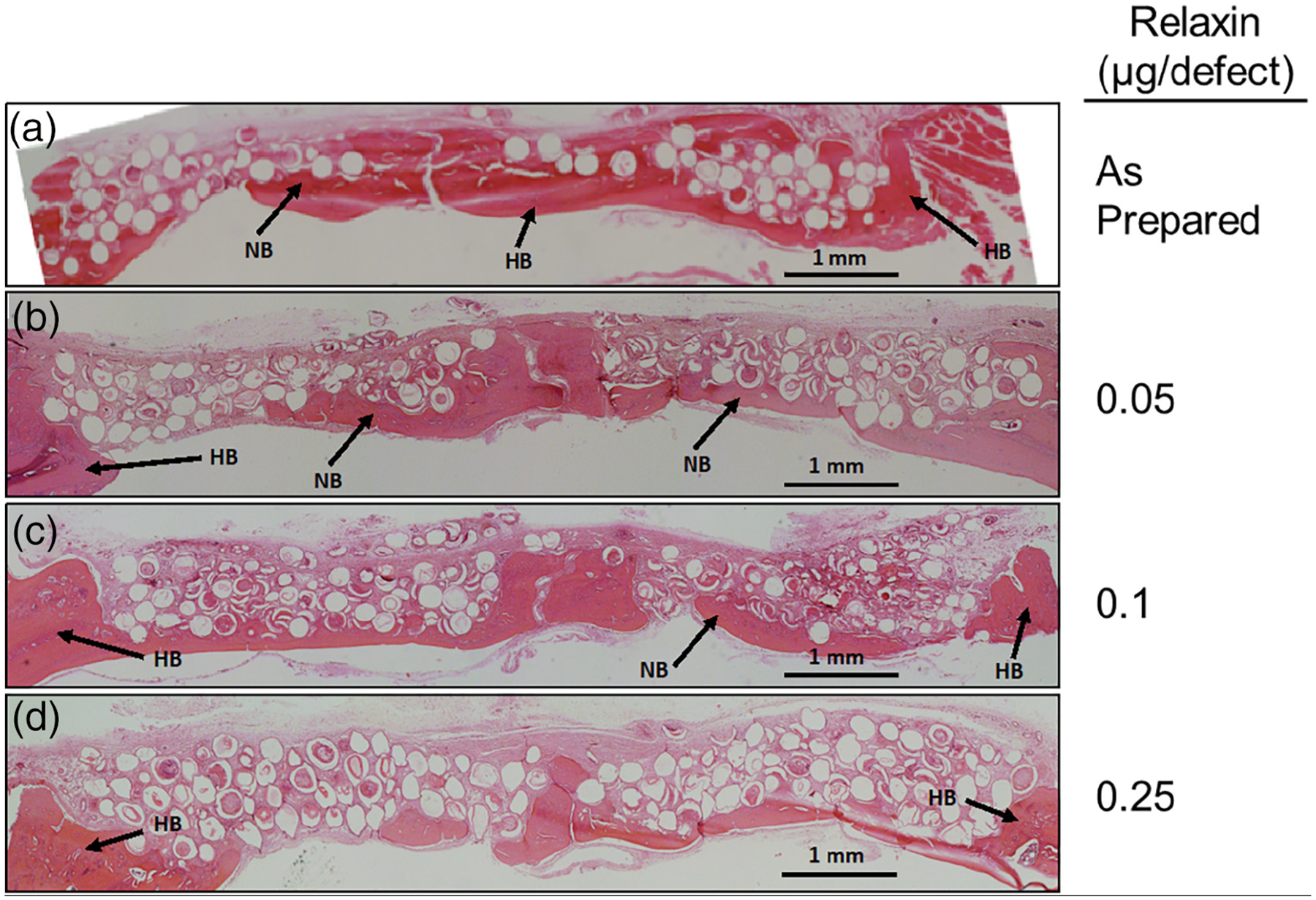
H&E stained sections of rat calvarial defects implanted with HA microspheres and various concentrations of relaxin alone for 6 weeks. HB, host bone; NB, new bone. Scale bar = 1 mm
Images of H&E stained sections of defects implanted with microspheres loaded with 0.25 μg of BMP-2 and varying amounts of relaxin are shown in Figure 5. All three concentrations of relaxin (0.05, 0.1, and 0.25 μg) in combination with 0.25 μg of BMP-2 produced no significant improvement in new bone growth compared to the group loaded with 0.25 μg of BMP-2 alone (Figure 5b–d; p = .105, .856, and .800, respectively).
FIGURE 5.
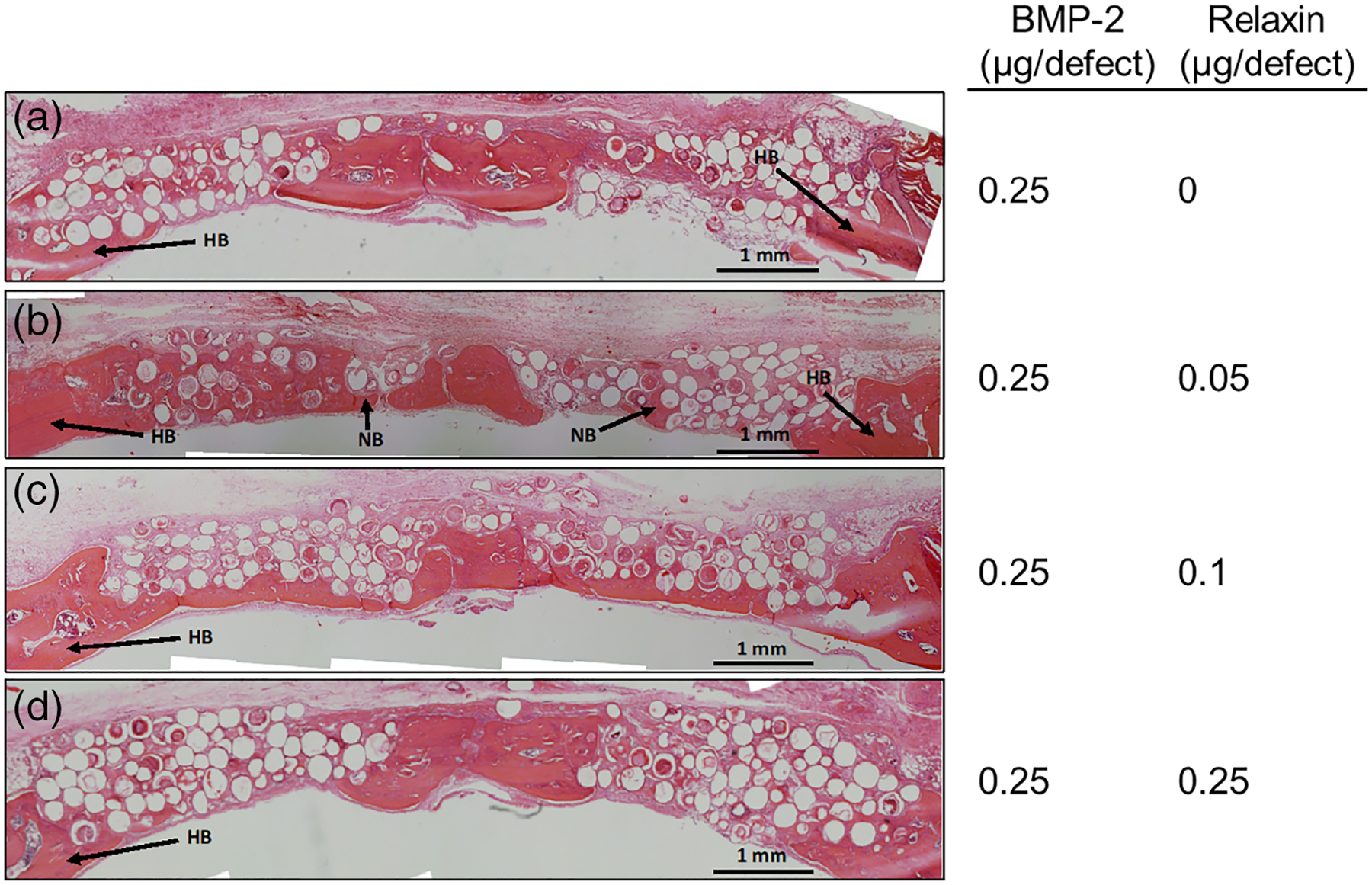
H&E stained sections of rat calvarial defects implanted with HA microspheres, 0.25 μg of BMP-2, and various concentrations of relaxin for 6 weeks. HB, host bone; NB, new bone. Scale bar = 1 mm
Figure 6 shows H&E stained sections of rat calvarial defects implanted with HA microspheres loaded with 0.5 μg of BMP-2 and different amounts of relaxin. All amounts of relaxin (0.05, 0.1, and 0.25 μg) showed new bone bridged the defect in each group. However, only defects loaded with 0.5 μg of BMP-2 and 0.05 μg of relaxin showed significant enhancement of bone growth compared to 0.5 μg of BMP-2 alone (Figure 6b–d; p = .002, .400, and .190, respectively).
FIGURE 6.

H&E stained sections of rat calvarial defects implanted with HA microspheres, 0.50 μg of BMP-2, and various concentrations of relaxin for 6 weeks. HB, host bone; NB, new bone. Scale bar = 1 mm
High magnification images of the H&E stained sections of select groups are shown in Figure 7. The image of a defect loaded with as-prepared HA microspheres confirmed that the defect area consisted mostly of fibrous connective tissue (Figure 7a). When compared to those loaded with 0.5 and 1 μg of BMP-2 alone (Figure 7b,c), more new bone growth is seen in defects loaded with a combination of 0.5 μg of BMP-2 and 0.05 μg of relaxin (Figure 7d). This confirmed relaxin enhanced BMP-2 at this amount. High magnification images of defects loaded with 1 μg of BMP-2 looked histologically comparable to defects loaded with a combination of 0.5 μg of BMP-2 and 0.05 μg of relaxin (Figure 7c vs. d).
FIGURE 7.
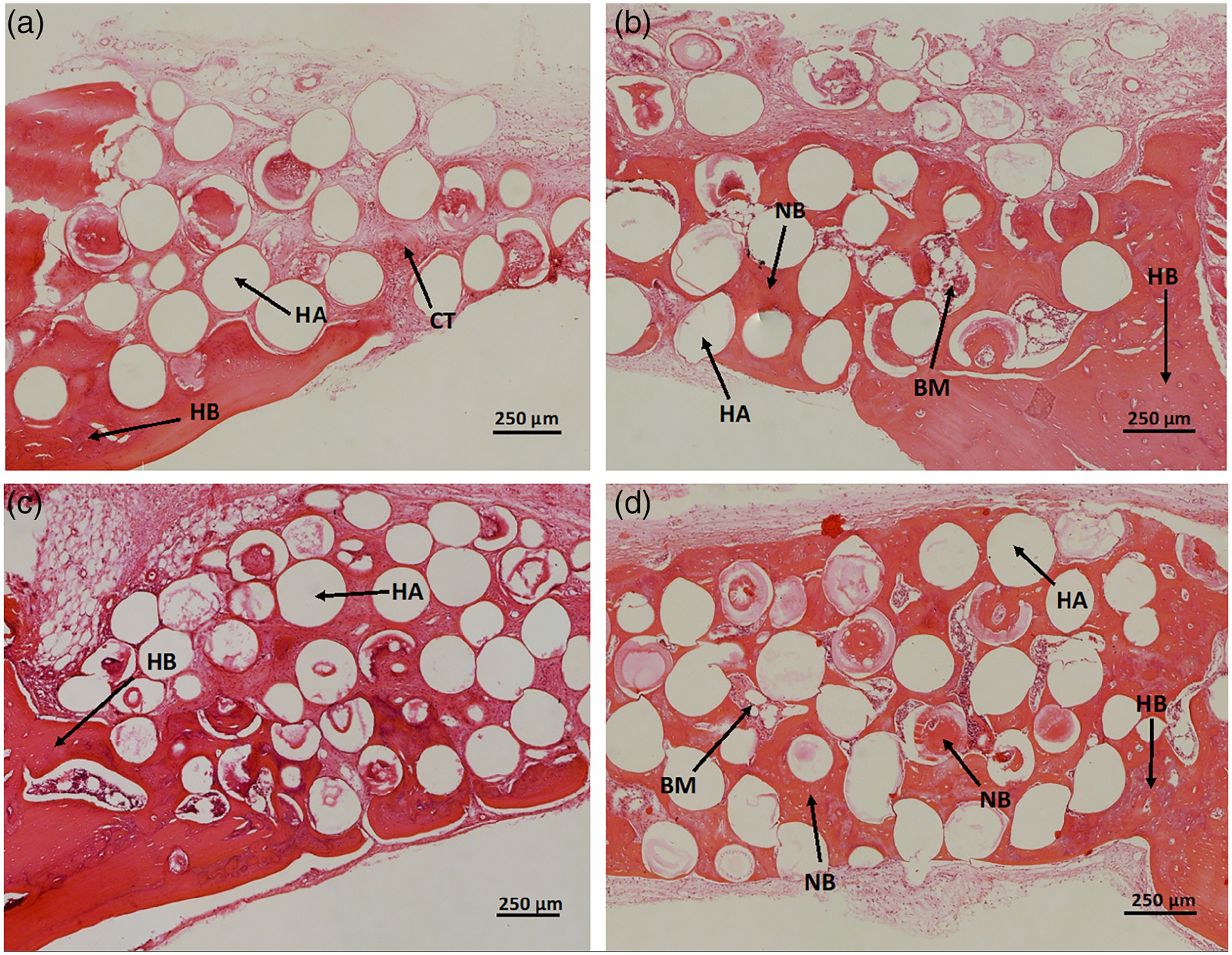
High magnification images of H&E stained sections of rat calvarial defects implanted for 6 weeks with (a) as prepared HA microspheres, (b) HA microspheres loaded with 0.5 μg of BMP-2, (c) HA microspheres loaded with 1 μg of BMP-2, (d) HA microspheres loaded with 0.5 μg of BMP-2 and 0.05 μg of relaxin. HB, host bone, NB, new bone, HA, hydroxyapatite, BM, bone marrow-like tissue, CT, fibrous connective tissue. Scale bar = 250 μm
Figure 8 shows the amount of new bone growth in the defects implanted with microspheres loaded with varying amounts of BMP-2 or relaxin. Bar graphs exhibiting the enhancement effects of 0.05 μg of relaxin on 0.25 and 0.5 μg of BMP-2 are shown in Figure 9. It should be noted that statistical significance indicated on the graph is in comparison to 0.25 (a) and 0.5 μg (b) of BMP-2 alone. The percentage area of new bone (mean ± SD) for all groups are summarized in Table 2.
FIGURE 8.
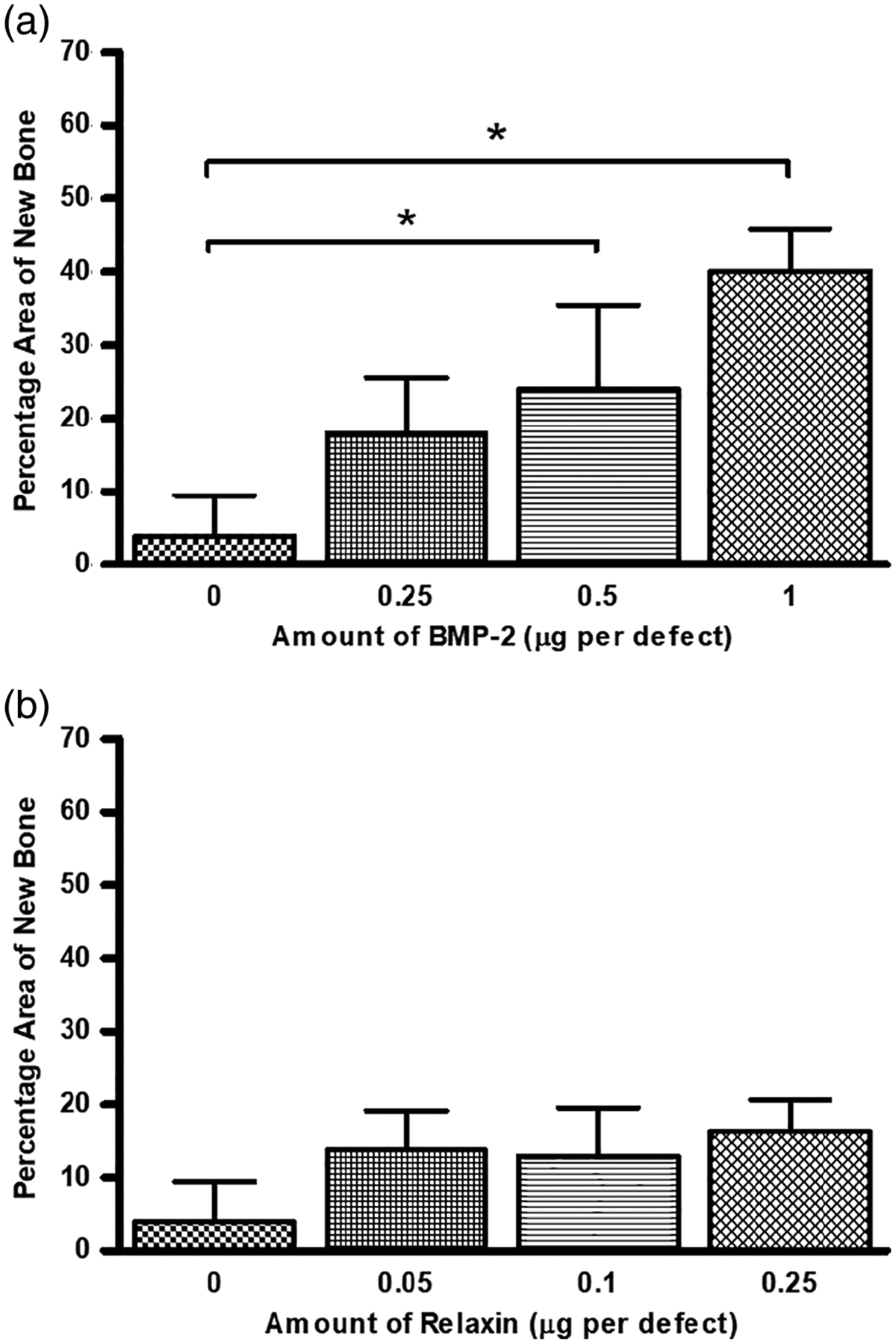
The percentage area of new bone formed in rat calvarial defects implanted for 6 weeks with HA microspheres loaded with (a) BMP-2 or (b) relaxin. Bars are the mean ± SD (*p < .05)
FIGURE 9.
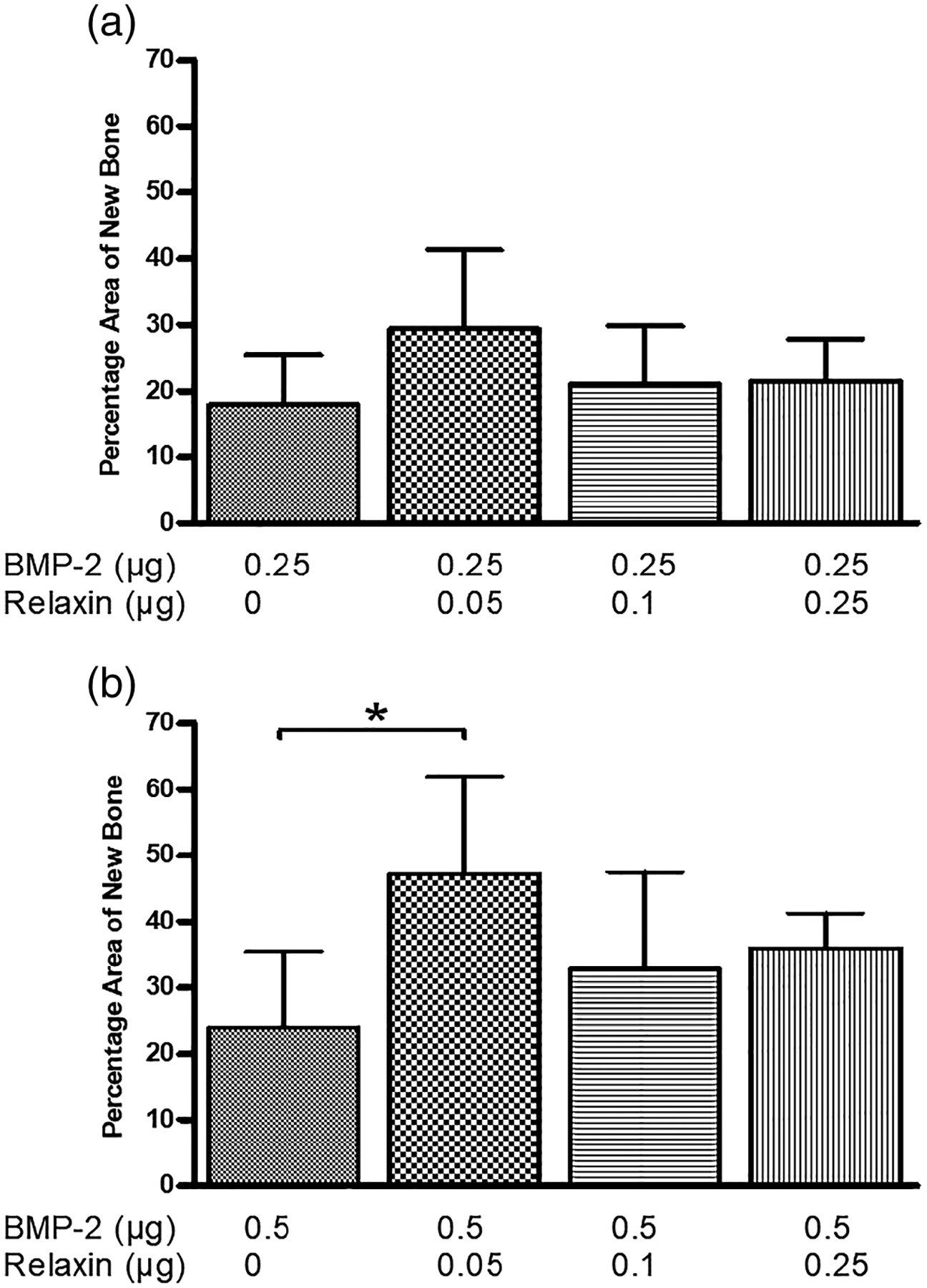
The enhancement effect of relaxin on rat calvarial defects implanted with (a) HA microspheres loaded with 0.25 μg of BMP-2 and (b) HA microspheres loaded with 0.5 μg of BMP-2. Data are shown as the mean ± SD (*p < .05)
TABLE 2.
Percentage area of new bone (mean ± SD) in rat calvarial defects with varying amounts of BMP-2, relaxin, and combinations of both
| Group | N | BMP-2 (μg) | Relaxin (μg) | New bone (% area) |
|---|---|---|---|---|
| 1 | 5 | 0 | 0 | 4.0 ± 6.2 |
| 2 | 8 | 0 | 0.05 | 16.3 ± 4.7 |
| 3 | 7 | 0 | 0.1 | 12.8 ± 7.3 |
| 4 | 8 | 0 | 0.25 | 13.7 ± 5.8 |
| 5 | 6 | 0.25 | 0 | 17.9 ± 8.4 |
| 6 | 7 | 0.5 | 0 | 23.9 ± 12.4 |
| 7 | 5 | 1 | 0 | 40.2 ± 6.3 |
| 8 | 6 | 0.25 | 0.05 | 29.4 ± 13.1 |
| 9 | 9 | 0.25 | 0.1 | 21.0 ± 9.4 |
| 10 | 9 | 0.25 | 0.25 | 21.5 ± 6.7 |
| 11 | 8 | 0.5 | 0.05 | 49.7 ± 14.7 |
| 12 | 8 | 0.5 | 0.1 | 32.9 ± 15.6 |
| 13 | 8 | 0.5 | 0.25 | 35.9 ± 5.8 |
3.4 |. Comparison to 1 μg of BMP-2 per defect
Figure 10 shows a comparison of the percentage area of new bone in the rat calvarial defects implanted with the BMP-2 and relaxin groups with 1 μg of BMP-2 per defect. No significant difference in the percentage area of new bone is seen when 0.5 μg of BMP-2 were loaded in HA microspheres in combination with 0.05, 0.1, and 0.25 μg of relaxin when compared to those loaded with 1 μg of BMP-2 (p = .458, .723, and .985, respectively). In addition, no significant difference in percentage area of new bone was observed between HA microspheres loaded with a combination of 0.25 μg of BMP-2 and 0.05 μg of relaxin when compared to the microspheres loaded with 1 μg of BMP-2 (p = .357). These data show that the addition of relaxin can reduce the BMP-2 dose required to regenerate a similar amount of new bone in a rat calvarial defect model.
FIGURE 10.
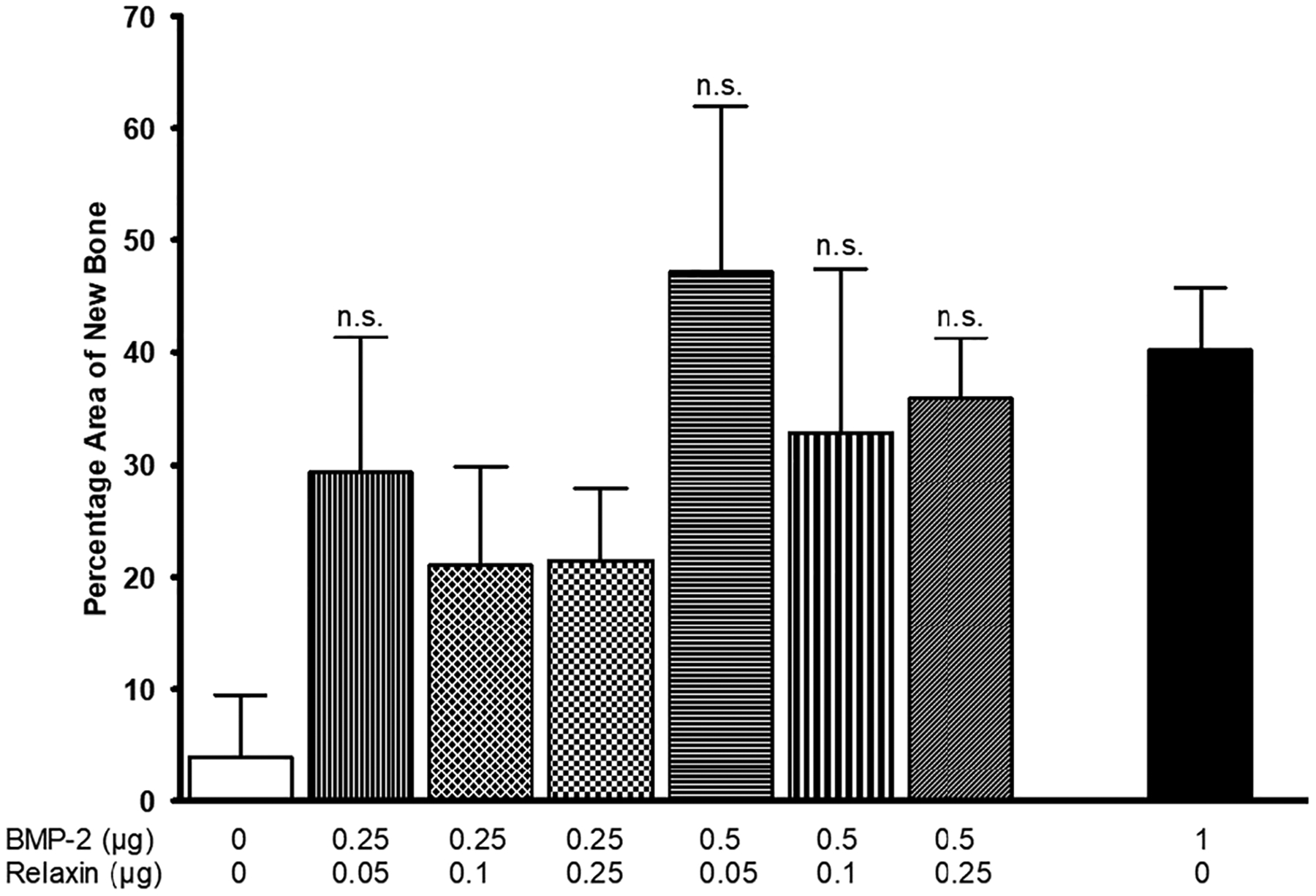
Comparison of the percent new bone formed in rat calvarial defects implanted with BMP-2 and relaxin to 1 μg of BMP-2 per defect. Data are shown as the mean ± SD (n.s., not significant compared to 1 μg of BMP-2; p > .05)
Optical images of von Kossa stained sections of rat calvarial defects implanted for 6 weeks with select groups of microspheres are shown in Figure 11. As the HA microspheres are composed of a phosphate material, both the HA microspheres and mineralized tissues (bone) are stained in this technique (black). New bone can be easily observed within the defect. These von Kossa stained sections complement the results from the H&E stained sections described earlier. The images qualitatively support the results from Figure 10 that the addition of relaxin can reduce the BMP-2 dose required to achieve a comparable amount of new bone growth.
FIGURE 11.
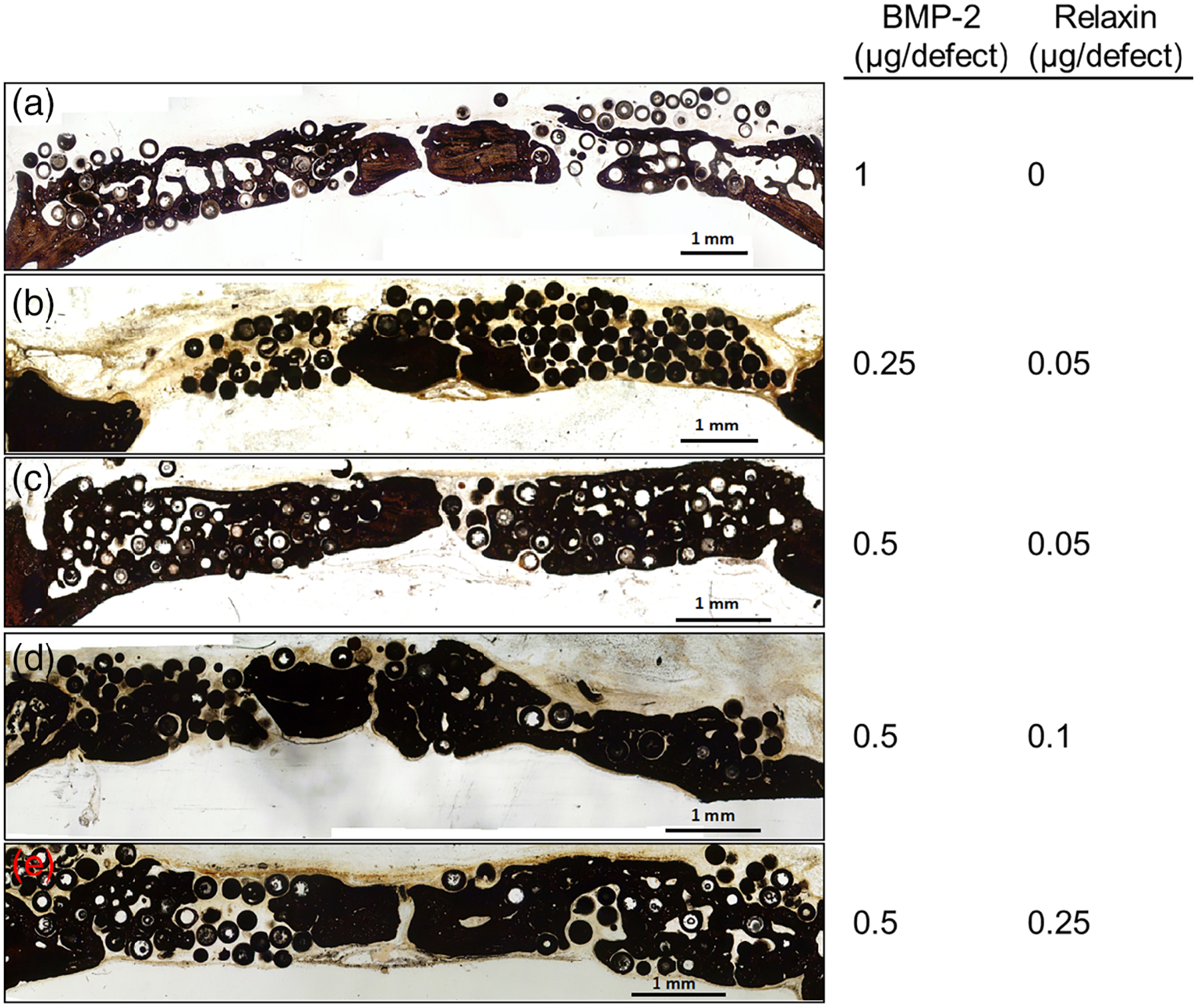
Von Kossa stained sections of rat calvarial defects implanted with various concentration combinations of BMP-2 and relaxin for 6 weeks. Comparisons against the group of 0.5 μg per defect of BMP-2. Scale bar = 1 mm
4 |. DISCUSSION
HA microspheres have been shown to be a carrier for growth factors while being osteoconductive and bioactive (Xiao et al., 2013). The present study aimed to test the ability of relaxin to enhance the osteoinductive effect of BMP-2 and to lower the required dose of BMP-2 for safer clinical use. HA microspheres were tested for their controlled release of both BMP-2 and relaxin alone as well as in combination. Studies were conducted in vivo to test the enhancement effect of relaxin and showed relaxin has an ability to lower the BMP-2 dose at specific concentrations.
The release profile of BMP-2 and relaxin from the hollow HA microspheres in vitro showed that more BMP-2 was released from the microspheres than relaxin. Further, when microspheres were loaded with both BMP-2 and relaxin, the release rates of BMP-2 or relaxin from the microspheres were faster than those of BMP-2 and relaxin when they were loaded alone. The isoelectric point of proteins has been shown to have an effect on adsorption and release of proteins from HA (Gorbunoff, 1984; Luo & Andrade, 1998). With an isoelectric point of 8.17 (BMP-2) and 9.12 (relaxin), the surfaces of BMP-2 and relaxin could be populated with positive ions in the neutral release buffer leading to an electrostatic attraction with the negatively charged HA surface. The zeta potentials of BMP-2 and relaxin in water (pH 7.4) being 6.11 mV and 21.20 mV, respectively, further supports our belief. It is expected that the release of BMP-2 in vivo would be faster than that in vitro due to a higher degradation rate of HA and the higher solubility of proteins in vivo (Xiao et al., 2013). In general, the gradual release profile observed in this study indicates that the HA microspheres can provide a controlled delivery system for BMP-2 and relaxin. Modification of the release rate of BMP-2 and relaxin, if needed for clinical purposes, can be achieved through modification of the HA microspheres themselves (Xiao et al., 2013) or the method used to incorporate the proteins into the HA microspheres (Gorbunoff, 1984).
As BMP-2 is an osteoinductive protein, increase in the amount of BMP-2 can lead to an increase in new bone formation. In the present study, 1 μg of BMP-2 per defect produced the largest amount of new bone (Figure 3). The amount of new bone in defects implanted with the HA microspheres loaded with 0.5 and 1 μg BMP-2 per defect was significantly higher than that in defects loaded with the as-prepared microspheres (i.e., with no BMP-2 loaded) (Figure 8a). In comparison, relaxin is known to be an enhancer of BMP-2 and does not have any osteoinductive properties on its own (Moon et al., 2014). In the present study, loading HA microspheres with relaxin alone (0.05 to 0.25 μg per defect) seemed to produce bone regeneration not statistically different from the as-prepared microspheres (Figure 4 and 8b; Table 2).
The effects of relaxin on 0.25 and 0.5 μg of BMP-2 were observed from the H&E stained sections (Figures 5 and 6) and confirmed by quantitative data (Figure 9). At an amount of 0.25 μg of BMP-2, the addition of relaxin (0.05 to 0.25 μg) did not enhance the ability of BMP-2 to stimulate new bone formation compared to 0.25 μg of BMP-2 alone. In comparison, the addition of 0.05 μg of relaxin to 0.5 μg of BMP-2 produced a significant improvement in bone regeneration compared to 0.5 μg of BMP-2 alone (Figure 9b). Interestingly, at 0.5 μg of BMP-2, no enhancement in bone regeneration was observed at any other amounts of relaxin. We speculated that relaxin contents higher than ~0.05 μg might have an inhibitory effect by unknown signaling pathways. Future studies may use relaxin additions lower than 0.05 μg to determine an optimal range to provide significant enhancement to bone regeneration. As relaxin did not enhance the bone regeneration capability of 0.25 μg of BMP-2, this might indicate that a sufficient amount of BMP-2 needs to be present for enhancement to occur. Future studies that employ less than 0.05 μg of relaxin, as discussed above, could reveal whether there is an enhancement effect at less than 0.5 μg of BMP-2.
The overall goal of this study was to determine whether the addition of relaxin could lower the previously published dose of BMP-2 (1 μg of BMP-2 per defect) for bone regeneration. Figure 10 depicts these comparisons. With the addition of 0.05 μg relaxin, the amount of BMP-2 required to produce the same amount of new bone in rat calvarial defects can be reduced by 50% (i.e., from 1 to 0.5 μg). The combination of 0.05, 0.1, and 0.25 μg of relaxin with 0.5 μg of BMP-2, as well as 0.05 μg of relaxin with 0.25 μg of BMP-2, all produced percentage areas of new bone that were not significantly different form 1 μg of BMP-2, indicating sufficient bone growth with dose reduction. The influence of the large within-group variance in the group of 0.25 μg of BMP-2 and 0.05 μg of relaxin on the statistical outcome is uncertain. The reasons for the combined effect are multiple. In addition to the enhancement effect mediated by relaxin, it is possible that the increase in bone formation seen with the combined microspheres could be due to the increased release of BMP-2 and relaxin.
Figure 7 shows high magnification images of H&E stained sections of rat calvarial defects implanted with select groups of microspheres. These images show no observable histological difference between a defect loaded with 1 μg of BMP-2 and a defect loaded with 0.5 μg of BMP-2 in combination with 0.05 μg of relaxin, further confirming that the addition of relaxin can lower the BMP-2 dose required for an equivalent amount of bone regeneration. This reduction supports the hypothesis that relaxin has the ability to lower BMP-2 doses with its enhancement effect, especially seen in the combination of 0.5 μg of BMP-2 with 0.05 μg of relaxin.
5 |. CONCLUSIONS
Hydroxyapatite microspheres can be used as a carrier for the controlled delivery of single or multiple proteins. Monitoring of BMP-2 and relaxin in vivo showed a slow and continuous release over the measured two-week period. When compared to the microspheres loaded with BMP-2 or relaxin alone, a higher amount of relaxin or BMP-2 was released from the microspheres loaded with a combination of relaxin and BMP-2. With the addition of 0.05 μg relaxin, the amount of BMP-2 required to produce the same amount of new bone in rat calvarial defects can be reduced by 50%. The reduction in BMP-2 dose when used in combination with relaxin implies theraperutic significance, as well as reduced adverse physiological effects.
ACKNOWLEDGMENTS
We would like to thank Richard Watters, the Missouri S&T Materials Research Center, and the Missouri S&T Animal Research Facility for providing technical support. We also thank Natalie Holl and Benjamin Barr for help with editing this manuscript. This project was funded by a grant from National Institute of Dental and Craniofacial Research (NIDCR) (grant # R15DE023987) and a seed grant from Missouri S&T Center for Biomedical Science and Engineering.
NOMENCLATURE
- BSA
Bovine Serum Albumin
- HA
Hydroxyapatite
- PBS
Phosphate Buffer Saline
- BMP-2
Bone Morphogenetic Protein-2
- H&E
Hematoxylin and Eosin
- FBS
Fetal Bovine Serum
- EDTA
Ethylenediaminetetraacetic Acid
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, & Summers RJ (2005). Receptors for relaxin family peptides. Annals of the New York Academy of Sciences, 1041(1), 61–76. [DOI] [PubMed] [Google Scholar]
- Bathgate RAD, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, & Summers RJ (2013). Relaxin family peptides and their receptors. Physiological Reviews, 93(1), 405–480. [DOI] [PubMed] [Google Scholar]
- Carragee EJ, Chu G, Rohatgi R, Hurwitz EL, Weiner BK, Yoon ST, … Kopjar B (2013). Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. The Journal of Bone & Joint Surgery, 95(17), 1537–1545. [DOI] [PubMed] [Google Scholar]
- Cholas R, Kunjalukkal Padmanabhan S, Gervaso F, Udayan G, Monaco G, Sannino A, & Licciulli A (2016). Scaffolds for bone regeneration made of hydroxyapatite microspheres in a collagen matrix. Materials Science and Engineering C, 63, 499–505. [DOI] [PubMed] [Google Scholar]
- Committee for the update of the guide for the care use of laboratory animals. (2011). Institute for laboratory animal research, Divison on earth life studies, national research concil. Guide for the care and use of laboratory animals. Washington, D.C: National Academies Press. [Google Scholar]
- Duarte C, Kobayashi Y, Kawamoto T, & Moriyama K (2014). Relaxin enhances differentiation and matrix mineralization through Relaxin/insulin-like family peptide receptor 2 (Rxfp2) in MC3T3-E1 cells in vitro. Bone, 65, 92–101. [DOI] [PubMed] [Google Scholar]
- Duarte C, Kobayashi Y, Morita J, Kawamoto T, & Moriyama K (2016). A preliminary investigation of the effect of relaxin on bone remodelling in suture expansion. European Journal of Orthodontics, 39(3), 227–234. [DOI] [PubMed] [Google Scholar]
- Ferlin A, Pepe A, Gianesello L, Garolla A, Feng S, Giannini S, … Agoulnik AI (2008). And others. Mutations in the insulin-like factor 3 receptor are associated with osteoporosis. Journal of Bone and Mineral Research, 23(5), 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Rahaman MN, Brown RF, & Day DE (2013). Evaluation of bone regeneration in implants composed of hollow HA microspheres loaded with transforming growth factor β1 in a rat calvarial defect model. Acta Biomaterialia, 9(3), 5718–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Rahaman MN, Day DE, & Brown RF (2011). Hollow hydroxyapatite microspheres as a device for controlled delivery of proteins. Journal of Materials Science: Materials in Medicine, 22(3), 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoudis PV, Dinopoulos H, & Tsiridis E (2005). Bone substitutes: An update. Injury, 36(3), S20–S27. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Traykova T, & Planell JA (2006). Calcium phosphate cements as bone drug delivery systems: A review. Journal of Controlled Release, 113(2), 102–110. [DOI] [PubMed] [Google Scholar]
- Gomes PS, & Fernandes MH (2011). Rodent models in bone-related research: The relevance of calvarial defects in the assessment of bone regeneration strategies. Laboratory Animals, 45(1), 14–24. [DOI] [PubMed] [Google Scholar]
- Gorbunoff MJ (1984). The interaction of proteins with hydroxyapatite. I. Role of protein charge and structure. Analytical Biochemistry, 136(2), 425–432. [DOI] [PubMed] [Google Scholar]
- Herford AS (2017). The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillofacial trauma. Chinese Journal of Traumatology, 20(1), 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangir AA, Nunley RM, Mehta S, Sharan A, & Fellows WHP. (2008). Bone-graft substitutes in orthopaedic surgery. American Academy of Orthopaedic Surgeons. [Google Scholar]
- Jeon O, Song SJ, Yang HS, Bhang SH, Kang SW, Sung MA, … Kim BS (2008). Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochemical and Biophysical Research Communications, 369(2), 774–780. [DOI] [PubMed] [Google Scholar]
- Kalfas IH (2001). Principles of bone healing. Neurosurgical Focus, 10 (4), E1. [DOI] [PubMed] [Google Scholar]
- Kisiel M (2013). Bone enhancement with BMP-2 for safe clinical translation. Acta Universitatis Upsaliensis. Sweden: Uppsala University. [Google Scholar]
- Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR, … Stupp SI (2013). Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials, 34(2), 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Porter RM, Wells J, Glatt V, Pilapil C, & Evans CH (2012). Evaluation of BMP-2 gene-activated muscle grafts for cranial defect repair. Journal of Orthopaedic Research, 30(7), 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Rahaman MN, Liu Y, Bal BS, & Bonewald LF (2013). Enhanced bone regeneration in rat calvarial defects implanted with surface-modified and BMP-loaded bioactive glass (13–93) scaffolds. Acta Biomaterialia, 9(7), 7506–7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuehl V, Meinel L, Merkle HP, & Gander B (2004). Localized delivery of growth factors for bone repair. European Journal of Pharmaceutics and Biopharmaceutics, 58(2), 197–208. [DOI] [PubMed] [Google Scholar]
- Luo Q, & Andrade JD (1998). Cooperative adsorption of proteins onto hydroxyapatite. Journal of Colloid and Interface Science, 200(1), 104–113. [Google Scholar]
- McGee-Russell S (1958). Histochemical methods for calcium. Journal of Histochemistry & Cytochemistry, 6(1), 22–42. [DOI] [PubMed] [Google Scholar]
- Moon JS, Kim SH, Oh SH, Jeong YW, Kang JH, Park JC, … Kim MS (2014). And others. Relaxin augments BMP-2-induced osteoblast differentiation and bone formation. Journal of Bone and Mineral Research, 29(7), 1586–1596. [DOI] [PubMed] [Google Scholar]
- Oryan A, Alidadi S, Moshiri A, & Bigham-Sadegh A (2014). Bone morphogenetic proteins: A powerful osteoinductive compound with nonnegligible side effects and limitations. BioFactors, 40(5), 459–481. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim JW, Kim DS, Kim EB, Park SJ, Park JY, … Oh SC (2008). The bone morphogenesis protein-2 (BMP-2) is associated with progression to metastatic disease in gastric cancer. Cancer Research and Treatment: Official Journal of Korean Cancer Association, 40(3), 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpalski C, Barr J, Wetterau M, Saadeh PB, & Warren SM (2010). Cranial bone defects: Current and future strategies. Neurosurgical Focus, 29(6), 1–11. [DOI] [PubMed] [Google Scholar]
- Tang W, Lin D, Yu Y, Niu H, Guo H, Yuan Y, & Liu C (2016). Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect. Acta Biomaterialia, 32, 309–323. [DOI] [PubMed] [Google Scholar]
- Xiao W, Fu H, Rahaman MN, Liu Y, & Bal BS (2013). Hollow hydroxyapatite microspheres: A novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomaterialia, 9(9), 8374–8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YT, Xiang LX, & Shao JZ (2007). Bone morphogenetic protein. Biochemical and Biophysical Research Communications, 362(3), 550–553. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zeng J, Yao A, Tu Q, Li J, Yan L, & Tang Z (2015). BMP2-loaded hollow hydroxyapatite microspheres exhibit enhanced osteoinduction and osteogenicity in large bone defects. International Journal of Nanomedicine, 10, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, He Q-F, Zhang T-H, Yu X-L, Liu Q, & Deng F-L (2012). Improvement in the delivery system of bone morphogenetic protein-2: A new approach to promote bone formation. Biomedical Materials, 7(4), 045002. [DOI] [PubMed] [Google Scholar]
- Zhou H, & Lee J (2011). Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomaterialia, 7(7), 2769–2781. [DOI] [PubMed] [Google Scholar]


