Abstract
In South Africa, less than half of children receiving antiretroviral therapy are virally suppressed. Adherence challenges include poor palatability of drugs and high pill burden. Subcutaneous implants offer a long-acting alternative to daily oral dosing regimens, which may improve outcomes in children living with HIV (CLWH). Qualitative in-depth interviews were conducted with 24 health care providers (HCPs) in Johannesburg, South Africa. Interviews were audio-recorded and transcribed. Data were coded and analyzed using NVivo 12 software and a Grounded Theory approach. Most HCPs welcomed an implant option for CLWH. Perceived benefits included fewer clinic visits, improved adherence, and “normalization” of the lives of CLWH. Concerns included painful insertion and removal, the potential for stigmatization, and caregivers' likely rejection of biodegradable implants. A single, small, non-transparent rod with some flexibility was preferred by most participants. HCP training and early outreach to mitigate potential misinformation about implants and caregivers' fears about biodegradable implants were emphasized. Further engagement with caregivers of CLWH is required and ongoing.
Keywords: HIV treatment, pediatric populations, qualitative end-user research, implants, health care providers
Introduction
Globally, just over 1 million children aged 0–9 were living with HIV in 2020, with around 90% of them in Sub-Saharan Africa.1 In South Africa, of the 47% of children living with HIV (CLWH) aged 0–14 who are receiving antiretroviral therapy (ART),2 around 45% are virally suppressed.3 Studies on pediatric adherence have identified a number of social barriers to successful pediatric HIV treatment, including HIV stigma, non-disclosure of the child's HIV status, low socio-economic status of households, lack of food, and poor supervision from caregivers, all of which are relevant in this setting.4–7 Many CLWH are also dependent on a caregiver who is likely dealing with their own complex health challenges, which may impact children's ART adherence and retention in care.
Across both high- and low-resource settings, the lack of pediatric-friendly formulations creates a further barrier to adherence.8 Challenges with existing HIV treatment formulations for children are multifaceted and include high pill burden, complex regimens, difficulties with measuring accurate doses, and poor palatability of drugs.4,8 The ART syrups, for example, are generally complicated to administer: they are bitter, require weight-banded dosing, and there are often large volumes of liquid to dispense and suspensions that require refrigeration (a particular problem in low-resource environments).9
Newer ART drug delivery platforms, such as powders, granules, pellets, and chewable or dispersible tablets, are increasingly child-friendly but still dependent on daily dosing.10 In many low-resource settings, including South Africa, some of these newer formulations are not yet available in the public sector, owing to lack of registration and/or prohibitive cost.11
To improve treatment success for young children facing a lifetime of ART, simplified dosing regimens and delivery platforms are critically needed. One solution may be long-acting, subcutaneous implants, which provide a longer therapeutic duration than current formulations, with highly controlled, sustained drug release and reversibility of treatment if needed. However, such an implant must be acceptable to end-users involved in the treatment of CLWH.
The perspectives of health care providers (HCPs) on implants for children would illuminate the social and clinical contexts into which this mode of treatment delivery will be introduced. Although implant acceptability among South African HCPs has been examined in relation to contraceptive implants12,13 and implants for pre-exposure prophylaxis (PrEP) for HIV,14 their perspectives on implants for pediatric use remain under-researched.
With this in mind, we assessed the acceptability and preferred characteristics of a new long-acting, potentially biodegradable implant among HCPs while it is still in the early and modifiable stages of the product development pipeline. Allowing end-users to state their preferred product characteristics remains an under-utilized approach that creates synergy between product development and socio-behavioral science.15,16 We present their perspectives here, with the aim of informing development of a pediatric implant that is ultimately acceptable for communities in South Africa.
Methods
In this qualitative, cross-sectional study, in-depth interviews (IDIs) were held with HCPs in Johannesburg, as part of a larger project, “Delivery of Antiretrovirals via Implantable System for Young children” (DAISY). The DAISY study protocol was approved by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand, as well as Salus, an independent institutional review board servicing RTI International, located in the United States. The study was overseen by the regulatory infrastructure of the Division of AIDS (DAIDS).
Sampling and recruitment
Using a quota sampling frame, we purposively recruited a range of HCPs from selected public sector children's clinics and research centers in Johannesburg, South Africa. Only HCPs who had worked with CLWH or had experience administering contraceptive implants were eligible for participation. Facility heads helped to identify eligible participants, and a recruitment pamphlet with information about study participation was distributed in study facilities, inviting interested HCPs to contact the study team.
Data collection
Trained social scientists held IDIs with participants in-person and virtually (due to Covid-19 restrictions). All interviews were in English, audio-recorded with participants' consent, and transcribed verbatim. A semi-structured topic guide was used, covering topics such as: experiences with current HIV treatment options, preferred implant characteristics, and delivery considerations for children 2–5 years (the age group targeted by product developers in the DAISY project).
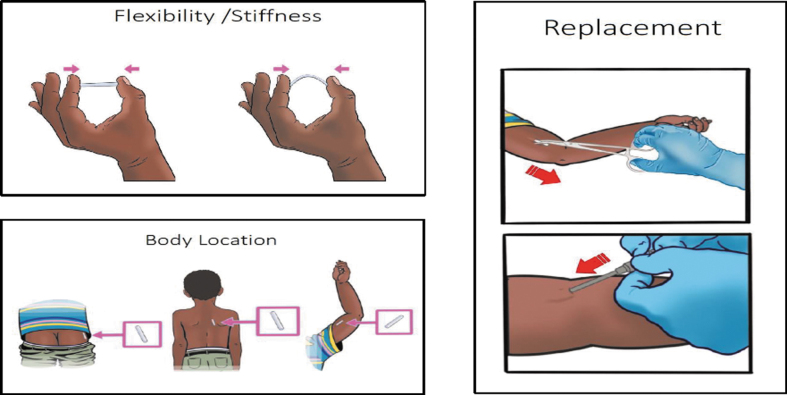
During in-person interviews, participants were invited to handle implant prototypes of varying lengths, flexibility, and colors, as they considered preferred attributes of implants (Fig. 1). Interviewers also displayed visual cue cards to clarify implant insertion and removal procedures (Fig. 2). After each interview, interviewers wrote detailed debriefing notes on a structured form, summarizing key points raised by the participant.
FIG. 1.
Implant prototypes given to participants to handle during interviews. Top to bottom: Flexible implant; small implant; colored implant; matchstick-size standard implant. Photo credit: Elizabeth T. Montgomery.
FIG. 2.
Examples of visual cue cards used in interviews to explain aspects of pediatric implant administration.
Data analysis
Data for analysis consisted of interviewers' debriefing notes and transcripts of audio-recorded interviews. Deductive themes in the debriefing notes were extracted and summarized in memos using Dedoose analysis software v.9. Alongside this analysis, four trained social scientists (two of whom had done the interviews) coded and analyzed interview transcripts in NVivo v.12, using a Grounded Theory approach encompassing open and axial coding17 and the constant comparative method.18 Text for the article was developed following a review of axial coding outcomes, which identified key themes and linkages between them.
Results
A total of 24 HCPs were interviewed, 18 in-person and 6 virtually. Table 1 summarizes the demographic characteristics of the sample. In terms of professional cadres, there was a roughly even split of medical doctors (n = 8), nurses (n = 7), and counselors (n = 7) and a smaller number of pharmacy staff. Some participants worked, in addition, as research staff in their institutions. Participants had spent a median of 11 years in their professions, and 8 years working with people living with HIV.
Table 1.
Socio-Demographic Characteristics of the Sample (n = 24)
| Variable | n (%) |
|---|---|
| Age, years, median (IQR) | 38 (30–44) |
| Gender | |
| Female | 18 (75) |
| Male | 6 (25) |
| Ethnicity | |
| Black | 14 (58.3) |
| Indian/Asian | 1 (4.2) |
| White | 8 (33.3) |
| Other | 1 (4.2) |
| Profession | |
| Doctor | 8 (33.3) |
| Nurse | 7 (29.2) |
| Counselor | 7 (29.2) |
| Pharmacist/pharmacy assistant | 2 (8.3) |
| Highest level of education | |
| Secondary school complete | 2 (8.3) |
| Skills training certificate | 1 (4.2) |
| College or university complete | 21 (87.5) |
| Has some experience administering implants | 10 (42) |
IQR, interquartile range.
In the sections that follow, we describe HCPs' appraisal of pediatric implants in terms of benefits, drawbacks, and health system implications of delivery and implementation.
“It allows them to be kids”: perceived benefits of pediatric ART implants
In general, all HCPs welcomed the idea of using implants to deliver ART to children. The syrups, pellets, and tablets that are currently the only ART modalities available for young children in the South African public sector were described as problematic, mainly in terms of cold chain maintenance, palatability, and the dispensing of large volumes at a time. Daily dosing and unpalatable syrups or tablets too large for children to swallow comprise an often emotionally draining and stressful experience for caregivers. As one participant put it, “having to force a child to drink medication each and every day, it is such a trauma” (Nurse with 10 years in the profession and implant experience).
The HCPs felt that the implant's long-acting characteristic would yield at least three concrete benefits in local pediatric treatment contexts. Firstly, follow-up clinic visits for viral load assessments or medication collection would be dramatically reduced. This would especially help families in remote rural areas or from low socio-economic backgrounds, for whom the cost of transport to clinics was often prohibitive. Second, this would also free up time for parents—who are often HIV positive themselves—to attend clinic appointments and take care of their own treatment needs.
Third, not only was reduced exposure to health facilities preferrable in a Covid-19 pandemic, but it was also thought to help “normalize” life for CLWH. One nurse said, “it allows them to be kids and enjoy their youth without thinking about taking medication” (Nurse with 10 years in the profession and implant experience). Several participants spoke about the importance of children being able to play, something that daily medication routines tended to interrupt.
Children like to play or play games or watch TV, so when their time to take treatment comes, the child starts to have moods… (Counsellor with 11 years in profession and no implant experience).
It was for this reason that most HCPs recommended a flexible rather than stiff implant, to avoid it inhibiting movement or poking out of the child's skin when playing.
Compared with existing ART delivery methods, an implant for children was considered potentially more “reliable” in the long run, leading to better monitoring of viral loads and improved clinical outcomes. When leaving their children with other family members or neighbors while at work, primary caregivers would not have to worry about whether their children were receiving their ART medication. Implants would also deliver precise dosages, circumventing the problem of skipped doses when daily medication runs out or is spat out by children.
Finally, HCPs touched on the persistence of HIV stigma in this social environment, and how taking chronic medication can reveal one's HIV status to others. Compared with tablets and syrups, most HCPs felt that the implant was more discreet and could even buy time for caregivers who were not ready to disclose to others. Overall, they saw the implant as a treatment method that would help to prevent stigmatization of the child.
“The minute they see needles they jump”: drawbacks to pediatric implants
Notwithstanding HCPs' strong support for implants, some concerns were expressed about this mode of delivering ART to children. The dominant concern was that insertion and removal would be painful for young children (even with an injection of lignocaine), and that HCPs would struggle to immobilize and comfort children sufficiently for the device to be properly administered. For many, it was a given that implant insertion in children would be more complex than in adults.
The health care workers are going to be nervous, I think… The surface area you are working with is smaller, it's often very traumatic for everyone because the children scream whether it is painful or not (Medical doctor with 9 years in profession and implant experience).
While older children could be “reasoned with,” HCPs pointed out that younger children would probably need to be physically restrained. Some worried that frightened children might even react violently to insertion and removal procedures and require additional people to restrain them. One nurse said,
You know, we fear children…they can kick you like nothing. Who's going to deal with these children? …You really need manpower because they have got that power to fight…So, you need someone to hold their legs, the other one holding the body, the other one the face (Nurse with 20 years in profession and implant experience)
She suggested restraining them by wrapping the child's body with a sheet, “as if you are bandaging the child” so that “that child will not have power.”
Many participants pointed out, in addition, that pain-free implant removal required technical expertise and dexterity on the part of HCPs. Those with personal experience of administering the contraceptive implant recalled instances where they had struggled with removing the Implanon NXT implant rod, which has a smooth surface that makes it slippery and difficult to grip with forceps, especially when wet. Some mentioned that the implant usually attached to fibrous tissue or embedded in fat (if the patient had gained weight since insertion), making removal potentially “more tricky.” For these reasons, most HCPs stated that non-transparent implants that are not too small or flexible would be less challenging to insert and remove.
Most HCPs recommended an implant duration of 6 months or longer, that would allow replacement of implants to be paired with biannual or annual clinic visits for viral load checks. Anything <6 months was deemed pointless and unnecessarily traumatic for the child, because “you are inflicting pain on this poor child [and] you can't be inflicting pain on a child 3-monthly” (Nurse with 12 years in profession and implant experience).
The HCPs considered a topical anesthetic essential and recommended a child-friendly trocar for insertion to minimize children's fear. Some advised that trocars be developed with a hidden needle, and be brightly colored, printed with images of comic-book heroes, or shaped like a toy gun, to not look “too medical or frightening” (Medical doctor with 14 years in profession and implant experience).
A second cluster of concerns about pediatric implants related to the potential for scarring and commensurate HIV status disclosure. Above, we described how HCPs saw the implant's elimination of daily dosing of ART medication as potentially preventing inadvertent HIV disclosure. Some felt this benefit would be undone, however, if implants led to visible scarring of the body. A doctor commented:
If we were to cut [into skin for] an implant every year, a few months, for five years, we could risk creating a scar which could identify the child as having had serial implants (Medical doctor with 11 years in profession and no implant experience).
Aside from scarring, HCPs concerned about stigma also worried about the visibility of implants themselves under the skin. They recommended that children's implants be smaller than contraceptive implants, and formulated as a single, thin rod (e.g., rather than a disc shape) that is skin-toned to minimize visibility through the skin. This set up a tension of sorts between the preference for implant visibility to facilitate removal, as described earlier, and their wish for invisibility of the implant under the skin, to avoid stigma.
These concerns also influenced preferences around appropriate locations on the body for insertion of the implant. Most HCPs preferred parts of the body that are seldom unclothed in public, such as the child's back or buttocks. Inserting the implant in a hard-to-reach location such as the back was also seen as deterring children from fiddling with it. Indeed, more than three-quarters of the sample preferred the back or the buttocks for these reasons. Just over half felt that the upper arm was a more suitable location, however, largely because this is where contraceptive implants are placed, and because insertion and removal were generally thought to be easier in this location.
Only a handful of HCPs raised concerns about implants migrating from their initial place of insertion. One nurse was emphatic that implant design needed to incorporate and address this possibility.
Maybe if it moves and gets lost somewhere, maybe in the blood vessels or travels somewhere where it should not travel, then I think maybe that will be, like, a big no-no for me. They must just make sure that it is fixed in one area (Nurse with 4 years in profession and implant experience).
Many HCPs believed such fears would also be held by caregivers, especially in relation to biodegradable implants, claiming that they would want to know “where did the implant go?” Participants thought caregivers would worry about the potential for old, biodegrading implants to get into the blood stream and lodge in critical organs, causing damage, or that as implants broke down over time, they would release toxic or carcinogenic substances into the body. This would lead to misattribution of future illnesses to the presence of old implants.
We will tell them that it absorbs into the body but one day a person will be coming and saying, “I've got chest pain, I think it's that thing you inserted in my arm.” Or “I've got a headache, I think that thing went to my brain” (Nurse with 12 years in profession and implant experience).
While HCPs themselves generally saw biodegradability in a positive light—eliminating the need for implant removal, thereby reducing trauma and scarring for the child—they had unanswered questions around dosing requirements and treatment management. One participant questioned whether therapeutic doses could be maintained with biodegradable implants, as some would break down faster than others. Another pointed out that non-biodegradable implants would become “proof” of a child's treatment history: if the child's family moves to a different province, for example, the absence of a palpable implant could endanger continuity of care.
Only two participants raised concerns that caregivers would attempt to remove the implant themselves rather than come to a health facility for this procedure—possibly influenced by local anecdotal evidence of contraceptive implants being removed by patients or by “implant robbers.”19 A pharmacist claimed, “you always get people who try to remove it themselves or get someone else to try and take it out. That would be risky” (Pharmacist with 12 years in profession and no implant experience).
Delivery and implementation considerations
Almost all participants believed that replacing current forms of treatment delivery with the implant would reduce HCPs' workload overall. Overall, participants felt that implant administration could be integrated into HCPs' existing responsibilities relatively easily, providing staff were well trained. Such training would need to be multi-purposed, building HCPs' technical skills and confidence to insert and remove implants, as well as their ability to deliver comprehensive patient education despite heavy workloads and under-staffing in health facilities. A few participants stressed that in addition to training medical practitioners, it would be important to train counselors, front-desk administrative staff, and health promotion staff, to support roll-out of the pediatric implant.
A key anticipated challenge to successful delivery of pediatric implants in the South African health system was denial of access. One participant described how this had happened with the contraceptive implant:
I feel like patients are being referred from one place to another [and] being dismissed. When you get to a [health] facility, they will tell you, “No, here we do not remove implants if we are not the ones that put the implant in you… you must go back to that facility” (Counsellor with 4 years in profession and no implant experience).
The perception that pediatric interventions are highly specialized, requiring only HCPs with advanced training, was identified as a further potential obstacle to access. One participant said,
If you don't have a specific nurse practitioner, for example… or doctor with pediatrics experience, in general people [healthcare providers] are scared of children and we have had children turned away from the clinic and [been told] “No, we don't treat children” (Medical doctor with 14 years in profession and implant experience).
When it came to implant storage, most participants were optimistic that this could be handled in health facilities of all levels—especially if cold-chain storage is not required. There was some anxiety about possible stock-outs during roll-out of the implant, however, especially as facilities are already experiencing regular stock-outs of other pediatric HIV medicines and trocars for contraceptive implants. The HCPs pointed out that implant stock-outs would impact management of patients' treatment plans, by necessitating repeated returns to tablets and syrups, and eroding patient trust built through careful education.
As one nurse put it, “our patients lose confidence in us and in the system, which is sad” (Nurse with 19 years in profession and implant experience). To avoid impractical stock-ordering “on a case-by-case basis,” which would be necessary with single drug implants and different implants for different weight bands, participants recommended fixed-dose drug combinations in a single rod, offered as first-line therapy. The question of the cost-effectiveness of pediatric implants was raised by a few participants, who felt that the likely higher cost of an implant would be mitigated by savings derived from improved adherence, fewer children on second- and third-line therapy, and fewer clinic visits.
Discussion
The HCPs in Johannesburg considered implants an acceptable drug delivery platform for HIV treatment in young children and endorsed its scientific development. They offered valuable insights on design and delivery considerations to increase acceptability among end-users and minimize burdens on health systems, young CLWH, and their caregivers. Participants highlighted that current delivery mechanisms for ART for young children were sub-optimal and believed that implants could improve adherence and clinical outcomes, by reducing clinic visits, delivering precise dosages, and potentially reducing HIV stigma. As with any new technology, however, potential drawbacks were also identified.
A primary concern was that children tend to fear needles much more than adults, and participants were reluctant to put children in their care through painful and traumatic procedures. Their message was clear: the pediatric implant must be designed and administered to be as pain-free as possible for young children, to not hinder children's daily activities, and to be discreet, lest they encourage stigma. At times, there were tensions between what HCPs preferred in terms of implant attributes and what they thought caregivers would want.
For example, while HCPs preferred a stiffer implant for ease of removal, they recognized that caregivers might consider a flexible implant to be more comfortable for the child. Similar tensions were noted also in a study on the acceptability of PrEP implants for adults in South Africa.14 For participants in our study, the need to minimize pain and trauma for children during implant insertion and removal trumped all other considerations.
Like many HCPs in the South African public sector context, most participants had considerable experience of health promotion and patient education, and they could anticipate the kinds of concerns and fears that caregivers in this setting might have in relation to medical devices or vaccines introduced into the body. The biodegradability of implants was one product attribute they felt caregivers would reject, as they would associate it with notions of dangerous decay, believing that dissolving implants move around the body or become carcinogenic over time.
Promoting alternative analogies that highlight the safety of medical devices as they biodegrade in the body may be essential for effective caregiver education. Obtaining perspectives from caregivers of CLWH would be important, not least of all for assessing whether they, indeed, hold the concerns that HCPs attributed to them.
Not surprisingly, there was a strong emphasis on the need for careful training and mentoring to secure HCPs' acceptance of this new delivery method and address their concerns about an implant for children. Participants called for lessons to be learned from the contraceptive implant experience, and for implant roll-out to include dedicated patient education to tackle myths and misunderstandings among caregivers. Similar recommendations have been made by studies of adult HIV implants in this setting,12,14,20 although these studies have stressed the importance of distinguishing between an ART implant for adults and the contraceptive implant—an issue that may be less relevant for pediatric implants, and which did not come up in our interviews. Additional research is needed on the cost-effectiveness of pediatric implants in low-resource settings and potential health systems barriers to uptake.
Limitations to the study included the non-randomness of participant recruitment and the small sample size (although acceptable for qualitative research). Findings may therefore not be generalizable to a larger and more diverse sample of HCPs in South Africa or in other similar settings. Second, given that participants knew that the research team was associated with product developers working on a pediatric implant, interviews may have been influenced by social desirability bias. Finally, in the six interviews carried out virtually using video communication technology, participants were not able to handle the implant prototypes, although they were able to view them remotely.
In conclusion, HCPs in this study recognized that implants would change the delivery landscape in pediatric ART programs but emphasized there would be trade-offs. Implants are a more complex delivery system for the health system to manage than tablets or syrups, yet they confer simplicity to the user and offer a route to greater adherence. This makes them less than “perfect” delivery mechanisms but very promising in terms of improving long-term treatment outcomes in young CLWH.
Acknowledgments
We are grateful to the health care providers who contributed their time and perspectives to this study. Thanks are also due to the DAISY product development and preclinical team members from RTI International and The University of North Carolina at Chapel Hill, who provided insight and guidance in the development of qualitative data collection tools. The DAISY study was funded by the National Institute of Allergy and Infectious Diseases (R61AI149499), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, all components of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authors' Contributions
F.S.: supervision and technical support to data collection (supporting); formal analysis (lead); writing—original draft (lead); writing—review and editing; I.H.: study conceptualization (supporting); supervision and technical support to data collection (lead); formal analysis (supporting); writing—original draft (supporting); L.F.: study conceptualization (supporting); writing—review and editing; S.P.: data collection; formal analysis (supporting); writing—review and editing; F.M.: formal analysis (supporting); writing—review and editing; R.M.: data collection; formal analysis (supporting); writing—review and editing; S.-J.L.: data collection; writing—review and editing; M.C.: study conceptualization (supporting); writing—review and editing; L.J.: funding acquisition (lead); study conceptualization (co-lead); writing—review and editing; E.T.M.: study conceptualization (lead); formal analysis (supporting); writing—review and editing.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The DAISY study was funded by the National Institute of Allergy and Infectious Diseases (R61AI149499), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, all components of the National Institutes of Health (NIH). The content is solely the responsibility for the authors and does not necessarily represent the official views of the NIH.
References
- 1. UNICEF. Global and Regional Trends: Although Strides Have Been Made in the HIV Response, Children Are Still Affected by the Epidemic 2021. Available at: https://data.unicef.org/topic/hivaids/global-regional-trends/ [Last accessed: January 31, 2022].
- 2. UNAIDS. Start Free, Stay Free, AIDS Free. Final Report on 2020 Targets. Geneva: UNAIDS; 2021. [Google Scholar]
- 3. Overmeyer R. Operation Puthuma: Report by Paediatric and Adolescent HIV Working Group. South African National Department of Health: South Africa; 2021. [Google Scholar]
- 4. Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep 2009;6:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Elsland SL, Peters RPH, Grobbelaar N, et al. Paediatric ART adherence in South Africa: A comprehensive analysis. AIDS Behav 2019;23:475–488. [DOI] [PubMed] [Google Scholar]
- 6. Reddington C, Cohen J, Baldillo A, et al. Adherence to medication regimens among children with human immunodeficiency virus infection. Pediatr Infect Dis J 2000;19:1148–1153. [DOI] [PubMed] [Google Scholar]
- 7. Fetzer BC, Mupenda B, Lusiama J, Kitetele F, Golin C, Behets F. Barriers to and facilitators of adherence to pediatric antiretroviral therapy in a sub-Saharan setting: Insights from a qualitative study. AIDS Patient Care STDS 2011;25:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlatter AF, Deathe AR, Vreeman RC. The need for pediatric formulations to treat children with HIV. AIDS Res Treat 2016;2016:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies MA, Boulle A, Fakir T, Nuttall J, Eley B. Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: A prospective cohort study. BMC Pediatr 2008;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beghin J-C, Yombi JC, Ruelle J, Van der Linden D. Moving forward with treatment options for HIV-infected children. Expert Opin Pharmacother 2018;19:27–37. [DOI] [PubMed] [Google Scholar]
- 11. Clinton Health Access Initiative. Five Things You Should Know About Pediatric DTG 2020. Available at: https://www.clintonhealthaccess.org/five-things-you-should-know-about-pediatric-dtg/ [Last accessed: July 7, 2022].
- 12. Humphries H, Upfold M, Mahlase G, Mdladla M, Gengiah TN, Abdool Karim Q. Implants for HIV prevention in young women: Provider perceptions and lessons learned from contraceptive implant provision. PLoS One 2022;17:e0262043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adeagbo O, Mullick S, Pillay D, et al. Uptake and early removals of Implanon NXT in South Africa: Perceptions and attitudes of healthcare workers. S Afr Med J 2017;107:822–826. [DOI] [PubMed] [Google Scholar]
- 14. Krogstad EA, Montgomery ET, Atujuna M, et al. Design of an implant for long-acting HIV pre-exposure prophylaxis: Input from South African health care providers. AIDS Patient Care STDS 2019;33:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brady M, Tolley E. Aligning product development and user perspectives: Social–behavioural dimensions of multipurpose prevention technologies. BJOG 2014;121(Suppl 5):70–78. [DOI] [PubMed] [Google Scholar]
- 16. Romano J, Van Damme L, Hillier S. The future of multipurpose prevention technology product strategies: Understanding the market in parallel with product development. BJOG 2014;121(Suppl 5):15–18. [DOI] [PubMed] [Google Scholar]
- 17. Glaser B, Strauss A.. The Discovery of Grounded Theory: Strategies for Qualitative Research. Mill Valley, CA: Sociology Press; 1967. [Google Scholar]
- 18. Glaser B. The constant comparative method of qualitative analysis. Soc Probl 1965;12:436–445. [Google Scholar]
- 19. Krogstad EA, Atujuna M, Montgomery ET, Minnis AM, Morroni C, Bekker LG. Perceptions matter: Narratives of contraceptive implant robbery in Cape Town, South Africa. Cult Health Sex 2021;23:383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krogstad EA, Atujuna M, Montgomery ET, et al. Perspectives of South African youth in the development of an implant for HIV prevention. J Int AIDS Soc 2018;21:e25170. [DOI] [PMC free article] [PubMed] [Google Scholar]