Abstract
Helicobacter pylori persists in the human stomach despite eliciting both cellular and humoral immune responses and inducing proinflammatory cytokines. To determine whether local humoral and cytokine responses are related to each other and to histologic responses, we studied 66 Japanese patients who underwent gastroscopy. Using specific enzyme-linked immunosorbent assays, we examined gastric antral mucosal-organ biopsy culture supernatants to assess interleukin-6 (IL-6) and interleukin-8 (IL-8) levels and antibody responses to H. pylori whole-cell antigens CagA, HspA, and HspB. Of the patients studied, 11 were H. pylori negative and 55 were H. pylori positive; by PCR, all strains were cagA+. As expected, compared to H. pylori-negative patients, H. pylori-positive patients had significantly higher humoral responses to all H. pylori antigens and had higher IL-8 (47.8 ± 3.5 versus 10.1 ± 4.3 ng/mg of biopsy protein; P < 0.001) and IL-6 levels (2.8 ± 0.3 versus 0.26 ± 0.2 ng/mg of protein; P < 0.001). Among the H. pylori-positive patients, supernatant anti-CagA immunoglobulin G (IgG) levels were significantly associated with H. pylori density (P < 0.005) and neutrophil infiltration (P < 0.005) scores. Anti-CagA immunoglobulin A levels were correlated with intestinal metaplasia (P < 0.05). Mononuclear cell infiltration scores were significantly associated with supernatant IL-6 levels (P < 0.005) and with IgG responses to whole-cell antigens (P < 0.05). Supernatant IL-8 levels were significantly associated with anti-CagA IgG (r = 0.75, P < 0.001). Anti-CagA responses correlated with neutrophil infiltration, intestinal metaplasia, H. pylori density, and IL-8 levels, suggesting that the absolute levels of these antibodies may be markers for gastric inflammation and premalignant changes in individual hosts.
Although essentially all colonized hosts have tissue responses to Helicobacter pylori (7, 19, 21), understanding of the mechanisms involved is not well established. Neutrophils may be present in both the epithelial cell layer and underlying lamina propria, and lymphocyte, macrophage, eosinophil, and plasma cell populations in the lamina propria are increased compared to those in H. pylori-negative persons (19). H. pylori produces chemotactic factors that attract neutrophils and mononuclear cells (17, 43, 49) and stimulate the production of chemoattractants from gastric epithelial cells (13, 18).
Among the cytokines present in the gastric mucosa of H. pylori-positive persons, interleukin-8 (IL-8) recruits and activates neutrophils (64), whereas interleukin-6 (IL-6) stimulates lymphocyte and macrophage function (32). Compared with persons not colonized by H. pylori, H. pylori-positive patients have elevated gastric mucosal IL-6 and IL-8 activity, as determined using tissue homogenates and in vitro organ culture (1, 2, 15, 25).
One H. pylori characteristic that has been linked to more intensive tissue responses is the high-molecular-mass (120- to 140-kDa) CagA protein (14, 53). Encoded by cagA (11, 65) and recognized by serum antibodies in persons carrying cagA+ strains (12, 16), serum and mucosal antibodies to CagA are significantly more prevalent among patients with peptic ulceration than among those with gastritis alone (9, 12, 16). Colonization by cagA+ strains induces more intense cellular infiltration in the gastric mucosa (35, 53) and increases the risk of development of atrophic gastritis (5, 37) and gastric cancer (8, 52).
Like other bacteria, H. pylori possesses highly conserved heat-shock proteins (chaparonins) that resemble homologous molecules in human cells (47). Heat-shock protein B (HspB) is a GroEL homolog with a molecular mass of 58 kDa (22, 41), to which virtually all H. pylori-positive persons produce a serum antibody response (55). H. pylori also possesses a GroES homolog (HspA) that has an H. pylori-specific carboxyl terminus. Both hspA and hspB, encoding HspA and HspB, respectively, have been cloned, expressed as fusion proteins with the maltose-binding protein (MBP), and purified in large scale. The MBP-HspA and MBP-HspB fusions are antigenically intact, yet preliminary studies have shown that the MBP-HspA fusion is recognized by sera from only about 40% of H. pylori-positive patients (30, 48, 55, 63).
We sought to determine whether there is a relationship between local gastric humoral immune responses, cytokine production, and histological parameters. To evaluate this association, we examined gastric antral mucosal-organ (biopsy) culture supernatants to assess immunoglobulin A (IgA) and immunoglobulin G (IgG) levels to H. pylori whole-cell antigens (WCA) and CagA, and IgG levels to HspA and HspB, in addition to levels of the cytokines IL-6 and IL-8. We hypothesized that local immune responses might affect the intensity of local cytokine production (as measured by IL-8 and IL-6 levels), reflect the intensity of the mucosal cellular infiltration and the H. pylori density, or both.
MATERIALS AND METHODS
Study groups and biopsies.
Sixty-six consecutive patients undergoing diagnostic upper gastrointestinal endoscopy (Q20 or Q200; Olympus, Tokyo, Japan) at Nagoya University Hospital were enrolled in this study. The indications for endoscopy in these patients were abdominal pain or discomfort, vomiting, and hematemesis. All endoscopies were done by the same endoscopist. Patients were considered to have duodenal ulcer (DU), gastric ulcer (GU), or no ulcer based on endoscopic findings (Table 1). There was no overlap with the patients in our previous studies (1, 2). The ulcer group was defined as patients having a circumscribed break in the mucosa in the duodenum (i.e., a DU) or in the stomach (i.e., a GU) with apparent depth covered by an exudate, as previously described (3, 53). None of these patients had taken nonsteroidal anti-inflammatory drugs, proton pump inhibitors, antibiotics, or bismuth compounds in the preceding 3 months. At the time of endoscopy, three biopsy specimens were obtained from adjacent areas of the gastric antrum with an Olympus biopsy forceps (FB-24KR [cap size, 6 mm]). When each biopsy specimen was taken, the forceps were fully opened and aimed at right angles to the gastric lumen to the extent possible to obtain uniformly sized biopsies. One biopsy each was used for bacterial culture of H. pylori, routine histological examination (hematoxylin-eosin and immunohistochemical analysis with anti-H. pylori serum), and in vitro organ culture. Biopsies were obtained from endoscopically intact mucosa distant from focal lesions such as ulcers and erosions. Samples were obtained with informed consent from all subjects in accordance with the Helsinki Declaration.
TABLE 1.
Characteristics of study population
| Endoscopic finding | No. of patients | % Male | Age (yr) (mean ± SD) | Histological scorea
|
|||
|---|---|---|---|---|---|---|---|
| Mononuclear cell infiltration | Polymorphonuclear cell infiltration | Atrophy | Metaplasia | ||||
| H. pylori-positive cases | |||||||
| Duodenal ulcer | 24 | 75 | 44 ± 8 | 2.29 ± 0.75c | 2.04 ± 0.81c | 0.88 ± 0.90 | 0.46 ± 0.72 |
| Gastric ulcer | 14 | 79 | 50 ± 11 | 1.93 ± 0.92c | 1.14 ± 1.03 | 1.71 ± 1.07d | 1.43 ± 1.16d |
| Non-ulcer | 17 | 41 | 49 ± 10 | 1.06 ± 0.90 | 0.82 ± 1.07 | 1.41 ± 0.94 | 0.65 ± 1.06 |
| All | 55 | 65 | 47 ± 9 | 1.82 ± 0.98 | 1.44 ± 1.08 | 1.25 ± 1.00 | 0.76 ± 1.02 |
| H. pylori-negative casesb | 11 | 55 | 44 ± 7 | 0.64 ± 0.81 | 0.82 ± 1.08e | 0.64 ± 0.92e | 0.27 ± 0.65e |
Histologic scores were assessed using the Sydney System (53); scores shown are means ± standard deviations.
All H. pylori-negative patients had NUD.
P < 0.05, compared with non-ulcer cases.
P < 0.05, compared with DU or non-ulcer cases.
P < 0.05, compared with the 55 H. pylori-positive cases.
Assessment of H. pylori status.
The H. pylori status of patients was determined by bacterial culture, identification of the organism in tissue sections using immunohistochemical analysis, and [13C]urea breath test (UBT) (33). Biopsy specimens were homogenized with a glass rod and incubated on brucella 10% newborn calf serum agar plates (BS agar; Intergen) for 5 to 7 days at 37°C in a 5% CO2 atmosphere. One colony was picked and streaked for isolation on a BS agar plate for 3 days. H. pylori colonies were identified by Gram staining, catalase, oxidase, and urease testing. The UBT was performed with 100 mg of [13C]urea. Breath samples were collected before the test meal was administered and again 20 and 40 min after ingestion of the urea. The ratio of 13CO2 to 12CO2 was measured by isotope ratio mass spectrometry, and a δ 13CO2 value of >5 per mil was considered positive for H. pylori. H. pylori-positive patients were defined by positive results in at least two of the diagnostic methods. H. pylori-negative patients had negative results in all three assays (histology, culture, and UBT) for H. pylori. A chloroform-phenol extraction method was used to obtain DNA from the H. pylori isolates as previously described (24). Analysis of the presence of cagA was done by PCR, using primers 5′-GATAACAGGCAAGCTTTTGAGG-3′ and 5′-CTGCAAAAGATTGTTTGGCAGA-3′, as previously described (64).
Immunohistochemical analysis for H. pylori colonization.
Antiserum to H. pylori was raised by injecting formalin-fixed H. pylori NCTC strain 11637 (5 × 109 bacteria) into the auricular vein of a rabbit 10 times every 4 to 6 days. Formalin-fixed biopsy tissues were incubated with a 1:10,000 dilution of the anti-H. pylori serum, washed, incubated with peroxidase-conjugated goat anti-rabbit immunoglobulins (Dakopatts, Glostrup, Denmark), and developed with 0.03% diaminobenzidine containing 10 mM H2O2. The specificity of this antiserum was confirmed by absorption with clinical H. pylori isolates.
Histology.
Neutrophil infiltration (activity), mononuclear cell infiltration, glandular atrophy, intestinal metaplasia, and H. pylori density were assessed on a scale of 0 to 3 corresponding to none, mild, moderate, and severe according to the Sydney System (20, 57), using formalin-fixed biopsy tissues stained with hematoxylin-eosin. All histologic evaluations were performed by one pathologist without knowledge of results from endoscopic diagnosis, 13C-labeled UBT, or serological tests.
Gastric biopsy culture.
Gastric antral mucosal biopsy tissues were weighed and cultured on a culture insert (Falcon, Oxnard, Calif.) over wells containing RPMI 1640 medium with 5% heat-inactivated fetal calf serum–HEPES buffer–100 U of penicillin G per ml–100 mg of streptomycin per ml (1.0 ml of medium/10 mg of tissue) in a 5% CO2 incubator for 24 h (38). Biopsies were positioned on the insert with mucosal surfaces up. At the conclusion of the incubation, the culture supernatant was collected from the wells, sterilized by passage through a 0.22-μm-pore-size filter, and stored at −70°C, until assayed for antibody, IL-8, and IL-6 levels. Biopsy tissues were homogenized in 1.0 ml of 3.3 mM CaCl2, and total protein was assayed by a modified Lowry method (56) to standardize cytokine and antibody results.
IL-8 and IL-6 assays.
Levels of IL-8 and IL-6 in the culture supernatant were assayed in duplicate using specific enzyme-linked immunosorbent assays (ELISA) (TFB, Tokyo, Japan) according to the manufacturer's instructions; the lower limits of detection were 3.0 pg/ml for IL-8 and 4.0 pg/ml for IL-6. The amounts of IL-8 and IL-6 in the organ cultures were expressed as nanograms per milligram of biopsy protein (1, 2).
Detection of antibodies.
Assay for H. pylori-specific IgG and IgA in the biopsy culture supernatants was performed using an ELISA, as previously described (54), with minor modifications. After preliminary checkerboard experiments, a 1:25 dilution was found to be the optimal dilution for subsequent antibody assays. Culture supernatants were diluted 1:25 in phosphate-buffered saline (PBS), incubated 1 h in a microtiter plate well containing 1.0 μg of sonicated pooled H. pylori WCA prepared as previously described (51, 54). Goat anti-human IgG and IgA were used at 1:4,000 and 1:2,000 dilutions, respectively. Color was developed, and optical densities were read as previously described (54). ELISA to detect anti-CagA IgG and IgA was performed using recombinant CagA antigen as previously described (8), with minor modifications. Culture supernatants were diluted 1:25 in PBS, incubated 1 h in a microtiter plate well containing 250 ng of recombinant CagA, and subsequently incubated with goat anti-human IgG (1:4,000) or goat anti-human IgA (1:2,000). The results for each sample are expressed as the ratio of the optical density (ODR) value of the sample to the four positive control sera (51), and normalized for biopsy protein (ODR per milligram of protein). Antibody responses to HspA and HspB of H. pylori were measured as previously described (55). In brief, antigen was purified from Escherichia coli strain MC1061 expressing HspA or HspB as a fusion protein with MBP (HspA-MBP or HspB-MBP) using large-scale amylose affinity. Purified MBP alone was used as a control antigen. For both fusion proteins, the optimal antigen concentration was 125 ng/well, and MBP alone was used at a concentration of 62.5 ng/well, as previously described. Culture supernatants were diluted 1:20 in PBS and incubated 1 h, and goat anti-human IgG was used at 1:4,000.
Statistical analysis.
Statistical analysis was performed by χ2, Fisher's exact, paired-T, or Mann-Whitney U tests, depending on the characteristics of the data set of concern. Variables with a P value of <0.15 on bivariate analysis were entered into a multivariate logistic regression model. A P value of <0.05 was considered statistically significant.
RESULTS
Assessment of H. pylori and cagA status of the study subjects.
In total, 66 patients were studied; 37 (67%) were male. Of the 66 patients, 55 (84%) were found to be H. pylori positive (Table 1). By definition, patients were defined as H. pylori positive if at least two diagnostic assays were positive. The sensitivity for H. pylori detection for UBT was 100%; that for histology was 94.3%, and that for bacterial culture was 92.5%. Of the 55 H. pylori-positive patients, 38 had peptic ulcer disease (GU, 14; DU, 24). All H. pylori isolates were found to be cagA+ by PCR. Each of the 11 H. pylori-negative patients had nonulcer dyspepsia (NUD). There were no clinical findings consistent with the presence of H. pylori.
Histological findings.
We first compared the intensity of antral histological findings (infiltration with mononuclear or polymorphonuclear cells, atrophy, and metaplasia) among H. pylori-positive and -negative patients (Table 1). The H. pylori-positive patients showed significantly higher scores than did the H. pylori-negative patients for all four histological features (Table 1). The 24 patients with DU disease had significantly higher mononuclear and polymorphonuclear cell infiltration scores than did the 17 patients with NUD. GU patients had higher atrophy and metaplasia scores than either DU or NUD patients and higher scores for mononuclear cell infiltration than NUD patients. No significant differences in mononuclear cell infiltration were observed between DU and GU patients.
Gastric biopsy culture antibody levels.
Next we examined the levels of antibodies present in the gastric biopsy culture supernatants from this same group of patients. As expected, all antibody responses to H. pylori antigens were significantly higher in H. pylori-positive patients than in H. pylori-negative patients (Table 2). However, there were no significant differences in any of the measured levels of antibodies in culture supernatants among H. pylori-positive patients with differing endoscopic diagnoses.
TABLE 2.
Gastric biopsy organ culture antibodies to H. pylori antigens in 66 Japanese patients, by patient H. pylori status and endoscopic findings
| Endoscopic finding | No. of patients | Level of antibody toa:
|
|||||
|---|---|---|---|---|---|---|---|
| WCA
|
CagA
|
HspA (IgG) | HspB (IgG) | ||||
| IgG | IgA | IgG | IgA | ||||
| H. pylori-positive cases | |||||||
| DU | 24 | 1.55 ± 1.84 | 0.94 ± 0.85 | 0.36 ± 0.28 | 0.15 ± 0.12 | 0.08 ± 0.25 | 0.22 ± 0.46 |
| GU | 14 | 1.69 ± 2.90 | 1.79 ± 2.51 | 0.23 ± 0.26 | 0.26 ± 0.37 | 0.18 ± 0.58 | 0.20 ± 0.40 |
| Nonulcer | 17 | 0.75 ± 2.59 | 0.68 ± 1.48 | 0.20 ± 0.28 | 0.20 ± 0.37 | 0.04 ± 0.12 | 0.23 ± 0.87 |
| All | 55 | 1.34 ± 2.37 | 1.08 ± 1.63 | 0.28 ± 0.28 | 0.19 ± 0.29 | 0.10 ± 0.34 | 0.22 ± 0.59 |
| H. pylori-negative casesb | 11 | 0.003 ± 0.008 | 0.020 ± 0.022 | 0.002 ± 0.003 | 0.015 ± 0.014 | 0.001 ± 0.003 | 0.000 ± 0.001 |
Antibody levels are shown as mean ± standard deviations of ODR per milligram of protein units for anti-WCA IgG and IgA and anti-CagA IgG and IgA assays and as mean ± standard deviations of net optical density for the anti-HspA and anti-HspB assays.
As shown in Table 1. For all values, P < 0.05 compared with the 55 H. pylori-positive cases.
Cytokine production.
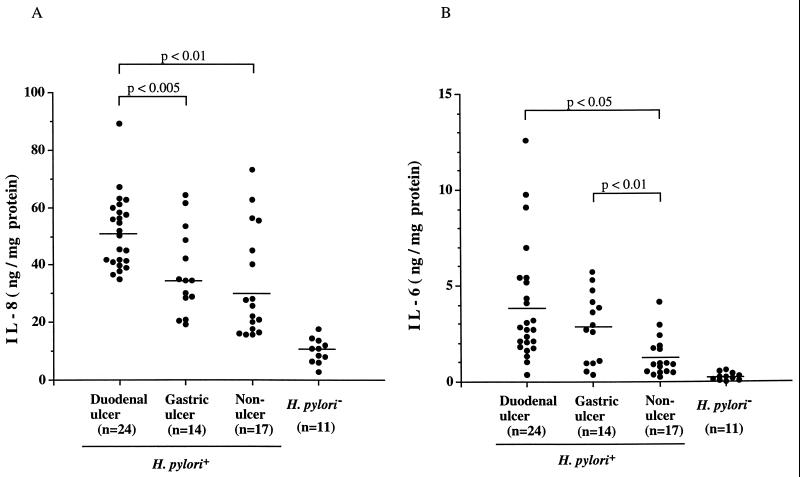
We next determined whether IL-6 and IL-8 levels present in the gastric tissue consistently vary among patients with particular endoscopic diagnoses (Fig. 1). Although supernatants of the gastric biopsy cultures contained measurable IL-8 (Fig. 1A) and IL-6 (Fig. 1B) levels in both H. pylori-positive and -negative subjects, as expected, the H. pylori-positive subjects had significantly higher IL-8 (42.0 ± 17.2 versus 10.1 ± 4.3 ng/mg of protein; P < 0.001) and IL-6 (2.8 ± 2.5 versus 0.3 ± 0.2 ng/mg of protein; P < 0.001) levels. However, there were considerable differences among the individual H. pylori-positive subjects. Supernatants from the patients with DU had significantly (P < 0.01) higher IL-8 levels (51.3 ± 12.7 ng/mg of protein) than did supernatants from patients with GU (37.3 ± 14.7 ng/mg of protein) or NUD (32.9 ± 18.8 ng/mg of protein). No significant differences in IL-8 levels were observed between GU and NUD patients. Specimens from patients with DU (3.9 ± 2.8 ng/mg of protein) or GU (2.8 ± 1.8) had significantly higher IL-6 levels than did NUD subjects (1.3 ± 1.0; P < 0.01). Thus, biopsy supernatant IL-8 and IL-6 levels but not antibody levels were strongly related to the clinical status.
FIG. 1.
Cytokine levels (nanograms per milligram of protein in culture supernatants) in gastric antral mucosal-organ cultures from 66 study patients. Patients were classified based on H. pylori status and, if positive, on endoscopic findings. (A) IL-8. Values for H. pylori-positive persons in each group were significantly (P < 0.001) higher than for H. pylori-negative persons. Patients with DU had the highest values. (B) IL-6. Values for H. pylori-positive persons in each group were significantly (P < 0.001) higher than for H. pylori-negative persons.
Correlations with immunological scores.
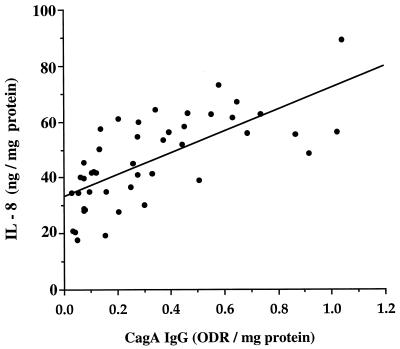
We asked whether, among the 55 H. pylori-positive patients, there was any relationship between biopsy culture supernatant antibody, cytokine levels, and histological findings. Culture supernatant IL-8 levels were strongly correlated (r = 0.75; P < 0.001) with levels of anti-CagA IgG (Fig. 2) but not with anti-CagA IgA, anti-WCA IgG, or IgA antibodies (data not shown). Culture supernatant IL-6 levels were not significantly correlated with levels of either IgA or IgG anti-CagA antibodies. There were no significant correlations between gastric biopsy culture supernatant cytokine levels and antibodies directed to H. pylori WCA, HspA, or HspB (data not shown).
FIG. 2.
Correlation between organ culture IL-8 levels and concentrations of IgG to CagA in 55 persons colonized with cagA+ H. pylori strains (r = 0.75, P < 0.0001).
We next examined whether cytokine and antibody levels were predictive of histologic findings. We categorized age and levels of IL-8, IL-6, anti-WCA IgG, anti-WCA IgA, anti-CagA IgG, and anti-CagA IgA by quartiles. Dichotomous outcome variables for mononuclear cell infiltration, neutrophil infiltration, atrophy, and H. pylori density were grouped as high (Sydney scale score, 2 or 3) or low (Sydney scale score, 0 or 1). Metaplasia was categorized as present (Sydney scale score, 1 to 3) or absent (Sydney scale score, 0). Multivariate analysis revealed several significant associations (Table 3). Female gender had an independent protective effect, with females having a significantly lower rate of neutrophil infiltration and H. pylori density than males. Older age correlated with a greater risk of gastric atrophy, and the risk increased linearly by quartile. Although levels of biopsy culture supernatant IL-8 did not predict histologic findings, high IL-6 levels were independent predictors of mononuclear cell infiltration, as were high biopsy culture supernatant levels of IgG to H. pylori WCA. Levels of gastric biopsy culture supernatant IgG antibodies to CagA were independently associated with increased neutrophil infiltration and H. pylori density. Levels of gastric biopsy culture supernatant IgA antibodies to CagA were associated with increased risk of metaplasia.
TABLE 3.
Predictors of histologic abnormalities of the gastric mucosa among patients carrying cagA+ H. pylori strains, by multivariate analysis
| Variable | OR (95% CI) for histologic findinga
|
||||
|---|---|---|---|---|---|
| Mononuclear cell infiltration | Neutrophil infiltration | Atrophy | Metaplasia | H. pylori density | |
| Female sex | NS | 0.2* (0.5–0.9) | NS | NS | 0.08+ (0.01–0.5) |
| Age | NS | NS | 2.1* (1.2–3.5) | NS | NS |
| IL-8 | NS | NS | NS | NS | NS |
| IL-6 | 2.2+ (1.2–4.1) | NS | NS | NS | NS |
| Whole-cell IgG | 2.5+ (1.3–5.0) | NS | NS | NS | NS |
| Whole-cell IgA | NS | NS | NS | NS | NS |
| CagA IgG | NS | 2.8+ (1.5–5.2) | NS | NS | 3.9+ (1.8–8.4) |
| CagA IgA | NS | NS | NS | 0.57* (0.3–0.9) | NS |
| HspA | NS | NS | NS | NS | NS |
| HspB | NS | NS | NS | NS | NS |
OR, odds ratio; 95% CI, 95% confidence interval; NS, not significant; ∗, P < 0.05; +, P < 0.005.
DISCUSSION
Humans colonized with H. pylori show a variety of responses to the organisms in their gastric tissues (6, 35, 44). The response can be considered to have an “acute inflammatory” component, characterized by intraepithelial and interstitial infiltration by polymorphonuclear leukocytes, and a “chronic inflammatory” component associated with increased numbers of mononuclear cells, including lymphocytes, monocytes/macrophages, and plasma cells in the lamina propria (19). However, among H. pylori-positive human populations, there is substantial heterogeneity in the intensity and distribution of these histological responses (59). The basis of this heterogeneity and its relation to clinical diagnoses represent important unsolved questions (6, 19).
The cytokines induced by H. pylori colonization, including tumor necrosis factor alpha, interleukin-1 (IL-1), IL-6, and IL-8, may play roles in regulating these tissue responses (1, 2, 15, 25, 50). IL-8 has been implicated in the pathogenesis of infectious and inflammatory conditions associated with neutrophil infiltration (34, 42, 60), whereas IL-6 may be involved in inflammation through its broad effects on growth, differentiation, and activation of mononuclear cells, including T- and B-lymphocytes and macrophages (26, 27, 38, 40), through the induction of other cytokines such as monocyte chemoattractant protein 1 (10).
In this study, as expected, in comparison with H. pylori-negative patients, H. pylori-positive patients had higher scores for mononuclear and polymorphonuclear cell infiltration, IL-8 and IL-6 levels, and H. pylori-specific antibodies in the gastric biopsy culture supernatants (1, 2, 15, 25). By multivariate analyses, we found that mucosal IL-6 but not IL-8 levels correlated with both mononuclear cell infiltration scores, and this is consistent with the prior literature (2, 65). We have previously reported that, in biopsy specimens from persons colonized with H. pylori, IL-8 mainly is present in gastric epithelial cells and macrophages (2), and IL-6 is present chiefly in macrophages (1). The absence of a correlation with IL-8 levels in multivariate analysis suggests that this cytokine does not exert an independent effect on histological change, and IL-8 may be chiefly produced by epithelial cells, which is consistent with other reports (13, 18). Compared to levels in patients with NUD, significantly elevated antral IL-8 and IL-6 levels were found in patients with DU, suggesting that the altered gastric secretory pathophysiology in DU patients (36, 45, 67) may be driven at least in part by these cytokines. These results are consistent with previous observations (4, 58) that DU patients have substantial infiltration with polymorphonuclear cells in gastric or duodenal mucosa. The elevated IL-6 levels in GU patients compared with NUD patients are consistent with differences in antral mononuclear cell scores (Table 1) and suggest potential mechanisms for the altered pathophysiology in GU patients. Patients with DU disease had significantly higher mononuclear and polymorphonuclear cell infiltration scores than did patients with NUD, whereas GU patients had higher atrophy and metaplasia scores than either DU or NUD patients and higher scores for mononuclear cell infiltration than NUD patients. These findings confirm that DU patients have more severe antral gastritis, and GU patients tend to have gastric atrophy, supporting previous work showing different H. pylori colonization patterns in DU and GU patients in relation to acid production and the presence of atrophy (39).
IL-8 induction is H. pylori strain specific in that, on average, cagA+ strains induce higher levels (13, 53, 61, 66, 69) than do cagA-negative strains. That all of our tested 55 strains were cagA+ is consistent with previous studies of Japanese H. pylori-positive patients (28, 46). Despite the universal presence of cagA+ strains, considerable variation of gastric biopsy culture supernatant IL-8 and IL-6 activity was present among individual H. pylori-positive patients, and importantly, the levels of IL-8 were strongly correlated with levels of anti-CagA IgG. The mechanisms underlying this association are not known, and this observation should be confirmed in other populations. Since H. pylori density also was significantly correlated with anti-CagA antibody, we hypothesize that the correlation between IL-8 and anti-CagA antibodies may reflect the local inflammatory response, which is dependent on the density of H. pylori. This is supported by our finding that H. pylori density is significantly correlated with anti-CagA IgG in gastric biopsy supernatant. Similarly, the specific antibody levels may reflect the intensity of the interaction of the H. pylori population with the host. Carriage of CagA-positive H. pylori strains has been associated with an increased prevalence and intensity of antral atrophy and intestinal metaplasia, in addition to higher degrees of cellular infiltration in gastric tissues (23, 37, 62, 68). The significant correlation between levels of anti-CagA IgA in organ culture supernatants and metaplasia scores could be due to a direct toxic effect by the antibodies or could reflect a more intense colonization by the particular strains in these patients or represent a phenomenon secondary to the metaplasia. That serum anti-CagA IgG levels vary in relation to variation of the 3′ region of cagA (70) might provide an intermediary mechanism for our observation concerning organ culture supernatant antibody levels. Regardless of the mechanisms involved, that anti-CagA antibody responses were correlated with IL-8 levels and intestinal metaplasia indicates their possible role as markers of gastric inflammation and premalignant lesions.
Bacterial Hsps may play an important role in inflammation (71), and human Hsp60 and H. pylori HspB have antigenic similarities (31). An Hsp60 epitope is detected on the surface of both human gastric cancer cells and human gastric biopsy specimens, and the intensity of cell surface Hsp60 correlated significantly with adhesion of H. pylori to human gastric cancer cells (29). Although we found that, as expected, levels of antibodies to HspA and HspB in the gastric biopsy culture supernatants were higher in H. pylori-positive than -negative patients, the lack of correlation between the particular levels and the histological findings suggests that these proteins may not be relevant to the induction of the specific patterns of gastric mucosal tissue responses related to disease outcome.
In conclusion, these findings indicate that quantitative evaluation of antibodies and cytokines in gastric antral biopsy culture supernatant, as well as histological scores, provides a means for examining individual variations in host responses to H. pylori. Such analyses can both raise and address hypotheses concerning the relationships between colonization and clinical outcomes. That anti-CagA responses correlated with intestinal metaplasia, H. pylori density, and IL-8 suggests that the absolute levels of these antibodies may be markers for gastric inflammation and premalignant changes in individual hosts.
ACKNOWLEDGMENTS
This study was supported in part by R01DK53707 from the National Institute of Health, by the Medical Research Service of the Department of Veterans Affairs, and by the Iris and Homer Akers Fellowship in Infectious Diseases.
REFERENCES
- 1.Ando T, Kusugami K, Ohsuga M, Ina K, Shinoda M, Konagaya T, Sakai T, Imada A, Kasuga N, Nada T, Ichiyama S, Blaser M J. Differential normalization of mucosal interleukin-8 and interleukin-6 activity after Helicobacter pylori eradication. Infect Immun. 1998;66:4742–4747. doi: 10.1128/iai.66.10.4742-4747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando T, Kusugami K, Ohsuga M, Shinoda M, Sakakibara M, Saito H, Fukatsu A, Ichiyama S, Ohta M. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91:1150–1156. [PubMed] [Google Scholar]
- 3.Atherton J C, Cao P, Peek R M, Jr, Tummuru M K, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 4.Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Infect Gastroenterol. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 5.Beales I L, Crabtree J E, Scunes D, Covacci A, Calam J. Antibodies to CagA protein are associated with gastric atrophy in Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1996;8:645–649. [PubMed] [Google Scholar]
- 6.Blaser M J. Ecology of Helicobacter pylori in the human stomach. J Clin Investig. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 8.Blaser M J, Perez-Perez G I, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 9.Ching C K, Wong B C, Kwok E, Ong L, Covacci A, Lam S K. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996;91:949–953. [PubMed] [Google Scholar]
- 10.Coletta I, Soldo L, Polentarutti N, Mancini F, Guglielmotti A, Pinza M, Mantovani A, Milanese C. Selective induction of MCP-1 in human mesangial cells by the IL-6/sIL-6R complex. Exp Nephrol. 2000;8:37–43. doi: 10.1159/000059327. [DOI] [PubMed] [Google Scholar]
- 11.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cover T L, Dooley C P, Blaser M J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990;58:603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crabtree J E, Farmery S M, Lindley I J, Figura N, Peichl P, Tompkins D S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994;47:945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crabtree J E, Figura N, Taylor J D, Bugnoli M, Armellini D, Tompkins D S. Expression of 120 kilodalton protein and cytotoxicity in Helicobacter pylori. J Clin Pathol. 1992;45:733–734. doi: 10.1136/jcp.45.8.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabtree J E, Shallcross T M, Heatley R V, Wyatt J I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree J E, Taylor J D, Wyatt J I, Heatley R V, Shallcross T M, Tompkins D S, Rathbone B J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 17.Craig P M, Territo M C, Karnes W E, Walsh J H. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut. 1992;33:1020–1023. doi: 10.1136/gut.33.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowe S E, Alvarez L, Dytoc M, Hunt R H, Muller M, Sherman P, Patel J, Jin Y, Ernst P B. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 19.Dixon M F. Pathophysiology of Helicobacter pylori infection. Scand J Gastroenterol. 1994;201:7–10. [PubMed] [Google Scholar]
- 20.Dixon M F, Genta R M, Yardley J H, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Dooley C P, Cohen H, Fitzgibbons P L, Bauer M, Appleman M D, Perez-Perez G I, Blaser M J. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 22.Dunn B E, Roop II R M, Sung C C, Sharma S A, Perez-Perez G I, Blaser M J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992;60:1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figura N, Vindigni C, Covacci A, Presenti L, Burroni D, Vernillo R, Banducci T, Roviello F, Marrelli D, Biscontri M, Kristodhullu S, Gennari C, Vaira D. cagA positive and negative Helicobacter pylori strains are simultaneously present in the stomach of most patients with non-ulcer dyspepsia: relevance to histological damage. Gut. 1998;42:772–778. doi: 10.1136/gut.42.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsyth M H, Atherton J C, Blaser M J, Cover T L. Heterogeneity in levels of vacuolating cytotoxin gene (vacA) transcription among Helicobacter pylori strains. Infect Immun. 1998;66:3088–3094. doi: 10.1128/iai.66.7.3088-3094.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gionchetti P, Vaira D, Campieri M, Holton J, Menegatti M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Miglioli M, Barbara L. Enhanced mucosal interleukin-6 and -8 in Helicobacter pylori-positive dyspeptic patients. Am J Gastroenterol. 1994;89:883–887. [PubMed] [Google Scholar]
- 26.Hirano T, Matsuda T, Turner M, Miyasaka N, Buchan G, Tang B, Sato K, Shimizu M, Maini R, Feldmann M, Kishimoto T. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988;18:1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- 27.Horii Y, Muraguchi A, Iwano M, Matsuda T, Hirayama T, Yamada H, Fujii Y, Dohi K, Ishikawa H, Ohmoto Y, Yoshizaki K, Hirano T, Kishimoto T. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989;143:3949–3955. [PubMed] [Google Scholar]
- 28.Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710–1714. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamiya S, Yamaguchi H, Osaki T, Taguchi H. A virulence factor of Helicobacter pylori: role of heat shock protein in mucosal inflammation after H. pylori infection. J Clin Gastroenterol. 1998;27:S35–S39. doi: 10.1097/00004836-199800001-00007. [DOI] [PubMed] [Google Scholar]
- 30.Kansau I, Guillain F, Thiberge J M, Labigne A. Nickel binding and immunological properties of the C-terminal domain of the Helicobacter pylori GroES homologue (HspA) Mol Microbiol. 1996;22:1013–1023. doi: 10.1046/j.1365-2958.1996.01536.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawahara Y, Yokota K, Mizuno M, Yunoki N, Uesu T, Okada H, Kobayashi K, Hirai Y, Oguma K, Tsuji T. Antibodies to human gastric epithelial cells and heat shock protein 60 in Helicobacter pylori positive mucosa associated lymphoid tissue lymphoma. Gut. 1999;45:20–23. doi: 10.1136/gut.45.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 33.Klein P D, Graham D Y. Minimum analysis requirements for the detection of Helicobacter pylori infection by the 13C-urea breath test. Am J Gastroenterol. 1993;88:1865–1869. [PubMed] [Google Scholar]
- 34.Ko Y C, Mukaida N, Ishiyama S, Tokue A, Kawai T, Matsushima K, Kasahara T. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993;61:1307–1314. doi: 10.1128/iai.61.4.1307-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolho K L, Karttunen R, Heikkila P, Lindahl H, Rautelin H. Gastric inflammation is enhanced in children with CagA-positive Helicobacter pylori infection. Pediatr Infect Dis J. 1999;18:337–341. doi: 10.1097/00006454-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Konturek P C, Konturek S J, Bobrzynski A, Kwiecien N, Obtulowicz W, Stachura J, Hahn E G, Rembiarz K. Helicobacter pylori and impaired gastric secretory functions associated with duodenal ulcer and atrophic gastritis. J Physiol Pharmacol. 1997;48:365–373. [PubMed] [Google Scholar]
- 37.Kuipers E J, Perez-Perez G I, Meuwissen S G, Blaser M J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 38.Kusugami K, Fukatsu A, Tanimoto M, Shinoda M, Haruta J, Kuroiwa A, Ina K, Kanayama K, Ando T, Matsuura T, Yamaguchi T, Morise K, Ieda M, Iokawa H, Ishihara A, Sarai S. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig Dis Sci. 1995;40:949–959. doi: 10.1007/BF02064182. [DOI] [PubMed] [Google Scholar]
- 39.Lee A, Dixon M F, Danon S J, Kuipers E, Megraud F, Larsson H, Mellgard B. Local acid production and Helicobacter pylori: a unifying hypothesis of gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995;7:461–465. [PubMed] [Google Scholar]
- 40.Linker-Israeli M, Deans R J, Wallace D J, Prehn J, Ozeri-Chen T, Klinenberg J R. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 41.Macchia G, Massone A, Burroni D, Covacci A, Censini S, Rappuoli R. The Hsp60 protein of Helicobacter pylori: structure and immune response in patients with gastroduodenal diseases. Mol Microbiol. 1993;9:645–652. doi: 10.1111/j.1365-2958.1993.tb01724.x. [DOI] [PubMed] [Google Scholar]
- 42.Mahida Y R, Ceska M, Effenberger F, Kurlak L, Lindley I, Hawkey C J. Enhanced synthesis of neutrophil-activating peptide-1/interleukin-8 in active ulcerative colitis. Clin Sci. 1992;82:273–275. doi: 10.1042/cs0820273. [DOI] [PubMed] [Google Scholar]
- 43.Mai U E, Perez-Perez G I, Allen J B, Wahl S M, Blaser M J, Smith P D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 45.McColl K E, el-Omar E, Gillen D. The role of H. pylori infection in the pathophysiology of duodenal ulcer disease. J Physiol Pharmacol. 1997;48:287–295. [PubMed] [Google Scholar]
- 46.Miehlke S, Go M F, Kim J G, Graham D Y, Figura N. Serologic detection of Helicobacter pylori infection with cagA-positive strains in duodenal ulcer, gastric ulcer, and asymptomatic gastritis. J Gastroenterol. 1998;33(Suppl. 10):18–21. [PubMed] [Google Scholar]
- 47.Negrini R, Lisato L, Zanella I, Cavazzini L, Gullini S, Villanacci V, Poiesi C, Albertini A, Ghielmi S. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991;101:437–445. doi: 10.1016/0016-5085(91)90023-e. [DOI] [PubMed] [Google Scholar]
- 48.Ng E K, Thompson S A, Perez-Perez G I, Kansau I, van der Ende A, Labigne A, Sung J J, Chung S C, Blaser M J. Helicobacter pylori heat shock protein A: serologic responses and genetic diversity. Clin Diagn Lab Immunol. 1999;6:377–382. doi: 10.1128/cdli.6.3.377-382.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nielsen H, Andersen L P. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992;33:738–742. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noach L A, Bosma N B, Jansen J, Hoek F J, van Deventer S J, Tytgat G N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 51.Parsonnet J, Blaser M J, Perez-Perez G I, Hargrett-Bean N, Tauxe R V. Symptoms and risk factors of Helicobacter pylori infection in a cohort of epidemiologists. Gastroenterology. 1992;102:41–46. doi: 10.1016/0016-5085(92)91782-y. [DOI] [PubMed] [Google Scholar]
- 52.Parsonnet J, Friedman G D, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peek R M, Jr, Miller G G, Tham K T, Perez-Perez G I, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA+Helicobacter pylori strains. Lab Investig. 1995;71:760–770. [PubMed] [Google Scholar]
- 54.Perez-Perez G I, Dworkin B M, Chodos J E, Blaser M J. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- 55.Perez-Perez G I, Thiberge J M, Labigne A, Blaser M J. Relationship of immune response to heat-shock protein A and characteristics of Helicobacter pylori-infected patients. J Infect Dis. 1996;174:1046–1050. doi: 10.1093/infdis/174.5.1046. [DOI] [PubMed] [Google Scholar]
- 56.Peterson G L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 57.Price A B. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 58.Satoh K, Kimura K, Yoshida Y, Kasano T, Kihira K, Taniguchi Y. Relationship between Helicobacter pylori colonization and acute inflammation of the duodenal mucosa. Am J Gastroenterol. 1993;88:360–363. [PubMed] [Google Scholar]
- 59.Satoh K, Kimura K, Yoshida Y, Kasano T, Kihira K, Taniguchi Y. A topographical relationship between Helicobacter pylori and gastritis: quantitative assessment of Helicobacter pylori in the gastric mucosa. Am J Gastroenterol. 1991;86:285–291. [PubMed] [Google Scholar]
- 60.Seitz M, Dewald B, Gerber N, Baggiolini M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J Clin Investig. 1991;87:463–469. doi: 10.1172/JCI115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma S A, Tummuru M K, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sozzi M, Valentini M, Figura N, De Paoli P, Tedeschi R M, Gloghini A, Serraino D, Poletti M, Carbone A. Atrophic gastritis and intestinal metaplasia in Helicobacter pylori infection: the role of CagA status. Am J Gastroenterol. 1998;93:375–379. doi: 10.1111/j.1572-0241.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 63.Suerbaum S, Thiberge J M, Kansau I, Ferrero R L, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function, and immunogenicity. Mol Microbiol. 1994;14:959–974. doi: 10.1111/j.1365-2958.1994.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 64.Taub D D, Oppenheim J J. Chemokines, inflammation and the immune system. Ther Immunol. 1994;1:229–246. [PubMed] [Google Scholar]
- 65.Tummuru M K, Cover T L, Blaser M J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tummuru M K, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 67.Walker M M, Crabtree J E. Helicobacter pylori infection and the pathogenesis of duodenal ulceration. Ann N Y Acad Sci. 1998;859:96–111. doi: 10.1111/j.1749-6632.1998.tb11114.x. [DOI] [PubMed] [Google Scholar]
- 68.Warburton V J, Everett S, Mapstone N P, Axon A T, Hawkey P, Dixon M F. Clinical and histological associations of cagA and vacA genotypes in Helicobacter pylori gastritis. J Clin Pathol. 1998;51:55–61. doi: 10.1136/jcp.51.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamaoka Y, Kodama T, Kashima K, Graham D Y, Sepulveda A R. Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol. 1998;36:2258–2263. doi: 10.1128/jcm.36.8.2258-2263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi Y, Zhong G, Brunham R C. Continuous B-cell epitopes in Chlamydia trachomatis heat shock protein 60. Infect Immun. 1993;61:1117–1120. doi: 10.1128/iai.61.3.1117-1120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]