Abstract
Connatal bacterial pneumonia is common in neonates. Animal studies and initial clinical reports indicate that surfactant dysfunction is involved in the pathophysiology of severe neonatal pneumonia. Since respiratory distress syndrome and connatal pneumonia may be difficult to differentiate in the first hours of life, neonates with respiratory failure due to bacterial infections might receive surfactant. Under such conditions surfactant components might be catabolized by bacteria and promote bacterial growth. We therefore investigated the influence of three modified natural (Curosurf, Alveofact, and Survanta) and two synthetic (Exosurf and Pumactant) surfactant preparations on the growth of bacteria frequently cultured from blood or tracheal aspirate fluid in the first days of life. Group B streptococci (GBS), Staphyloccocus aureus, and Escherichia coli were incubated in a nutrient-free medium (normal saline) for 5 h at 37°C, together with different surfactants at concentrations of 0, 1, 10, and 20 mg/ml. With the exception of E. coli, incubation in saline alone led to a variable decrease in CFU. In the presence of Alveofact, Exosurf, and Pumactant the decline in bacterial numbers was less marked than in saline alone. Curosurf was bactericidal in a dose-dependent fashion for GBS and had a strong negative impact on the growth of a GBS subtype that lacked the polysaccharide capsule. In contrast, Survanta (10 and 20 mg/ml) significantly promoted the growth of E. coli, indicating that surfactant components may actually serve as nutrients. We conclude that bacterial growth in different surfactant preparations is influenced by microbial species and the composition and dose of the surfactant. Further studies are necessary to elucidate the mechanisms behind our findings and to evaluate the effects of surfactant on bacterial growth in vivo.
Pulmonary surfactant is a complex mixture of phospholipids and specific proteins that line the alveolar surface of the lung. Its major function is to reduce surface tension, thereby protecting the alveoli against collapse at the end of expiration (8, 11, 39). In addition, the role of surfactant in host defense against inhaled bacteria has been recognized over the last several years (for a review, see reference 38).
Since the observation that surfactant deficiency in lungs of premature newborns is responsible for the respiratory distress syndrome (RDS), exogenous surfactant therapy has become an established treatment of RDS (17). However, surfactant dysfunction has been described in other pulmonary diseases. Surfactant inactivation probably plays a key role in the pathophysiology of acute RDS due to pneumonia in neonates, infants, and adults (32). The infection provokes an influx of inflammatory cells with a resulting release of cytokines, enzymes, and reactive oxygen metabolites. Disruption of the epithelial-endothelial barrier leads to leakage of plasma proteins into the airspaces with consequent inhibition of surfactant function. The detrimental effects of plasma proteins on surfactant function can be overcome in vitro by increasing the surfactant concentration (7, 15).
Group B streptococci (GBS), Staphylococcus aureus, and Escherichia coli are responsible for most cases of early-onset infections in the neonatal period (1, 29). Neonates with severe respiratory failure due to pneumonia caused by these organisms may therefore receive exogenous surfactant. It has been speculated that surfactant given under such circumstances might serve as a nutrient for bacteria and thereby promote microbial growth (31). Only a few reports, with diverg results, have been published concerning the direct influence of surfactant on bacterial growth (3, 16, 19, 23). Either these studies evaluated the effects of low phospholipid concentrations (<1 mg/ml) or the authors did not specify the phospholipid concentration of the surfactant material as crude extracts of bronchoalveolar lavage fluid from animal sources were used. Only two studies have analyzed bacterial growth in the presence of commercially available surfactant preparations currently used for replacement therapy in newborns. Sherman et al. (31) reported that complete natural surfactant derived from human amniotic fluid or natural sheep bronchial lavage fluid promoted the growth of GBS, whereas Exosurf, a synthetic surfactant containing two alcohols as spreading agents, was bactericidal. Intermediate effects were observed for modified natural surfactants derived from bovine, porcine, or calf lungs. Neumeister et al. (27) observed that the modified bovine surfactant Survanta significantly promoted the growth of E. coli. However, these observations were either limited to one bacterial strain (31) and/or one phospholipid concentration (27, 31).
The purpose of this study was to examine the effects of different phospholipid concentrations of three modified natural (Curosurf, Alveofact, and Survanta) and two synthetic (Exosurf and Pumactant) surfactant preparations on the in vitro proliferation of GBS, S. aureus, and E. coli.
MATERIALS AND METHODS
Bacterial strains.
By repeated gradient centrifugation (9), we produced a low-density and a high-density phase variant (LD and HD, respectively) from the GBS strain 090 Ia Colindale. The strain was a gift from Stellan Håkansson, University of Umeå, Umeå, Sweden, and was originally isolated from a neonate with early-onset septicemia. We also studied two GBS wild-type strains (Lancefield serotypes Ib and III) isolated from two neonates with GBS septicemia. The serotypes Ia, Ib, and III account for most neonatal infections, whereas subtype II is more commonly observed in GBS-infected adults. The GBS LD subpopulation is abundantly encapsulated, whereas GBS HD lacks a polysaccharide capsule.
S. aureus 25923 and E. coli 25922 were obtained from the American Type Culture Collection, Rockville, Md. These strains were used to facilitate comparison with a previous study (27).
All strains were stored at −70°C in 1-ml aliquots of nutrient broth (Standard I; Merck, Darmstadt, Germany) composed of the following (concentrations are in milligrams per milliliter): peptone, 15.0; sodium chloride, 6.0; yeast extract, 3.0; and d(+)-glucose, 1.0. Before use bacteria were transferred to 11.5 ml of broth and cultured for 16 h at 37°C. Working cultures were made by growing the bacteria to the mid-logarithmic phase. For this, the starter culture was diluted 1:7 with fresh prewarmed broth and incubated for 3 h at 37°C. Subsequently, the bacteria were harvested by centrifugation at 1,800 × g for 10 min, washed twice with sterile isotonic saline, and resuspended in saline at a final concentration of 8 × 108 CFU/ml. Bacterial suspensions were adjusted to the desired concentrations by determination of the optical density at 595 nm (Ultrospec III; Pharmacia Biotech, Freiburg, Germany) with reference to standard curves established by plating 10-fold dilutions of the suspension on Columbia agar plates with 5% sheep blood (Oxoid, Wesel, Germany).
Surfactant preparations.
Curosurf (batch no. 96/0052; Chiesi Farmaceutici, Parma, Italy) is produced from minced pig lungs and consists of 99% phospholipids and 1% surfactant proteins. Alveofact (SF-RI 1, July 1997; Dr. Karl Thomae, Ltd., Biberach, Germany), a compound obtained from bovine lung lavage, is composed of 90% phospholipids, about 1% proteins, 3% cholesterol, 0.5% free fatty acids, and other components, including triglycerides. Survanta (batch no. 95-896Z7; Abbott, Ltd., Wiesbaden, Germany) is prepared by lipid extraction of minced bovine lungs and contains approximately 84% phospholipids, 1% proteins, and 6% free fatty acids. To this preparation dipalmitoylphosphatidylcholine (DPPC), palmitic acid, and tripalmitin are added to standardize the composition. Exosurf (batch no. T4798A; Wellcome, Burgwedel, Germany), a totally synthetic, protein-free surfactant, is composed of DPPC (∼84%), cetyl alcohol, and tyloxapol. Pumactant (ALEC [artificial lung expanding compound] batch no. 82; Britannia Pharmaceuticals, Redhill, Surrey, United Kingdom) is an artificial, protein-free compound composed to 100% of a mixture of DPPC and phosphatidylglycerol at a weight ratio of 7:3.
Table 1 demonstrates the relative differences in composition of each of the commercially available surfactant preparations in comparison with human natural surfactant obtained from amniotic fluid. Human surfactant contains a small proportion of carbohydrates, antioxidants, and antibacterial peptides. Such components (e.g., the antibacterial peptide prophenin [36] detected in Curosurf) or platelet-activating factor (detected in Survanta and Curosurf [26]) are contained in trace amounts in modified natural surfactants.
TABLE 1.
Composition of different surfactant preparations compared to human natural surfactant
| Component | % Composition (wt/wt)a
|
|||||
|---|---|---|---|---|---|---|
| Alveofact | Curosurf | Survanta | Exosurf | Pumactant | Natural surfactant | |
| Phospholipidsb | 88 | 99 | 84 | 82 | 100 | 81 |
| PC | 72 | 78 | 62 | 82 | 70 | 63 |
| Lyso-PC | <1 | <1 | 1 | 0 | 0 | 0.5 |
| PG | 8 | 3.5 | 2.5 | 0 | 30 | 5 |
| Cholesterol and neutral lipids | 4 | 0 | Not stated | 0 | 0 | 5–10 |
| Free fatty acids | 0.5 | <0.5 | 6 | 0 | 0 | 1.5 |
| Proteins | 1.5 (only SP-B and SP-C) | 1 (only SP-B and SP-C) | 0.5–1 (only SP-B and SP-C) | 0 | 0 | 5–10 (SP-A, SP-B, SP-C, SP-D) |
| Other components | Not statedc | Not statedc | DPPC, neutral lipids are addedc | 11 (cetyl-alcohol), 7 (tyloxapol)c | None | 1 (carbohydrates, antioxidants, anionic peptides, etc.) |
Values are modified from references 30 and 37 and from product information. Source: Alveofact, bovine lung lavage; Curosurf, porcine lung homogenate; Survanta, bovine lung homogenate; Exosurf, synthetic; Pumactant, synthetic; natural surfactant, human amniotic fluid. SP, surfactant protein.
PC, phosphatidylcholine; PG, phosphatidylglycerol.
All preparations contain various amounts of sodium chloride and/or other stabilizing agents (e.g., sodium hydrogen carbonate).
Bacterial growth in surfactant.
To determine the effects of different surfactant preparations on bacterial growth, we incubated a bacterial suspension containing 7 × 107 CFU/ml with different concentrations of surfactant (1, 10, and 20 mg/ml) or without surfactant (control) in saline at a final volume of 1 ml. Saline was used to mimic a nutrient-free medium that would allow the bacteria to use surfactant components as nutrients. Due to carbon dioxide exchange the pH in the alveolar lining fluid is as low as 6.9 and the potassium concentration is higher than in serum (28). However, adjustment of the pH of our samples to 6.9 with a buffer, or adapting the electrolyte content to that of alveolar fluid (sodium, 135 mmol/liter; chloride, 103 mmol/liter; potassium, 7.3 mmol/liter; calcium, 3.2 mmol/liter) had no effect on the survival of GBS LD in comparison with incubation in normal saline alone (Table 2). Since the metabolic rate of bacteria incubated in a nutrient-free medium is low, the pH of GBS suspended in saline was nearly constant throughout the 5-h experiment. Since the surfactant phospholipids are suspended in saline the pH of Curosurf (pH 5.7) is virtually identical to that of saline (pH 5.8) so that the addition of different amounts of Curosurf did not alter pH of the suspension.
TABLE 2.
Growth and/or survival of GBS strain 090 la LD
| Medium (mg/ml) | Mean CFU/ml (107) ± SD at time (h):
|
|||
|---|---|---|---|---|
| 0 | 1 | 3 | 5 | |
| Normal saline | 5.3 ± 0.9 | 5.9 ± 1.7 | 4.8 ± 1.3 | 4.0 ± 1.5 |
| Electrolyte solution | 5.2 ± 0.7 | 5.1 ± 0.8 | 6.4 ± 0.9 | 4.7 ± 0.6 |
| Nutrient-rich medium | ||||
| Without surfactant | 6.4 ± 0.6 | 15.2 ± 3.9 | 52.4 ± 11.4 | 59.3 ± 4.9 |
| With Curosurf (1) | 6.2 ± 2.0 | 13.5 ± 0.6 | 47.4 ± 7.8 | 66.1 ± 1.6 |
| With Curosurf (10) | 6.1 ± 1.6 | 14.8 ± 2.6 | 47.9 ± 4.0 | 58.8 ± 3.9 |
| With Curosurf (20) | 6.0 ± 0.8 | 14.2 ± 2.1 | 51.7 ± 6.7 | 59.8 ± 7.1 |
Growth and/or survival was measured in sterile saline in an electrolyte solution (28) mimicking alveolar fluid electrolyte content (sodium, 135 mmol/liter; chloride, 103 mmol/liter; potassium, 7.3 mmol/liter; calcium, 3.2 mmol/liter) and in a nutrient-rich medium (Standard I nutrient broth) containing 1, 10 or 20 mg of Curosurf per ml or no surfactant. Values are the mean CFU counts from four experiments.
When we incubated GBS in a nutrient-rich medium in the presence or absence of Curosurf (1, 10, or 20 mg/ml) a 10-fold increase in bacterial numbers occurred within 5 h and no effect of surfactant could be seen (Table 2). Based on these results, we used normal sterile saline for our final comparative studies.
The tubes containing saline, bacteria, and surfactant were placed on a horizontal shaker (Thermoshake TH 05; Gerhardt, Bonn, Germany) at 120 rpm for 5 h at 37°C. At 0 and 5 h an aliquot of 100 μl was taken from each tube, serially diluted in saline, and spread onto blood agar plates. The number of colonies after 24 h of incubation at 37°C was counted, and the CFU per milliliter were calculated from the colony counts and the respective dilutions.
Statistical analysis.
All data represent the mean and standard deviation (SD) of six repeated experiments. For analysis, a logarithmic transformation of the data (log10 CFU/ml) was calculated, since bacterial growth follows a logarithmic growth curve. Data points in figures were calculated as follows: the log10 CFU/ml at time zero was subtracted from that at 5 h, so that a positive log10 difference [Δ(5 h, 0 h) log10 CFU/ml] indicates the bacterial growth, and a negative log10 difference represents a decrease in the number of bacteria. The statistical significance between the growth rate of bacteria incubated with surfactant versus the saline control group was tested by Dunnett's multiple comparison test for analysis of variance using GraphPad software (GraphPad Software, Inc., San Diego, Calif.). Statistical significance was accepted at P values of <0.05.
RESULTS
Effects of modified natural surfactants on bacterial survival. (i) Curosurf.
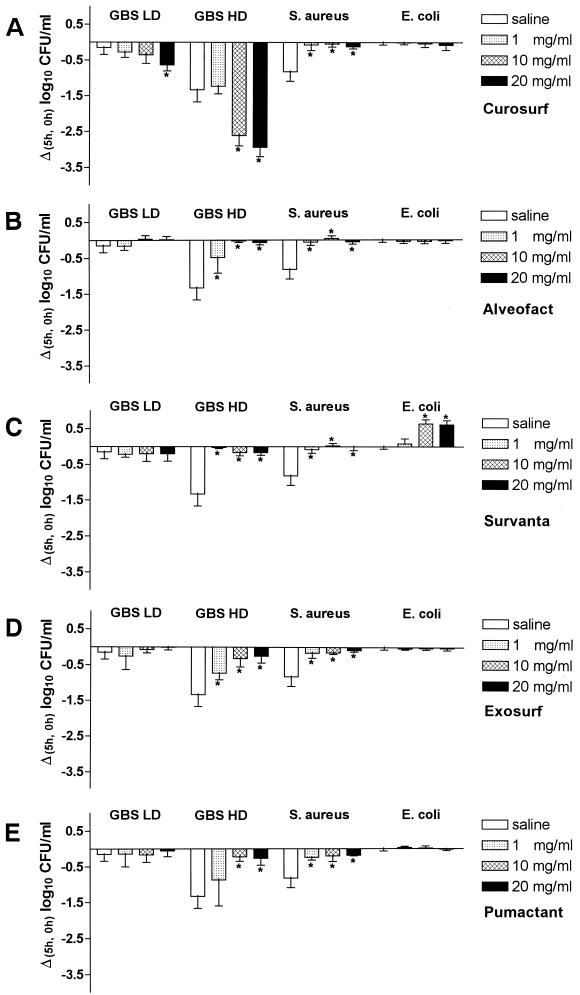
Curosurf in normal saline reduced the growth of both GBS with (LD) or without (HD) polysaccharide capsule (Fig. 1A). The number of viable HD GBS after 5 h of incubation was significantly decreased at Curosurf concentrations of 10 and 20 mg/ml compared to saline controls without Curosurf. No such decrease in bacterial numbers was observed for S. aureus or E. coli. Compared to S. aureus incubated in saline, the numbers of CFU per milliliter were slightly higher in solutions containing surfactant (Fig. 1A), but there was no increase in bacterial numbers compared to the number of CFU inoculated into the medium at the beginning of the experiments (0 h).
FIG. 1.
Effects of Curosurf (A), Alveofact (B), Survanta (C), Exosurf (D), and Pumactant (E) on the in vitro growth of different bacterial strains. A total of 7 × 107 CFU of bacteria per ml were incubated with different concentrations of surfactant (1, 10, and 20 mg/ml) or without surfactant (saline) for 5 h at 37°C. Values are mean [Δ(5 h, 0 h)] log10 CFU/ml ± the SD obtained from six experiments. ∗, P < 0.01 versus saline.
(ii) Alveofact.
In the presence of Alveofact, all gram-positive bacteria demonstrated increased viability compared to GBS HD, GBS LD, or S. aureus incubated in saline alone (Fig. 1B). In contrast to the findings with Curosurf, no inhibitory effect was observed on the growth of both phase variants of GBS. No differences were observed between the effects of Curosurf and Alveofact when the surfactants were incubated with S. aureus. The survival of E. coli was not influenced by Alveofact (Fig. 1B).
(iii) Survanta.
The addition of different concentrations of Survanta to GBS LD did not alter bacterial survival over the 5-h period, whereas Survanta seemed to protect GBS HD and S. aureus against the bactericidal effect of 5 h of incubation in saline alone (Fig. 1C). Unlike all other tested surfactant preparations, Survanta significantly promoted the growth of E. coli. At phospholipid doses of ≥10 mg/ml, the numbers of CFU per milliliter were increased 4.5 times compared to the bacterial count at the beginning of the experiments (0 h).
Effects of synthetic surfactants on bacterial survival: Exosurf and Pumactant.
Incubation of bacteria in Exosurf (Fig. 1D) or Pumactant (Fig. 1E) resulted in a similar growth pattern. No effects were observed on GBS LD and E. coli. However, compared to the incubation in saline alone, both surfactants protected GBS HD and S. aureus from the negative impacts of incubation on cell viability in a nutrient-free medium.
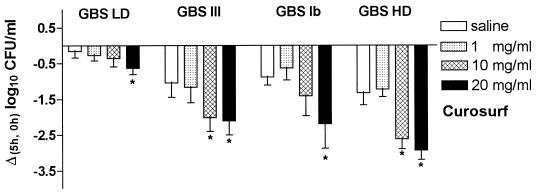
Bacterial survival of different GBS subtypes in the presence of Curosurf.
Figure 2 shows the survival of different GBS subtypes incubated in saline or in saline containing 1, 10, or 20 mg of Curosurf per ml. The nonencapsulated HD variant of GBS was most sensitive to the negative impact of surfactant on survival. At Curosurf concentrations of 10 or 20 mg/ml, <1% of the initial number of bacteria were viable after 5 h of incubation. In contrast, the abundantly encapsulated strain GBS LD survived 5 h of incubation in saline without a significant decrease in the number of CFU. The effects observed following incubation of GBS LD with 20 mg of Curosurf per ml were rather moderate compared to incubation of GBS HD and the GBS wild-type strains with the same surfactant dose.
FIG. 2.
Effects of Curosurf on in vitro growth of different GBS subtypes. A total of 7 × 107 CFU of the encapsulated GBS LD (serotype Ia), wild-type GBS serotype III, wild-type GBS serotype Ib, or nonencapsulated GBS HD per ml were incubated with different concentrations of surfactant (1, 10, and 20 mg/ml) or without surfactant (saline) for 5 h at 37°C. Values are mean [Δ (5 h, 0 h)] log10 CFU/ml ± the SD from six experiments. ∗, P < 0.01 versus saline.
DISCUSSION
We evaluated bacterial proliferation under the influence of different natural and synthetic surfactant preparations that are in clinical use in Europe and/or the United States. For our studies we chose GBS, S. aureus, and E. coli since more than 75% of cases of early-onset neonatal septicemia are caused by these organisms. Connatal infections often trigger premature birth and might be followed by respiratory failure within the first hours of life. Studies of surfactant treatment in infants with “idiopathic” RDS reveal that up to 20% of surfactant-treated neonates demonstrate signs of infection within the first days of life (for a review, see reference 12). All of the surfactant preparations investigated in this study have therefore been used in newborns with respiratory failure due to pneumonia.
Recommended doses for surfactant replacement therapy vary between 50 and 200 mg/kg of body weight for the initial treatment of babies with RDS. It has been recognized that doses of 300 mg of surfactant per kg (body weight) may be needed to overcome surfactant inhibition in pneumonia (35) or meconium aspiration syndrome (6). Such high doses would, even in babies devoid of endogenous surfactant, probably result in phospholipid concentrations well above 10 mg/ml in the alveolar lining layer, at least after resorption of fetal lung liquid (21). We speculate, on the basis of our present findings, that the surfactant layer on the alveolar surface might be an important part of the pulmonary defense system.
We found that Curosurf inhibited survival of GBS in saline in a dose-related fashion. For S. aureus, incubation in saline alone had a bactericidal effect. When Curosurf was added S. aureus was protected to some extent against the negative impact of the nutrient-free medium on microbial viability. In contrast, the viability of E. coli was unaffected by Curosurf, as well as by Alveofact, Exosurf, and Pumactant. These results probably reflect variations in the metabolic demands of different bacteria. In our in vitro assay system the bacteria were incubated in sterile saline, a nutrient-free medium. However, our own results of incubation of GBS and Curosurf in a culture medium containing glucose and protein (Table 2), as well as similar studies by Neumeister et al. (27), demonstrated that incubation of bacteria in nutrient-rich growth-promoting broth might mask the effects of surfactant that were observed when saline alone was used as a medium. Under normal physiological circumstances the alveolar lining fluid may be considered to be relatively poor in nutrient content. However, in the course of pneumonia and mechanical ventilation, serum components, including albumin and glucose, may leak into the bronchoalveolar space and increase the amount of nutrients available for bacterial proliferation.
Both the nonencapsulated GBS HD and S. aureus showed a slight decline in CFU during the 5-h incubation in sterile saline alone. GBS HD, the nonencapsulated phase variant, demonstrated a strong decline in viability when incubated with Curosurf, whereas GBS LD was clearly less susceptible under similar conditions, probably protected by the polysaccharide capsule. The capsule is an important virulence factor in GBS infections, and wild-type strains often contain a mixture of both encapsulated and nonencapsulated bacteria (9). These findings demonstrate that different subtypes of one bacterial species might differ in their interactions with surfactant. When we tested different clinical isolates from infants with GBS septicemia, the observed variation was small compared to the differences between different bacterial species. A similar observation was made by Neumeister et al. (27), who compared the influence of surfactant on different reference strains and several clinical isolates of GBS, S. aureus, and E. coli.
Several years ago, Coonrod and Yoneda (3) demonstrated that the surfactant fraction of rat alveolar lining material caused lysis of Streptococcus pneumoniae and several other gram-positive bacteria (Streptococcus viridans, Streptococcus pyogenes, and Streptoccoccus bovis). These authors speculated that the observed bactericidal effect was due to free fatty acids contained in the lung lavage preparation (5). More recently, Brogden et al. (2) described an anionic bactericidal peptide in bovine pulmonary surfactant, and prophenin-1, an antibacterial peptide that might be associated with surfactant lipids, has been isolated from porcine leukocytes (10). This antibacterial peptide has also been found in Curosurf, a surfactant extracted from porcine lung homogenate (36). Part of the observed effects may thus be due to direct negative effects of these bactericidal peptides on the bacterial cell wall. The polarity of these peptides seems to be most important for their antibacterial activity. It has been shown that changes in, for example, the sodium, zinc, calcium, or phosphorus content of the incubation medium can modify the in vitro bactericidal activity of such peptides (2, 10, 36).
We found that all of the investigated surfactant preparations protected S. aureus from the negative effects of saline on bacterial growth. This might indicate that staphylococci can catabolize surfactant lipids to some extent. The production and release of phospholipases by S. aureus has been described (24). LaForce et al. (22) reported increased growth of S. aureus after incubation with complete natural rabbit surfactant. Apparently, the bacteria can use surfactant components as nutrients. Natural surfactant isolated by lung lavage and subsequent sucrose gradient centrifugation contains a small proportion of carbohydrates (<1%) and ca. 10% proteins, including the specific surfactant-associated proteins (SP-A, SP-B, SP-C, and SP-D) (18). The hydrophilic proteins SP-A and SP-D are potent stimulators of macrophage function and are generally believed to serve as important components of the pulmonary host defense system against invading microorganisms (34). However, these proteins are removed by extraction with organic solvents and therefore absent in all of the industrially produced modified natural surfactants examined in the present study. SP-B, present in small amounts in all modified natural surfactants, may in itself have a bacteriostatic effect (see below).
The relative resistance of gram-negative E. coli to each of the investigated surfactants may reflect the failure of the surfactant molecules to penetrate the lipopolysaccharide layer. In fact, incubation of E. coli with Survanta significantly promoted bacterial growth. This has also been reported by other investigators (27). Recently, it has been shown that proliferation of E. coli is inhibited by mature human pulmonary SP-B or, more specifically, by residues 12 to 34 of SP-B (20). The reason for the observed proliferation of E. coli is unclear, but Survanta contains relatively little SP-B (25, 30) and, in contrast to the other modified natural surfactants examined, it is enriched with artificial lipids. Increased growth of E. coli has also been reported after exposure to a crude surfactant preparation obtained from dog lungs (16). In the present study, synthetic surfactants containing lipids only (Pumactant) or lipids plus spreading agents (Exosurf) had no effect on the proliferation of E. coli. Although differences in surfactant composition might explain some of these seemingly conflicting results, species differences may also play a role. For example, the clearing rate of inhaled pneumococci varies between different animals (4).
Our finding that some surfactant preparations may enhance bacterial survival or even promote bacterial proliferation (as observed for Survanta and E. coli) is certainly alarming and should be further studied in animal experiments. So far most studies on surfactant for treatment of inflammatory lung disease have focused on gas exchange. Song et al. demonstrated improved lung function in rats with E. coli pneumonia treated with Curosurf (33). Unfortunately, no attempts were made to examine bacterial growth in that study. Interestingly, the present in vitro data obtained with Curosurf and GBS are in keeping with our previous observations made with GBS-infected newborn rabbits, showing mitigation of bacterial proliferation in lung homogenate following treatment with this particular surfactant preparation (13).
Clinical and radiological signs do not differentiate with certainty between pneumonia and RDS in the first hours of life. Since we and others have observed that some surfactant preparations might promote bacterial growth, infants with severe respiratory failure treated with surfactant should receive antibiotic therapy until infection can be ruled out by culture and laboratory findings.
We conclude that bacterial growth in the presence of surfactant depends on the bacterial species and the origin and concentration of the applied surfactant preparation. Except for cultures of E. coli in Survanta, most surfactants do not seem to promote bacterial growth. However, E. coli is now rarely isolated from blood cultures or tracheal aspirate fluid in the neonatal period. Curosurf significantly diminished the proliferation of GBS, the organism that accounts for most cases of early-onset septicemia. Initial clinical observations give us no reason to believe that treatment with surfactant should have serious adverse effects in neonates with connatal pneumonia (14) but, clearly, further in vivo studies are necessary to clarify the relevance of the effects observed here. Even if exogenous surfactant obtained from animal lungs influences bacterial growth in vitro, the effects of exogenous phospholipids on the microbiology of the human lung remain unclear. Careful follow-up of babies with bacterial infections treated with surfactant therefore seems mandatory.
ACKNOWLEDGMENTS
We thank Docent Connie Jarstrand, Department of Immunology, Microbiology, Pathology, and Infectious Diseases, Karolinska Institute, Huddinge Hospital, Stockholm, Sweden, for technical advice and critical discussion.
This study was supported by the Deutsche Forschungsgemeinschaft (DFG He 2072/2-2), the Swedish Medical Research Council (project 3351), and a collaborative project of the German Academic Exchange Service and the Swedish Institute (313/S-PPP).
REFERENCES
- 1.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. pp. 980–1054. [Google Scholar]
- 2.Brogden K A, De Lucca A J, Bland J, Elliot S. Isolation of an bovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc Natl Acad Sci USA. 1996;93:412–416. doi: 10.1073/pnas.93.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coonrod J D, Yoneda K. Detection and partial characterization of antibacterial factors in alveolar lining material of rats. J Clin Investig. 1983;71:129–141. doi: 10.1172/JCI110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coonrod J D, Varble R, Jarrells M C. Species variation in the mechanism of killing inhaled pneumococci. J Lab Clin Med. 1990;116:354–362. [PubMed] [Google Scholar]
- 5.Coonrod J, Lester R L, Hsu L C. Characterization of the extracellular bactericidal factors of rat alveolar lining material. J Clin Investig. 1984;74:1269–1279. doi: 10.1172/JCI111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Findlay R D, Taeusch H W, Walther F J. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996;97:48–52. [PubMed] [Google Scholar]
- 7.Fuchimukai T, Fujiwara T, Takahashi A, Enhorning G. Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol. 1987;62:429–437. doi: 10.1152/jappl.1987.62.2.429. [DOI] [PubMed] [Google Scholar]
- 8.Goerke J. Pulmonary surfactant: functions and molecular composition. In: van Golde L M G, editor. Pulmonary surfactant: from surface chemistry via biochemistry to clinical practice. BBA-Molecular Basis of Disease Section. Amsterdam, The Netherlands: Elsevier Science Publishers; 1998. pp. 79–89. [Google Scholar]
- 9.Håkansson S, Granlund-Edstedt M, Sellin M, Holm S E. Demonstration and characterization of buoyant-density subpopulations of group B streptococcus type III. J Infect Dis. 1990;161:741–746. doi: 10.1093/infdis/161.4.741. [DOI] [PubMed] [Google Scholar]
- 10.Harwig S S L, Kokryakov V N, Swiderek K M, Aleshina G M, Zhao C, Lehrer R I. Prophenin-1, an exceptionally proline-rich antimicrobial peptide from porcine leukocytes. FEBS Lett. 1995;362:65–69. doi: 10.1016/0014-5793(95)00210-z. [DOI] [PubMed] [Google Scholar]
- 11.Hawgood S, Clements J A. Pulmonary surfactant and its apoproteins. J Clin Investig. 1990;86:1–6. doi: 10.1172/JCI114670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herting E. Surfactant therapy for neonatal pneumonia and for meconium aspiration syndrome. In: Wauer R R, editor. Surfactant therapy: basic principles, diagnosis, therapy. Stuttgart, Germany: Thieme; 1998. pp. 124–132. [Google Scholar]
- 13.Herting E, Jarstrand C, Rasool O, Curstedt T, Sun B, Robertson B. Experimental neonatal group B streptococcal pneumonia: effect of a modified porcine surfactant on bacterial proliferation in ventilated near-term rabbits. Pediatr Res. 1994;36:784–791. doi: 10.1203/00006450-199412000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Herting, E., O. Gefeller, M. Land, L. van Sonderen, K. Harms, B. Robertson, and members of the Collaborative European Multicenter Study Group. Surfactant treatment of neonates with respiratory failure and group B streptococcal infection. Pediatrics, in press. [DOI] [PubMed]
- 15.Holm B A, Enhorning G, Notter R H. A biophysical mechanism by which plasma proteins inhibit lung surfactant activity. Chem Phys Lipids. 1988;49:49–55. doi: 10.1016/0009-3084(88)90063-1. [DOI] [PubMed] [Google Scholar]
- 16.Jalowayski A A, Giammona S T. The interaction of bacteria with pulmonary surfactant. Am Rev Respir Dis. 1972;105:236–241. doi: 10.1164/arrd.1972.105.2.236. [DOI] [PubMed] [Google Scholar]
- 17.Jobe A H. Pulmonary surfactant therapy. N Engl J Med. 1993;328:861–868. doi: 10.1056/NEJM199303253281208. [DOI] [PubMed] [Google Scholar]
- 18.Johansson J. The proteins of the surfactant system. Eur Respir J. 1994;7:372–391. doi: 10.1183/09031936.94.07020372. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson S, Musher D, Goree A, Lawrence E C. Human alveolar lining material and antibacterial defenses. Am Rev Respir Dis. 1986;133:136–140. doi: 10.1164/arrd.1986.133.1.136. [DOI] [PubMed] [Google Scholar]
- 20.Kaser M R, Skouteris G G. Inhibition of bacterial growth by synthetic SP-B1-78 peptides. Peptides. 1997;18:1441–1444. doi: 10.1016/s0196-9781(97)00211-8. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Shido A, Nitta K, Inui S, Ganzuka M, Robertson B. The critical concentration of surfactant in fetal lung liquid at birth. Respir Physiol. 1990;80:181–192. doi: 10.1016/0034-5687(90)90082-a. [DOI] [PubMed] [Google Scholar]
- 22.LaForce F M. Effect of alveolar lining material on phagocytic and bactericidal activity of lung macrophages against Staphylococcus aureus. J Lab Clin Med. 1976;88:691–699. [PubMed] [Google Scholar]
- 23.LaForce F M, Boose D S. Sublethal damage of Escherichia coli by lung lavage. Am Rev Respir Dis. 1981;124:733–737. doi: 10.1164/arrd.1981.124.6.733. [DOI] [PubMed] [Google Scholar]
- 24.Matos J E, Harmon R J, Langlois B E. Lecithinase reaction of Staphylococcus aureus strains of different origin on Baird-Parker medium. Lett Appl Microbiol. 1995;21:334–335. doi: 10.1111/j.1472-765x.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno K, Ikegami M, Chen C M, Ueda T, Jobe A H. Surfactant protein-B supplementation improves in vivo function of a modified natural surfactant. Pediatr Res. 1995;37:271–276. doi: 10.1203/00006450-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Moya F R, Hoffman D R, Zhao B, Johnston J M. Platelet activating factor in surfactant preparations. Lancet. 1993;345:858–860. doi: 10.1016/0140-6736(93)93062-6. [DOI] [PubMed] [Google Scholar]
- 27.Neumeister B, Woerndle S, Bartmann P. Effects of different surfactant preparations on bacterial growth in vitro. Biol Neonate. 1996;70:128–134. doi: 10.1159/000244357. [DOI] [PubMed] [Google Scholar]
- 28.Nielsson D W. Electrolyte composition of pulmonary alveolar subphase in anesthetized rabbits. J Appl Physiol. 1986;60:972–979. doi: 10.1152/jappl.1986.60.3.972. [DOI] [PubMed] [Google Scholar]
- 29.Philip A G S. The changing face of neonatal infection: experience at a regional medical center. Pediatr Infect Dis J. 1994;13:1098–1102. doi: 10.1097/00006454-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Seeger W, Grube C, Günther A, Schmidt R. Surfactant inhibition by plasma proteins: differential sensitivity of various surfactant preparations. Eur Respir J. 1993;6:971–977. [PubMed] [Google Scholar]
- 31.Sherman M P, Campbell L A, Merritt T A, Long W A, Gunkel J H, Curstedt T, Robertson B. Effect of different surfactants on pulmonary group B streptococcal infection in premature rabbits. J Pediatr. 1994;125:939–947. doi: 10.1016/s0022-3476(05)82013-x. [DOI] [PubMed] [Google Scholar]
- 32.Somerson N L, Kontras S B, Pollack J D, Weiss H S. Pulmonary compliance: alteration during infection. Science. 1971;171:66–68. doi: 10.1126/science.171.3966.66. [DOI] [PubMed] [Google Scholar]
- 33.Song G W, Robertson B, Curstedt T, Gan X Z, Huang W X. Surfactant treatment in experimental Escherichia coli pneumonia. Acta Anaesthesiol Scand. 1996;40:1152–1159. doi: 10.1111/j.1399-6576.1996.tb05580.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Golde L M G. Potential role of surfactant proteins A and D in innate lung defense against pathogens. Biol Neonate. 1995;67(Suppl. 1):2–17. doi: 10.1159/000244202. [DOI] [PubMed] [Google Scholar]
- 35.Walmrath D, Günther A, Ghofrani H A, Schermuly R, Schneider T, Grimminger F, Seeger W. Bronchoscopic surfactant administration in patients with severe adult respiratory distress syndrome and sepsis. Am J Respir Crit Care Med. 1996;154:57–62. doi: 10.1164/ajrccm.154.1.8680699. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Griffiths W J, Curstedt T, Johansson J. Porcine pulmonary surfactant preparations contain the antibacterial peptide prophenin and a C-terminal 18-residue fragment thereof. FEBS Lett. 1999;460:257–262. doi: 10.1016/s0014-5793(99)01363-0. [DOI] [PubMed] [Google Scholar]
- 37.Wauer R. Respiratory distress syndrome. In: Wauer R R, editor. Surfactant therapy: basic principles, diagnosis, therapy. Stuttgart, Germany: Thieme; 1998. pp. 2–19. [Google Scholar]
- 38.Wright J R. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 39.Wright J R, Clements J A. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987;135:426–444. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]