Abstract
Adipose tissue is characterized as an endocrine organ that acts as a source of hormones and paracrine factors. In diseases such as cancer, endocrine and paracrine signals from adipose tissue contribute to cancer progression. Young individuals with estrogen receptor-alpha positive (ER-α+) breast cancer (BC) have an increased resistance to endocrine therapies, suggesting that alternative estrogen signaling is activated within these cells. Despite this, the effects of stromal age on the endocrine response in BC are not well defined. To identify differences between young and aged ER-α+ breast tumors, RNA sequencing data were obtained from The Cancer Genome Atlas. Analysis revealed enrichment of matrix and paracrine factors in young (≤40 years old) patients compared to aged (≥65 years old) tumor samples. Adipose-derived stromal/stem cells (ASCs) from noncancerous lipoaspirate of young and aged donors were evaluated for alterations in matrix production and paracrine secreted factors to determine if the tumor stroma could alter estrogen signaling. Young and aged ASCs demonstrated comparable proliferation, differentiation, and matrix production, but exhibited differences in the expression levels of inflammatory cytokines (Interferon gamma, interleukin [IL]-8, IL-10, Tumor necrosis factor alpha, IL-2, and IL-6). Conditioned media (CM)-based experiments showed that young ASC donor age elevated endocrine response in ER-α+ BC cell lines. MCF-7 ER-α+ BC cell line treated with secreted factors from young ASCs had enhanced ER-α regulated genes (PGR and SDF-1) compared to MCF-7 cells treated with aged ASC CM. Western blot analysis demonstrated increased activation levels of p-ER ser-167 in the MCF-7 cell line treated with young ASC secreted factors. To determine if ER-α+ BC cells heightened the cytokine release in ASCs, ASCs were stimulated with MCF-7-derived CM. Results demonstrated no change in growth factors or cytokines when treated with the ER-α+ secretome. In contrast to ER-α+ CM, the ER-α negative MDA-MB-231 derived CM demonstrated increased stimulation of pro-inflammatory cytokines in ASCs. While there was no observed change in the release of selected paracrine factors, MCF-7 cells did induce matrix production and a pro-adipogenic lineage commitment. The adipogenesis was evident by increased collagen content through Sirius Red/Fast Green Collagen stain, lipid accumulation evident by Oil Red O stain, and significantly increased expression in PPARγ mRNA expression. The data from this study provide evidence suggesting more of a subtype-dependent than an age-dependent difference in stromal response to BC, suggesting that this signaling is not heightened by reciprocal signals from ER-α+ BC cell lines. These results are important in understanding the mechanisms of estrogen signaling and the dynamic and reciprocal nature of cancer cell-stromal cell crosstalk that can lead to tumor heterogeneity and variance in response to therapy.
Keywords: breast cancer, adipose-derived stromal/stem cells, endocrine response, paracrine signaling
Introduction
In the United States, approximately one in eight women will develop invasive breast cancer (BC) during her lifetime [1,2]. The risk of BC increases with age, as a large percentage occurs in women 50 and over [3]. Fewer than 5% of BCs occur in women under the age of 40; however, BC is the leading cause of cancer death among women between 20 and 39 years old [4]. Roughly 11 out of 100,000 women between the ages of 15–39 years have estrogen receptor-α positive (ER-α+) tumors, making endocrine therapy the treatment of choice [5]. Although initially responsive to endocrine therapy, ∼25%–30% of all BC patients are at risk of recurrence due to acquired or de novo resistance to endocrine therapies [6].
Mechanisms of endocrine resistance are composed of alterations to estrogen-mediated signaling, including mutations in drug targets such as ER-α or activation of signaling pathways independent of ER-α [7–14]. In particular, ER-α acts through several mechanisms such as direct genomic, nongenomic, and ligand-independent signaling [1,15]. Direct genomic signaling is the traditional mechanism of estrogen signaling. The binding of the dimerized estrogen receptors leads to nuclear translocation and binding to estrogen response element sequences of target genes [16]. Estrogen can also induce rapid, nongenomic signaling in which estrogen induces gene transcription and protein synthesis by alternative mechanisms. The activation of signaling cascades, including the PKC pathway, RAS/RAF/MAPK pathway, PI3K/AKT pathway, and the cAMP/PKA pathway, leads to subsequent activation and binding of a variety of transcription factors (i.e., C/EBPβ, NFκB, STAT) to alternative DNA response elements [17–22].
Estrogen receptors can also be activated in the absence of estrogens or other receptor agonists, adding to the complexity of estrogen signaling [23]. Ligand-independent ER-α activation occurs through the phosphorylation of specific residues (i.e., serine or tyrosine) in the receptors themselves or by coregulators such as PKA, MAPK, PKC, inflammatory cytokines (i.e., interleukin [IL]-2), and peptide growth factors (i.e., insulin-like growth factor 1 [IGF-1], TGF-β, EGF). Despite the identification of multiple mechanisms of endocrine resistance, young individuals in the ER-α+ cohort do not have an identified mechanism of action for ER-α activation and signaling.
Young individuals with luminal ER-α+ BC have an observed increase in resistance to endocrine therapies such as tamoxifen [24]. Currently, there is neither a specific focus on mechanisms of tumorigenesis in young ER-α+ patient populations nor are current therapies designed to combat endocrine resistance evaluated in young ER- α+ BC patients [24,25]. Recently, attention has been directed to the tumor microenvironment (TME) as an active participant and/or contributor in tumorigenesis and metastasis and the progression toward hormone independence and endocrine therapy resistance [26–29].
Tumors are composed of a diverse population of stromal cells such as adipose-derived stromal/stem cells (ASCs), endothelial cells, fibroblasts, adipocytes, and immune cells [30,31]. The interaction between tumor cells and the microenvironment can be characterized as reciprocal. This interplay between normal cells, cancer cells, and the extracellular matrix contributes to the hallmarks of cancer, including immunomodulation, angiogenesis, invasion and metastasis, and apoptotic resistance [32]. Although the stem cell response becomes damaging in light of tumor function and progression, it contrastingly exhibits regenerative properties observed in chronic wounds [33–36].
ASCs, a multipotent cell population found in breast tissue, are integral to the TME and wound healing response. Although initially characterized based on their multilineage differentiation and proactive role in tissue regeneration, ASCs have been linked to BC progression and endocrine signaling [31,37–40]. ASCs express and secrete multiple growth factors, cytokines, chemokines, and inflammatory biomarkers such as stromal-derived factor 1 (SDF-1) and IGF-1 that aid in cancer development and progression [41–43]. These signaling molecules also enhance BCBC progression and regulate resistance to endocrine therapies in ER-α+ BCBC. Furthermore, studies show that ASCs create a pro-inflammatory TME that promotes tumorigenic activity by releasing cytokines such as tumor necrosis factor alpha (TNF-α), IL-6, and IL-8 [44]. In addition, ASC phenotype exhibits variance based on donor-specific characteristics, thus contributing to tumor heterogeneity [45,46].
Despite the suggested role of stromal age in cancer, current research is yet to focus on the impact on hormone independence and endocrine therapy resistance. In the present study, the effects of ASC age on the ER-α+ MCF-7 cell line were assessed to investigate the underlying mechanism(s) for the impact of stromal age on the endocrine response to ER-α+ BC. In addition, the reciprocal response of ASCs to ER-α+ BC was evaluated through the analysis of ER-α+ BC conditioned media (CM) on ASCs.
Materials and Methods
Cell culture
Human estrogen receptor-alpha positive (ER-α+; MCF-7) and triple negative breast cancer (TNBC; MDA-MB-231) human BC cell lines were obtained from ATCC. TUBcX-41C was obtained as previously described [47]. They were maintained in Dulbecco's modified Eagle's medium (DMEM, Catalog No.: 11965-092; Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Catalog No.: SH30087.03; Cytiva, Logan, UT, USA), 1% Nonessential amino acids (NEAA, Catalog No.: 11140-050; Gibco), Minimal essential amino acids (MEMAA, Catalog No.: 11130-051; Gibco), sodium pyruvate (100 mM, Catalog No.: 11360-070; Gibco), L-glutamine (Catalog No.12561-056; Gibco), Antibiotic-Antimycotic (Anti-Anti, Catalog No.:15240062; Gibco), and insulin (4 mg/mL, Catalog No.: 12585-014; Gibco) at 37°C in humidified 5% carbon dioxide [CO2].
MCF-7 cells were seeded at 3000 cells/cm2 and grown in the exponential phase. Human ASCs obtained from Obatala Sciences (New Orleans, LA, USA) were isolated from noncancerous abdominal lipoaspirate tissue. When possible ASCs were purchased and obtained from a commercially available source. IRB exemption number E11468. Young donors (24–34 years old [YO], n = 3) and aged donors (50–68 YO, n = 3) were grouped by age, body mass index (BMI), and race (Supplementary Table S1). Cultures of ASCs (3000 cells/cm2) were maintained with Alpha-MEM with L-glutamine (Catalog No.: 12561-056; Gibco) supplemented with 10% FBS (Catalog No.: 43602-500; JR Scientific, Woodland, CA, USA) and 1% Anti-Anti (Catalog No.: 15240062; Gibco) until attaining 80% confluence. The procedure implemented for ASC expansion was described previously by Bunnell et al. [48].
Conditioned media
ASC conditioned media
ASCs were cultured until they attained 80% confluence in 10% Alpha-MEM with L-glutamine (Catalog No.: 12561-056; Gibco) supplemented with 10% FBS (Catalog No.: 43602-500; JR Scientific) and 1% Anti-Anti (Catalog No.:15240062; Gibco). Cells were washed with sterile 1× phosphate buffer saline (PBS, Catalog No.: 10010023; Gibco). To remove exogenous signaling from hormones and growth factors, ASCs were then cultured in phenol-free DMEM (Catalog No.: 31053-028; Gibco), 5% dextran/charcoal stripped FBS (Catalog No.: SH3006803; GE Healthcare), 1% penicillin/streptomycin (P/S, Catalog No.: 15140-122; Gibco), 1% MEMAA, 1% NEAA, 1% GlutaMAX (Catalog No.: 35050-061; Gibco), and 1% sodium pyruvate for 24 h.
The CM was collected, filtered, and stored briefly at −20°C until use on MCF-7 cells. MCF-7 cells were seeded at 50,000 cells/cm2 in 10 cm2 dishes. Cells were maintained in 10% DMEM at 37°C in humidified 5% CO2 until 80% confluent. Once confluent, cells were washed with 1 × PBS and maintained in CM obtained from aged and young ASCs for 24 h (polymerase chain reaction [PCR]/proliferation) or 15 min (western blot). Cells were then collected and stored at −80°C for RNA extraction, Western blot, and cytokine array analysis.
Breast cancer conditioned media
MCF-7, MDA-MB-231, and TU-BCx-4IC cells were cultured until they attained 95%–100% confluence in DMEM supplemented with 10% FBS, NEAA, MEM amino acids, Anti-Anti (100 U/mL), sodium pyruvate, and human recombinant insulin (1 × 10−10 mol/L). To remove exogenous signaling from hormones and growth factors, cancer cells were washed with 1× PBS and placed in 5% media consisting of phenol-red free DMEM supplemented with 5% CS FBS, GlutaMAX, and P/S (Gibco) for 24 h. After 24 h, the CM was collected and centrifuged to reduce cellular debris then stored at −20°C until use. Timepoints for experiments that required CM stimulation are found in Supplementary Figure S1.
Proliferation assay
ASCs were seeded in 96 well plates at 3000 cells/cm2. Three days after plating, wells were maintained with culture medium or AdipoQual™ Adipogenic differentiation medium (OS-002; Obatala Sciences). Cell density at days 1, 3, 7, and 10 was assessed using the Cell Counting Kit-8 (CCK-8; Dojindo, Rockville, MD, USA). Absorbance was measured using a cell imaging multimode reader (Cytation 3, BioTek, Winooski, VT, USA) and Gen5 Microplate Reader and Imager Software (BioTek). MCF-7 cells were seeded at 50 cells/cm2 in 96-well plates. Cells were maintained in 10% DMEM at 37°C in humidified 5% CO2 and treated with CM after 24 h. At days 0, 1, 5, and 7, cells were fixed with 95% methanol for 10 min. They were washed thrice with 1 × PBS before crystal violet staining. Cells were stained with 3% crystal violet solution and incubated at room temperature for 1 h. The wells were washed with water then allowed to dry before imaging. After dissolving in 33% acetic acid, absorption was determined at 595 nm using the Cytation 3 and Gen5 Microplate Reader and Imager Software.
Differentiation assays
ASCs were cultured until they attained 80% confluence in 10% alpha-MEM with L-glutamine supplemented with 10% FBS and 1% Anti-Anti. For ASC differentiation based on donor age, ASCs were cultured for 14 days in adipogenic differentiation medium. For ASC differentiation following exposure to cancer condition media, ASCs were washed with 1 × PBS, treated with CM for 3 days, and then cultured for 7 days in adipogenic differentiation media. For both experiments, at end point, ASCs were either stained for lipid droplet formation using Oil Red O stain (Catalog No.: A1298914; Alfa Aesar, Haverhill, MA, USA, 0.3% ORO in Isopropyl alcohol and PBS) or collected and stored at −80°C for RNA extraction and quantitative real time (qRT)-PCR analysis. Controls for all differentiation studies were ASCs grown in stromal media. Additional controls for ASCs pretreated with cancer CM include pretreatment with 5% dextran stripped FBS and full stromal media.
Quantitative real time PCR
RNA was extracted from cell pellets using QIAshredders (Catalog No.: 79656; Qiagen, Germantown, MD, USA) and RNeasy Mini Kit (Catalog No.: 74106; Qiagen) as per manufacturer instructions. Complementary DNA (cDNA) was synthesized using qScript cDNA SuperMix (Catalog No.: 101414-106; VWR, Radnor, PA, USA) and 1 μg total RNA. Quantitative PCR was performed using the BioRad iCycler (v4.006; BioRad) and Quanta SYBR green (VWR) as per manufacturer's protocol. Expression was calculated using the ΔΔ(Ct) method and reference genes GAPDH (ASCs) and ß-Actin (MCF-7). Primer sequences are presented in Supplementary Table S2. For ASCs, normalization was to aged ASC gene expression designated as 1. For MCF-7 cells, normalization was to vehicle control gene expression designated as 1. Biological replicates were n = 3 independent aged and young donor sets.
WES automated immunoassays Immuno (Blot-free)
MCF-7 cells were seeded at 50,000 cells/cm2 in 10 cm2 dishes. Cells were maintained in 10% DMEM at 37°C in humidified 5% CO2 until 80% confluent. At 80% confluency, 10% DMEM was removed and either 5% charcoal stripped media, 5% charcoal stripped media with 1 ng 17β estradiol (Catalog No.: E2758; Sigma), or CM from aged and young ASC donors was added to the dishes. After 15 min at 37°C in humidified 5% CO2, the media was removed, cells were washed, and cells were collected on ice and stored at −80°C. Cell pellets were lysed in 100 μL of mammalian protein extraction reagent (M-PER, Catalog No.:78501; ThermoFisher Scientific, Waltham, MA, USA) supplemented with 1 × Halt protease and phosphatase inhibitor (Catalog No.:78444; ThermoFisher Scientific). Bicinchoninic acid protein assays were run on the cell extracts, and 1 μg of total protein was mixed with a master mix (Catalog No.: SM-W004; ProteinSimple, San Jose, CA, USA) to give a final concentration of 0.2 mg/mL total protein.
Samples were heated at 95°C for 5 min. Samples, blocking solution, primary antibodies (ER-α, 1:50, No. sc-543 [Santa Cruz]; actin, 1:500, No. NB600–501H [Novus Biologicals]; phospho-ER α serine 167, 1:25, No. 64508 [Cell Signaling Technology, Danvers, MA, USA]; phospho-ER α serine 118, 1:25, No. 2511 [Cell Signaling]; phospho-ERK1/2, 1:25, No. 4307 [Cell Signaling]; total ERK, 1:50, No. 4695 [Cell Signaling]; phospho-AKT, 1:25, No. 4060 [Cell Signaling]; total AKT, 1:50, No. 4691 [Cell Signaling]), horseradish peroxidase-conjugated secondary antibodies (Catalog No.: DM-002; ProteinSimple), chemiluminescent substrate (Catalog No.: DM-002, ProteinSimple), and separation and stacking matrices (Catalog No.: SM-W004, ProteinSimple) were loaded into designated wells in a 384 well plate according to the manufacturer's instructions. After plate loading, fully automated electrophoresis and immunodetection took place in the WES capillary system (ProteinSimple). Proteins were separated by molecular weight at 375 V for 25 min and primary and secondary antibodies incubated for 30 min.
Chemiluminescence was captured by a charge-coupled device camera and the digital image analyzed using Compass software (ProteinSimple). The amount of each protein was calculated based on peak area. A virtual image is shown based on peaks from the electropherogram and represented as a blot.
Western blot analysis (traditional blot)
ASCs were seeded in growth medium until attaining 90% confluence. Once confluent, cells were washed with 1 × PBS and then treated with cancer CM for 30 min. ASCs were then collected on ice for total protein extraction using M-PER lysis buffer (Catalog No.: 78501, Thermo Fisher Scientific) supplemented with 0.1% protease inhibitor and 0.1% phosphatase inhibitor (Catalog No.: 1862209 and 1862495, Thermo Fisher Scientific). Total protein was loaded for all samples. Samples were prepared for electrophoresis and run at 150 V on Invitrogen Bolt 4%–12% Bis-Tris Plus electrophoresis gels (Catalog No.: NW04120BOX, Invitrogen, Carlsbad, CA, USA) as per manufacturer's specifications.
Protein was transferred to iBlot 2 Transfer Stack using the iBlot 2 Gel Transfer Device (Invitrogen) as per the manufacturer's specifications. Samples were blocked in 3% milk for 1 h. Primary antibodies were used, including p-AKT Serine/Threonine Kinase 1 (AKT1), p-Mitogen-Activated Protein Kinases 3/1 (ERK1/2), and p-Mitogen-Activated Protein Kinases 8/9 (c-Jun N-Terminal Kinase [JNK]1/2), and were diluted 1:1000. Loading control was Rho-GDIα (Cell Signaling Technology) and diluted 1:50. Infrared-tagged secondary antibodies (LiCor Biosciences, Lincoln, Nebraska) were diluted 1:10,000 in 5% Bovine serum albumin-Tris-buffered saline-Tween. Western blots were analyzed using LiCor Odyssey Infrared Imaging System (LiCor Biosciences).
Cytokine array analysis
Samples were prepared using the same procedure as the CM experiments; CM was collected from ASCs after 24 h. Cytokine array analysis procedures followed the manufacturer's protocol (Catalog No.: ARY005B; R&D Systems, Minneapolis, MN, USA).
Fibril collagen quantification
ASCs were maintained in culture medium until they attained 90% confluence. Cells were washed with 1 × PBS then stimulated with cancer cell-derived CM. Following 3 days of CM stimulation, ASCs were returned to culture medium for 3 days. This process was repeated until the samples reached timepoints of 1 and 2 weeks. At the sample timepoints, fibril collagen content was determined using a Sirius Red/Fast Green Collagen Staining Kit (Catalog No.: 9046; Chondrex Inc., Redmond, WA, USA). The samples were stained as per the manufacturer's protocol. Collagen quantification was performed on the Cytation 3 and Gen5 Microplate Reader and Imager Software and calculated using empirical formulas provided by the Chondrex.
Prostaglandin E2 ASC stimulation
ASCs were maintained in culture medium until they attained 100% confluence. Cells were then stimulated with 10 nM of prostaglandin E2 (PGE2) for 24 h. After stimulation, cells were washed with 1 × PBS and cultured in phenol-free DMEM (Catalog No.:31053-028; Gibco), 5% dextran/charcoal stripped FBS (Catalog No.: SH3006803; GE Healthcare), 1% P/S (Catalog No.: 15140-122; Gibco), 1% MEMAA, 1% NEAA, 1% GlutaMAX (Catalog No.: 35050-061; Gibco), and 1% sodium pyruvate for 19 h. Cells were then collected for RNA extraction and qRT-PCR gene expression analysis.
xCell cell enrichment analysis
Ductal and lobular neoplasms were selected from the case set of The Cancer Genome Atlas (TCGA)-Breast Cancer Gene (BRCA) project (phs000178.v11.p8) derived from the National Institute of Health (NIH) Genomic Data Commons (GDC) Data Portal [49]. Based on the clinical information provided with TCGA-BRCA project, tumors were grouped into young (≤45 years old) and aged (≥65 years old) categories. These selected cases were matched with precalculated TCGA data provided by the xCell webtool. Immune and stromal cell infiltrate from young and aged ER-α+ BC patients was analyzed using the xCell webtool.
BC data sources and analysis
Invasive breast carcinoma data set (IlluminaHiSeq) from TCGA research network was evaluated for differences in gene expression between young and aged patients based on ER status. Database for Annotation, Visualization and Integrated Discovery v6.8 (DAVID v6.8) was used to identify enriched functional-related gene groups found of interest in TCGA dataset [50–52].The Breast Cancer Gene-Expression Miner v4.4 (bc-GenExMiner v4.4) was used to perform an age-dependent comparison in gene expression of significant genes from TCGA data [53]. The Kaplan–Meier Plotter was used to assess the effect of targeted genes on the survival of ER-α+ BC patients [54]. Cell enrichment analysis of young and aged ER-α+ breast tumors was performed using the xCell webtool [55].
Biostatistics
All values are presented as mean ± standard error of the mean (SEM). Statistical analyses among two groups were performed using the Student's t-test. Statistical significance was set at P < 0.05. Analysis was performed using Prism 8 (GraphPad Software, San Diego, CA, USA).
Results
TME composition is altered in young ER-α+ BC
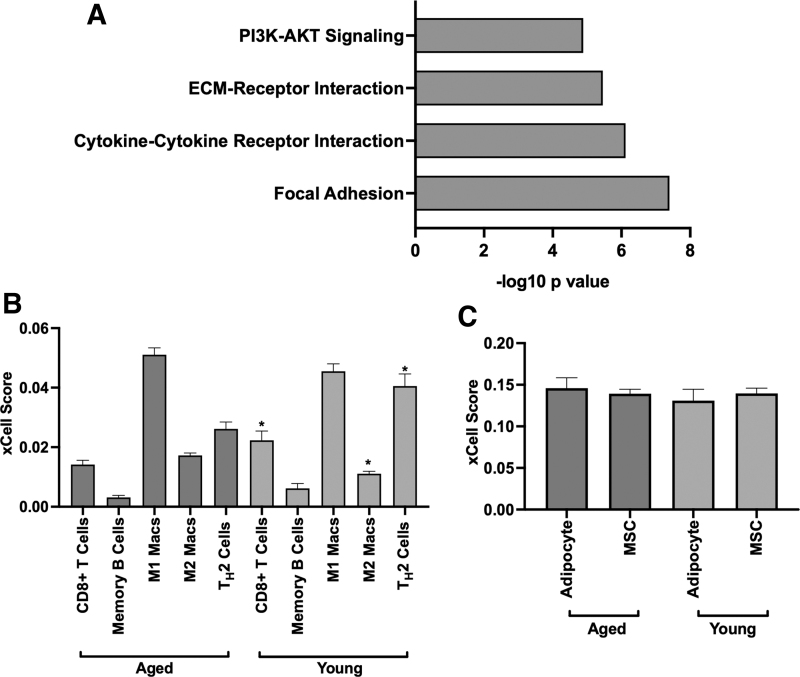
According to the Young Survival Coalition, compared to older women, young women face more aggressive cancers with lower survival rates and a BC subtype that is biologically distinct from that of older women [56–58]. To better identify distinguishing features and underlying mechanisms observed in young ER-α+ patients, previously aligned and published data derived from TCGA IlluminaHiSeq invasive BC were analyzed for differences between aged and young patient populations [59–61]. Tumor samples were separated based on BC ER-α+ receptor status and age (young individuals ≤40 years old and aged individuals ≥65 years old), and changes in gene expression were evaluated. Unbiased pathway analysis was performed using the DAVID functional annotation tool to identify if a central mechanism was uniquely altered in young ER-α+ tumor samples. Results demonstrated that key pathways enriched in young ER-α+ tumor samples were those associated with the extracellular matrix, focal adhesions, cytokine–cytokine interactions, and PI3K/AKT pathways (Fig. 1A). Observed changes include enhanced pro-inflammatory cytokines and growth factors suggesting that the tumors may be eliciting support from surrounding cell types, specifically stem cells [62,63].
FIG. 1.
Tumor microenvironment is differentially regulated and exhibits cellular heterogeneity in young and aged ER-α+ breast cancer patients. (A) DAVID functional annotation tool was used to determine enriched pathways of tumors derived from young ER-α+ breast cancer patients. Genes with a fold change ≥1.3 were selected from TCGA data analyzed in this study. Findings show significant P values (*P < 0.05) for matrix, focal adhesion, cytokine, and PI3K/AKT pathways. (B) xCell cell type enrichment analysis of young (<45 years old) and aged (>65 years old) ER-α+ breast cancer patients revealed significant P values (*P < 0.05) for M2 macrophages, CD8+ T cells, and TH2 cells. Both CD8+ T cells and TH2 cells are enriched in young tumors, while M2 macrophages exhibit a lower quantity compared to aged. (C) Both adipocytes and MSCs exhibit similar quantities in young and aged ER-α+ breast tumors. AKT, protein kinase B; CM, conditioned medium; ER-α+, estrogen receptor-alpha positive; M1 Macs, M1 macrophage; M2 Macs, M2 macrophage; MSC, mesenchymal stem cell; PI3K, phosphoinositide 3-kinase; TCGA, The Cancer Genome Atlas; TH2, T helper 2.
To further determine differences in surrounding noncancer cell infiltrate, TCGA invasive BC data set was next analyzed for differences in stromal cell composition between aged and young ER-α+ breast tumors. Comparison of breast tumor cell infiltration revealed significant differences (P < 0.05) in CD8+ T cells, T helper 2 (TH2) cell, and M2 macrophages between young and aged ER-α+ breast tumors (Fig. 1B). Young tumors exhibited higher xCell scores for CD8+ T cells (0.02 ± 0.003) and TH2 cells (0.04 ± 0.004), but a lower score for anti-inflammatory M2 macrophages (0.01 ± 0.0008). In contrast to immune cell infiltration, both MSC and adipocyte xCell scores were similar for young and aged ER-α+ breast tumors (Fig. 1C).
TCGA data comparing gene expression of young and aged breast tumors showed that the greatest altered genes in young tumors include cytokines, chemokines, growth factors, matrix, and those from the tumor necrosis superfamily (Table 1). With an increase in specific stromal components observed in TCGA enrichment data, we next sought to determine the role of these specific components in patient survival. Genes elevated in young tumors did not have an observed association with poor patient survival using Kaplan–Meier plot (Supplementary Fig. S1). These data suggest that while young ER-α+ tumors demonstrated increased expression of cytokines, chemokines, and matrix compared to aged tumors, singular expression of one factor alone may not be sufficient to modulate patient outcome. Although data suggest no significant difference in the stromal cell infiltrate between young and aged tumors, stromal cell populations should be further investigated as the sources for the expression of the trophic and matrix factors observed in young ER-α+ tumors.
Table 1.
Enriched Gene Expression in Young Estrogen Receptor-Alpha Positive Breast Cancer Tumors
| Gene | Fold change | Gene | Fold change |
|---|---|---|---|
| Matrix | Paracrine | ||
| COL17A1 | 2.3 | CCL5 | 1.4 |
| COL3A1 | 1.3 | CD27 | 1.5 |
| COL5A2 | 1.3 | CD40 | 1.1 |
| COL6A3 | 1.4 | CD40LG | 1.4 |
| COL6A6 | 1.5 | CXCL12 | 1.2 |
| COL7A1 | 1.6 | EGFR | 1.7 |
| FN1 | 1.3 | FGF1 | 1.7 |
| ITGAD | 1.9 | IGF1 | 1.4 |
| ITGA1 | 1.4 | IL12B | 2.0 |
| LAMA1 | 1.8 | IL16 | 1.3 |
| LAMA2 | 1.5 | IL32 | 1.3 |
| LAMA3 | 1.4 | IL8 | 1.2 |
| LAMB1 | 1.3 | PDGFB | 1.3 |
| LAMB3 | 1.6 | TNFAIP1 | 1.2 |
| LAMC1 | 1.4 | TNFAIP3 | 1.4 |
| LAMC2 | 1.7 | TNFAIP6 | 1.4 |
| LAMC3 | 1.6 | TNFRSF13B | 1.9 |
Proliferation, adipogenic differentiation, and matrix gene expression of ASCs are consistent between young and aged
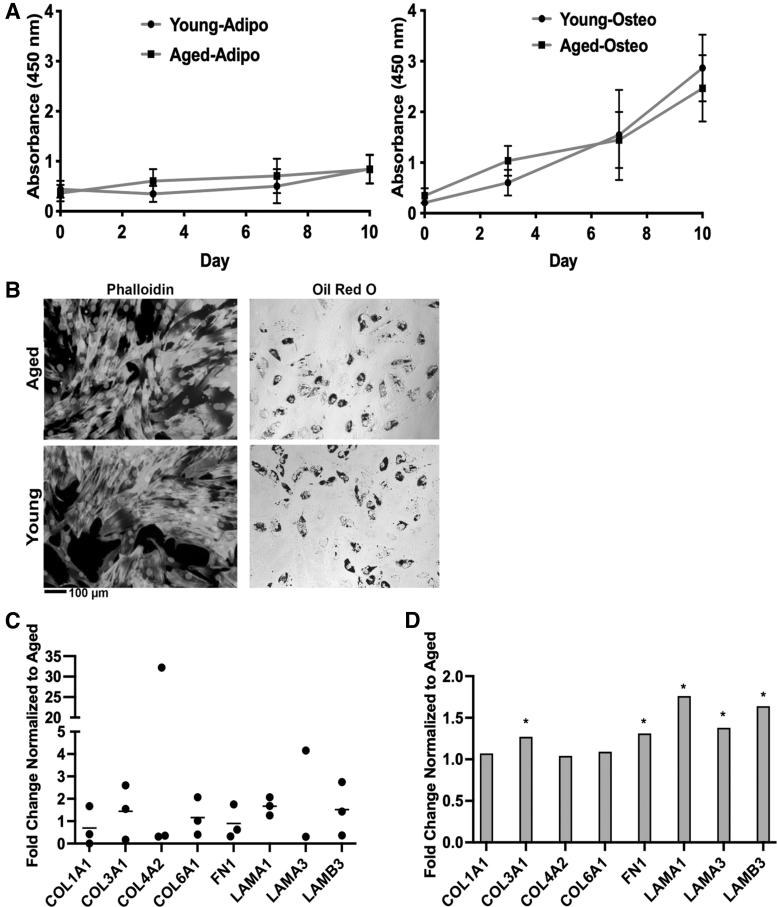
Due to suggested differences in paracrine and matrix factors in young ER-α+ tumors, we next sought to determine if stromal/stem cell age was a mediator for these observed differences by comparing the proliferation, differentiation, and gene expression (matrix and paracrine) of aged (50–70 years old, mean age 62 ± 7.8) and young ASC donors (24–34 years old, 27.8 ± 4.1). Proliferation results revealed no significant difference in the proliferation of young and aged ASCs (Fig. 2A). ASCs were differentiated into adipogenic lineages using lineage-specific induction factors to determine the effect of age on differentiation capacity. The number of young ASCs capable of adipogenic differentiation paralleled that of aged ASCs (Fig. 2B). Furthermore, ASCs were differentiated to adipogenic lineage, and proliferation was measured during the course of differentiation. Results demonstrated no significant differences between young and aged proliferation in a differentiated state. In addition, phalloidin actin staining exhibited no difference between young and aged ASC morphology (Fig. 2B). To determine if ASCs could be the source of altered matrix and/or cytokine profiles observed in young and aged tumors, ASCs were evaluated for differences in the matrix and cytokine expression altered in our TCGA tumor data. Gene expression of extracellular matrix (ECM) components was similar between aged and young donors (Fig. 2C). Both COL4A2 (10.95 ± 10.62 SEM) and LAMA3 (6.82 ± 4.72 SEM) were elevated in young ASCs; however, the expression was variable within the donor cohorts and was not significantly different across donor cohorts. In contrast, TCGA data revealed significant differences (P < 0.05) in COL3A1, FN1, LAMA1, LAMA3, and LAMB3 expression between young and aged tumors (Fig. 2D). Some ECM genes observed in our TCGA data were not detected using PCR and thus excluded from the final analysis.
FIG. 2.
Young and aged ASCs display similar proliferation, differentiation, and matrix gene expression (A–C) (A) Young and aged ASC proliferation cultured in basal and adipogenic medium was assessed at days 1, 3, 7, and 10 using the CCK-8. Young and aged proliferation was comparable in both basal and adipogenic conditions. (B) Young and aged ASCs exhibit comparable differentiation capacity and morphology in two-dimensional culture. Young and aged ASCs were maintained in CM for 7 days, stained with Alexa Fluor 488 Phalloidin, and counterstained with DAPI. Adipogenic-differentiated young and aged ASCs were maintained for 14 days in adipogenic differentiation medium. Oil Red-O was used to stain for lipid rich vacuoles. (C) Young and aged ASCs exhibit comparable matrix gene expression. Young and aged ASCs were cultured in basal medium and collected at 80% confluency for qRT-PCR analysis. The gene expression of extracellular matrix elements of young ASCs was normalized to aged ASC gene expression. N = 3 biological replicates (all assays), N = 3 technical replicates (proliferation), N = 2 technical replicates (differentiation and qRT-PCR), error bars = SEM (D) TCGA data of young ER-α+ breast tumors exhibit significantly elevated matrix gene expression (*P < 0.05) compared to aged. ASC, adipose-derived stromal/stem cell; CCK-8, Cell Counting Kit-8; COL1A1, collagen type I alpha 1; COL3A1, collagen type III alpha 1; COL4A2, collagen type IV alpha 2; COL6A1, collagen type VI alpha 1; FN1, fibronectin-1; LAMA1, laminin subunit alpha 1; LAMA3, laminin subunit alpha 3; LAMB3, laminin subunit beta 3; qRT-PCR, quantitative real time polymerase chain reaction; SEM, standard error of the mean.
Age alters paracrine signaling of ASCs
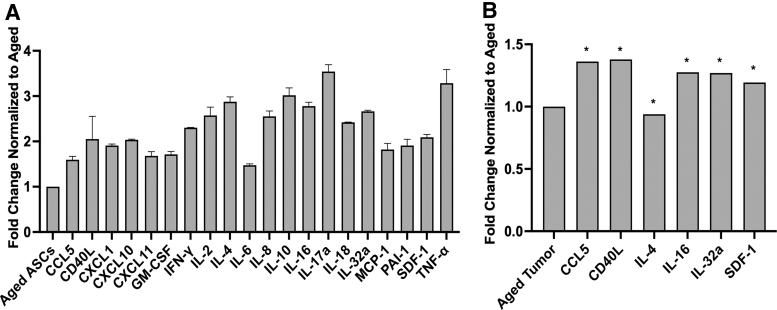
Following the evaluation of ECM gene expression, we next performed cytokine array analysis to detect differences in protein expression of young and aged ASCs. Results revealed elevated levels of CCL5, SDF-1, CD40LG, CXCL1, IFN-γ, IL-16, IL-32a, and TNF-α in young ASC CM (Fig. 3A). Due to altered cytokine expression in noncancerous young ASCs, we next assessed those specific cytokines in TCGA invasive BC data set comparing young and aged ER-α+ tumors. When young ASC cytokine expression was compared with young ER-α+ tumor cytokine expression, CCL5, CD40L, CXCL1, IL-4, IL-16, IL-32a, and SDF-1 were also significantly elevated (P < 0.05) in young breast tumors (Fig. 3B).
FIG. 3.
Young tumors and ASCs exhibit a comparable inflammatory profile. (A) Young and aged ASC cytokine profile was assessed using cytokine array analysis. Young ASC cytokine expression is normalized to aged, N = 2 biological replicates. (B) TCGA data of young ER-α+ tumors parallel young ASC cytokine expression. Genes, including CCL5, CD40L, CXCL1, CXCL12, IFN-γ, IL-16, IL-32, and TNF-α, are elevated in both young ER-α+ tumors and young ASCs. Genes, including CCL5, CD40L, GM-CSF, IL-4, IL-16, IL-32a, and SDF-1, show significance (*P < 0.05) between aged and young tumors. Young tumor gene expression is normalized to aged. CCL5, C-C motif chemokine ligand 5; CD40L, CD40 ligand; CXCL1, C-X-C motif chemokine ligand 1; CXCL10, C-X-C motif chemokine ligand 10; CXCL11, C-X-C motif chemokine ligand 11; GMCSF, granulocyte macrophage colony stimulating factor; IFN-γ, interferon gamma; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; PAI-1, plasminogen activator inhibitor 1; TNF-α, tumor necrosis factor alpha.
To determine if the elevated cytokine profile observed to be altered in our cytokine array was specifically only in the young patient cohort, we next evaluated cytokine expression in breast tumors across three age groups: young (<40 years old), middle aged (40–70 years old), and aged (>70 years old). Only CXCL1 was significantly elevated (P < 0.05) in young compared to aged breast tumors (Data not included). Paracrine factors observed in the cytokine array data were both pro-inflammatory (IL-8, TNF-α) and anti-inflammatory (IL-4, IL-10) suggesting that further studies evaluating ASC paracrine secretion in the young ER-α+ patient TME are needed.
Young ASCs induce an endocrine response in ER-α+ BC
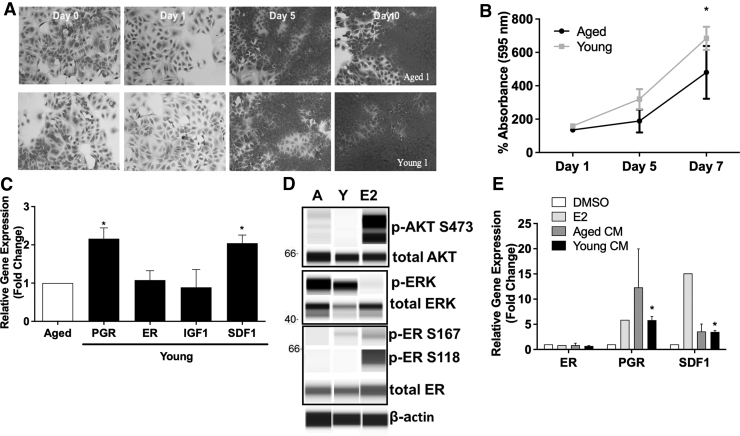
Due to the observed differences in aged and young ASC paracrine factors, we investigated the effect of the ASC secretome on ER-α+ BC. To determine if young ASC secretome altered ER-α+ BC proliferation, the MCF-7 cancer cell line was treated with CM obtained from young and aged ASCs. After 7 days, the total cell number was evaluated. Young ASC secretome increased proliferation of MCF-7 cells (684.5 ± 69.78 SEM) compared to MCF-7 cells treated with aged CM (480.4 ± 158.2 SEM) at day 7 (Fig. 4A, B). The proliferation of MCF-7 cells treated with young CM showed a significant change from day 1 to 7 (P < 0.05), whereas those treated with aged CM showed no significant difference in proliferation. To determine if the increased proliferation was mediated through endocrine signals, ER-α response genes were evaluated in MCF-7 cell line following treatment with either young or aged CM for 24 h. Results show that ER-α regulated genes PGR and SDF-1 were increased by 2 ± 0.44 SEM (P < 0.05) and 2 ± 0.35 SEM (P < 0.05), respectively, in MCF-7 cells treated with young ASC CM compared to those treated with aged CM (Fig. 4C).
FIG. 4.
Young ASC secretome enhances proliferation and estrogen signaling in MCF-7 cell lines. MCF-7 cells were treated with condition media from aged or young ASCs, and proliferation using crystal violet was evaluated at days 0, 1, 5, and 7. MCF-7 cells treated with young CM showed greater proliferation through (A) visual staining and (B) absorption. N = 3 biological and technical replicates, error bars = SEM (C) MCF-7 cells were cultured with aged and young ASC CM and collected for qRT-PCR. Both PGR and SDF-1 expression were significantly different (*P < 0.05) from aged expression. N = 3 biological and N = 2 technical replicates, error bars = SEM (D) MCF-7 cells were cultured with aged and young ASC CM and evaluated for total and phosphorylated AKT, ERK, and ER-α protein expression using WES capillary system. Results show an increase at p-ER ser-167 in MCF-7 cells stimulated with young CM. (E) MCF-7 cells were treated with tamoxifen for 4 h before being treated with either young or aged ASC CM. MCF-7 cells were collected after 24 h for qRT-PCR to evaluate ER regulated gene expression. Results confirm that ASC signaling is mediated by ER-α. N = 3 biological and N = 2 technical replicates, error bars = SEM. ERK, extracellular signal related kinase; IGF-1, insulin like growth factor 1; PGR, progesterone receptor.
Protein expression of mediators of rapid estrogen signaling (ERK and AKT) and ER-α phosphorylation sites S118 and S167 was next evaluated to determine how the ASC secretome converged with ER-α signaling cascades. Following stimulation with CM for 15 min, MCF-7 cells were collected for Western blot analysis. Results revealed an increase at p-ER 167 in MCF-7 cells exposed to young CM, suggesting that ASC signaling is mediated through the ER-α (Fig. 4D). There was no observed activation of p-ER S118 or increased activity of p-AKT S473 or p-ERK Thr202/Tyr204 (Fig. 4D). Due to the suggested activation of p-ER S167 with young condition media, we next sought to determine if the observed increase in ER-α mediated gene signatures (SDF-1, PGR) required the ER-α for activation. To do this, MCF-7 cells were treated with tamoxifen for 4 h before stimulation with young or aged ASC CM. MCF-7 cells were collected after 24 h, and ER response genes were evaluated with qRT-PCR.
Pretreatment with tamoxifen prevented the induction of PGR and SDF-1 in MCF-7 cells treated with young ASC CM, which suggests that the increased PGR gene expression observed in young ASC CM treated MCF-7 cells is mediated by the ER-α (Fig. 4E). To rule out increased estrogen production as an underlying cause for the ER-α activity in young ASC CM stimulated MCF-7 cells, we evaluated the gene expression of hydroxysteroid 17-β dehydrogenase (HSD17) and cytochrome P450 family 19 subfamily A member 1 (CYP19A1) in young and aged ASCs. The comparison of HSD17 and CYP19A1 expression in young and aged ASCs revealed no statistical significance, indicating that elevated ER-α activity is not a response to an increase in estrogen synthesis in stem cells (Supplementary Fig. S2).
ER-α+ BC induced pro-adipogenic differentiation and matrix production in ASCs are independent of donor age
The TME is fluid and results from reciprocal stimuli between cancer and stromal cells. To determine if ASCs were modulated by ER-α+ BC, CM from the MCF-7 BC cell line was used to stimulate ASCs. Following stimulation, ASCs were evaluated for alterations in growth factor and cytokine production. Results demonstrated no significant increase in growth factor or cytokine gene expression in ASCs treated with MCF-7 CM. Moreover, IL-8 was significantly repressed (0.51 ± 0.10) by MCF-7 CM (Fig. 5A, B). Young and aged ASCs responded similarly to ER+ CM with no observed trends in age-dependent alterations to cytokine or growth factor expression (Supplementary Fig. S3A). The loss of response of ASCs to ER-α+ CM was unexpected, as stromal cells such as fibroblasts and adipocytes can acquire a cancer-associated phenotype.
FIG. 5.
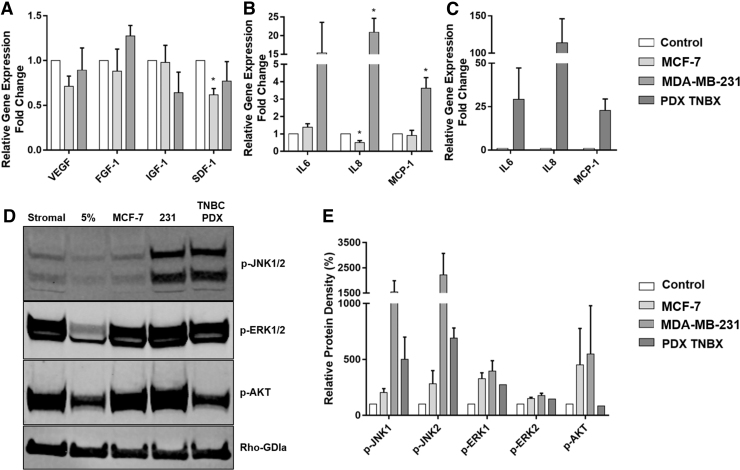
Triple negative breast cancer enhances ASC inflammatory cytokine expression. (A–C) ASCs were stimulated with MCF-7, MDA-MB-231, and TU-BcX-4IC CM for 72 h and harvested for qRT-PCR analysis. Findings show significant P values (*P < 0.05) in SDF-1 and IL-8 expression in MCF-7 stimulated ASCs and IL-8 and MCP-1 expression in MDA-MB-231 stimulated ASCs. N = 3 biological (MCF-7 CM, MDA-MB-231 CM, N = 2 TU-BcX-4IC) and N = 2 technical replicates, error bars = SEM (D, E) ASCs were stimulated with culture medium, MCF-7 CM, or TNBC CM for 30 min and harvested for western blot analysis of selected inflammatory and cytokine pathways. FGF-1, fibroblast growth factor 1; JNK, Janus kinase; VEGF, vascular endothelial growth factor.
To determine if growth factor and cytokine expression in ASCs could be modulated by BC cells of other subtypes, ASCs were exposed to CM from two triple-negative BC cell lines (MDA-MB-231 and primary TU-BcX-4IC) [47]. ASCs stimulated with the TNBC MDA-MB-231 CM had significantly elevated expression of IL-8 (20.89 ± 3.76) and MCP1 (3.63 ± 0.60) in ASCs. IL-6 was also consistently elevated in ASCs with TNBC CM (15.38 ± 8.19); however, donor variability affected significance (Fig. 5A–C). This effect was further amplified with stimulation from primary TNBCs. Both young and aged ASCs demonstrated elevated expression of pro-inflammatory cytokines with TNBC CM. In addition, there was a suggested elevated response in young ASCs, and further studies are required to demonstrate this conclusively (Supplementary Fig. S3B).
To further clarify the observed inflammatory response in ASCs stimulated with TNBC CM, ASCs were stimulated for 30 min with stromal media, 5% media, MCF-7 CM, or TNBC CM, and pathways associated with inflammation and cytokine stimulation were evaluated. Initial pathways downstream of inflammatory stimulation include TLR4 induction of SMAD3, ERK1/2, JNK, AKT, and NF-κB induction of mTORC1. Results demonstrated that classically activated inflammatory cascades TLR4/SMAD3 and NF-κB/mTORC1 were not elevated in ASCs alone or with CM (results not shown). Phosphorylation of both Mitogen-Activated Protein Kinases 8/9 (JNK1/2) was increased in only TNBC lines MDA-MB-231 (1547 ± 443.1 and 2221 ± 854, respectively) and TU-BcX-4IC CMs (Fig. 5D, E; 499.6 ± 141.7 and 690 ± 64.77, respectively). There was no observed difference in JNK activation based on ASC donor age (Supplementary Fig. S3B), suggesting that ASC response to BC stimulation is primarily subtype mediated.
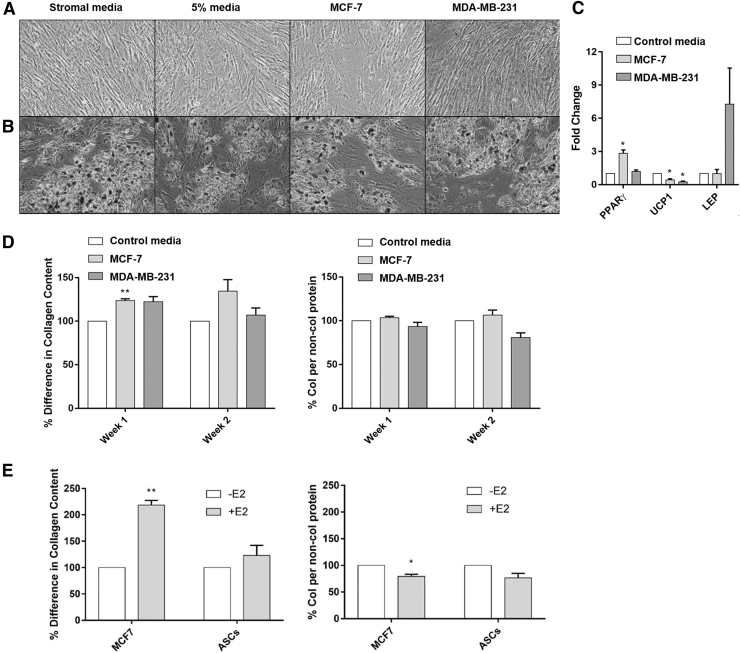
To verify if ASCs are more reactive to pathways that could mediate an inflammatory response such as JNK, young and aged ASCs were stimulated with 10 nm PGE2 for 24 h and expression of inflammatory cytokines was evaluated. Results revealed that young ASCs had significantly (P < 0.05) elevated IL-8 expression compared to aged ASCs, suggesting that young ASCs may be more responsive to the inflammatory mediator PGE2 than aged ASCs (Supplementary Fig. S4). We next sought to determine if BC cell CM regulated ASC adipogenic differentiation. To do this, ASCs were stimulated for 3 days in MCF-7 CM or MDA-MB-231 CM and then differentiated to an adipogenic phenotype for 7 days. Again, unstimulated full stromal media, 5% pretreatment, and unstimulated adipogenic differentiation media were used as controls. Following adipogenic differentiation (Fig. 6A, B), all pretreatments demonstrated equivalent adipogenic potential, and no differences were noted in MCF-7 CM stimulated adipogenesis. Furthermore, CM from TNBC did not enhance adipogenesis. These data suggest that adipogenic potential in ASCs is similarly regulated by BC receptor status.
FIG. 6.
ASC adipogenesis is independent of breast cancer subtype, while fibril ECM production is dependent on breast cancer subtype. (A, B) ASCs were stimulated with MCF-7 and MDA-MB-231CM for 3 days followed by culture in adipogenic medium for 7 days. Oil Red O staining revealed no difference in adipogenesis between cohorts. N = 3 biological replicates and N = 2 technical replicates (C) qRT-PCR analysis of MCF-7 and MDA-MB-231 stimulated ASCs revealed significantly elevated expression (*P < 0.05) of PPAR-γ in MCF-7 CM stimulated ASCs. MCF-7 and MDA-MB-231 stimulated ASCs also exhibited significantly lower expression of UCP-1 compared to the control. N = 3 biological replicates and N = 2 technical replicates, error bars = SEM (D) ASCs were initially stimulated with MCF-7 CM and estrogen, maintained by alternating CM and full culture medium for 2 weeks, and stained with Sirius Red/Fast Green for fibril collagen and noncollagenous protein content. Significantly increased (*P < 0.05) fibril collagen content was observed in MCF-7 CM stimulated ASCs in week 1 but was not observed in TNBC CM stimulated ASCs at either timepoint. (E) Further treatment of MCF-7 cells and ASCs with 17β-estradiol revealed significantly elevated (*P < 0.05; **P < 0.01) expression of fibril collagen in MCF-7 cells only. N = 3 biological and technical replicates, error bars = SEM. LEP, Leptin; PPARγ, peroxisome proliferator activated receptor gamma; UCP1, uncoupling protein 1.
To determine if early transcriptional changes were associated with stem cell adipogenic differentiation (PPARγ, LEP, UCP1), qRT-PCR analysis of ASCs was conducted following 3 days of MCF-7 or TNBC CM stimulation. A significant fold increase (2.84 ± 0.31) was observed in PPARγ mRNA expression following stimulation with MCF-7 CM (Fig. 6C). To determine if the estrogenic activity observed in young ASCs is specific to undifferentiated ASCs, we next induced young and aged ASCs to an adipogenic lineage and evaluated ER mediated gene changes in the MCF-7 cell line. CM was collected from young and aged ASCs that had undergone adipogenic differentiation. MCF-7 cells were treated with aged and young adipocyte CM for 24 h and then evaluated for ER-α mediated gene expression changes. Results demonstrated that there were no significant increases in ER responsive genes in MCF-7 cells treated with young adipocyte CM compared to aged adipocyte CM, suggesting a stem specific effect (Supplementary Fig. S5).
ER status alters fibril ECM production in ASCs over an extended period
To determine if the ER/estrogen BC axis regulates matrix production in ASCs, changes in the production of fibril ECM components were investigated. ASCs were stimulated with MCF-7 CM and estrogen followed by an alternation of CM and full media for 2 weeks. At the 1- and 2-week timepoints, ASCs were stained with a Sirius Red/Fast Green stain to quantify the amount of fibril collagen and noncollagenous protein present. The MCF-7 CM at both timepoints increased the fibril collagen content compared to the 5% treatment (Fig. 6D). MCF-7 CM increased fibril collagen production in week 1 (123.6 ± 2.117) and week 2 (134.4 ± 13.25). In contrast, ASCs stimulated with TNBC CM did not have an observed significant change in collagen production. Further treatment of either ASCs or MCF-7 cells with 17β-estradiol resulted in increased estrogen-stimulated matrix production in only MCF-7 stimulated cells (Fig. 6E); further matrix protein increased independent of increased total protein suggesting that these effects are matrix specific. This suggests that matrix production in ER-α+ tumors may be driven by estrogen mediated effects.
Discussion
The BC microenvironment, defined as a combination of cells within the tumor and its stroma, the ECM, and surrounding signaling molecules [64], plays a pivotal role in directing cell behavior. Strong evidence supporting the significance of the microenvironment on cell behavior is demonstrated in heterochronic parabiosis studies [65]. In recent years, an overwhelming amount of evidence supports the contribution of the TME to BC progression and metastasis [66–73]. Furthermore, studies have shown that the TME actively participates in the progression to hormone independence and endocrine therapy resistance [27,28]. Specific age-related changes to the microenvironment have been linked to tumor progression [74]. Although it is well-documented that specific extracellular matrix gene profiles are associated with the prognosis and treatment of BC, little evidence is known about how local cellular function, specifically the ASC secretome, contributes to endocrine resistance in young ER-α+ BC patients [75–79]. The extent to which ASC age affects BC progression and response to treatment is yet to be fully elucidated.
ASCs are no longer solely used for cell restoration in regenerative medicine but rather for the benefits derived from their secretome. While beneficial in regenerative medicine, these same paracrine actions of ASCs may enable tumor formation, growth, and maintenance. The current study presents evidence indicating that the age-dependent contribution of ASCs to the breast TME is mediated predominantly through their paracrine secretome rather than their independent ECM production. While there were observed differences in ECM gene expression between young and aged ER-α+ tumors, matrix production was not observed to be driven by ASCs. Instead ECM production was primarily enhanced through stimulation of ER-α+ BC cells with estrogen. This finding is in accordance with a recent study from our laboratory where we show that fibril collagen is enriched primarily in ER-α+ breast cancers [80].
The expression of paracrine factors between young and aged BC tumors is strikingly different in our analysis of TCGA data. Elevated expression of the chemokine IL-8 was observed in young ASC CM; however, this was not observed in TCGA data from young ER-α+ BC tumors (Table 2, Fig. 3). In contrast, a significant decrease in IL-8 expression was observed in ER-α+ CM stimulated ASCs, suggesting that ASC inflammatory response in the TME is regulated by ASC age and not necessarily tumor subtype. IL-8, a pro-inflammatory chemokine that promotes neutrophil chemotaxis and degranulation, is linked to a more invasive phenotype by promoting angiogenesis and metastasis, particularly in triple-negative BC subtypes [81]. Our results demonstrate that TNBC CM stimulated ASCs exhibited significantly elevated IL-8 expression (Fig. 5A–C).
Table 2.
Comparison of Young Tumors and Young Adipose-Derived Stromal/Stem Cell Cytokine Expression and Their Role in Wound Healing
| ASC-secreted factor | Role in wound healing | Elevated in young tumors | Elevated in young ASCs |
|---|---|---|---|
| MCP-1 | Macrophage chemoattractant Possible macrophage polarization |
No | Yes |
| CXCL12 | Macrophage polarization | Yes | Yes |
| IL-10 | Anti-inflammatory cytokine | No | Yes |
| IL-8 | Neutrophil recruitment | No | Yes |
| TNF | Conflicting-shown to accelerate vascularization but also delays wound healing | Yes | Yes |
| CCL5 | Macrophage recruitment | Yes | Yes |
ASCs, adipose-derived stromal/stem cells.
While our study demonstrated that IL-8 expression in ASCs is mediated by patient age and TNBC receptor status, others have shown that ASCs coinjected with ER-α+ MCF7 cells have elevated IL-8 [82]. Differences in the aforementioned studies may be due to ASC donor background (BMI, race) or the growth dependent on two- or three-dimensional culture conditions. In addition, IL-8 is associated with drug resistance observed in the ER-α+ MCF-7 cell line [83]. To date, a role for IL-8 and ER-α+ endocrine resistance has not been established for patient age.
The macrophage attracting chemokine CCL5 was also upregulated in young ASCs. Not only has CCL5 been shown to recruit CCR5-expressing macrophages to tumors but also has been shown to promote collagen deposition using macrophages [84–86]. Studies have shown that overexpression of CCL5 in breast tumor cells results in increased invasiveness [87]. SDF-1, a chemokine naturally secreted by human MSCs, is an essential mediator of MSC chemotaxis and primary breast tumorigenesis [87,88]. Furthermore, SDF-1 has been shown to promote hormone-independent growth in the hormone-dependent MCF-7 line [89].
As an ER-α regulated gene, SDF-1 is suggested to play a role in the progression of breast carcinoma cells [90,91]. SDF-1 signaling also contributes to chemotherapy resistance in cancer cells by modulating survival pathways such as AKT, NOTCH, and the BCL-2 family [92,93]. Interestingly, while we saw an increase in SDF-1 in young ASCs and MCF-7 treated with young ASC secretome, we did not observe AKT activation change. The SDF-1 secretion by MSCs has shown variance based on microenvironmental factors, one of which could be age [26,94].
Many of the cytokines observed to be increased in young ASCs (and with stimulation from the TNBC cell line) have a pro-inflammatory role. Specifically, we have demonstrated that IL-8 was significantly elevated in TNBC CM stimulated ASCs (Fig. 5A–C) and that the downstream cytokine signaling pathway, JNK 1/2, had increased phosphorylation in TNBC CM stimulated ASCs (Fig. 5D, E). PGE2 is an upstream activator of the JNK signaling pathway and mediator of IL-8 expression. These findings are suggestive of a possible mechanism, and additional studies are needed to confirm the cytokine responsive pathway specifically activated in young ASCs. Inflammation has a significant role in tumor development. While normal inflammation is self-limiting, neoplasms seem to involve inflammatory profiles in which regulation fails. For example, monocyte chemotactic protein chemokines recruit monocytes that later develop into tumor-associated macrophages (TAMs) [95]. TAMs produce angiogenic growth factors and IL-10 interferons that disrupt the antitumor response by cytotoxic T cells [95].
BC comprises a large cellular array: ASCs, adipocytes, fibroblasts, immune cells, and endothelial cells. The cell infiltration analysis in young and aged breast tumors has revealed significant differences between several immune cell populations, including CD8+ T cells, M2 macrophages, and Th2 cells (Fig. 1B, C). Previous studies have shown that CD8+ T cells, Th1 cells, M1 macrophages, and natural killer cells exhibit antitumor activities, whereas T-regulatory cells and M2 macrophages exhibit immune-inhibitory, pro-tumor activities [96]. Interestingly, M2 macrophage infiltrate was significantly lower in young compared to aged tumors, and CD8+ T cells were significantly higher in young compared to aged tumors. Although Jin and Hu determined that CD8+ T cells are associated with disease-free survival in young BC patients compared to aged, Ali et al. showed that CD8+ T cell infiltrate predicts a more favorable outcome for ER- tumors than ER+ tumors [97,98]. Based on the literature, there is still no conclusive evidence identifying significant biological differences between young and aged BC patients [98].
With discrepancies in immune cell infiltration as prognostic indicators, other stromal cells have been exploited as possible prognostic indicators for BC. Although MSC infiltration in young and aged breast tumors was not significantly different, MSCs may influence age-dependent tumorigenesis alternatively. Rather than directly infiltrating the tumor, it is suggested that MSCs prime the site for tumor cell growth through the secretion of high levels of multiple growth factors, cytokines, and chemokines [89]. Younger patients may have MSCs that secrete higher volumes of pro-tumorigenic factors than those aged, thus resulting in poor prognoses in young patients with specific subtypes, including ER-α+ and TNBC. Further studies evaluating breast tumor derived MSCs will be needed to validate this hypothesis.
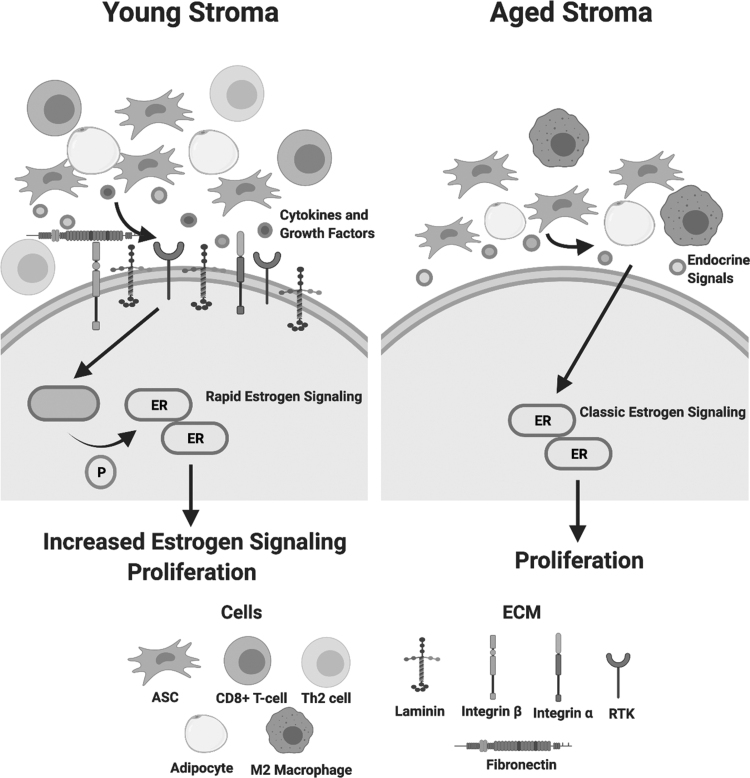
Patient age has been shown to correlate with stem cell potency, inflammatory secretion, and differentiation capacity [31,99,100]. Studies on bone marrow-derived stem cells noted that aged stem cells lacked receptors for cytokines and chemokines, including TNFR. The loss of these receptors resulted in an inability to respond to immune signals from the surrounding environment [101]. Together this suggests that young stroma, specifically young ASCs, may be more reactive to pro-inflammatory signals in a tumor environment than aged stroma (Fig. 7). Our preliminary results are in accordance with these studies as we show that young ASCs have elevated IL-8 expression following PGE2 treatment, while aged ASCs do not have an increase in IL-8 (Supplementary Fig. S4). Prior studies evaluating differences in young and aged ASCs demonstrate that young ASCs have increased levels of pro-inflammatory signaling (MCP1, IL-8, TNF) and adipogenic markers (PPARγ) compared to aged ASCs [102].
FIG. 7.
Young ER-α+ breast tumor stroma exhibits significant differences in specific matrix components and immune cell types. Young tumor stroma (<45 years old) revealed significantly higher levels of matrix components (fibronectin, integrin α and β, collagen III, collagen V, collagen VI, collagen VII, and laminins A, B, and C) and immune cell types (CD8+ T cells and TH2 cells) (*P < 0.05). Aged tumor stroma (>65 years old) revealed a significantly higher M2 macrophage infiltrate (*P < 0.05) compared to young stroma. The young and aged tumor stroma exhibit comparable numbers for MSC and adipocyte infiltrate; however, young MSCs may exhibit a greater potency and stimulate a rapid estrogen signaling pathway through cytokine and growth factor secretions. The activation of ER-α through ser-167 phosphorylation by another signaling pathway may be an underlying factor for the increased ER-α signaling and increased proliferation observed in MCF-7 cells stimulated with young CM compared to those stimulated with aged CM. Created with Biorender.com
Conclusion
This study has observed that the ASC secretome enhances estrogen signaling in the ER-α+ MCF-7 cell line. However, we also observed that the ER-α+ MCF-7 cell line does not robustly stimulate ASCs. Data suggest that the tumor-stromal response is more driven by BC subtype than patient age. Data also suggest that an age-dependent effect in cytokine expression occurs with TNBC CM stimulation, but more replicates will be needed to validate this observation. Further investigation of the role of stromal age on ER-α+ estrogen signaling will require expansion to other ER-α+ BC cell lines and in vivo murine xenograft studies.
Supplementary Material
Acknowledgment
The authors thank Dr. Jeffrey Gimble for providing valuable feedback in the writing of this article.
Disclaimer
The content is solely the authors' responsibility and does not necessarily represent the official views of the National Institutes of Health.
Young and aged ER+ breast tumor comparison is in whole or part based upon data generated by TCGA Research Network: https://www.cancer.gov/tcga
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This publication was supported by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. This publication was also supported by funding from the NIH-RCMI grant No. 2U54MD007595 from the National Institute on Minority Health and Health Disparities.
Supplementary Material
References
- 1. Marino M, Galluzzo P and Ascenzi P. (2006). Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 7:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Cancer Society. (2020). Cancer Facts & Figures 2020. American Cancer Society, Atlanta, GA. [Google Scholar]
- 3. Breast Cancer Facts and Figures 2017–2018. American Cancer Society, Atlanta, GA. [Google Scholar]
- 4. Siegel RL, Miller KD and Jemal A. (2019). Cancer statistics, 2019. CA Cancer J Clin 69:7–34. [DOI] [PubMed] [Google Scholar]
- 5. SEER*Explorer: an interactive website for SEER cancer statistics. Surveillance Research Program, National Cancer Institute. [Google Scholar]
- 6. Haque MM and Desai KV. (2019). Pathways to endocrine therapy resistance in breast cancer. Front Endocrinol 10:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camidge DR, Pao W and Sequist LV. (2014). Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 11:473–481. [DOI] [PubMed] [Google Scholar]
- 8. Hanker AB, Brewer MR, Sheehan JH, Koch JP, Sliwoski GR, Nagy R, Lanman R, Berger MF, Hyman DM, et al. (2017). An acquired HER2(T798I) gatekeeper mutation induces resistance to neratinib in a patient with HER2 mutant-driven breast cancer. Cancer Discov 7:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin JJ, Riely GJ and Shaw AT. (2017). Targeting ALK: precision medicine takes on drug resistance. Cancer Discov 7:137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG and Varmus H. (2005). Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russo M, Misale S, Wei G, Siravegna G, Crisafulli G, Lazzari L, Corti G, Rospo G, Novara L, et al. (2016). Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discov 6:36–44. [DOI] [PubMed] [Google Scholar]
- 12. Arteaga CL and Engelman JA. (2014). ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25:282–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cocco E, Schram AM, Kulick A, Misale S, Won HH, Yaeger R, Razavi P, Ptashkin R, Hechtman JF, et al. (2019). Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med 25:1422–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, Chodon T, Guo R, Johnson DB, et al. (2014). Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 4:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuentes N and Silveyra P. (2019). Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 116:135–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klinge CM. (2001). Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marino M, Pallottini V and Trentalance A. (1998). Estrogens cause rapid activation of IP3-PKC-alpha signal transduction pathway in HEPG2 cells. Biochem Biophys Res Commun 245:254–258. [DOI] [PubMed] [Google Scholar]
- 18. Dos Santos EG, Dieudonne MN, Pecquery R, Le Moal V, Giudicelli Y and Lacasa D. (2002). Rapid nongenomic E2 effects on p42/p44 MAPK, activator protein-1, and cAMP response element binding protein in rat white adipocytes. Endocrinology 143:930–940. [DOI] [PubMed] [Google Scholar]
- 19. Watters JJ, Campbell JS, Cunningham MJ, Krebs EG and Dorsa DM. (1997). Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology 138:4030–4033. [DOI] [PubMed] [Google Scholar]
- 20. Marino M, Acconcia F and Trentalance A. (2003). Biphasic estradiol-induced AKT phosphorylation is modulated by PTEN via MAP kinase in HepG2 cells. Mol Biol Cell 14:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu Q and Moss RL. (1996). 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci 16:3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Picotto G, Massheimer V and Boland R. (1996). Acute stimulation of intestinal cell calcium influx induced by 17 beta-estradiol via the cAMP messenger system. Mol Cell Endocrinol 119:129–134. [DOI] [PubMed] [Google Scholar]
- 23. Bennesch MA and Picard D. (2015). Minireview: tipping the balance: ligand-independent activation of steroid receptors. Mol Endocrinol 29:349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fletcher SJ, Sacca PA, Pistone-Creydt M, Coló FA, Serra MF, Santino FE, Sasso CV, Lopez-Fontana CM, Carón RW, Calvo JC and Pistone-Creydt V. (2017). Human breast adipose tissue: characterization of factors that change during tumor progression in human breast cancer. J Exp Clin Cancer Res 36:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeh WL, Tsai CF and Chen DR. (2017). Peri-foci adipose-derived stem cells promote chemoresistance in breast cancer. Stem Cell Res Ther 8:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhowmick NA, Neilson EG and Moses HL. (2004). Stromal fibroblasts in cancer initiation and progression. Nature 432:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanahan D and Weinberg RA. (2000). The hallmarks of cancer. Cell 100:57–70. [DOI] [PubMed] [Google Scholar]
- 28. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL and Weinberg RA. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348. [DOI] [PubMed] [Google Scholar]
- 29. Recouvreux S, Sampayo R, Bessone MI and Simian M. (2015). Microenvironment and endocrine resistance in breast cancer: friend or foe? World J Clin Oncol 6:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dvorak HF. (1986). Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659. [DOI] [PubMed] [Google Scholar]
- 31. Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, et al. (2009). Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis 30:589–597. [DOI] [PubMed] [Google Scholar]
- 32. Hanahan D and Weinberg RA. (2011). Hallmarks of cancer: the next generation. Cell 144:646–674. [DOI] [PubMed] [Google Scholar]
- 33. Prockop DJ and Oh JY. (2012). Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther 20:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharpless NE and DePinho RA. (2007). How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8:703–713. [DOI] [PubMed] [Google Scholar]
- 35. Lähteenvuo J and Rosenzweig A. (2012). Effects of aging on angiogenesis. Circ Res 110:1252–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Donato AJ, Black AD, Jablonski KL, Gano LB and Seals DR. (2008). Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. [DOI] [PubMed] [Google Scholar]
- 38. Lee CC, Ye F and Tarantal AF. (2006). Comparison of growth and differentiation of fetal and adult rhesus monkey mesenchymal stem cells. Stem Cells Dev 15:209–220. [DOI] [PubMed] [Google Scholar]
- 39. Choudhery MS, Badowski M, Muise A and Harris DT. (2013). Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy 15:330–343. [DOI] [PubMed] [Google Scholar]
- 40. Choudhery MS, Badowski M, Muise A, Pierce J and Harris DT. (2014). Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freese KE, Kokai L, Edwards RP, Philips BJ, Sheikh MA, Kelley J, Comerci J, Marra KG, Rubin JP and Linkov F. (2015). Adipose-derived stems cells and their role in human cancer development, growth, progression, and metastasis: a systematic review. Cancer Res 75:1161–1168. [DOI] [PubMed] [Google Scholar]
- 42. Nowicka A, Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, Broaddus R, Kolonin M, Mok SC, et al. (2013). Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One 8:e81859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Razmkhah M, Jaberipour M, Hosseini A, Safaei A, Khalatbari B and Ghaderi A. (2010). Expression profile of IL-8 and growth factors in breast cancer cells and adipose-derived stem cells (ASCs) isolated from breast carcinoma. Cell Immunol 265:80–85. [DOI] [PubMed] [Google Scholar]
- 44. Eterno V, Zambelli A, Pavesi L, Villani L, Zanini V, Petrolo G, Manera S, Tuscano A and Amato A. (2014). Adipose-derived mesenchymal stem cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget 5:613–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parsons AM, Ciombor DM, Liu PY and Darling EM. (2018). Regenerative potential and inflammation-induced secretion profile of human adipose-derived stromal vascular cells are influenced by donor variability and prior breast cancer diagnosis. Stem cell rev Rep 14:546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prieto Gonzalez E. (2019). Heterogeneity in adipose stem cells In: Stem Cells Heterogeneity-Novel Concepts. Birbrair A, ed. Springer International Publishing: Nature Switzerland AG, pp 119–150. [Google Scholar]
- 47. Matossian MD, Chang T, Wright MK, Burks HE, Elliott S, Sabol RA, Wathieu H, Windsor GO, Alzoubi MS, et al. (2022). In-depth characterization of a new patient-derived xenograft model for metaplastic breast carcinoma to identify viable biologic targets and patterns of matrix evolution within rare tumor types. Clin Transl Oncol 24:127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bunnell BA, Flaat M, Gagliardi C, Patel B and Ripoll C. (2008). Adipose-derived stem cells: isolation, expansion and differentiation. Methods 45:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grossman RL, Heath AP, Ferretti V, Varmus HE, Lowy DR, Kibbe WA and Staudt LM. (2016). Toward a Shared Vision for Cancer Genomic Data. N Engl J Med 375:1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dennis G Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC and Lempicki RA. (2003). DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4:P3. [PubMed] [Google Scholar]
- 51. Huang da W, Sherman BT and Lempicki RA. (2009). Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang da W, Sherman BT and Lempicki RA. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. [DOI] [PubMed] [Google Scholar]
- 53. Jézéquel P, Frénel JS, Campion L, Guérin-Charbonnel C, Gouraud W, Ricolleau G and Campone M. (2013). bc-GenExMiner 3.0: new mining module computes breast cancer gene expression correlation analyses. Database (Oxford) 2013:bas060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nagy Á, A Lánczky, Menyhárt O and Győrffy B. (2018). Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep 8:9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aran D, Hu Z and Butte AJ. (2017). xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol 18:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N and Perou CM. (2011). Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 29:e18–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, et al. (2008). Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26:3324–3330. [DOI] [PubMed] [Google Scholar]
- 58. Partridge AH, Goldhirsch A, Gelber S, et al. (2010). Breast cancer in young women. In: Diseases of the Breast. ed 4th. Harris JR, ME Lippman, CE Osborne, et al., eds. Philadelphia, PA: Lippincott Williams & Wilkins, pp 1073–1082. [Google Scholar]
- 59. Goldman M, Craft B, Swatloski T, Ellrott K, Cline M, Diekhans M, Ma S, Wilks C, Stuart J, Haussler D and Zhu J. (2013). The UCSC Cancer Genomics Browser: update 2013. Nucleic Acids Res 41:D949–D954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sanborn JZ, Benz SC, Craft B, Szeto C, Kober KM, Meyer L, Vaske CJ, Goldman M, Smith KE, et al. (2011). The UCSC Cancer Genomics Browser: update 2011. Nucleic Acids Res 39:D951–D959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vaske CJ, Benz SC, Sanborn JZ, Earl D, Szeto C, Zhu J, Haussler D and Stuart JM. (2010). Inference of patient-specific pathway activities from multi-dimensional cancer genomics data using PARADIGM. Bioinformatics 26:i237–i245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rhodes LV, Antoon JW, Muir SE, Elliott S, Beckman BS and Burow ME. (2010). Effects of human mesenchymal stem cells on ER-positive human breast carcinoma cells mediated through ER-SDF-1/CXCR4 crosstalk. Mol Cancer 9:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Renema N, Navet B, Heymann MF, Lezot F and Heymann D. (2016). RANK-RANKL signalling in cancer. Biosci Rep 36:e00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC and Jung JP. (2018). Engineering breast cancer microenvironments and 3D bioprinting. Front Bioeng Biotechnol 6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Conboy IM and Rando TA. (2012). Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle 11:2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, et al. (2011). Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1:54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gilbert LA and Hemann MT. (2010). DNA damage-mediated induction of a chemoresistant niche. Cell 143:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Condeelis J and Pollard JW. (2006). Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124:263–266. [DOI] [PubMed] [Google Scholar]
- 69. Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E and Joyce JA. (2011). Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev 25:2465–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM and Karin M. (2011). Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Oskarsson T. (2013). Extracellular matrix components in breast cancer progression and metastasis. Breast 22 Suppl 2:S66–S72. [DOI] [PubMed] [Google Scholar]
- 72. Insua-Rodríguez J and Oskarsson T. (2016). The extracellular matrix in breast cancer. Adv Drug Deliv Rev 97:41–55. [DOI] [PubMed] [Google Scholar]
- 73. Lu P, Weaver VM and Werb Z. (2012). The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sprenger CC, Plymate SR and Reed MJ. (2010). Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int J Cancer 127:2739–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Helleman J, Jansen MP, Ruigrok-Ritstier K, van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn JG, Sleijfer S, Foekens JA and Berns EM. (2008). Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clin Cancer Res 14:5555–5564. [DOI] [PubMed] [Google Scholar]
- 76. van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, et al. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536. [DOI] [PubMed] [Google Scholar]
- 77. Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, et al. (2005). Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365:671–679. [DOI] [PubMed] [Google Scholar]
- 78. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, et al. (2000). Molecular portraits of human breast tumours. Nature 406:747–752. [DOI] [PubMed] [Google Scholar]
- 79. Bergamaschi A, Tagliabue E, Sørlie T, Naume B, Triulzi T, Orlandi R, Russnes HG, Nesland JM, Tammi R, et al. (2008). Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol 214:357–367. [DOI] [PubMed] [Google Scholar]
- 80. Byrne CE, Decombe J-B, Bingham GC, Remont J, Miller LG, Khalif L, King CT, Hamel K, Bunnell BA, Burow ME and Martin EC. (2021). Evaluation of extracellular matrix composition to improve breast cancer modeling. Tissue Eng Part A 27:500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C and Huang R-P. (2004). Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer 109:507–515. [DOI] [PubMed] [Google Scholar]
- 82. Wang Y, Liu J, Jiang Q, Deng J, Xu F, Chen X, Cheng F, Zhang Y, Yao Y, et al. (2017). Human adipose-derived mesenchymal stem cell-secreted CXCL1 and CXCL8 facilitate breast tumor growth By promoting angiogenesis. Stem Cells 35:2060–2070. [DOI] [PubMed] [Google Scholar]
- 83. Shi Z, Yang W-M, Chen L-P, Yang D-H, Zhou Q, Zhu J, Chen J-J, Huang R-C, Chen Z-S and Huang R-P. (2012). Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat 135:737–747. [DOI] [PubMed] [Google Scholar]
- 84. Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, Shenoy A, Bassat E, Halpern Z, et al. (2016). Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med 213:2315–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mantovani A, Marchesi F, Malesci A, Laghi L and Allavena P. (2017). Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Walens A, DiMarco AV, Lupo R, Kroger BR, Damrauer JS and Alvarez JV. (2019). CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. Elife 8:e43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mira E, Lacalle RA, González MA, Gómez-Moutón C, Abad JL, Bernad A, Martínez AC and Mañes S. (2001). A role for chemokine receptor transactivation in growth factor signaling. EMBO Rep 2:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Orimo A and Weinberg RA. (2006). Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5:1597–1601. [DOI] [PubMed] [Google Scholar]
- 89. Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, Tilghman SL, Salvo VA, Fonseca JP, et al. (2010). Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat 121:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hall JM and Korach KS. (2003). Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol 17:792–803. [DOI] [PubMed] [Google Scholar]
- 91. Sauvé K, Lepage J, Sanchez M, Heveker N and Tremblay A. (2009). Positive feedback activation of estrogen receptors by the CXCL12-CXCR4 pathway. Cancer Res 69:5793–5800. [DOI] [PubMed] [Google Scholar]
- 92. Cristino S, Piacentini A, Manferdini C, Codeluppi K, Grassi F, Facchini A and Lisignoli G. (2008). Expression of CXC chemokines and their receptors is modulated during chondrogenic differentiation of human mesenchymal stem cells grown in three-dimensional scaffold: evidence in native cartilage. Tissue Eng Part A 14:97–105. [DOI] [PubMed] [Google Scholar]
- 93. Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ and Heeschen C. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1:313–323. [DOI] [PubMed] [Google Scholar]
- 94. Corcoran KE, Trzaska KA, Fernandes H, Bryan M, Taborga M, Srinivas V, Packman K, Patel PS and Rameshwar P. (2008). Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS One 3:e2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Coussens LM and Werb Z. (2002). Inflammation and cancer. Nature 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pruneri G, Vingiani A and Denkert C. (2018). Tumor infiltrating lymphocytes in early breast cancer. Breast 37:207–214. [DOI] [PubMed] [Google Scholar]
- 97. Ali HR, Chlon L, Pharoah PD, Markowetz F and Caldas C. (2016). Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLoS Med 13:e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jin YW and Hu P. (2020). Tumor-infiltrating CD8 T cells predict clinical breast cancer outcomes in young women. Cancers (Basel) 12:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pandey AC, Semon JA, Kaushal D, O'Sullivan RP, Glowacki J, Gimble JM and Bunnell BA. (2011). MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res Ther 2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schipper BM, Marra KG, Zhang W, Donnenberg AD and Rubin JP. (2008). Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 60:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ, Kaminski N, et al. (2014). Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med 189:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Antoon JW, Nitzchke AM, Martin EC, Rhodes LV, Nam S, Wadsworth S, Salvo VA, Elliott S, Collins-Burow B, Nephew KP and Burow ME. (2013). Inhibition of p38 mitogen-activated protein kinase alters microRNA expression and reverses epithelial-to-mesenchymal transition. Int J Oncol 42:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.