Abstract
Background:
Tumor grade is a new validated prognostic factor for medullary thyroid cancer (MTC). Calcitonin doubling time can predict MTC recurrence. We aimed to describe the association of tumor grade with calcitonin doubling and its effect on disease-specific outcomes times after resection.
Methods:
A retrospective analysis of MTC patients who underwent resection at a single tertiary-care cancer center between 1986 and 2017 were evaluated. Tumors were designated as high-grade MTC if two head and neck pathologists identified mitotic index ≥5 per 2 mm2, tumor necrosis, or a Ki67 proliferative index ≥5% within the tumor. Calcitonin doubling time was calculated using a validated calculator with at least three consecutive levels. Using Cox proportional hazards models, outcomes evaluated included locoregional relapse-free survival (LRFS), distant metastasis-free survival (DMFS), and overall survival (OS).
Results:
Among 117 patients, 95 were low grade and 22 high grade. Median follow-up was 70.2 months. High-grade patients demonstrated significantly faster calcitonin doubling times when compared with low-grade patients (8.51 ± 3.22 months vs. 38.42 ± 11.19 months; p < 0.001). In addition, most high-grade patients (66.7%) had calcitonin doubling times less than 1 year compared with fewer low-grade patients (1.0%; p < 0.001). High- and low-grade patients were further stratified by those who had calcitonin doubling times less than or greater than 2 years—a previously validated prognostic cutoff point. For patients with calcitonin doubling times less than 2 years, 70% were high grade, while 30% were low grade (p < 0.001). On multivariate analysis comparing grade and calcitonin doubling times, high-grade patients had significantly worse LRFS (hazards ratio [HR] 4.77 [confidence interval; CI 1.19–8.81]), DMFS (HR 7.25 [CI 2.36–22.28]), and OS (HR 6.04 [CI 1.85–19.72]; p < 0.05 for all), while calcitonin doubling times less than 2 years had worse DMFS (HR 7.22 [CI 1.05–49.75]). High-grade patients with calcitonin doubling times less than 2 years had associated worse LRFS and OS (both p < 0.05) compared with low-grade patients.
Conclusions:
The majority of high-grade MTC patients have calcitonin doubling times less than 2years. Close monitoring should be advocated for patients assessed to have high-grade tumors as they are at risk for poor disease-specific outcomes and structural recurrence.
Keywords: calcitonin doubling times, IMTCGS, medullary thyroid carcinoma, MTC grading, MTC surveillance
Introduction
Medullary thyroid cancer (MTC) is a neuroendocrine tumor that originates from parafollicular calcitonin producing cells of the thyroid.1 While some MTCs are aggressive tumors and others more indolent, surgical resection remains the only treatment modality that can be curative.2,3 Factors associated with worse prognosis have included age, nodal status, stage three disease, vascular invasion, and calcitonin levels.4–7 Recently, our group established a grading system based on mitotic rate and tumor necrosis that also demonstrated associations with disease-specific outcomes.8 Subsequently, an international grading system demonstrating enhanced prognostic predictability was established using mitotic rate, Ki-67 proliferation rate, and necrosis.9 The two-tiered grading system classified MTC as either low- or high-grade with the latter associated with worse local recurrence, distant metastatic recurrence, and survival.
Calcitonin levels are validated biomarkers to assess MTC recurrence.10,11 In the postoperative setting, persistently high calcitonin levels suggest recurrent or residual disease.12 Similarly, trending of biomarker levels and calculation of calcitonin and carcinoembryonic antigen (CEA) doubling times have been promoted for detection of tumor recurrence and have correlated with disease-specific outcomes.13,14 A calcitonin doubling time of less than 2 years has been recognized as a prognostic cutoff point predictive of worse local and distant recurrence.14
More recently, calcitonin doubling times have been used to monitor patients with advanced MTCs being treated with RET inhibitors.15,16 As a result, the American Thyroid Association (ATA) guidelines advocate for the measurement of calcitonin levels every 6 months to determine respective doubling times (Grade B recommendation).2 While both have demonstrated an association with disease progression, the impact of grade on calcitonin doubling times could further characterize MTCs and identify patients at higher risk of recurrence and deserving of close surveillance.
In this study, our object was to determine how calcitonin doubling times were different in high- versus low-grade MTC. We hypothesized that high-grade patients would have more rapid observed calcitonin doubling times compared with low-grade patients after surgery. In addition, high-grade patients with rapid calcitonin doubling times would consequently be associated with worse tumor-specific outcomes.
Materials and Methods
Study design and study population
To study the role of surveillance markers in MTC grade, a retrospective cohort study was designed to evaluate patients previously diagnosed with MTC. One hundred forty-four patients who were diagnosed with MTC and underwent thyroidectomy between 1986 and 2017 were identified. Type and extent of surgical intervention was selected by the treating surgeon and influenced by preoperative diagnostics and surgeon preference. Patients with distant metastatic disease at presentation (10 patients) were excluded to focus analysis on patients who had curative resection (i.e., those without known residual disease).
Since tumor grading could only be performed after thyroidectomy, those patients with metastatic disease who had their primary tumor resected were a highly selected cohort of patients and were thus excluded. Patients with missing follow-up/surveillance data (17 patients) were also excluded, leaving a final cohort of 117 nondistant metastatic surgically treated MTC patients (Fig. 1). The study was approved by the Institutional Review Board of MSKCC (IRB Protocol #17-103) and a waiver for participant consent was granted by the IRB. The study was reported in concordance with the STROBE guidelines.17
FIG. 1.
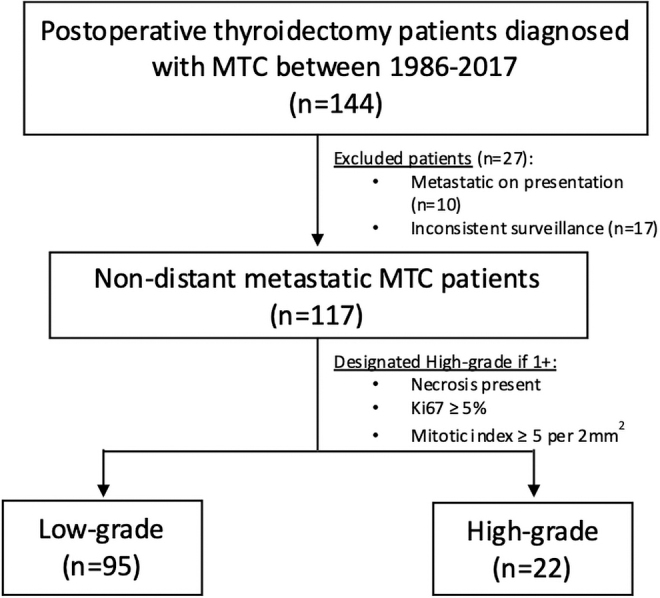
Flowchart visualizing inclusion and exclusion criteria of the 117 nondistant metastatic surgically treated MTC patients. MTC, medullary thyroid cancer.
Histopathological examination and determination of grade
MTC grading was classified by two pathologists specialized in thyroid pathology (R.A.G. and B.X.) using tumor necrosis, mitotic rate, and Ki-67 proliferation index.9 Tumors were determined to be high-grade if pathological review demonstrated tumor necrosis, a mitotic rate ≥5 per 2 mm2, or Ki-67 proliferation index ≥5%. For 55 patients, Ki-67 immunohistochemistry was not performed due to specimen block availability. These patients were graded on necrosis and mitotic rate alone8 as Ki-67 proliferation index rarely altered grade (1 of 64 patients).
Germline RET mutation status is determined by performing next-generation sequencing of blood specimens (Quest® Diagnostics, San Juan Capistrano, CA, USA). Additional pathological characteristics evaluated included lymphovascular invasion (LVI), size, margin status, extrathyroidal extension (ETE), extrathyroidal vascular invasion, extrathyroidal structural invasion, TNM staging, and amyloid deposition. LVI was defined as an invasive focus present in the vascular lumen covered by endothelial cells as previously defined.8 ETE was defined as invasion of peri-thyroid adipose tissue, skeletal muscle, or adjacent organs. Calcitonin expression was measured by immunohistochemistry and assessed on a scale of 0–100% based on pathology review of the tumor specimen.
Calcitonin/CEA doubling time calculation and use
Calcitonin and CEA levels were recorded preoperatively and postoperatively. Levels were assessed immediately preoperatively, immediately postoperatively, 6 months postoperatively, 12 months postoperatively, and then every 6 months (where applicable). Calcitonin and CEA doubling times were calculated using the previously validated Kuma Hospital Doubling Time Calculator.18,19
After the immediate postoperative period, successive calcitonin/CEA levels were inputted starting at least 6 months postoperatively with at least three successive values needed to model and calculate doubling times for each patient. Patients who had undetectable calcitonin/CEA levels or had detectable values that did not double were designated as “never doubled” and not included in the calculation for median calcitonin or CEA doubling times. In patients with undetectable, but nonabsolute values (e.g., “<5”) values inputted into the Kuma calculator were the upper limits of the assay. For example, if the noted level was “<5” then the value used for doubling time determination was “5.”
Statistical analysis and outcomes
Statistical analysis was performed using SPSS Statistics (IBM Corporation, Armonk, NY, USA). Disease status was categorized as alive with disease, alive without disease, dead with disease, or dead of other causes, and documented at the time of last chart review on December 1, 2021. Disease outcomes evaluated included locoregional relapse-free survival (LRFS), distant metastasis-free survival (DMFS), and overall survival (OS).
LRFS was defined as disease recurrence in the neck as determined by diagnostic imaging. DMFS was defined as radiographic recurrence noted outside of the neck. Mortality was defined as patients who died of disease, died with disease, or died of other causes. LRFS, DMFS, and mortality were calculated using Cox regression modeling as time-to-event analyses from time of initial surgery. High- and low-grade patients with calcitonin doubling times less than 2 years were compared using a univariate survival analysis using log-rank tests. Outcomes relative to CEA were not evaluated due to less patients with complete data. p-Values <0.05 were considered to be statistically significant.
Results
Descriptive analysis of patients with nondistant metastatic low- and high-grade MTC patients
Among 117 MTC patients, 95 were classified as low-grade and 22 high-grade tumors (Table 1). Median follow-up was for the entire cohort was 70.2 months [confidence interval; CI 84.7–111.0 months]. Sex (p = 0.09), age (p = 0.15), bilateral disease (p = 0.84), and germline RET mutation status (p = 0.68) were not significantly different between the two groups. Pathological characteristics differed by grade with more high-grade patients having significantly greater LVI (86.4% vs. 33.7%; p < 0.001), median tumor size (4.0 cm vs. 2.0 cm; p < 0.05), ETE (50.0% vs. 18.9%; p < 0.05), and positive lymph node metastasis (50.0% vs. 34.7%; p < 0.01).
Table 1.
Characteristics of the High- and Low-Grade Medullary Thyroid Cancer Patients Undergoing Curative Resection
| Characteristics | Low grade (n = 95) | High grade (n = 22) | p |
|---|---|---|---|
| Patient characteristics | |||
| Sex | |||
| Male | 42 (44.2%) | 15 (68.1%) | 0.09 |
| Female | 53 (55.8%) | 7 (31.8%) | |
| Age at diagnosis (years) (range) | 49 [3–80] | 55 [15–77] | 0.15 |
| Median follow-up after surgery (months) (min, max) | 70.4 (9.4, 315.9) | 48.0 (2.5, 333.8) | 0.35 |
| Bilateral disease present | |||
| Yes | 14 (14.7%) | 3 (13.6%) | 0.84 |
| No | 80 (84.2%) | 18 (81.8%) | |
| Unknown | 1 (1.1%) | 1 (4.5%) | |
| Last known status | |||
| Alive w/o disease | 67 | 2 | — |
| Alive with disease | 23 | 9 | |
| Dead with disease | 2 | 10 | |
| Dead of other causes | 3 | 1 | |
| Structural disease present preoperatively | 16 (16.8%) | 12 (54.5%) | <0.01 |
| RET status | 19 (20%) | 4 (18.2%) | 0.68 |
| Surgical and tumor characteristics | |||
| Surgery performed | |||
| Thyroid lobectomy | 1 (1.1%) | 0 (0%) | <0.05 |
| Thyroidectomy alone | 20 (21.1%) | 2 (9.1%) | |
| Single-stage thyroidectomy/nodal dissection | 59 (62.1%) | 11 (50.0%) | |
| Staged thyroidectomy + nodal dissection | 14 (14.7%) | 9 (40.9%) | |
| Median preoperative CEA (ng/mL) (min, max) | 16.8 (1.4, 980) | 26.0 (1.4, 573) | 0.53 |
| Median preoperative calcitonin (pg/mL) (min, max) | 650.0 (9, 85000) | 4325.0 (312, 44190) | 0.18 |
| LVI | |||
| Positive | 32 (33.7%) | 19 (86.4%) | <0.001 |
| Negative | 51 (53.7%) | 2 (9.1%) | |
| Unknown/nondiagnostic | 12 (12.6%) | 0 (4.5%) | |
| Size (cm) [min, max] | 2.01 [0.2, 7.2] | 4.01 [0.4, 9.5] | <0.05 |
| ETE | |||
| Positive | 18 (18.9%) | 11 (50.0%) | <0.05 |
| Negative | 74 (77.9%) | 6 (27.2%) | |
| Unknown/nondiagnostic | 3 (3.2%) | 5 (22.7%) | |
| Margins | |||
| Positive | 18 (18.9%) | 9 (40.9%) | 0.09 |
| Negative | 69 (72.6%) | 11 (50.0%) | |
| Unknown/nondiagnostic | 8 (8.4%) | 2 (9.1%) | |
| pT stage | |||
| 1 | 43 (45.2%) | 1 (4.5%) | <0.001 |
| 2 | 13 (13.7%) | 2 (9.1%) | |
| 3 | 16 (16.8%) | 7 (31.8%) | |
| 4 | 4 (4.2%) | 3 (13.6%) | |
| Unknown | 19 (20.0%) | 9 (40.9%) | |
| pN stage | |||
| Positive | 33 (34.7%) | 11 (50.0%) | <0.01 |
| Negative | 44 (46.3%) | 2 (9.1%) | |
| Unknown | 18 (18.9%) | 9 (40.9%) | |
| pM stage | |||
| Positive | 0 (0%) | 0 (0%) | — |
| Negative | 95 (100%) | 22 (100%) | |
| Median percentage of cells with calcitonin expression, by IHCa (min, max) | 100% (10–100%) | 100% (90–100%) | 0.4 |
| Amyloid present | 54 (56.8%) | 13 (59.1%) | 0.96 |
| Necrosis present | 0 (0%) | 21 (95.4%) | <0.001 |
| Mitosis present | 25 (26.3%) | 17 (77.3%) | <0.01 |
Bold values signify statistical significance.
No. of patients: low grade = 69; high grade = 13.
CEA, carcinoembryonic antigen; ETE, extrathyroidal extension; IHC, immunohistochemistry; LVI, lymphovascular invasion.
Median preoperative CEA (high grade: 26.0 ng/mL, range 1.4–573.0 vs. low grade: 16.8 ng/mL, range 1.4–980; p = 0.53) and calcitonin (high grade: 4325.0 ng/mL, range 3.5–44190.0 vs. low grade: 650.0 ng/mL, range 9–85,000; p = 0.18) were not statistically different. Median percentage of cells with calcitonin expression did not significantly differ between high-grade (100%, range 90–100%) and low-grade (100%, range 10–100%) patients (p = 0.4).
At last evaluation, disease status was noted to be different between the high- and low-grade patients. In particular, 67/95 (70.5%) of low-grade patients were alive without disease compared with 2/22 (9.1%) of high-grade patients. In addition, 2/95 (2.1%) low-grade compared with 10/22 (45.4%) high-grade patients were dead with disease.
Evaluation of CEA and calcitonin as surveillance markers
Calcitonin and CEA levels were analyzed as postoperative surveillance markers (Table 2). Postoperative CEA levels at 6 (p = 0.11) and 12 (p = 0.25) months were higher in high-grade patients compared with low-grade patients, but this was not significantly different. Doubling of CEA levels were observed in 12/95 (12.6%) low-grade and 12/22 (54.5%) high-grade patients with average CEA doubling time 36.8 months (range 21.2–111.2) in low-grade and 8.8 months (range 3.2–24.4) in high-grade patients (p < 0.001). Owing to fewer patients having postoperative CEA values, further analysis was focused on calcitonin.
Table 2.
Evaluation of Calcitonin and Carcinoembryonic Antigen as Markers of Recurrence After Resection in High- and Low-Grade Medullary Thyroid Cancer
| Tumor marker value | n | Low grade (n = 95) | High grade (n = 22) | p |
|---|---|---|---|---|
| CEA | ||||
| Median CEA 6 months postoperative (ng/mL) (min, max) | 71 | 2.5 (0.6, 33.4) | 4.1 (1.0, 59.0) | 0.11 |
| Median CEA 12 months postoperative (ng/mL) (min, max) | 85 | 2.3 (0, 34.0) | 4.0 (0.8, 2657) | 0.25 |
| CEA doubling time | ||||
| Median time if doubled (months) (min, max) | 97 | 36.8 (21.2, 111.2) | 8.8 (3.2, 24.4) | <0.001 |
| <1 Year | 0/95 (0%) | 11/22 (50.0%) | ||
| 1–2 Years | 1/95 (1.1%) | 0/22 (0%) | ||
| 2 or more years | 11/95 (11.6%) | 1/22 (4.5%) | ||
| Never doubled | 67/95 (70.5%) | 6/22 (27.2%) | ||
| Unknown | 16/95 (16.8%) | 4/22 (18.2%) | ||
| Calcitonin | ||||
| Median calcitonin 6 months postoperative (pg/mL) (min, max) | 111 | 1.5 (0, 14,800) | 210.0 (0, 8900) | 0.21 |
| Median calcitonin 12 months postoperative (pg/mL) (min, max) | 112 | 0.1 (0, 10,400) | 415.0 (3, 36,800) | 0.20 |
| Calcitonin doubling time | ||||
| Median time if doubled (months) (min, max) | 115 | 31.6 (15.6, 111.4) | 8.7 (2.6, 23.5) | <0.001 |
| <1 Year | 0/95 (0%) | 16/22 (72.7%) | ||
| 1–2 Year | 6/95 (6.3%) | 4/22 (18.2%) | ||
| 2 or more years | 23/95 (24.2%) | 0/22 (0%) | ||
| Never doubled | 65/95 (68.4%) | 1/22 (4.5%) | ||
| Unknown | 1/95 (1.1%) | 1/22 (4.5%) | ||
Bold values signify statistical significance.
Similarly, postoperative calcitonin levels were higher in high-grade patients but not significantly different at 6 (p = 0.21) and 12 (p = 0.20) months. Among low-grade patients, 6/95 (6.3%) calcitonin levels doubled within 1–2 years, 23/95 (24.4%) at 2 or more years, and 65/95 (68.4%) never doubled. Conversely with high-grade patients, 16/22 (72.7%) doubled within 1 year, 4/22 (18.2%) within 1–2 years, and 1/22 (4.5%) never doubled. For two patients, one low grade and one high grade, doubling times were not evaluated due to variable follow-up and inconsistent calcitonin and CEA measurements. No high-grade patients had calcitonin doubling times greater than 2 years. Among patients with observed doubling, median calcitonin doubling time was 31.6 months (range 15.6–111.4) in low-grade and 8.7 months (range 2.6–23.5) in high-grade patients (p < 0.001).
Evaluation of outcomes based on calcitonin doubling times
High-grade (n = 21) and low-grade (n = 94) patients were stratified by calcitonin doubling times to assess patient status and outcomes at last chart review (Table 3). Within the cohort, 20/21 (95.2%) high-grade and 6/94 (29.9%) low-grade patients were observed to have calcitonin doubling times <2 years. Among the cohort of high-grade patients with calcitonin doubling times <2 years, 15/20 experienced locoregional recurrence, 10/20 distant recurrence, and 9/20 died. In low-grade patients with calcitonin doubling times <2 years, 2/6 had locoregional recurrence, 1/6 distant recurrence, and 1/6 died. Within the cohort, 87 patients were identified to have had intrathyroidal disease. Of the patients in this cohort, 13 experienced local recurrence with 8/13 (62%) being high grade and 5/13 (38%) being low grade.
Table 3.
Evaluation of Outcomes Based on Grade and Stratified by Doubling Time
| Outcomes |
Low grade (n = 94) |
High grade (n = 21) |
||||
|---|---|---|---|---|---|---|
| Calcitonin doubling time | <2 Years | >2 Years | Never doubled | <2 Years | >2 Years | Never doubled |
| Disease status | ||||||
| Alive w/o disease | 0/6 | 12/23 | 55/65 | 2/20 | — | 0/1 |
| Alive with disease | 5/6 | 11/23 | 6/65 | 8/20 | 1/1 | |
| Dead with disease | 1/6 | 0/23 | 1/65 | 9/20 | 0/1 | |
| Dead other causes | 0/6 | 0/2 | 3/65 | 1/20 | 0/1 | |
| Locoregional recurrence | 2/6 | 4/23 | 6/65 | 15/20 | — | 1/1 |
| Distant recurrence | 1/6 | 11/23 | 1/65 | 10/20 | — | 1/1 |
| Mortality | 1/6 | 0/23 | 1/23 | 9/20 | — | 0/1 |
Of the 26 patients with observed calcitonin doubling times less than 2 years, 6 (23.1%) were low-grade and 20 (76.9%) were high-grade patients. With a high proportion of high-grade patients with calcitonin doubling times <2 years, we next assessed the relative associations of grade and calcitonin doubling times on outcomes (Table 4). A multivariate survival analysis was performed using Cox proportional hazards models comparing patients with high-grade tumors and rapid calcitonin doubling time as independent factors. High-grade categorization was associated with significantly worse LRFS (hazard ratio [HR] 7.07 [CI 2.08–24.04]), DMFS (HR 6.58 [CI 1.29–33.56]), and OS (HR 5.23 [CI 1.47–18.68]; all p < 0.05). Conversely, patients with calcitonin doubling times less than 2 years were associated with worse DMFS (HR 7.22 [CI 1.05–49.78]; p < 0.05) but not LRFS (HR 1.58 [CI 0.47–5.37]; p = 0.46) or OS (HR 1.58 [CI 0.44–5.67]; p = 0.49).
Table 4.
Multivariate Analysis of Survival Outcomes after Curative Resection for Medullary Thyroid Cancer
| Factors | LRRFS |
DMFS |
OS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | CI |
p | HR | CI |
p | HR | CI |
p | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| High grade | 7.07 | 2.08 | 24.04 | <0.05 | 6.58 | 1.29 | 33.56 | <0.05 | 5.23 | 1.47 | 18.68 | <0.05 |
| Calcitonin doubling time <2 years | 1.58 | 0.47 | 5.37 | 0.46 | 7.22 | 1.05 | 49.78 | <0.05 | 1.58 | 0.44 | 5.67 | 0.49 |
Bold values signify statistical significance.
CI, confidence interval; DMFS, distant metastasis-free survival; HR, hazard ratio; LRRFS, locoregional recurrence-free survival; OS, overall survival.
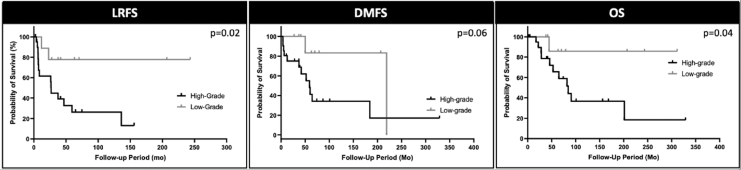
Among patients with calcitonin doubling times less than 2 years, univariate survival analysis using log-rank tests were used to determine the impact of grade on LRFS, DMFS, and OS (Fig. 2). High-grade tumors were associated with worse LRFS and OS (both p < 0.05), while DMFS was not significantly different (p = 0.06).
FIG. 2.
Univariate survival analysis comparing high- and low-grade patient among patients with calcitonin doubling time less than 2 years using log-rank tests. High-grade patients in this cohort were associated with worse LRFS (left panel) and OS (right panel) when compared with low-grade patients (p < 0.05 for both). DMFS (middle panel) among patients was not statistically different between high- and low-grade patients (p = 0.06). DMFS, distant metastasis-free survival; LRFS, locoregional relapse-free survival; OS, overall survival.
Discussion
Tumor grade is an important prognostic factor after resection in patients with MTC. Compared with low-grade patients, both CEA and calcitonin doubling times were observed to be significantly more rapid among high-grade patients, consistent with the associated worse LRFS and DMFS seen in high-grade patients. When taken together, the study found that high-grade patients with rapid calcitonin doubling times to be a high-risk cohort with worse LRFS and OS compared with low-grade patients. These findings suggest that consideration of patient grade and calcitonin trends are critical aspects for predicting recurrence among resected MTC patients.
Resection of all identified disease remains the standard of care for patients with MTC. Despite being the only option for cure, recurrence rates have been reported to occur in ∼50% of patients and associated with worse outcomes.20–22 Previous reports have described the correlation of absolute calcitonin and CEA levels with aggressive disease23; however, this was not demonstrated in this study due to the wide range of postoperative values. In our cohort of patients, determination of calcitonin and CEA doubling times was instead a useful surveillance adjunct that could be utilized by clinicians to predict tumor recurrence.
Consistent with their aggressive biology and high recurrence rates, 90% of high-grade patients had calcitonin doubling times less than 2 years. Conversely, the majority of low-grade patients either had prolonged doubling times greater than 2 years (24.2%) or never doubled (68.4%). This is consistent with previous reports that demonstrated doubling times less than 25 months to be associated with worse recurrence and disease-specific mortality.2,14,24 Similarly, findings from this study not only demonstrate that short calcitonin doubling times (<2 years) as a biomarker for tumor recurrence, but also an indicator suggestive of more aggressive high-grade disease.
The study identified high-grade patients with rapid calcitonin doubling times to be an especially high-risk cohort with poor disease-specific outcomes. When compared with low-grade patients, high-grade patients with doubling times less than 2 years were associated with worse LRFS and OS (p < 0.05 for both). While these results highlight the significance of close surveillance for high-grade patients, it also suggests consideration could be given for adjuvant therapy trials in this high-risk cohort. Worse outcomes indicate that surgical resection may not completely cure many patients with high-grade tumors.
Similar to differentiated thyroid cancer,25 ovarian cancer,26 and prostate cancer,27 this study suggests that grading and biomarker surveillance are important components of risk stratifying MTC patients who can impact disease management. Tyrosine kinases inhibitors (Vendatinib and Cabozantinib) and RET inhibitors (LOXO-292 and Pralsetinib) have demonstrated encouraging results in the setting of metastatic MTC.28–31 In particular, with reported efficacious response rates in the ARROW trial32 and recent FDA approval of Pralsetinib for advanced metastatic MTC, this study establishes a high-risk cohort that could be considered in the design of an adjuvant therapy trial.
Finally, regardless of grade, the study found that early and frequent follow-up is needed for MTC patients to assess calcitonin doubling times. In our study, we found that high-grade patients were associated with more rapid calcitonin and CEA doubling times, which is consistent with greater propensity of high-grade patients to experience local and distant recurrence. Notably, grade was a better predictor of LRRFS and OS when compared with rapid calcitonin doubling times. While most low-grade patients demonstrated prolonged doubling times or never doubled, rapid calcitonin doubling time independently was associated with DMFS (HR 7.22 [CI 1.05–49.78]; p < 0.05) regardless of grade.
In addition, DMFS was not significantly different among high- and low-grade patients with rapid calcitonin doubling times (p = 0.06). Thus, this study suggests that patients with low-grade tumors who have prolonged calcitonin doubling times are at less risk for recurrence and can be followed with longer interval surveillance. While further investigations evaluating the timing of recurrence relative to MTC risk factors are needed to determine the optimal surveillance intervals dependent on grade and calcitonin doubling times, the study demonstrates the importance of grade and tumor marker surveillance as important components of stratifying patients.
The study has several limitations. Owing to the retrospective nature of the study, postoperative calcitonin and CEA levels were subject to provider preference and variably measured potentially affecting determination of doubling times in cases where biomarkers were not measured every 6 months. In addition, CEA levels were inconsistently measured postoperatively, which limited a more in-depth analysis of CEA doubling times and outcomes.
Furthermore, the retrospective nature of the study prevented consistent CEA and calcitonin assays from being used, thus affecting doubling time determination. Owing to the rarity of MTC, assessment of outcomes was limited in certain patient cohorts in this study. This was relevant to high-grade patients as this cohort had fewer patients and impeded a more extensive analysis. In addition, it should be noted that Ki-67 proliferation index was unavailable for 44 low-grade patients and 11 high-grade patients. However, in this cohort we found that in nearly all patients Ki-67 did not impact grade determination as only 1 out of 64 was reclassified from low grade to high grade after Ki-67 determination.
Conclusion
The majority of high-grade MTC patients have observed calcitonin doubling times less than 2 years with grade and calcitonin doubling times found to be independent predictors of disease-specific outcomes. Therefore, determination of calcitonin/CEA doubling times and tumor grade should be a part of the postoperative assessment of patients with MTC. High-grade patients with rapid calcitonin doubling times are associated with worse LRFS and OS, and should be closely monitored for structural disease recurrence.
Authors' Contributions
Conceptualization, investigation, formal analysis, funding acquisition, methodology, visualization, writing—original draft, and writing—review and editing by A.N. Conceptualization, investigation, formal analysis, methodology, validation, visualization, and writing—review and editing by B.X. Conceptualization, investigation, methodology, supervision, and writing—review and editing by I.G. and P.M.S. Conceptualization, methodology, supervision, and writing—review and editing by R.M.T., R.J.W., A.R.S., and R.A.G. Conceptualization, formal analysis, methodology, supervision, writing—original draft, and writing—review and editing by B.R.U.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This project was funded in part thanks to NIH/NCI Cancer Center Support Grant (Grant No. P30CA008748) and NIH T32 Research Fellowship Grant.
References
- 1. Kebebew E, Ituarte PH, Siperstein AE, et al. . Medullary thyroid carcinoma: Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88(5):1139–1148; doi: . [DOI] [PubMed] [Google Scholar]
- 2. Wells Jr SA, Asa SL, Dralle H, et al. . Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25(6):567–610; doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filetti S, Durante C, Hartl D, et al. . Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 2019;30(12):1856–1883; doi: 10.1093/annonc/mdz400. [DOI] [PubMed] [Google Scholar]
- 4. Modigliani E, Cohen R, Campos JM, et al. . Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: Results in 899 patients. The GETC Study Group. Groupe d'etude des tumeurs a calcitonine. Clin Endocrinol (Oxf) 1998;48(3):265–273; doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 5. Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 2006;107(9):2134–2142; doi: 10.1002/cncr.22244. [DOI] [PubMed] [Google Scholar]
- 6. Kwon H, Kim WG, Jeon MJ, et al. . Dynamic risk stratification for medullary thyroid cancer according to the response to initial therapy. Endocrine 2016;53(1):174–181; doi: 10.1007/s12020-015-0849-6. [DOI] [PubMed] [Google Scholar]
- 7. Ho AS, Wang L, Palmer FL, et al. . Postoperative Nomogram for predicting cancer-specific mortality in medullary thyroid cancer. Ann Surg Oncol 2015;22(8):2700–2706; doi: 10.1245/s10434-014-4208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alzumaili B, Xu B, Spanheimer PM, et al. . Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod Pathol 2020;33(9):1690–1701; doi: 10.1038/s41379-020-0532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu B, Fuchs TL, Ahmadi S, et al. . International medullary thyroid carcinoma grading system: A validated grading system for medullary thyroid carcinoma. J Clin Oncol 2022;40(1):96–104; doi: 10.1200/JCO.21.01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basuyau JP, Mallet E, Leroy M, et al. . Reference intervals for serum calcitonin in men, women, and children. Clin Chem 2004;50(10):1828–1830; doi: 10.1373/clinchem.2003.026963. [DOI] [PubMed] [Google Scholar]
- 11. Wells Jr. SA, Haagensen Jr. DE, Linehan WM, et al. . The detection of elevated plasma levels of carcinoembryonic antigen in patients with suspected or established medullary thyroid carcinoma. Cancer 1978;42(3 Suppl):1498–1503; doi: . [DOI] [PubMed] [Google Scholar]
- 12. Pellegriti G, Leboulleux S, Baudin E, et al. . Long-term outcome of medullary thyroid carcinoma in patients with normal postoperative medical imaging. Br J Cancer 2003;88(10):1537–1542; doi: 10.1038/sj.bjc.6600930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laure Giraudet A, Al Ghulzan A, Auperin A, et al. . Progression of medullary thyroid carcinoma: Assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol 2008;158(2):239–246; doi: 10.1530/EJE-07-0667. [DOI] [PubMed] [Google Scholar]
- 14. Barbet J, Campion L, Kraeber-Bodere F, et al. . Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 2005;90(11):6077–6084; doi: 10.1210/jc.2005-0044. [DOI] [PubMed] [Google Scholar]
- 15. Yeh T, Yeung M, Sherman EJ, et al. . Structural doubling time predicts overall survival in patients with medullary thyroid cancer in patients with rapidly progressive metastatic medullary thyroid cancer treated with molecular targeted therapies. Thyroid 2020;30(8):1112–1119; doi: 10.1089/thy.2019.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brandenburg T, Tiedje V, Muchalla P, et al. . Continued Discontinuation of TKI Treatment in Medullary Thyroid Carcinoma—Lessons from individual cases with long-term follow-up. Front Endocrinol (Lausanne) 2021;12:718418; doi: 10.3389/fendo.2021.718148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007;335(7624):806–808; doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyauchi A, Kodo T.. Doubling Time, Doubling Rate, and Progression Calculator, Version 2. 2nd ed. Kuma Hospital, Center of Excellence in Thyroid Care: Kobe, Japan; 2019. [Google Scholar]
- 19. Sabra MM, Sherman EJ, Tuttle RM. Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell-derived thyroid carcinoma. Cancer 2017;123(15):2955–2964; doi: 10.1002/cncr.30690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Machens A, Hinze R, Thomusch O, et al. . Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg 2002;26(1):22–28; doi: 10.1007/s00268-001-0176-3. [DOI] [PubMed] [Google Scholar]
- 21. van Heerden JA, Grant CS, Gharib H, et al. . Long-term course of patients with persistent hypercalcitoninemia after apparent curative primary surgery for medullary thyroid carcinoma. Ann Surg 1990;212(4):395– 400; discussion 400–391; doi: 10.1097/00000658-199010000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rougier P, Parmentier C, Laplanche A, et al. . Medullary thyroid carcinoma: Prognostic factors and treatment. Int J Radiat Oncol Biol Phys 1983;9(2):161–169; doi: 10.1016/0360-3016(83)90093-7. [DOI] [PubMed] [Google Scholar]
- 23. Park H, Park J, Choi MS, et al. . Preoperative serum calcitonin and its correlation with extent of lymph node metastasis in medullary thyroid carcinoma. Cancers (Basel) 2020;12(10):2894; doi: 10.3390/cancers12102894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Machens A, Hauptmann S, Dralle H.. Medullary thyroid cancer responsiveness to pentagastrin stimulation: An early surrogate parameter of tumor dissemination? J Clin Endocrinol Metab 2008;93(6):2234–2238; 10.1210/jc.2007-2792. [DOI] [PubMed] [Google Scholar]
- 25. Tuttle RM, Alzahrani AS. Risk stratification in differentiated thyroid cancer: From detection to final follow-up. J Clin Endocrinol Metab 2019;104(9):4087–4100; doi: 10.1210/jc.2019-00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Menon U, Skates SJ, Lewis S, et al. . Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol 2005;23(31):7919–7926; doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 27. D'Amico AV, Whittington R, Malkowicz SB, et al. . Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280(11):969–974; doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 28. Elisei R, Schlumberger MJ, Muller SP, et al. . Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31(29):3639–3646; doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wells Jr. SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J Clin Oncol 2012;30(2):134–141; doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wirth LJ, Sherman E, Robinson B, et al. . Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020;383(9):825–835; DOI: 10.1056/NEJMoa2005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Subbiah V, Hu MI, Wirth LJ, et al. . Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol 2021;9(8):491–501; doi: 10.1016/S2213-8587(21)00120-0. [DOI] [PubMed] [Google Scholar]
- 32. Gainor JF, Curigliano G, Kim DW, et al. . Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol 2021;22(7):959–969; doi: 10.1016/S1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]