Abstract
Since the beginning of the COVID-19 (Coronavirus Disease of 2019) pandemic, myocarditis has received much attention and controversy as one of the more worrisome cardiovascular complications. After the availability of highly effective COVID-19 mRNA vaccines in late 2020, myocarditis was also appreciated as an important vaccine-related adverse event. Though the overall frequency of clinically evident viral myocarditis is rare in the general population, young males show a higher predilection for COVID vaccine-induced myocarditis. The severity of COVID-19 viral myocarditis is variable, ranging from very mild to severe, while vaccine-induced myocarditis is usually mild, and rarely a severe or fatal disease. The diagnosis of either COVID-19 or vaccine-induced myocarditis is based on typical clinical features, laboratory investigations, and imaging, preferably with cardiac magnetic resonance. The management of COVID-19 myocarditis is supportive care for mild or moderate disease. For the rare patient who develops severe disease, advanced heart failure therapies such as mechanical circulatory support devices may have to be employed and can be lifesaving. Avoidance of strenuous exercise during the bout of myocarditis and its recovery phase is important. Despite the small but finite risk of vaccine-induced myocarditis, the benefits of protection against COVID-19 disease and its attendant complications far outweigh the risks.
Keywords: COVID-19, SARS-CoV-2, Myocarditis, Vaccine induced myocarditis, Cardiac magnetic resonance
Introduction
First reported in December 2019, SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) or COVID-19 disease was declared a pandemic by the World Health Organization (WHO).1 As of January 2022, >380 million people had been infected with SARS-CoV-2 worldwide with 5.71 million deaths.2 Coronaviruses are enveloped, single-stranded, positive-sense RNA viruses of the Coronaviridae.3 They mainly cause respiratory infections in humans and animals. There have been 2 prior epidemics due to coronavirus - SARS-CoV in 2002, and MERS-CoV (Middle Eastern Respiratory Syndrome) in 2012.4 , 5 Even though key features of infection with SARS-CoV-2 are predominately respiratory, it is now well established that it can also cause cardiovascular (CV), gastrointestinal, neurological, and hematological manifestations. Since the beginning of the pandemic, there has been a plethora of reports and research on CV involvement by the SARS-CoV-2 virus – this includes myocardial injury, acute coronary syndromes, arrhythmias, myocarditis, thromboembolism, heart failure, hypotension, cardiogenic shock and even cardiac arrest.6, 7, 8, 9, 10 Furthermore, symptomatic COVID-19 infection is more likely to occur in patients with common CV comorbidities or risk factors such as those with hypertension, diabetes, obesity, smoking, chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), heart failure (HF) or arrhythmias.6, 7, 8, 9, 10 Messenger RNA (mRNA) based vaccines were authorized for emergency use against COVID-19 by the FDA in December 2020. The two common vaccines commercially available in 2021 in the USA were both mRNA vaccines - BNT162b2 (Pfizer) and mRNA-1273 (Moderna) and are highly effective and safe.11 , 12 A third vaccine, manufactured by Janssen had a recombinant, replication-incompetent adenovirus type 26 expressing the SARS-CoV-2 spike protein, and was given as a single dose. Due to the higher risk of Thrombosis with Thrombocytopenia syndrome (TTS) associated with this vaccine, its use was curtailed to special situations only and the mRNA vaccines were deemed preferable.13 Another rare complication seen after COVID-19 or vaccination involves low platelet counts and thrombosis, especially cerebral venous thrombosis.14 Unlike COVID-19 vaccine-induced myocarditis which typically has involved younger males, this condition has a predilection for younger women. Vaccine-induced thrombocytopenia and thrombosis (VITT) has been reported after both the Janssen and AstraZeneca vaccines, both of which are adenoviral vector-based vaccines. Autoantibodies to platelet factor-4 may play a role, similar to heparin-induced thrombocytopenia.15 At this time, no association with myocarditis has been reported in conjunction with VITT.
While myocarditis was not seen in the initial safety trials of these vaccines, many large-scale post-vaccination datasets to date have reported myocarditis as one of the most important adverse effects. Myocarditis is defined as inflammation of the myocardium, characterized by infiltration by immune cells and ensuing myocardial tissue damage.16 The WHO defines myocarditis as an inflammatory disease of the cardiac muscle, which may be diagnosed clinically by biomarkers and imaging, in the appropriate clinical context and confirmed if needed by histological, immunological, and immunohistochemical criteria.17 Clinically, myocarditis presents variably ranging from mild chest pain or dyspnea to severe heart failure and cardiogenic shock.6 , 16 , 18 There are many causes of myocarditis, and the reader is referred to various recent comprehensive reviews on this topic for a detailed description, nonetheless, in clinical practice, the major causative agents of myocarditis are viruses, such as enteroviruses (Coxsackievirus B), adenovirus and parvovirus B19.1 , 18, 19, 20, 21, 22, 23, 24, 25, 26 Apart from viruses, other microbiological agents causing myocarditis include bacterial and protozoal infections27, 28, 29 and drugs such as immune checkpoint inhibitors, anticonvulsants, antibiotics, and antipsychotics.30, 31, 32 Systemic diseases including autoimmune diseases such as lupus and rheumatoid arthritis can sometimes present as fulminant myocarditis.33, 34, 35, 36, 37, 38 Vaccine-induced myocarditis has also been rarely reported after vaccination against other viruses (smallpox, hepatitis B) and bacteria (meningococcus).39, 40, 41
The initial diagnosis of either COVID-19 or its vaccine-induced myocarditis is based on clinical suspicion, laboratory investigations, and imaging tests such as echocardiography or fluorodeoxyglucose positron emission tomography (PET) or cardiovascular magnetic resonance (CMR) – the latter is the non-invasive gold standard for diagnosis of inflammatory cardiomyopathies. Endomyocardial biopsy may rarely be needed to confirm the diagnosis, especially if there is consideration of giant cell myocarditis or the clinical picture includes cardiogenic shock with a rapid hemodynamic decline and/or incessant ventricular arrhythmias and may reveal myocardial infiltration by lymphocytes and mononuclear cells. In this review, we will discuss myocarditis as one of the uncommon CV complications of both COVID-19 infection and its mRNA vaccines. Less data is available on myocarditis due to other COVID-19 vaccines available worldwide (adenoviral platforms), thus this paper will focus mainly on the 2 mRNA vaccines available in the US. Worldwide, as of June 2022, approximately 66% of people have received at least 1 dose of a vaccine and >6 million people get a vaccine daily.
Methods
We performed a detailed literature search of PUBMED/MEDLINE, EMBASE, medRxiv, and Cochrane electronic medical databases containing reports of either COVID-19 myocarditis or its vaccine-related myocarditis. The following medical search (MeSH) terms: COVID-19, SARS-CoV-2, myocarditis, myopericarditis, myocardial injury, inflammation, cardiomyopathy, cardiovascular complication, COVID-19 Vaccination, mRNA COVID-19 vaccine, troponin, cardiac magnetic resonance. The search duration was from December 2019 to May 2022 and was limited to English-language publications.
Incidence and prevalence
COVID-19 myocarditis incidence and prevalence
The true incidence of COVID-19 myocarditis is probably unknown. In retrospective studies, the incidence has been reported anywhere from as low as 0.01 % in large population studies to as high as 7.7 % in hospitalized patients.42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 In a Centers for Disease Control and Prevention (CDC) study using a US-based database of >900 hospitals, the risk of myocarditis was 0.146% in 1,452,773 patients with COVID-19 versus 0.009% in patients without COVID-19 [Risk Ratio (RR) = 15.7, 95% Confidence interval (CI) 14.1–17.2].42 Males were at higher risk than females (0.187% vs. 0.109%). Increasing age also increased the risk (>75 years old: 0.238%; ages 65–74: 0.186%; ages 50–64: 0.155%; age < 16 years: 0.133%). Compared to the pre-COVID-19 year (2019), the pandemic year (2020) saw a 42% higher incidence of myocarditis in hospitalized patients. In a large registry (using the TriNetX platform with anonymized electronic medical record (EMR) data coming mostly from about 50 sites, mostly from the USA), researchers found that out of 718,365 COVID-19 patients studied in the first 6 months of 2020, 35,820 (5.0%) had a new diagnosis of myocarditis. The study also reported increased mortality in patients with a diagnosis of COVID-19 and myocarditis (Odd ratio (OR) = 1.36, CI 1.21-1.53).43 Male sex was identified to have a higher incidence of myocarditis compared to females (OR = 1.41, CI 1.21-1.64). Another US-based study of almost 260,000 COVID-19 patients found that COVID-19 was significantly associated with myocarditis (OR = 8.17, 95% CI 3.58–18.62), though the overall incidence of myocarditis in COVID-19 patients was only 0.2%.44 In a nationwide Israeli study, the incidence of myocarditis was 0.011% in COVID-19 positive patients (RR compared to COVID-19 negative patients = 18.28; 95% CI, 3.95 to 25.12).46 Table 1 shows selected studies with the incidence and risk of myocarditis in patients with COVID-19.
Table 1.
| Study | Design/Database | Country | Total COVID-19 patients | Myocarditis incidence total (%) | Risk/Odds ratio |
|---|---|---|---|---|---|
| Boehmer et al. 2021 | Retrospective (Premier Healthcare Database Special COVID-19 Release) | USA | 1,452,773 | 2116 (0.146) | RR 15.7; 95% CI 14.1–17.2) |
| Buckley et al. 2021 | Retrospective (Global federated health research network) | International | 718,365 | 35,820 (5.0) | RR, 18.28; 95% CI, 3.95 to 25.12 |
| Barda et al. 2021 | Retrospective (Clalit Health Services) | Israel | 173,106 | 19 (0.01) | NA |
| Annie et al. 2021 | Retrospective (TriNetX platform) | USA | 259,352 | 383 (0.2) | NA |
| Murk et al. 2021 | Retrospective (Health Verity's Marketplace data set) | USA | 70,288 | 70 (0.1) | OR 8.17; 95% CI 3.58-18.62 |
| Linschoten et al. 2020 | Registry | International | 3011 | 3 (0.1) | NA |
| Jalali et al. 2021 | Retrospective (Single Center) | Iran | 196 | 15 (7.7) | NA |
| Sang III et al. | Autopsy | USA | 50 | 2 (4) | NA |
| Kunal et al. 2020 | Single Center Retrospective | India | 108 | 3 (2.8) | NA |
| Bhatia et al. 2021 | Registry | Australia | 644 | 2 (0.31) | NA |
| Daniels et al. 2021 | Registry (US Universities, Athletes only) | USA | 1597 | 37 (2.3) | NA |
COVID-19 vaccine-induced myocarditis incidence and prevalence
Vaccine-induced myocarditis had been documented in the past (36–38). In an analysis of US vaccine adverse event reporting system (VAERS) maintained by the CDC, in the years 1990 to 2018, myopericarditis was noted in 0.1% of all vaccinations. Of these affected patients, 79% were male and symptom onset was usually ≤2 weeks post-vaccination.53 Myocarditis is now also known to be a rare adverse effect of COVID-19 mRNA vaccination.10 , 54, 55., 56, 57, 58, 59, 60, 61, 62., 63, 64 The reported incidence of vaccine-induced myocarditis varies among different studies. VAERS had 1226 reports of probable cases of myocarditis between December 2020 to June 2021 in ∼300 million mRNA vaccine doses administered. Of these cases, 67% were after the second dose and the median time to symptom onset was 3 days (range: 0–79).55 , 56 Of those affected, 79% were male, the median patient age was 26 (range: 12–94 years) and most of the patients were <30 years old. In this study, the incidence was noted to be 40.6 per million in males and 4.2 per million in females aged 12–29, while in the ≥30 age group the incidence was 2.4 and 1 case per million in males and females, respectively.55 , 56 In a retrospective study from Israel, of 5.1 million fully vaccinated patients with the BNT162b2 mRNA vaccine (Pfizer-BioNTech), 142 cases of myocarditis were reported after vaccination. The vast majority (95%) of cases were mild, and there was only 1 reported death. As described in other studies also, an increased risk of myocarditis was seen after the second vaccine dose, with the highest incidence being in males between the ages of 16 to 19 years.57 In a second study from Israel, out of 2.5 million patients vaccinated with at least one dose of the same vaccine, only 54 cases were identified yielding an incidence of 2.13 per 100,000 patients. Most cases (76%) were mild in severity, 22% were moderate and only 1 patient developed cardiogenic shock. Again, the highest risk was noted in males aged 16 to 29 years.58 In another retrospective study, myocarditis was noted to be one of the most common vaccine-related adverse events with 2.7 events per 100,000. In comparison, patients with SARS-CoV-2 infection in this study had an ∼4-fold higher risk of myocarditis (11 events per 100,000).46
In the VAERS report as of September 2021, of the more than ∼350 million mRNA vaccine doses administered, 1626 verified myocarditis cases were recorded. Males and younger individuals were more likely to be affected (82% males) with a median age of 21 years.59 In a US Kaiser Permanente study of 2,392,924 patients who received at least 1 dose of the mRNA vaccine, 15 cases of myocarditis were reported with 2 cases occurring after the 1st dose and 13 cases presenting after the second dose.60 All patients were hospitalized with improvement in symptoms with conservative management. The incidence was 0.8 and 5.8 cases per million after the 1st and 2nd dose, respectively. While most reported cases of COVID-19 vaccine myocarditis come from large databases of the 2 available mRNA vaccines, the large National English Immunization Database from the UK also showed cases occurring after a non-mRNA-based vaccine (in the period from December 2020 to August 2021). In this study, the risk of myocarditis was an extra 2, 1, 6 events per million, respectively within 28 days after the first dose of the ChAdOx1 vaccine (Chimpanzee Adenoviral vaccine developed by the University of Oxford, AstraZeneca), BNT162b2 (Pfizer) vaccine and mRNA-1273 (Moderna) vaccine. This rate increased to 10 events per million after the 2nd dose of mRNA-1273. These rates were still lower than the incidence of myocarditis after de novo initial COVID-19 infection (40 cases per million).61 In another Kaiser Permanente vaccine study, investigators compared the two-mRNA vaccines head-to-head for their risk of myocarditis and pericarditis in the 18–39-year-old age group. The authors found that the risk of myocarditis was higher in the mRNA-1273 group with 9.7 extra cases per million with the mRNA-1273 (Moderna) vaccine (59). However, there was no difference in the myocarditis severity between the two vaccines with all cases being mild and none requiring critical care.62 Table 2 shows selected studies with the incidence and risk of myocarditis in patients who received COVID-19 Vaccines.
Table 2.
| Study | Design/Database | Country | Type of vaccine | Total COVID-19 vaccine patients | Myocarditis incidence total (1st + 2nd dose) | Incidence rate ratio |
|---|---|---|---|---|---|---|
| Simone et al. 2021 | Retrospective (Kaiser Permanente) | USA | BNT162b2 or mRNA-1273 | 2,392,924 | 15 (2 + 13) | 1st Dose: 0.38 (0.05-1.40) 2nd Dose: 2.7 (1.4-4.8) |
| Patone et al. | Retrospective | England | ChAdOx1, BNT162b2 or mRNA-1273 | 38,615,491 ChAdOx1 (n = 20,615,911), BNT162b2 (n = 16,993,389) or mRNA-1273 (n = 1,006,191) |
ChAdOx1: 142 + 84, BNT162b2: 94 + 64, mRNA-1273: 9 |
1st Dose: ChAdOx1 (IRR 1.29, 95% CI 1.05, 1.58), BNT162b2 (IRR 1.31, 95% CI 1.03, 1.66) mRNA-1273 (IRR 2.97; 95% CI 1.34, 6.58) 2nd Dose: mRNA-1273 (IRR 9.84, 95% CI 2.69, 36.03) BNT162b2 (IRR 1.30, 95% CI 0.98, 1.72). |
| Mevorach et al. 2021 | Retrospective | Israel | BNT162b2 | 5,442,696 | 136 (19 + 117) | NA |
| Witberg et al. 2021 | Retrospective (Clalit Health Services) | Israel | BNT162b2 | 2,558,421 | 54 | 2.13 (1.56 to 2.70) per 100,000 |
| Diaz et al. 2021 | Retrospective (Providence health care system) | USA & Canada | BNT162b2 (Pfizer) or mRNA-1273 (Moderna) | 2,000,287 | 20 | 1.0 (0.61-1.54) per 100,000 |
| Husby et al. 2021 | Retrospective (Danish Vaccination Register) | Denmark | BNT162b2 (Pfizer) or mRNA-1273 (Moderna) | 4,155,361 | 269 | 1.7 (1.3 to 2.2) per 100,000 |
Pathophysiology
COVID-19 myocarditis pathophysiology
SARS-CoV-2 is an enveloped single-stranded, positive-sense RNA virus. Numerous studies have shown that SARS-CoV-2 uses its spike protein to bind to human angiotensin-converting enzyme 2 (ACE-2) on the plasma membrane to allow entry into the host cell. ACE-2 is expressed in alveolar type-2 cells of the lungs, in the small intestine, as well as in arterial and venous endothelial cells of the heart and kidneys.16 , 65 , 66 The “spike protein” is one of the structural proteins of SARS-CoV-2 - it has 2 subunits, S1 and S2. S1 binds to ACE2 on targeted cells meanwhile, the S2 subunit is responsible for membrane fusion between the virus and the host cell. After the fusion, viral RNA enters the cell nucleus for replication.66
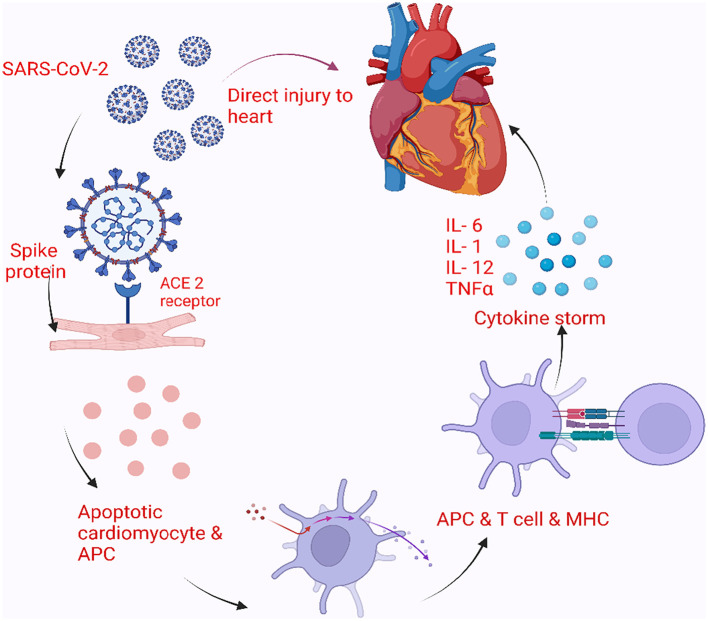
The precise mechanism of COVID-19 myocarditis is not well known. Proposed mechanisms include direct myocardial injury, immune-mediated injury, or very likely, a combination of the two (Fig. 1 ). It likely enters cardiac myocytes using specific receptors as mentioned and then damages the cell and leads to apoptosis. Many viruses and bacterial infections trigger innate immunity either directly due to tissue damage or indirectly via toll-like receptors and pattern-recognition receptors, which then recruit and activate macrophages, killer T cells, and suppress regulatory T cells. In a majority of cases, this kind of temporary response can clear the initial infection, however, excessive stimulation due to specific viral proteins can lead to further release of inflammatory mediators like cytokines and result in persistent activation of macrophages, natural killer cells, and T lymphocytes causing prolonged myocyte injury.16 ACE-2 receptors are also found in the myocardium, SARS-CoV-2 can bind to these ACE-2 entry receptors and gain access to the myocardium causing direct cell injury and myocarditis.65, 66, 67 In murine models infected with SARS-CoV-2, myocardial infection was noted with severe down-regulation of ACE-2 expression.66 In an autopsy study of 39 cases, 24 had SARS-CoV-2 in cardiac tissue and 16 had a high viral load. These 16 cases had increased cytokine expression panels when compared to the 15 patients without any sign of SARS-CoV-2 in cardiac tissue. However, there was no difference in the density of inflammatory cells.68 Another proposed route of direct cellular injury may occur via damage to the endothelial cells in the myocardium. ACE-2 receptors have also been found in pericytes and it has been hypothesized that infection of these pericytes will cause endothelial dysfunction and subsequent myocardial damage.69 In another autopsy study of COVID-19 infected patients, electron microscopy found an increased concentration of SARS-CoV-2 virus in cardiac endothelial cells.70 However, similar findings could not be confirmed with either electron microscopy or in situ hybridization techniques by other investigators.71 Finally, a hyperimmune response and the systemic inflammatory pathway are also thought to contribute to myocarditis. This heightened systemic inflammation is associated with increased release of cytokines, interleukins, tumor necrosis factor, and other chemokines and can cause direct myocardial injury - this mechanism is likely operational in patients with so-called cytokine storm in severe COVID-19 illness.71 , 72 In another autopsy study of 21 patients with death attributed to COVID-19 infection, the investigators found 3 cases of lymphocytic myocarditis. Two of these patients had CD4+ predominant T lymphocytes, whereas the other patient had CD8+ lymphocytes. The remainder of the 18 cases had diffuse macrophage infiltration of the myocardium without any evidence of lymphocytes or myocardial cell injury.72 In a different pathway involving the immune response, the initial endothelial or myocardial damage attracts further inflammatory mediators including macrophages, killer T cells and leads to a decrease in regulatory T cells, all of which potentiate myocardial inflammation. An increased level of CD68+ cells had been found in COVID-19 cardiac biopsies. The highest concentration of CD68+ cells was seen in the endothelial layer – these can damage the myocardium directly and cause microvascular thrombosis.73 , 74 In a case study, 2 patients with symptoms and signs suggestive of myocarditis, with prior mild respiratory illness suggestive of COVID-19 and negative nasopharyngeal COVID-19 test at presentation, underwent endomyocardial biopsy. Biopsy revealed myocardial inflammation without necrosis, increased lymphocytes, macrophages, cytotoxic T cells, and along with thickened arterioles. Interestingly, SARS-CoV-2 nucleic acid was identified in the myocardium using real-time polymerase chain reaction (RT-PCR).75 While the actual mechanism of myocarditis remains elusive, a possible combination of all these mechanisms is likely the case which varies depending on individual patient risk factors and genetic predisposition.71
Fig. 1.
Pathophysiology of myocarditis due to COVID-19.
COVID-19 myocarditis involves direct myocardial injury, immune-mediated injury, or a combination of the two. Heightened systemic inflammation is associated with increased release of cytokines, tumor necrosis factor-α, interleukins (IL-1,6,12), and other chemokines causing direct myocardial injury. ACE-2, Angiotensin-Converting Enzyme 2; APC, Antigen Presenting Cells.
COVID-19 vaccine induced myocarditis pathophysiology
mRNA-based COVID-19 vaccines have a strand of nucleoside-modified mRNA which encodes the viral spike glycoprotein of SARS-CoV-2. These mRNA strands are encapsulated in lipid nanoparticles which act as the delivery vehicle inside the cell. Once inside the host cell, mRNA encodes the translation to the spike protein which acts as an antigen and stimulates the adaptive immune response in the vaccinee. Should such an individual now encounter infection with the SARS-CoV-2 virus, its spike-protein is unable to bind to the ACE-2 receptor to enter cells due to inhibition by vaccine-induced spike-protein antibodies.
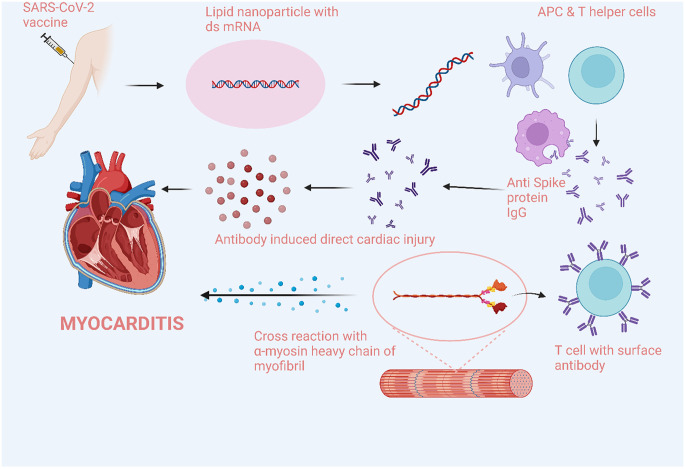
The exact mechanism of COVID-19 vaccine-induced myocarditis is not well known. Some theories include immune activation from the mRNA, molecular mimicry between viral and self-antigens, immune dysregulation induced by COVID-19, and host genetic, age and sex predispositions (Fig. 2 ).10 , 54 , 76 mRNA molecules can themselves be immunogenic and activate the innate immune system which can lead to their destruction even before they enter host cells. Nucleoside modifications of mRNA have been shown to reduce the activation of innate immunity.77 Nonetheless, in genetically predisposed individuals, these nucleoside-modified mRNA vaccines can still stimulate innate immunity and activate the systemic inflammatory response and damage the myocardium. However, this should not be limited to myocarditis only and affect other organs. Another possibility is the activation of immune response post vaccination led to increased formation of cardiac autoantibodies which then contribute to myocarditis.54 Molecular mimicry between the spike protein of SARS-CoV-2 and host self-antigens has been proposed as another theory.78 Host antibody production in response to the spike protein of the mRNA vaccine can cross-react with other host proteins like myocardial α-myosin heavy chain and cause myocardial damage (Fig. 2). Vaccine-induced myocarditis is predominantly reported in young males, which supports a significant role of genetic and phenotypic predisposition to immune dysregulation and myocarditis. In murine models, testosterone has been noted to inhibit anti-inflammatory cells and stimulate immune responses mediated by Th1 cells.76 , 79 , 80 The pathophysiology of COVID-19 vaccine-induced myocarditis remains completely unsolved but with ongoing research, more information will likely be available in the future.
Fig. 2.
Pathophysiology of myocarditis due to COVID-19 mRNA vaccines.
COVID-19 vaccine-induced myocarditis involves immune activation by the mRNA vaccines followed by molecular mimicry between viral and self-antigens, and immune dysregulation. APC, Antigen Presenting Cells.
Clinical presentation and prognosis
COVID-19 myocarditis presentation and prognosis
Patients with COVID-19 myocarditis can vary in presentation from asymptomatic myocardial involvement, mild left and/or right ventricular dysfunction to full-blown cardiogenic shock requiring mechanical circulatory support.39 Initial presenting symptoms of COVID-19 myocarditis are somewhat similar to COVID-19 pneumonia, with dyspnea as one of the predominant symptoms. Other commonly reported symptoms include chest pain, fever, cough, and tachycardia. In some reports, dyspnea had been the presenting symptom in 80% of patients, while chest pain had been reported in slightly >50% of patients, while others had reported equal distribution.81, 82, 83, 84, 85 The mean age of the affected patient was reported between 45 and 55 years, while extremes of both age groups had been reported in the literature. Some studies reported both sexes are at the same risk while others reported a male predominance. Commonly reported co-morbidities include hypertension, diabetes, obesity, asthma, or COPD.81, 82, 83, 84, 85
The prognosis of COVID-19 myocarditis varies from mild symptoms with full recovery to long-term symptoms and in many cases death. Myocarditis was more often associated with severe disease and patients with a diagnosis of myocarditis are more likely to be hospitalized than seen in the office setting. Patients with severe COVID-19 myocarditis can present either with cardiogenic shock or with septic shock or vasodilatory shock.86 The true incidence of morbidity and mortality is difficult to assess, and most reports are from hospitalized patients with a complicated course, which likely skewed the data. In a retrospective study of US hospital claims data, patients with COVID-19 had a strong association but overall low absolute risk of myocarditis (OR 8.17, 95% CI 3.58–18.62, absolute risk 0.1%).45 In another large retrospective review, the presence of myocarditis in COVID-19 patients was associated with an increased risk of mortality compared to patients without myocarditis (OR 1.36, p5% CI 1.21-1.53, p < 0.001).43 Retrospective data from the TriNetX research network, reported a 30-day mortality of 13.4% in COVID-19 myocarditis patients compared to 4.2% propensity-matched COVID-19 only patients (p < 0.001).44 Similarly, systematic reviews from case reports and series reported mortality from 13.7% to as high as 33%.81, 82, 83, 84, 85
COVID-19 vaccine-induced myocarditis presentation and prognosis
In case series and retrospective data, the most common initial presenting symptom in patients with COVID-19 vaccine myocarditis was chest pain. Other reported symptoms were tachycardia, dyspnea, fever, fatigue, and myalgia.54 , 55 The median age was reported from 17 to 30 years in case series, while in the CDC VAERS data median age was 26. Most patients were male, while in VAERS data it was close to 90%. Similarly, other population studies have reported median age of 27 years and male sex in >90%.54 , 55
Overall prognosis in vaccine-induced myocarditis is usually good and the majority of patients had mild to intermediate symptoms with full recovery.54 Although the majority of patients from the VAERS database were admitted to the hospital (96%), most of them had a mild course, and no reported mortality (52). In the nationwide Israeli study, the BNT162b2 (Pfizer–BioNTech) was associated with 136 cases of myocarditis out of which 129 were mild cases with a short hospital stay, only 1 patient died from fulminant myocarditis.57 In another Israeli study, mild cases of myocarditis were noted in 76% of patients, intermediate disease in 22% and 1 patient died from cardiogenic shock.58 In the updated descriptive study reporting from the US VAERS from December 2020 to August 2021, out of 676 cases only 12 patients required intensive care therapies like vasopressors, while mechanical ventilation was used in only 2 patients.59
Diagnostic workup
COVID-19 myocarditis diagnostic workup
Initial laboratory workup involves routine testing for COVID-19 including, RT-PCR, complete blood count, complete metabolic panel, coagulation testing, and inflammatory markers including c-reactive protein, erythrocyte sedimentation rate, ferritin, and others.
Cardiac biomarkers which are commonly tested and usually positive in COVID-19 myocarditis include cardiac troponin, brain natriuretic peptide (BNP), and creatinine kinase-myocardial band (CK-MB). Studies had shown persistent elevation in cardiac troponin (both troponin-I and troponin-T) in patients with myocarditis.81, 82, 83, 84 Diagnostic workup in patients suspected of having COVID-19 myocarditis includes an electrocardiogram (ECG), echocardiogram, and CMR. Chest radiography and/or computed tomography is utilized in all individuals. Common ECG findings with COVID-19 myocarditis include ST elevation, T wave inversion, and non-specific ST-T wave changes. Other reported findings include acute ischemia, new-onset bundle branch, atrioventricular (AV) blocks, premature ventricular contractions, and ventricular tachycardia/fibrillation.81, 82, 83, 84, 85 Echocardiography findings reported normal to decreased left ventricular ejection fraction, pericardial effusion or cardiomegaly, or hypertrophy.81, 82, 83, 84, 85 Endomyocardial biopsy remains the gold standard for tissue/histopathological diagnosis of myocarditis, though its use had now been limited due to its invasive nature and increased availability of CMR. Commonly reported findings in endomyocardial biopsy include lymphocytic infiltrates of the myocardium and endothelium, myocardial edema, and necrosis, while a few cases with normal biopsies had also been reported.81, 82, 83, 84, 85 Coronary angiography is usually unremarkable or may show nonobstructive coronary artery disease which would not explain the patient's presentation.85 CMR remains a useful test in patients suspected of myocarditis and can provide structural and functional evaluation of the heart along with detailed tissue characterization.87 , 88 It can also be used to rule out other etiologies like ischemia and cardiomyopathy while looking for myocarditis.10 CMR findings in patients with myocarditis include edema, inflammation, fibrosis, and T2 late gadolinium enhancement.89 Furthermore, CMR can be used to monitor the long-term effects of myocarditis52 , 90 (Fig. 3 ). CMR had been extensively used in college athletes for diagnosis and monitoring of sequelae of COVID-19 myocarditis.52 , 90, 91, 92, 93, 94, 95 Table 3A shows selected studies evaluating the role of CMR in COVID-19 myocarditis.52 , 92, 93, 94, 95, 96, 97, 98, 99
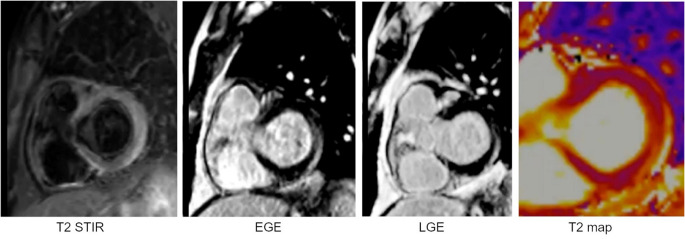
Fig. 3.
Cardiac MRI (CMR) findings in a patient with acute myocarditis due to COVID-19. T2-weighted short tau inversion recovery (STIR) image with fat suppression at the basal short axis of the heart at the level of the atrioventricular grooves shows hyperintensity in the lateral wall indicative of edema. EGE (early gadolinium enhancement) image taken at the same slice level shows inferolateral wall hyperenhancement that reflects hyperemia. LGE (late gadolinium enhancement) image shows gadolinium retention in the same myocardial segment in an epicardial pattern which is suggestive of nonischemic injury or necrosis or scar. Acutely, this usually reflects necrosis whereas in chronic cases, LGE size is used as a surrogate for scar or focal fibrosis. T2 mapping is done at the same slice location and confirms an elevated T2 value in the lateral segment (71 ms vs. normal control of 48 ms). Overall, the CMR findings in the appropriate clinical context are diagnostic of nonischemic injury or non-inflammatory cardiomyopathy,89 in this case, COVID-19 myocarditis.
Table 3A.
| Author | Total COVID +/ underwent CMR | Myocarditis + | Cardiac markers | CMR findings | Conclusion |
|---|---|---|---|---|---|
| Daniels et al. | 1597/1597 | 37 | Troponin elevation, ECG changes | Increased T1/T2, and LGE | 7.4-fold increased detection of myocarditis via MRI |
| Hendrickson et al. | 137/5 | 0 | Troponin elevation, coronary artery ectasia | Unremarkable | NA |
| Clark et al. | 59/59 | 2 | Normal | Increase T1/T2, and LGE | 3% prevalence of myocarditis in COVID + |
| Starekova et al. | 145/145 | 2 | Troponin elevation | Increased T2, and LGE | 1.4% prevalence of myocarditis in COVID + patients |
| Martinez et al. | 789/27 | 3 | Troponin elevation, ECG/echo changes | Increased T2, LGE | 0.4% prevalence of myocarditis in COVID + |
| Brito et al. | 48/48 | 0 | ECG changes and troponin elevation | Pericardial LGE | NA |
| Vago et al. | 12/12 | 0 | NA | No Involvement | NA |
| Malek et al. | 26/26 | 0 | Normal cardiac biomarkers | 5 patients with borderline myocardial edema | NA |
| Rajpal et al. | 26/26 | 4 | Normal cardiac biomarkers and ECG | Increased T2 and LGE | 0.15 % prevalence of myocarditis in COVID + |
COVID-19 vaccine-induced myocarditis diagnostic workup
Elevated troponin was seen in almost all patients with vaccine-induced myocarditis. Other laboratory workups include elevated BNP and C-reactive protein (CRP).54 The most common ECG findings include ST elevation, while AV blocks, T wave changes, PR depression, and non-specific ST changes had been reported.54 Echocardiography usually shows normal to mildly reduced ejection fraction, though severely reduced ejection fraction had also been documented.54 , 57 Endomyocardial biopsy in patients with COVID-19 vaccine-induced myocarditis showed monocytes, neutrophils, and lymphocytic infiltrates, with interstitial edema.57 The overall use of CMR was higher in COVID-19 vaccine-induced myocarditis patients with many case series reporting almost 100% use in all patients.39 , 54 , 57 Common findings include myocardial or sub-epicardial edema, late gadolinium enhancement, increased T1 and T2 intensity, and wall motion abnormalities. Table 3B shows selected studies evaluating the role of CMR in COVID-19 vaccine-induced myocarditis.100, 101, 102, 103, 104, 105, 106, 107, 108, 109
Table 3B.
Role of CMR in patients with COVID-19 vaccine-induced myocarditis.100, 101, 102, 103, 104, 105, 106, 107, 108, 109
| Author | Total patients/Myocarditis | Type of vaccine/dose | Cardiac investigations | Major findings |
|---|---|---|---|---|
| Shiyovich et al. | 4/4 | Pfizer-BNT162b2/Booster | ECG changes, troponin elevation | Increased T1/T2 and LGE |
| Nunn et al. | 4/4 | 3 Pfizer BNT162b2,1 mRNA-1273/Primary | ECG changes, troponin & BNP elevation | Increased T1/T2 and LGE |
| Manfredi et al. | 6/6 | 4 Pfizer-BNT162b2,2 mRNA-1273/Primary | Elevated troponins, atrial tachycardia | Increased T2 and LGE |
| Montgomery et al. | 23/23 | 7 Pfizer-BNT162b2,16 mRNA-1273/Primary | ECG changes, troponin elevation & echo changes | Increased T1/T2 and LGE |
| Kim et al. | 7/4 | 2 Pfizer-BNT162b2,2 mRNA-1273/Primary | ECG changes, biomarker elevation & echo changes | Increased T1/T2 and LGE |
| Dickey et al. | 6/6 | 5 Pfizer-BNT162b2,1 mRNA-1273/Primary | ECG changes, biomarker elevation & echo changes | Increased T2 signal and LGE |
| Mansour et al. | 2/2 | mRNA-1273/Primary | ECG changes, biomarker elevation | Increased T1, T2 signal and LGE |
| Marshall et al. | 7/7 | Pfizer-BNT162b2/Primary | ECG changes, biomarker elevation | LGE |
| Rosner et al. | 7 /7 | 5 Pfizer-BNT162b2,1 mRNA-1273, 1 adenovirus vaccine)/Primary | ECG changes, biomarker elevation | LGE in all patients increased T2 signal intensity in 3 patients |
| Larson et al. | 8/8 | 5 Pfizer-BNT162b2, 3 mRNA-1273/Primary | ECG changes, biomarker elevation & echo changes | LGE in all patients, edema in 6 patients |
Management
Management of COVID-19 myocarditis
Treatment is usually dictated by the clinical presentation of the disease. Patients should be counseled to avoid strenuous exercise even if asymptomatic but with a clinical picture consistent with myocarditis (e.g., elevated troponin and abnormal CMR and/or PET). Although evidence-based guidelines are not yet available, data have been extrapolated from other cases of viral myocarditis – an expert consensus document by the American College of Cardiology (ACC) recommends 3–6 months of no vigorous exercise.110 After this time, risk stratification (using symptoms, echocardiography and advanced imaging if indicated) may be performed again to evaluate fitness for return to play which is especially important in athletes.111 On the other hand, regular, aerobic non-strenuous exercise may boost the immunologic response in healthy individuals receiving the vaccine112 – thus, recommendations to curtail exercise should be reserved only for those with either confirmation or strong suspicion of myocarditis.
Patients who are asymptomatic with normal left ventricular function and no arrhythmias can be observed and followed up in the outpatient setting with close follow-up and consideration of repeat testing for cardiovascular manifestation based on the persistence of symptoms. It is essential to recognize that many patients may have cardiac manifestations related to COVID-19 infection, but they may not have myocarditis.10 In a systemic review, various treatments have been tried in patients with COVID-19 myocarditis - some of these treatments are mainly directed toward COVID-19 infection.81, 82, 83, 84, 85 Common treatments for more severe cases have included intravenous steroids, intravenous immunoglobulins, colchicine, Interferon beta, lopinavir/ritonavir, and remdesivir. Tocilizumab was used infrequently.81, 82, 83, 84, 85, 86
Patients who have symptomatic myocarditis should be hospitalized if it is moderate or worse in severity. Cardiac monitoring should be performed, and consideration should be made to discharge patients with remote monitoring if there is a concern for any arrhythmias. If not in cardiogenic shock, the use of beta-blockers is recommended to prevent ventricular as well as supraventricular arrhythmia. If there is left ventricular dysfunction, ace inhibitors or angiotensin receptor blockers should be initiated as well. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in myocarditis should be avoided as there is evidence of sodium retention and renal injury associated with it. NSAIDs can be considered for the treatment of pericarditis. Patients can develop bradyarrhythmia or tachyarrhythmia and may require antiarrhythmic agents for ventricular arrhythmias, and temporary cardiac pacing for bradyarrhythmia or advanced heart block.113 Patients with fulminant myocarditis usually present with hemodynamic instability and require critical care. Cases have been reported where methylprednisolone and intravenous immunoglobulin were used to treat fulminant myocarditis.114 In a case series, among 14 patients diagnosed with COVID-19 myocarditis, steroid therapy was used in 58% of patients and was associated with favorable outcomes.115 Fulminant myocarditis should be managed akin to cardiogenic shock, with the use of inotropic, vasoactive support as well as mechanical circulatory support devices if indicated in consultation with the advanced heart failure team as these patients may require a durable left ventricular assist device or cardiac transplantation.10 Once recovered all patients should be considered for guideline-directed medical therapy and repeat imaging and evaluation after discharge.
Management of COVID-19 vaccine-induced myocarditis
Management of vaccine-induced myocarditis involves the same principles as for the management of COVID-19 infection. Patients with symptoms like chest pain after the vaccine should be evaluated and if required hospitalized for possible myocarditis. In the majority of patients, symptoms are mild to moderate, and treatment can be considered with NSAIDs, colchicine, and corticosteroids.10 , 54 In one US-based retrospective study, the use of NSAIDs was noted to be in almost 87% of patients with myocarditis.60 Severe disease especially fulminant myocarditis, though rare after the mRNA COVID-19 vaccines, should be treated emergently with hemodynamic support similar to COVID-19 myocarditis. As mentioned, an expert consensus pathway by the ACC recommends avoiding strenuous activity for 3–6 months, however, a return to activity needs to be individualized and depends on the initial severity of the disease and follow-up imaging.10 , 54
COVID-19 disease versus COVID-19 vaccine-induced myocarditis: comparison of risks and benefits
Despite the small risk of myocarditis with vaccines, the overall benefits of COVID-19 vaccines far outweigh these risks. Most cases of vaccine-induced myocarditis are mild, and the majority of patients recover without any sequelae.54 , 55 , 57, 58, 59 Several studies had reported a comparison of the risk of COVID-19 disease versus vaccine-induced myocarditis. In an Israeli study assessing the safety of the BNT162b2 vaccine the risk of myocarditis after vaccination was 2.7 events per 100,000 persons, while in the same study the risk of myocarditis from COVID-19 infection was 11.0 events per 100,000 persons.46 In a UK-based study, the risk of myocarditis was estimated at around 1–6 events after the first dose and 10 events per million after the second dose which was much lower when compared to 40 events per million following COVID-19 infection.61 Similarly, another study from the same group reported the overall risk of myocarditis was higher with COVID-19 infection when compared to vaccine-induced myocarditis even after the third dose, the only exception was younger males.116 In a CDC report, the ACIP (Advisory Committee on Immunization Practices) reported that the benefits of COVID-19 vaccines significantly outweigh the risk of myocarditis in all populations. The greatest benefits were noted in patients >30 years old in both sexes. The risk of vaccine-induced myocarditis was highest in males 12–29 years old with 39–47 cases per million vaccines administered however in the same population an estimated 11,000 COVID-19 cases, 560 hospitalization, 139 ICU admissions, and 6 deaths were prevented with vaccination.56 Given the extensive risk of complicated COVID-19 infection in all populations, vaccination against COVID-19 remains the mainstay in prevention.
Conclusions
COVID-19 viral myocarditis and vaccine-induced myocarditis are two of the important but fortunately rare cardiovascular complications of COVID-19 illness. Cardiac magnetic resonance is an indispensable tool for diagnosis and management. Despite the small risk of vaccine-induced myocarditis, the benefits of protection against COVID-19 disease far outweigh the risks.
Declaration of Competing Interest
None of the authors has any relevant conflicts.
References
- 1.Timeline: WHO's COVID-19 Response, Accessed March 30th 2022. 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline/ Accessed March 29.
- 2.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King A.M.Q., Lefkowitz E., Adams M.J., Carstens E.B. Elsevier Inc.; 2012. Virus Taxonomy. [DOI] [Google Scholar]
- 4.Severe Acute Respiratory Syndrome (SARS) https://www.who.int/health-topics/severe-acute-respiratory-syndrome#tab=tab_1 Accessed May 24, 2022.
- 5.Al-Tawfiq J.A., Memish Z.A. Emerging respiratory viral infections: MERS-CoV and influenza. Lancet Respir Med. 2014;2(1):23–25. doi: 10.1016/S2213-2600(13)70255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/circulationaha.120.046941. [DOI] [PubMed] [Google Scholar]
- 7.Chung M.K., Zidar D.A., Bristow M.R., et al. COVID-19 and cardiovascular disease. Circ Res. 2021;128(8):1214–1236. doi: 10.1161/circresaha.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. 2020 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cenko E., Badimon L., Bugiardini R., et al. Cardiovascular disease and COVID-19: a consensus paper from the ESC working group on coronary pathophysiology & microcirculation, ESC working group on thrombosis and the association for acute cardiovascular care (ACVC), in collaboration with the European heart rhythm association (EHRA) Cardiovasc Res. 2021;117(14):2705–2729. doi: 10.1093/cvr/cvab298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee W., Ty J., MMF Gluckman, et al. ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022 doi: 10.1016/j.jacc.2022.02.003. Published online May 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/nejmoa2034577/suppl_file/nejmoa2034577_protocol.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baden L.R., el Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/nejmoa2035389/suppl_file/nejmoa2035389_data-sharing.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen COVID-19 Vaccine | FDA. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine Accessed July 2, 2022.
- 14.Gupta A., Sardar P., Cash M.E., Milani R.V., Lavie C.J. Covid-19 vaccine- induced thrombosis and thrombocytopenia-a commentary on an important and practical clinical dilemma. Prog Cardiovasc Dis. 2021;67:105–107. doi: 10.1016/j.pcad.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizk J.G., Gupta A., Sardar P., et al. Clinical characteristics and pharmacological management of COVID-19 vaccine–induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis: a review. JAMA Cardiol. 2021;6(12):1451–1460. doi: 10.1001/jamacardio.2021.3444. [DOI] [PubMed] [Google Scholar]
- 16.Cooper L.T. Myocarditis. N Engl J Med. 2009;360(15):1526–1538. doi: 10.1056/nejmra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson P., McKenna R.W., Bristow M., et al. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93(5):841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 18.Kindermann I., Barth C., Mahfoud F., et al. Update on myocarditis. J Am Coll Cardiol. 2012;59(9):779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 19.Pollack A., Kontorovich A.R., Fuster V., Dec G.W. Viral myocarditis–diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12(11):670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 20.Tschöpe C., Ammirati E., Bozkurt B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18(3):169–193. doi: 10.1038/S41569-020-00435-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez F., Kühl U., Pieske B., Garcia-Pavia P., Tschöpe C. Update on myocarditis and inflammatory cardiomyopathy: reemergence of endomyocardial biopsy. Rev Esp Cardiol (Engl Ed) 2016;69(2):178–187. doi: 10.1016/j.rec.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Kühl U., Pauschinger M., Noutsias M., et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111(7):887–893. doi: 10.1161/01.cir.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 23.Kindermann I., Kindermann M., Kandolf R., et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118(6):639–648. doi: 10.1161/circulationaha.108.769489. [DOI] [PubMed] [Google Scholar]
- 24.Matsumori A., Shimada T., Chapman N.M., Tracy S.M., Mason J.W. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail. 2006;12(4):293–298. doi: 10.1016/j.cardfail.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Chen F., Shannon K., Ding S., et al. HIV type 1 glycoprotein 120 inhibits cardiac myocyte contraction. AIDS Res Hum Retroviruses. 2002;18(11):777–784. doi: 10.1089/08892220260139512. [DOI] [PubMed] [Google Scholar]
- 26.Barbaro G. HIV-associated cardiomyopathy. Herz Kardiovaskuläre Erkrankungen. 2005;30(6):486–492. doi: 10.1007/s00059-005-2728-z. [DOI] [PubMed] [Google Scholar]
- 27.McAlister H.F., Klementowicz P.T., Andrews C., Fisher J.D., Feld M., Furman S. Lyme carditis: an important cause of reversible heart block. Ann Intern Med. 1989;110(5):339–345. doi: 10.7326/0003-4819-110-5-339. [DOI] [PubMed] [Google Scholar]
- 28.Hidron A., Vogenthaler N., Santos-Preciado J.I., Rodriguez-Morales A.J., Franco-Paredes C., Rassi A. Cardiac involvement with parasitic infections. Clin Microbiol Rev. 2010;23(2):324–349. doi: 10.1128/cmr.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rassi A., Rassi A., Little W.C., et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med. 2006;355(8):799–808. doi: 10.1056/nejmoa053241. [DOI] [PubMed] [Google Scholar]
- 30.Hu J.R., Florido R., Lipson E.J., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115(5):854–868. doi: 10.1093/cvr/cvz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilian J.G., Kerr K., Lawrence C., Celermajer D.S. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841–1845. doi: 10.1016/S0140-6736(99)10385-4. [DOI] [PubMed] [Google Scholar]
- 32.Taliercio C.P., Olney B.A., Lie J.T. Myocarditis related to drug hypersensitivity. Mayo Clin Proc. 1985;60(7):463–468. doi: 10.1016/S0025-6196(12)60870-2. [DOI] [PubMed] [Google Scholar]
- 33.Corradi D., Vaglio A., Maestri R., et al. Eosinophilic myocarditis in a patient with idiopathic hypereosinophilic syndrome: insights into mechanisms of myocardial cell death. Hum Pathol. 2004;35(9):1160–1163. doi: 10.1016/j.humpath.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Corssmit E.P.M., Trip M.D., Durrer J.D. Löffler’s endomyocarditis in the idiopathic hypereosinophilic syndrome. Cardiology. 1999;91(4):272–276. doi: 10.1159/000006923. [DOI] [PubMed] [Google Scholar]
- 35.Vinit J., Bielefeld P., Muller G., et al. Heart involvement in Churg-Strauss syndrome: retrospective study in French Burgundy population in past 10 years. Eur J Intern Med. 2010;21(4):341–346. doi: 10.1016/j.ejim.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Caforio A.L.P., Adler Y., Agostini C., et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European society of cardiology working group on myocardial and pericardial disease. Eur Heart J. 2017;38(35):2649–2662. doi: 10.1093/eurheartj/ehx321. [DOI] [PubMed] [Google Scholar]
- 37.Nunes H., Freynet O., Naggara N., et al. Cardiac sarcoidosis. Semin Respir Crit Care Med. 2010;31(4):428–441. doi: 10.1055/S-0030-1262211. [DOI] [PubMed] [Google Scholar]
- 38.Cooper L.T. Giant cell and granulomatous myocarditis. Heart Fail Clin. 2005;1(3):431–437. doi: 10.1016/j.hfc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Murphy J.G., Wright R.S., Bruce G.K., et al. Eosinophilic-lymphocytic myocarditis after smallpox vaccination. Lancet. 2003;362(9393):1378–1380. doi: 10.1016/S0140-6736(03)14635-1. [DOI] [PubMed] [Google Scholar]
- 40.Arness M.K., Eckart R.E., Love S.S., et al. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004;160(7):642–651. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- 41.Barton M., Finkelstein Y., Opavsky M.A., et al. Eosinophilic myocarditis temporally associated with conjugate meningococcal C and hepatitis B vaccines in children. Pediatr Infect Dis J. 2008;27(9):831–835. doi: 10.1097/inf.0b013e31816ff7b2. [DOI] [PubMed] [Google Scholar]
- 42.Boehmer T.K., Kompaniyets L., Lavery A.M., et al. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckley B.J.R., Harrison S.L., Fazio-Eynullayeva E., Underhill P., Lane D.A., Lip G.Y.H. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur J Clin Invest. 2021;51(11) doi: 10.1111/eci.13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Annie F.H., Alkhaimy H., Nanjundappa A., Elashery A. Association between myocarditis and mortality in COVID-19 patients in a large registry. Mayo Clin Proc Innov Qual Outcomes. 2022;6(2):114–119. doi: 10.1016/j.mayocpiqo.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murk W., Gierada M., Fralick M., Weckstein A., Klesh R., Rassen J.A. Diagnosis-wide analysis of COVID-19 complications: an exposure-crossover study. CMAJ. 2021;193(1):E10–E18. doi: 10.1503/cmaj.201686/tab-related-content. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/nejmoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linschoten M., Peters S., van Smeden M., et al. Cardiac complications in patients hospitalised with COVID-19. Eur Heart J Acute Cardiovasc Care. 2020;9(8):817–823. doi: 10.1177/2048872620974605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jalali F., Hatami F., Saravi M., et al. Characteristics and outcomes of hospitalized patients with cardiovascular complications of COVID-19. J Cardiovasc Thorac Res. 2021;13(4):355–363. doi: 10.34172/jcvtr.2021.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sang C.J., Burkett A., Heindl B., et al. Cardiac pathology in COVID-19: a single center autopsy experience. Cardiovasc Pathol. 2021:54. doi: 10.1016/j.carpath.2021.107370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunal S., Sharma S.M., Sharma S.K., et al. Cardiovascular complications and its impact on outcomes in COVID-19. Indian Heart J. 2020;72(6):593–598. doi: 10.1016/j.ihj.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatia K.S., Sritharan H.P., Chia J., et al. Cardiac complications in patients hospitalised with COVID-19 in Australia. Heart Lung Circ. 2021;30(12):1834–1840. doi: 10.1016/j.hlc.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daniels C.J., Rajpal S., Greenshields J.T., et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA Cardiol. 2021;6(9):1078–1087. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su J.R., McNeil M.M., Welsh K.J., et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990–2018. Vaccine. 2021;39(5):839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 54.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/circulationaha.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ACIP Presentation Slides | Immunization Practices | CDC. June 2021. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html Accessed March 29, 2022.
- 56.Gargano J.W., Wallace M., Hadler S.C., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mevorach D., Anis E., Cedar N., et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385(23):2140–2149. doi: 10.1056/nejmoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witberg G., Barda N., Hoss S., et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/nejmoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simone A., Herald J., Chen A., et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181(12):1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patone M., Mei X.W., Handunnetthi L., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/S41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Myocarditis Analyses in the Vaccine Safety Datalink : Rapid Cycle Analyses and “Head-to-Head” Product Comparisons. 2022. https://stacks.cdc.gov/view/cdc/110921 Accessed March 30.
- 63.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Husby A., Hansen J.V., Fosbøl E., et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021:375. doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goulter A.B., Goddard M.J., Allen J.C., Clark K.L. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004:2. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oudit G.Y., Kassiri Z., Jiang C., et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625. doi: 10.1111/J.1365-2362.2009.02153.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindner D., Fitzek A., Bräuninger H., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fox S.E., Li G., Akmatbekov A., et al. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142(11):1123–1125. doi: 10.1161/circulationaha.120.049465. [DOI] [PubMed] [Google Scholar]
- 71.Kawakami R., Sakamoto A., Kawai K., et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(3):314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wenzel P., Kopp S., Gobel S., et al. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res. 2020;116(10):1661–1663. doi: 10.1093/cvr/cvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Basso C., Leone O., Rizzo S., et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox S.E., Falgout L., Vander Heide R.S. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol. 2021:54. doi: 10.1016/j.carpath.2021.107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heymans S., Cooper L.T. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75–77. doi: 10.1038/S41569-021-00662-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Caso F., Costa L., Ruscitti P., et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5) doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazaros G., Klein A.L., Hatziantoniou S., Tsioufis C., Tsakris A., Anastassopoulou C. The novel platform of mRNA COVID-19 vaccines and myocarditis: clues into the potential underlying mechanism. Vaccine. 2021;39(35):4925–4927. doi: 10.1016/j.vaccine.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huber S.A., Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68(8):5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kariyanna P.T., Sutarjono B., Grewal E., et al. A systematic review of COVID-19 and myocarditis. Am J Med Case Rep. 2020;8(9):299. doi: 10.12691/ajmcr-8-9-11. [DOI] [Google Scholar]
- 82.Haussner W., DeRosa A.P., Haussner D., et al. COVID-19 associated myocarditis: a systematic review. Am J Emerg Med. 2022;51:150–155. doi: 10.1016/j.ajem.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaiswal V., Sarfraz Z., Sarfraz A., et al. COVID-19 infection and myocarditis: a state-of-the-art systematic review. J Prim Care Community Health. 2021:12. doi: 10.1177/21501327211056800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rathore S.S., Rojas G.A., Sondhi M., et al. Myocarditis associated with Covid-19 disease: a systematic review of published case reports and case series. Int J Clin Pract. 2021;75(11) doi: 10.1111/ijcp.14470. [DOI] [PubMed] [Google Scholar]
- 85.Ho J.S., Sia C.H., Chan M.Y., Lin W., Wong R.C. Coronavirus-induced myocarditis: a meta-summary of cases. Heart Lung. 2020;49(6):681–685. doi: 10.1016/j.hrtlng.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castiello T., Georgiopoulos G., Finocchiaro G., et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27(1):251–261. doi: 10.1007/S10741-021-10087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelle S., Bucciarelli-Ducci C., Judd R.M., et al. Society for cardiovascular magnetic resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J Cardiovasc Magn Reson. 2020;22(1) doi: 10.1186/S12968-020-00656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clark D.E., Aggarwal S.K., Phillips N.J., Soslow J.H., Dendy J.M., Hughes S.G. Cardiac magnetic resonance in the evaluation of COVID-19. Card Fail Rev. 2022:8. doi: 10.15420/cfr.2021.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 90.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moulson N., Petek B.J., Drezner J.A., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144(4):256–266. doi: 10.1161/circulationaha.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clark D.E., Parikh A., Dendy J.M., et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR) Circulation. 2021;143(6):609–612. doi: 10.1161/circulationaha.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hendrickson B.S., Stephens R.E., Chang J.V., et al. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation. 2021;143(19):1926–1928. doi: 10.1161/circulationaha.121.053982. [DOI] [PubMed] [Google Scholar]
- 94.Starekova J., Bluemke D.A., Bradham W.S., et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6(8):945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martinez M.W., Tucker A.M., Bloom O.J., et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6(7):745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brito D., Meester S., Yanamala N., et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2021;14(3):541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vago H., Szabo L., Dohy Z., Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. JACC Cardiovasc Imaging. 2021;14(6):1279. doi: 10.1016/j.jcmg.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Małek Ł.A., Marczak M., Miłosz-Wieczorek B., et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: a magnetic resonance study. J Magn Reson Imaging. 2021;53(6):1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiyovich A., Witberg G., Aviv Y., Kornowski R., Hamdan A. A case series of myocarditis following third (booster) dose of COVID-19 vaccination: magnetic resonance imaging study. Front Cardiovasc Med. 2022:9. doi: 10.3389/fcvm.2022.839090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nunn S., Kersten J., Tadic M., et al. Case report: myocarditis after COVID-19 vaccination - case series and literature review. Front Med (Lausanne) 2022:9. doi: 10.3389/fmed.2022.836620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manfredi R., Bianco F., Bucciarelli V., et al. Clinical profiles and CMR findings of young adults and pediatrics with acute myocarditis following mRNA COVID-19 vaccination: a case series. Vaccines (Basel) 2022;10(2) doi: 10.3390/vaccines10020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ryan M., Montgomery J., Engler R., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim H.W., Jenista E.R., Wendell D.C., et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6(10):1196. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dickey J.B., Albert E., Badr M., et al. A series of patients with myocarditis following SARS-CoV-2 vaccination with mRNA-1279 and BNT162b2. JACC Cardiovasc Imaging. 2021;14(9):1862–1863. doi: 10.1016/j.jcmg.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mansour J., Short R.G., Bhalla S., et al. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin Imaging. 2021;78:247. doi: 10.1016/j.clinimag.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marshall M., Ferguson I.D., Lewis P., et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148(3) doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 108.Rosner C.M., Genovese L., Tehrani B.N., et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/circulationaha.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Larson K.F., Ammirati E., Adler E.D., et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/circulationaha.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gluckman T.J., Bhave N.M., Allen L.A., et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;79(17):1717–1756. doi: 10.1016/j.jacc.2022.02.003/suppl_file/mmc1.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Phelan D., Kim J.H., Chung E.H. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol. 2020;5(10):1085–1086. doi: 10.1001/jamacardio.2020.2136. [DOI] [PubMed] [Google Scholar]
- 112.Ghram A., Moalla W., Lavie C.J. Vaccine and physical activity in the era of COVID-19 pandemic. Prog Cardiovasc Dis. 2021;67:33–34. doi: 10.1016/j.pcad.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42(2):206. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sawalha K., Abozenah M., Kadado A.J., et al. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patone M., Mei X.W., Handunnetthi L., et al. Risk of myocarditis following sequential COVID-19 vaccinations by age and sex. medRxiv. 2021 doi: 10.1101/2021.12.23.21268276. Published online December 25. 2021.12.23.21268276. [DOI] [Google Scholar]