Abstract
An enzyme-linked immunosorbent assay (ELISA) using four recombinant antigens of Toxoplasma gondii (rP22, rP25, rP29, and rP35) was used in an attempt to differentiate pregnant women with toxoplasma serologic profiles (TSPs) indicative of recently acquired infections (acute profile) from those with TSPs indicative of infections acquired in the distant past (chronic profile). In general, immunoglobulin G antibodies in sera from women with the acute profile reacted more strongly with the recombinant antigens than did those in sera from women with the chronic profile. However, reactivities differed significantly between antigens that reacted with a single serum and between sera that reacted with a single antigen. Because of these variations, we employed a combination of the four antigens in an ELISA (Comb-ELISA) and evaluated its ability to distinguish pregnant women with the acute profile from those with the chronic profile. Eighteen of 20 (90%) sera from acute-profile women were positive in the Comb-ELISA, whereas 69 of 70 (98.6%) sera from the chronic-profile women were negative. Thus, the Comb-ELISA may be useful for diagnosis of toxoplasmosis in pregnant women and for differentiation between recently acquired infections and infections acquired in the more distant past.
Accurate diagnosis of recently acquired infection with Toxoplasma gondii is important for the proper clinical management of pregnant women, since the parasite can be transmitted from a recently infected mother to her fetus (18, 23, 28). In the United States, the decision as to whether a woman was recently infected, thereby placing her fetus at risk, is often made from serologic test results obtained with a single sample of serum. Thus, it is critical to determine as accurately as possible whether the infection was acquired prior to or during gestation (21, 24). A number of assays, based on chemically modified antigen preparations of T. gondii (5), recombinant toxoplasma antigens (1, 3, 10, 12, 13, 16, 22, 25), immunoglobulin classes of toxoplasma antibodies (7, 24, 27), and the avidity of immunoglobulin G (IgG) antibodies for toxoplasma antigens (8, 18), have been developed to attempt to diagnose recently acquired infections and to differentiate acute from chronic infections. The limitations of these tests and their frequent inability to accurately differentiate recently acquired infections from those acquired long before conception have been an impetus for further research to improve diagnosis of the infection in pregnant women.
In previous studies (19, 20), a 35-kDa antigen was detected in immunoblots of T. gondii tachyzoites that were probed with serum from individuals soon after they became infected with the parasite. The results of these studies led us to postulate that the 35-kDa antigen might be useful for the detection of the acute stage of the infection. Recently, antibodies to the recombinant P35 antigen (rP35) were detected by rP35 enzyme-linked immunosorbent assay (rP35 ELISA) in sera of 85.3% of pregnant women with toxoplasma serologic profiles (TSPs) consistent with recently acquired infections with T. gondii but not in sera of pregnant women with TSPs consistent with infections acquired in the distant past (13).
The objective of the present study was to examine three additional recombinant antigens of T. gondii, rP22, rP25, and rP29, to compare their ability to detect IgG antibodies with that of the rP35 antigen and to determine whether the rP35 ELISA that differentiates recent from past infections could be improved by using rP22, rP25, rP29, and rP35 in combination.
MATERIALS AND METHODS
Serum samples.
Sera were provided by the Toxoplasma Serology Laboratory of the Research Institute, Palo Alto Medical Foundation, Palo Alto, Calif. Because the main objective of the study was to determine whether a recently acquired infection with T. gondii could be differentiated from an infection acquired in the distant past using a single serum sample from a pregnant woman, all sera used in the study were from pregnant women. The sera were divided into three groups: group I sera were from 26 women with TSPs consistent with recently acquired T. gondii infections (acute profile), group II sera were from 70 women with TSPs consistent with infections acquired in the distant past (chronic profile), and group III sera were from 20 women who were seronegative for T. gondii antibodies. The 20 women in group III were healthy pregnant women with no reported illness, and their ages were in the same range as those of the other groups. The serologic profile of each sample was based on the results of the following serologic tests performed in the Toxoplasma Serology Laboratory: Sabin-Feldman dye test (DT), IgM ELISA, IgA ELISA, and AC/HS test (14, 15, 28). The results of these tests comprise the TSP (14). Sera from women in group I had high DT titers, positive IgM and IgA ELISA results, and acute patterns in the AC/HS test. Sera from women in group II had low DT titers, negative IgM and IgA ELISA results, and chronic patterns in the AC/HS test. The classification of acute or chronic profile was based on the results of the TSP in combination with the individual's clinical history (14).
Ten sera from each group were used to determine the reactivity of each serum with individual antigens and the optimal concentration of each antigen. Ten sera from each of the three groups were randomly chosen and coded, and then they were used in a blind study to determine the effectiveness of the ELISA with combined recombinant antigens (Comb-ELISA) to differentiate sera from the three groups. Thirteen of these 30 sera were used further in an experiment to determine the reproducibility of the Comb-ELISA.
Infections, including human immunodeficiency virus infection, other than with T. gondii were not reported for any of the women from whom the serum samples were obtained.
Immunoblot analysis.
T. gondii lysate antigen (TLA) was prepared from tachyzoites of the RH strain (9). TLA, nonrecombinant maltose-binding protein (MBP), glutathione S-transferase (GST), and rP22, rP25, rP29, and rP35 were separated by electrophoresis in a sodium dodecyl sulfate–10% polyacrylamide gel and transferred to nitrocellulose membranes (12), which were then incubated with the sera, as previously described (17, 21). Thereafter, the immunoblots were incubated with horseradish peroxidase-conjugated goat anti-human IgG (Caltag Laboratories, Burlingame, Calif.) at an optimal dilution of 1:8,000 in phosphate-buffered saline (PBS) with 3% bovine serum albumin (BSA) at room temperature for 1 h. After being washed with PBS, the membranes were incubated with 3,3′-diaminobenzidine tetrahydrochloride (Sigma Chemicals) at a concentration of 0.1 mg/ml in PBS. Controls to determine the reactivity of the conjugate with recombinant antigens, nonrecombinant antigens, or TLA did not reveal any bands.
Three pools of serum samples from five individuals per group were used in immunoblots to determine the reactivities of the antibodies with each recombinant antigen and with the MBP and GST protein controls.
Recombinant antigens.
Four recombinant antigens, corresponding to the P22 (21), P25 (11), P29 (6), and P35 (3) genes, were used in the present study. The DNA sequences of the P22 (accession number M33572), P25 (accession numbers M57302 and Y09782), P29 (accession numbers Y13863 and UU79158), and P35 (accession numbers A19564, AF150729, AA012298, and N60667) genes were obtained from the GenBank database. Portions of the open reading frames of these genes were amplified from the total cDNA of tachyzoites from the RH strain of T. gondii by PCR with gene-specific primers. A cDNA fragment of the P22 gene, corresponding with amino acids 27 to 172, was amplified using primers P22A (5′-GGGAATTCTCGTCCACCACCGAGACGCCAGC-3′) and P22B (5′-GGGAAGCTTACTTGCCCGTGAGAGACACAG-3′). A cDNA fragment of the P25 gene, corresponding with amino acids 16 to 231, was amplified using primers P25A (5′-GGGTCTAGATCTCGTGAGACCGTG-3′) and P25B (5′-GGGAAGCTTCTATGCGAGTTTCACCTC-3′). A cDNA fragment of the P29 gene, corresponding with amino acids 26 to 237, was amplified using primers P29A (5′-GGGAATTCGCGGCCACCGCGTCAG-3′) and P29B (5′-GGGTCTAGACTACTGGCGGGCATCCTC-3′). A cDNA fragment of the P35 gene, corresponding with amino acids 1 to 135, was amplified using primers P35A (5′-GGGAATTCATGAACGGTCCTTTGAGT-3′) and P35B (5′-GGGAAGCTTAGAATAGTAGTTTGCGGG-3′). The PCR products from the P22, P25, and P29 genes were cloned in frame with the MBP gene in the pMAL-C2 expression vector (New England Biolabs). The P35 gene PCR product was cloned in frame with the GST gene in the pGEX-5X-1 expression vector (Pharmacia).
Production and purification of recombinant proteins.
For expression of recombinant pMAL-P22, -P25, or -P29 (rP22, rP25, rP29) or nonrecombinant MBP, Escherichia coli strain JM101 (Stratagene, La Jolla, Calif.), transformed with each plasmid, was grown in Luria Bertani (LB) medium supplemented with 50 μg of ampicillin per ml and 0.2% glucose at 37°C overnight. Two-liter culture flasks containing 400 ml of LB medium supplemented with 50 μg of ampicillin per ml and 0.2% glucose were inoculated with 10 ml of the overnight culture. The cultures were grown at 37°C with vigorous shaking until the optical density at 600 nm reached 0.5 to 0.8. Isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added to a final concentration of 0.5 mM, and growth was continued for 4 h at 37°C. The cells were centrifuged at 4,000 × g for 20 min at 4°C. The pellets were resuspended in 50 ml of column buffer (20 mM Tris-HCl [pH 7.4], 1 mM EDTA, 200 mM NaCl). Samples were frozen at −20°C overnight. The cells were sonicated eight times in an ice-water bath in pulses of 15 s each). The sonicated sample was centrifuged at 9,000 × g and 4°C for 30 min. The fusion proteins were purified from the supernatants (crude extracts) by affinity chromatography according to the manufacturer's instructions (New England Biolabs). Briefly, the crude extracts were diluted 1:5 with column buffer and applied to a 10-ml amylose resin column at a flow rate of 1 ml/min. After extensive washes with column buffer, fusion proteins were eluted with column buffer containing 10 mM maltose.
For expression of recombinant pGEX-5X-1-P35 (rP35) or GST protein, E. coli strain BL21 (Pharmacia Biotech, Piscataway, N.J.), transformed with the recombinant plasmids, was grown in LB medium supplemented with 50 μg of ampicillin per ml at 37°C overnight. Two-liter culture flasks containing 400 ml of LB medium supplemented with 50 μg of ampicillin per ml were inoculated with 5-ml samples of the overnight cultures. The cultures were grown at 30°C with vigorous shaking until the optical density at 600 nm reached 0.8 to 1.0. IPTG was added to a final concentration of 1.0 mM, and growth was continued for 4 h at 30°C. The cells were pelleted at 7,700 × g and 4°C for 10 min. The pellets were resuspended in 20 ml of 1× Tris-buffered saline (TBS)–1% Triton X-100. Samples were frozen at −20°C overnight. Resuspended cell pellets were sonicated as described above and centrifuged at 12,000 × g for 10 min at 4°C. Recombinant proteins were purified from the supernatants (crude extracts) using a batch purification protocol (Pharmacia Biotech). Briefly, 50% glutathione-Sepharose 4B slurry was prepared according to the protocol. Two milliliters of slurry was added to crude extracts, and they were incubated with gentle agitation at room temperature for 30 min. The resins were pelleted at 500 × g at room temperature for 5 min. Pellets were washed two times with 50 ml of 1× TBS–1% Triton X-100 followed by two washes with 50 ml of 1× TBS. Recombinant proteins were eluted with glutathione elution buffer (10 mM glutathione, 50 mM Tris-HCl [pH 8.0]).
ELISA with individual recombinant antigens for demonstration of IgG antibodies.
The optimal concentration of the recombinant antigens for ELISA was determined using the checkerboard method (4). Three sera from each group were used. Different concentrations of each recombinant antigen in 0.1 ml of carbonate buffer were used to coat the wells of microtiter plates. The optimal concentrations, which provided the greatest difference between the absorbencies noted with sera from group I and from group II, were 3 μg/ml for rP22, rP25, and rP29 and 5 μg/ml for rP35. These concentrations, in a volume of 100 μl of 0.05 M carbonate buffer, pH 9.6, were used to examine the antigens individually. Control wells were coated with 3 μg of MBP per ml or with 5 μg of GST per ml in 100 μl of carbonate buffer. Coating was performed at 4°C overnight. Thereafter, plates were washed with PBS-Tween and postcoated with 200 μl of 3% BSA per well in PBS at 37°C for 2 h. After washing, 100 μl of serum diluted 1:50 in 3% BSA in PBS was applied to each well. Plates were incubated at 37°C for 1 h and then washed, and 100 μl of horseradish peroxidase-conjugated goat anti-human IgG (Caltag Laboratories) diluted 1:8,000 was added to each well. After 1 h of incubation at 37°C, the plates were washed, 100 μl of 0.03% O-phenylenediamine in H2O2 was added to each well, and then the plates were incubated at room temperature for 10 min. The test was read using an automatic ELISA reader (Dynatech Laboratories, Chantilly, Va.). Each sample was run in duplicate. Results were determined for each serum by calculating the mean value of the absorbency readings for duplicate wells. The final results were reported after the MBP or GST control protein reading was subtracted from the sample reading for each of the recombinant antigens.
Comb-ELISA for detection of IgG antibodies.
To evaluate the ability of the Comb-ELISA to distinguish recently infected women from women who acquired the infection in the distant past, the wells of the microtiter plates were coated with 100 μl of carbonate buffer containing 3 μg each of rP22, rP25, and rP29 per ml and 5 μg of rP35 per ml. Control wells were coated with 100 μl of carbonate buffer containing 9 μg of MBP per ml plus 5 μg of GST per ml. Thereafter, the plates were incubated with sera and conjugate as described above.
RESULTS
Reactivity of T. gondii IgG antibodies in immunoblots with recombinant antigens.
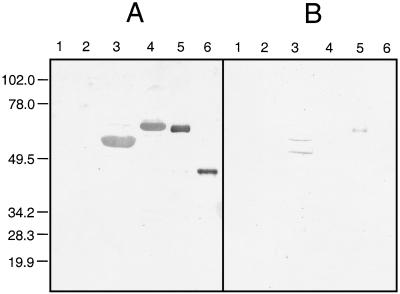
Three pools of sera, each consisting of five sera from group I, II, or III, were examined in immunoblots to determine the reactivities of IgG antibodies to TLA, recombinant antigens, and nonrecombinant control proteins. Whereas IgG antibodies in group I and group II sera reacted strongly with TLA, IgG antibodies in group III sera did not react with TLA or with any of the recombinant antigens (data not shown). Figure 1 shows that IgG antibodies in the five pooled group I sera reacted strongly with each of the recombinant antigens, whereas IgG antibodies in group II sera reacted weakly with rP22 and rP29 and did not react with rP25 or rP35. IgG antibodies in each serum pool did not react with the MBP or GST control. Gel electrophoresis to determine the molecular weights of the recombinant antigens revealed that the positions of the bands corresponding to each recombinant antigen on the gel were consistent with the molecular weights calculated from the sizes of the gene fragments encoding each antigen (data not shown).
FIG. 1.
Immunoblots to determine reactivities of IgG antibodies to recombinant antigens and control proteins. Nonrecombinant control proteins MBP (lanes 1) and GST (lanes 2) and recombinant antigens rP22 (lanes 3), rP25 (lanes 4), rP29 (lanes 5), and rP35 (lanes 6) were electrophoresed in a sodium dodecyl sulfate–10% polyacrylamide gel and transferred to nitrocellulose paper. Duplicate blots were reacted with a pool of either group I (A) or group II (B) sera. Numbers on the left are molecular masses, in kilodaltons.
Reactivities of IgG antibodies to T. gondii in ELISA with individual recombinant antigens.
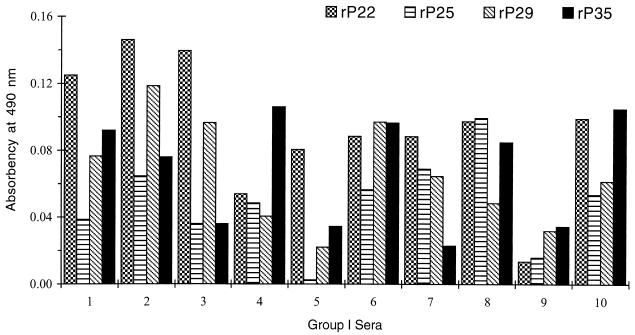
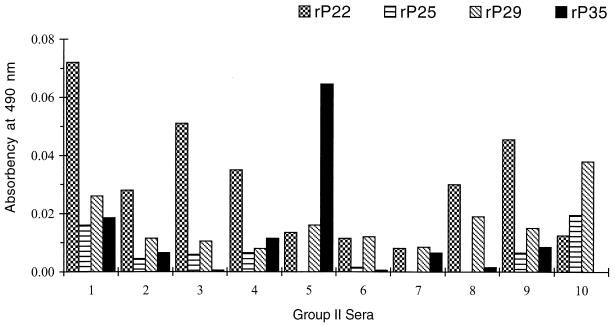
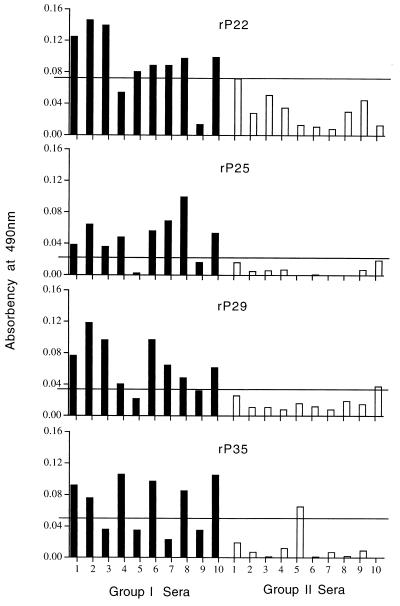
Ten sera from each group were examined. None of the sera from group III had a positive reading with any of the recombinant antigens after the absorbency readings of the control proteins were subtracted (data not shown). All 10 sera from group I (Fig. 2) and 6 of 10 sera from group II (Fig. 3) had absorbency readings indicative of a positive result with each recombinant antigen. The degree of antibody reactivity within the same serum differed for each antigen. To differentiate group I from group II sera, a cutoff value for each recombinant antigen was established as the mean plus 2 standard deviations of the absorbency readings obtained with the 10 sera from group II. Using these cutoff values (Fig. 4), two sera from group I (numbers 4 and 9) were negative with rP22, two (numbers 5 and 9) were negative with rP25 and rP29, and four (numbers 3, 5, 7, and 9) were negative with rP35. One serum from group II had readings slightly above the cutoff value with rP29 (number 10) and rP35 (number 5).
FIG. 2.
ELISA with individual rP22, rP25, rP29, and rP35 antigens in group I sera. Absorbency readings are those obtained after the reading from the nonrecombinant protein control for each antigen was subtracted from the readings for the recombinant antigen.
FIG. 3.
ELISA with individual rP22, rP25, rP29, and rP35 antigens in group II sera. Absorbency readings are as described in the legend for Fig. 2.
FIG. 4.
ELISA with individual rP22, rP25, rP29, and rP35 antigens in sera from groups I and II. The cutoff value (horizontal line) represents the mean plus 2 standard deviations for the readings from group II sera.
Reactivities of IgG antibodies to T. gondii in the Comb-ELISA.
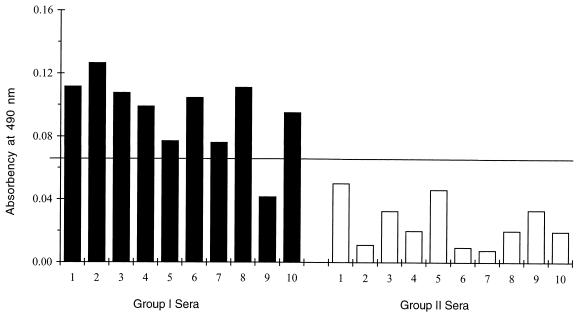
The 20 sera from groups I and II used in the ELISA with individual recombinant antigens were used in the experiments to evaluate the Comb-ELISA (Fig. 5). The cutoff value was calculated as described above, using results obtained with sera from group II. Whereas none of the sera from this group had readings above the cutoff value, 9 of 10 sera from group I had readings above the cutoff value. It was interesting that sera 3, 4, 5, and 7 from group I, which were negative with one or more antigens when tested with individual recombinant antigens (Fig. 4), were positive when tested in the Comb-ELISA. Only serum 9 remained negative.
FIG. 5.
Comb-ELISA in 10 group I and 10 group II sera. The cutoff value (horizontal line) represents the mean plus 2 standard deviations for the readings from 10 group II sera.
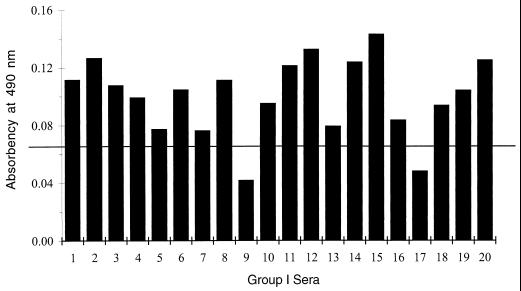
To better differentiate group I from group II sera, we examined 60 samples from the latter group in the Comb-ELISA. The results for these sera, combined with the results for the 10 sera from the same group as described above, were used to establish a cutoff value that was more representative of the reactivities of sera from group II (chronic profile) in the Comb-ELISA. The cutoff value was 0.065 and represented the reactivities of 70 sera from group II in the Comb-ELISA. Only 2 (2.8%) of the 70 group II sera had absorbency readings above the cutoff value (data not shown). In contrast, 18 of 20 (90%) sera from group I (acute profile) had readings above the established cutoff value (Fig. 6).
FIG. 6.
Comb-ELISA in 20 group I sera from 20 pregnant women. The cutoff value of 0.065 (horizontal line) represents the mean plus 2 standard deviations for readings from 70 group II sera.
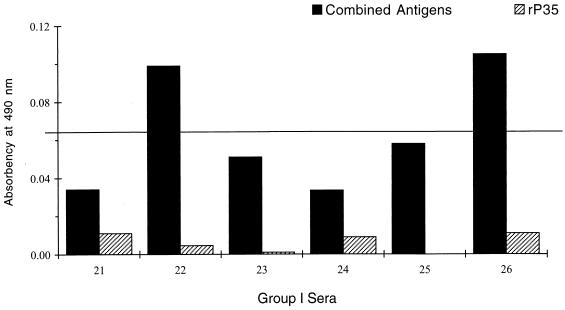
In a previous study employing ELISA with a single recombinant antigen (rP35) (13), it was found that IgG antibodies to rP35 were not detected in six sera with TSPs consistent with the acute profile. When the same sera were examined in the Comb-ELISA, two of the six sera gave readings suggestive of the acute profile (Fig. 7).
FIG. 7.
Comparison of Comb-ELISA and rP35 ELISA with six group I sera which were negative in the rP35 ELISA. Readings with Comb-ELISA and rP35 ELISA are shown for each of six group I sera. The cutoff value (horizontal line) is as defined in the legend for Fig. 6.
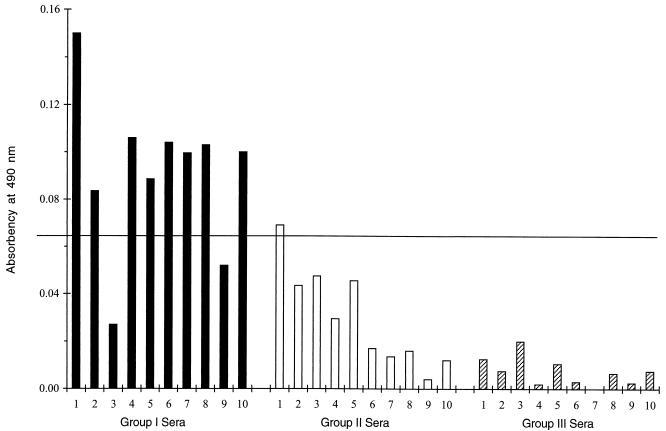
In the blind study (Fig. 8), 100% of sera from women who were not infected had absorbency readings far below the cutoff value (Fig. 8, group III sera). Of 10 sera with TSPs compatible with past infection, one had an absorbency reading minimally above the cutoff value, and the remaining 9 had absorbency readings well below the cutoff value (Fig. 8, group II sera). Of 10 sera with TSPs compatible with recent infection, 2 had absorbency readings below the cutoff value, and the remaining 8 had readings well above the cutoff value (Fig. 8, group I sera).
FIG. 8.
Blind study using the Comb-ELISA. Thirty sera were tested, and then the sera were grouped according to their original TSPs. The cutoff value (horizontal line) is as defined in the legend for Fig. 6.
Statistical analysis (by analysis of variance) of the results of the reproducibility experiment revealed no significant variation among either the means (P = 0.77) or the standard deviations (P = 0.48).
DISCUSSION
Because T. gondii can be transmitted from a recently infected mother to her fetus, a rapid and accurate diagnosis of the infection is critical for establishing proper clinical care. Frequently, only a single sample of serum is available for determination of whether the infection was acquired recently or in the distant past. In the present study, we compared the reactivities of IgG antibodies to four recombinant antigens of T. gondii in sera from pregnant women with acute and chronic TSPs. IgG antibodies in sera from women with acute TSPs reacted to the four recombinant antigens more strongly than did antibodies in sera from those with chronic TSPs. Immunoblots also revealed remarkable differences in the reactivities of IgG antibodies from these two groups of women with the individual recombinant antigens.
Significant variations in the reactivities of IgG antibodies were noted when sera from the pregnant women were tested in an ELISA with individual recombinant antigens. When the reactivities of IgG antibodies from each serum were analyzed, the differences were more evident. For example, one serum reacted with rP25, rP29, and rP35, but not with rP22; another reacted only with rP22. These variations indicate the difficulty in developing a serologic test using only a single recombinant antigen.
Because of the problems encountered when a single recombinant antigen was used to distinguish women with acute TSPs from those with chronic TSPs during gestation, we attempted to differentiate these two groups of individuals using the Comb-ELISA. The Comb-ELISA proved effective in differentiating acute from chronic TSPs; the sensitivity and specificity were 90 and 97%, respectively.
It was interesting that five sera with acute TSPs were negative when tested with the individual recombinant antigens. However, four of these sera were positive when tested using the Comb-ELISA. Moreover, in a previous study in which rP35 ELISA was employed (13), six sera from pregnant women with TSPs indicative of recent infection were negative. When these six sera were tested in the Comb-ELISA, two were positive for recently acquired infection.
By combining several recombinant antigens that present multiple different epitopes, the probability of detecting T. gondii antibodies during different stages of the infection will likely be increased (2, 10, 12).
A number of reports (17, 22, 25) have described the successful use of recombinant antigens for detection of antibodies to T. gondii. In some of them (12, 25), the antigens were used to attempt to distinguish between acute and chronic infections. However, it is difficult to compare the results of these studies because the criteria for acute and chronic infections vary among investigators. For most, the presence of specific IgG and IgM antibodies was sufficient to define serum samples as being from acutely infected individuals, while the absence of specific IgM antibodies was sufficient to define serum samples as being from chronically infected individuals. However, because of the demonstrated persistence of IgM antibodies during the chronic stage of infection with T. gondii (14, 26), this criterion alone cannot be used to distinguish acute from chronic infections (23). In the United States, only a single serum sample is usually available with which to determine if the infection was acquired during pregnancy. This problem was the impetus for our studies on such sera to attempt to differentiate recent from past infections with T. gondii. Our results suggest that combinations of recombinant antigens will be useful for serologic diagnosis of toxoplasmosis in pregnant women and for differentiation between a recently acquired infection and one acquired in the distant past.
ACKNOWLEDGMENTS
We thank Greg Maine and Sean Nowland for their critical discussions and Xiulan Zhou for technical assistance.
This work was supported by Public Health Service grant AI04717.
REFERENCES
- 1.Andrews C D, Dubey J P, Tenter A M, Webert D W. Toxoplasma gondii recombinant antigens H4 and H11: use in ELISAs for detection of toxoplasmosis in swine. Vet Parasitol. 1997;70:1–11. doi: 10.1016/s0304-4017(96)01154-5. [DOI] [PubMed] [Google Scholar]
- 2.Aubert D, Maine G T, Villena I, Hunt J C, Howard L, Sheu M, Brojanac S, Chovan L E, Nowlan S F, Pinon J M. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol. 2000;38:1144–1150. doi: 10.1128/jcm.38.3.1144-1150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey K L, Donahue C G, Ward G E. Identification and molecular characterization of GRA8, a novel, proline rich, dense granule protein of Toxoplasma gondii. Mol Biochem Parasitol. 2000;105:25–37. doi: 10.1016/s0166-6851(99)00160-7. [DOI] [PubMed] [Google Scholar]
- 4.Crowther J R. Methods in molecular biology. 42. ELISA: theory and practice. Totowa, N.J: Humana Press; 1995. [DOI] [PubMed] [Google Scholar]
- 5.Dannemann B R, Vaughan W C, Thulliez P, Remington J S. Differential agglutination test for diagnosis of recently acquired infection with Toxoplasma gondii. J Clin Microbiol. 1990;28:1928–1933. doi: 10.1128/jcm.28.9.1928-1933.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer H G, Stachelhaus S, Sahm M, Meyer H E, Reichmann G. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol Biochem Parasitol. 1998;91:251–262. doi: 10.1016/s0166-6851(97)00227-2. [DOI] [PubMed] [Google Scholar]
- 7.Gross U, Keksel O, Dardé M L. Value of detecting immunoglobulin E antibodies for the serological diagnosis of Toxoplasma gondii infection. Clin Diagn Lab Immunol. 1997;4:247–251. doi: 10.1128/cdli.4.3.247-251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedman K, Lappalainen M, Seppala I, Makela O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 9.Huskinson J, Stepick-Biek P N, Araujo F G, Thulliez P, Suzuki Y, Remington J S. Toxoplasma antigens recognized by immunoglobulin G subclasses during acute and chronic infection. J Clin Microbiol. 1989;27:2031–2038. doi: 10.1128/jcm.27.9.2031-2038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs D, Vercammen M, Saman E. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin Diagn Lab Immunol. 1999;6:24–29. doi: 10.1128/cdli.6.1.24-29.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson A M, Illana S. Cloning of T. gondii gene fragments encoding diagnostic antigens. Gene. 1991;99:127–132. doi: 10.1016/0378-1119(91)90044-c. [DOI] [PubMed] [Google Scholar]
- 12.Johnson A M, Roberts H, Tenter A M. Evaluation of a recombinant antigen ELISA for the diagnosis of acute toxoplasmosis and comparison with traditional antigen ELISAs. J Med Microbiol. 1992;37:404–409. doi: 10.1099/00222615-37-6-404. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Maine G, Suzuki Y, Araujo F G, Galvan G, Remington J S, Parmley S. Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. J Clin Microbiol. 2000;38:179–184. doi: 10.1128/jcm.38.1.179-184.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liesenfeld O, Press C, Flanders R, Ramirez R, Remington J S. Study of Abbott Toxo IMx system for detection of immunoglobulin G and immunoglobulin M toxoplasma antibodies: value of confirmatory testing for diagnosis of acute toxoplasmosis. J Clin Microbiol. 1996;34:2526–2530. doi: 10.1128/jcm.34.10.2526-2530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liesenfeld O, Press C, Montoya J G, Gill R, Isaac-Renton J L, Hedman K, Remington J S. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J Clin Microbiol. 1997;35:174–178. doi: 10.1128/jcm.35.1.174-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin V, Arcavi M, Santillan G, Amendoeira M R R, De Souza Neves E, Griemberg G, Guarnera E, Garberi J C, Angel S O. Detection of human Toxoplasma-specific immunoglobulins A, M, and G with a recombinant Toxoplasma gondii Rop2 protein. Clin Diagn Lab Immunol. 1998;5:627–631. doi: 10.1128/cdli.5.5.627-631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmley S F, Sgarlato G D, Mark J, Prince J B, Remington J S. Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J Clin Microbiol. 1992;30:1127–1133. doi: 10.1128/jcm.30.5.1127-1133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelloux H, Brun E, Vernet G, Marcillat S, Jolivet M, Guergour D, Hidalgo H, Fleuret A, Ambroise-Thomas P. Determination of anti-Toxoplasma gondii immunoglobulin G avidity: adaptation to the Vidas system (BioMerieux) Diagn Microbiol Infect Dis. 1998;32:69–73. doi: 10.1016/s0732-8893(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 19.Potasman I, Araujo F G, Desmonts G, Remington J S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986;154:650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- 20.Potasman I, Araujo F G, Thulliez P, Desmonts G, Remington J S. Toxoplasma gondii antigens recognized by sequential samples of serum obtained from congenitally infected infants. J Clin Microbiol. 1987;25:1926–1931. doi: 10.1128/jcm.25.10.1926-1931.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince J B, Auer K L, Huskinson J, Parmley S F, Araujo F G, Remington J S. Cloning, expression, and cDNA sequence of surface antigen P22 from Toxoplasma gondii. Mol Biochem Parasitol. 1990;43:97–106. doi: 10.1016/0166-6851(90)90134-8. [DOI] [PubMed] [Google Scholar]
- 22.Redlich A, Muller W A. Serodiagnosis of acute toxoplasmosis using a recombinant form of the dense granule antigen GRA6 in an enzyme-linked immunosorbent assay. Parasitol Res. 1998;84:700–706. doi: 10.1007/s004360050473. [DOI] [PubMed] [Google Scholar]
- 23.Remington J S, McLeod R, Desmonts G. Toxoplasmosis. 4th ed. Philadelphia, Pa: W. B. Saunders; 1995. [Google Scholar]
- 24.Stepick-Biek P, Thulliez P, Araujo F G, Remington J S. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J Infect Dis. 1990;162:270–273. doi: 10.1093/infdis/162.1.270. [DOI] [PubMed] [Google Scholar]
- 25.Tenter A M, Johnson A M. Recognition of recombinant T. gondii antigens by human sera in an ELISA. Parasitol Res. 1991;77:197–203. doi: 10.1007/BF00930858. [DOI] [PubMed] [Google Scholar]
- 26.Wilson M, Remington J S, Clavet C, Varney G, Press C, Ware D the FDA Toxoplasmosis Ad Hoc Working Group. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. J Clin Microbiol. 1997;35:3112–3115. doi: 10.1128/jcm.35.12.3112-3115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong S Y, Hajdu M-P, Ramirez R, Thulliez P, McLeod R, Remington J S. Role of specific immunoglobulin E in diagnosis of acute toxoplasma infection and toxoplasmosis. J Clin Microbiol. 1993;31:2952–2959. doi: 10.1128/jcm.31.11.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong S Y, Remington J S. Toxoplasmosis in pregnancy. Clin Infect Dis. 1994;18:853–862. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]