Abstract
Free-living amoebas (FLAs) are microorganisms, unicellular protozoa widely distributed in nature and present in different environments, such as water or soil; they are maintained in ecosystems and play a fundamental role in the biological control of bacteria, other protozoa, and mushrooms. In particular circumstances, some can reach humans or animals, promoting several health complications. Notably, FLAs are characterized by a robust capacity to survive in extreme environments. However, currently, there is no updated information on the existence and distribution of this protozoan in inhospitable places. Undoubtedly, the cellular physiology of these protozoan microorganisms is very particular. They can resist and live in extreme environments due to their encysting capacity and tolerance to different osmolarities, temperatures, and other environmental factors, which give them excellent adaptative resistance. In this review, we summarized the most relevant evidence related to FLAs and the possible mechanism, which could explain their adaptative capacity to several extreme environments.
1. Introduction
Amoebas are unicellular protist microorganisms belonging to the genus amoeba of the family Amoebidae that contains five described genera. Free-living amoebas (FLAs) develop their lives in the environment and are characterized by the lack of a cell wall in the trophozoite stage, which allows them to extend their cytoplasm to mobilize, resulting in the formation of pseudopods, further enabling them to feed on smaller microorganisms, mainly bacteria or decaying particles. Therefore, it plays an essential biological role in the control of bacterial populations. [1, 2]. Amoebas are found in various environments; however, environmental conditions can affect their survival, such as pH, temperature, hydrogen sulfide concentration, and salinity. These conditions directly influence the structure of amoeba communities, mainly in aquatic-type environments [3]. These microorganisms survive in adverse environments using osmoregulation to control the water inside to cell through vacuoles [4]. This mechanism is regulated by the expression of aquaporins in the contractile vacuole membrane. The expression of V-ATPase (vacuolar H+-ATPase) in the vacuolar membrane has been reported. V-ATPases move H+ inside, lowering the vacuole's pH relative to the cytosol, promoting an osmosis gradient across the vacuole membrane, facilitating water entry through aquaporins in the vacuole membrane [5, 6].
The first description of an amoeba was in 1826 as a membranous type of microorganism whose shape is modified to move in the environment [7]. However, the first published evidence of this microorganism dates back to 1755 by the German naturist Rösel von Rosenhof AJ in Insecten-Belustigung (extracted from [8]). Currently, there are two classes of amoebae that differ from each other; FLA are those that live free in the environment that do not strictly need a host, and parasitic that needs a host and are mostly pathogenic to humans with the ability to generate serious diseases [8].
Until 2002, amoebas, such as eukaryotic microorganisms, have been studied mainly for their morphology, characterizing them as testate amoebas; however, few differences and morphological structures led to the lack of classification and differentiation between the diverse species of amoebas [9]. With the advances in molecular biology, a new classification era of these organisms arises, with the development of sequencing of specific genes based initially on a small sequence of the 18 s gene, or SSU-rDNA [10]. After, universal and conserved genes were used (cytoskeleton actin and tubulin protein genes) that have brought with them greater resolution in the identification and classification of supergroups [11]. Accordingly, three supergroups that have been identified were, Amoebozoa supergroup brings together most of the organisms capable of producing lobed pseudopods, Rhizaria, which is characterized by creating filamentous pseudopods; and the Excavata, which brings together a large part of the flagellate heterotrophs [11, 12].
With all current information, it is possible to speculate that the differences in amoebas could be associated with the presence or absence of flagella, developed with the expansion of these organisms to different substrates to seek favorable conditions promoting their survival, searching for nutrients from bacteria. The evolution of these microorganisms is not entirely clear; however, fossil evidence of Arcellinida testate proves the first appearance of eukaryotic cells for more than 700 million years [13, 14].
As aforementioned, these cosmopolitan organisms can be found across environments where life is carried to the maximum of its survival capabilities (extreme environments), with extremely high or low ranges of temperature, radiation, pressure, acidity, alkalinity, salinity, sulfur, and among others. FLAs, similar to other species, have developed several strategies to survive in hostile environments through the formation of cysts [15]. Indeed, cyst-forming FLAs can survive in several environments for many years. Thus, it has been shown that survival can range between 2 and 21 years, even in arid and cold climates (~4°C), offering high viability, particularly those belonging to the genus Acanthamoeba [16–18]. Indeed, through their robust adaptability [19, 20], FLAs could play an essential epidemiological role as a reservoir and vehicle for a wide variety of microorganisms; in addition, they contribute to plant growth, soil mineralization, and nutrient cycling [21]. Of note, it has been attributed to the genera Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris, Sappinia pedata, Vermamoeba vermiformis, and Paravahlkamfia francinae (all free-living amoebas) as etiologic factors in fatal central nervous system infections and other human serious diseases [8, 22–24]. These are amphizoid amoebas because they live as parasites on hosts and in their natural environment [25, 26].
Antibodies against Acanthamoeba are present in approximately 80% of the human population [27], suggesting that this FLA is an organism that comes into contact with humans regularly [28]. Acanthamoeba species do not require a host to survive; they can settle in tissues and cause serious illness [29–31]. Some species belonging to the genera Acanthamoebae, Naegleria, Balamuthia mandrillaris, and Sappinia pedata are carriers of other pathogens such as Legionella pneumophilia, Listeria monocytogenes, Mycobacterium avium, Pseudomonas aeruginosa, Pseudomona saccharophilia, and among others [32–36]. In addition to being associated with human diseases, these amoebas play an essential role in ecosystems, acting as predators and hosts of microorganisms such as Cryptosporidium and Toxoplasma [37–39]. Thus, the present review is focused on summaries of the more critical evidence related to amoebas living in extreme environments, and we will propose some hypothetical mechanisms which could help to explain, in part, this characteristic of the amoebas residing in harsh environments for several time. Accordingly, we could arise how these organisms survived for so many years on the earth.
1.1. Free-Living Amoebae
FLAs maintain their life cycle in the environment. Still, some species act as opportunistic and nonopportunistic pathogens that can affect different hosts, such as animals and humans, and thus carry out a monoxene life cycle. Acanthamoeba and Naegleria are referred to as amphizoid organisms because they can exist as both FLA and pathogenic parasites (Centers for Disease Control and Prevention [40]. Amoebas of the genus Acanthamoeba present two stages during their life cycle: (a) trophozoite (13–40 μm in diameter) or metabolically active vegetative form, which feeds on bacteria and smaller organisms and multiplies by binary fission, giving rise to two identical daughter cells and (b) cysts (6–10 μm in diameter) or forms of resistance [41–44]. Other amoebas, such as those in the genus Naegleria, have three morphological stages, one of which is a temporary amoeboflagellar stage in which the organism does not feed or reproduce and only serves to move to a better microenvironment [1, 45]. The cysts originate from the production of a protective covering by the trophozoite when it is under extreme environmental conditions such as changes in temperature, humidity, pH, nutrients, osmotic pressure, and among others, which covers it and turns it into a form of resistance. It generally has two layers: an external or ectocyst and an internal or endocyst; a third layer, the mesocyst, is present in some species. These cystic structures may explain why the cyst is so resistant to various disinfection methods, such as chlorination and sterilization of water systems, and mainly how they can survive extreme environments, such as the lagoons of the Atacama Desert see Figure 1. Inside the cyst, the trophozoite remains inactive until it is in a favorable environment, which initiates the process of disembedding [8]. Thus, the evidence is robust to show the resistance of the amoeba to survival in extreme environments, characterized by changes in humidity, temperature, and nutrients, which could affect its activity, quantity, and diversity [8].
Figure 1.
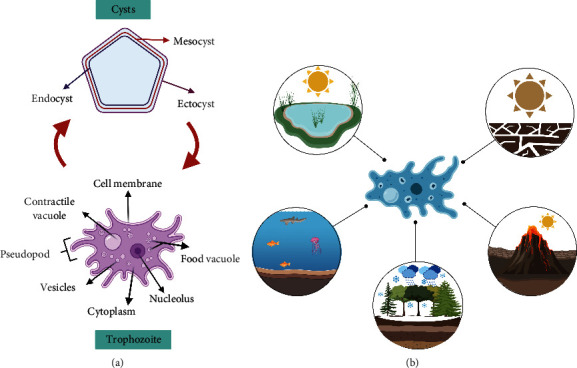
Free-living amoeba life cycle diagram. (a) Structure of the cyst, composed of three-layer: endocyst, mesocyst, and ectocyst. The presence of layer mesocyst is dependent on the genus. The trophozoite is the metabolically active vegetative form. (b) Diagram of environments where FLA can be found, created with http://BioRender.com/.
1.2. Taxonomy of Free-Living Amoebas
The International Society of Protozoologists proposed a new classification based on morphological, biochemical, and phylogenetic approaches. Eukaryotes are classified into six supergroups: Amoebozoa, Opisthokonta, Rhizaria, Archaeplastida, Chromalveota, and Excavata [46]. Three of these six groups correspond to FLA groups, such as the Amoebozoa supergroup, which has noneruptive pseudopods called lobopods that can be branched. The gymnamoebas, naked amoebas and a significant number of testate amoebas are grouped here. The Rhizaria supergroup amoebae have very fine pseudopods that can be simple, branched, anastomosing, or microtubule support (axopods). They have a very diverse way of life; some species are photosynthetic or parasites of plants and other organisms; they are fresh and saltwater aquatic amoebas, but they can also be found in soils [46, 47]. In many cases, a group of amoebae in the Excavata supergroup can have a flagellated phase of their life cycle. Although they differ from amebozoans, eruptive pseudopodia predominate in these organisms [1]. Acanthamoeba and Balamuthia are members of the Acanthamoebidae family, which is part of the Amoebozoa supergroup. The Excavata supergroup of the Vahlkampfiidae family includes Naegleria fowleri, Vahlkampfia, and Willaertia Sappinia, a family member of Thecamoebidae in the supergroup Amoebozoa [46, 48, 49].
Fowler and Carter discovered the Acanthamoeba and Naegleria genera in 1965, respectively. Some Acanthamoeba species were initially divided into three morphological groups (I, II, and III) [50, 51]. However, at the Acanthamoeba species level, this morphological classification is incorrect [52, 53]. The genus taxonomy is primarily based on morphological characteristics such as cyst size and morphology [19]. The disadvantage of this type of diagnosis based on morphological characteristics of the cyst varies depending on the method used [36]. Therefore, it is necessary to use cultures and molecular identification from specific primers for correct identification [43, 53]. Commonly used primers are based on the amplification of the particular genes for both species and genera of amoebae, such as FLA, which corresponds to a universal primer of the 18S rDNA gene [54], JDP, BAL, ITS, and NFITSFW name the specific primers for the genus Acanthamoeba spp. [54], Balamuthia mandrillaris [55], Naegleria spp. [56], and Naegleria fowleri [57, 58], respectively. In addition, it has been proposed that FLA diversity could be associated with developed places [59] it is believed such extreme environments, which could confer different properties to the FLA, a summary of the FLA taxonomy is shown in Figure 2.
Figure 2.
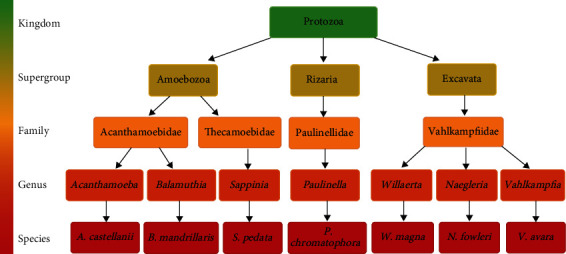
Taxonomic classification of free-living amoebas. The figure shows the taxonomic classification of the species corresponding to free-living amoebas. The figure was made based on the following authors [45, 47, 106–108]), created with http://BioRender.com/.
Currently, 22 genotypes for the genus Acanthamoeba have been identified, ranging from T1 to T22, based on slight differences detected in regions of the rDNA gene, of which less than 5% are recognized as a single genotype [53, 60–64].
1.3. Pathogenicity Promoted by Free-Living Amoebas and Possible Treatment
It has been demonstrated that FLAs can promote several serious diseases. Among the most abundant amoebas in nature are the Acanthamoeba and signalment B. mandrillaris, which have been recognized as opportunistic human pathogens enabling blindness by Acanthamoeba keratitis and rare but fatal granulomatous encephalitis (GAE) by signalment B. mandrillaris [24, 65]. Naegleria fowleri is another pathogen FLA, known to cause central nervous system infection and primary amoebic meningoencephalitis, a pathophysiological state with a poor survival rate [24]. Of note, FLAs are not only recognized as disease causing, if not, which also act as vehicles for pathogenic bacteria [66]. All this evidence depicted the relevant role of FLA in promoting several human pathophysiological conditions.
Although it is very well known that FLAs can promote several serious diseases, most medical solutions have been effectively used. Indeed, current treatments combining drugs, such as amphotericin B and miltefosine, have been to be effective in treating Primary Amoebic Meningoencephalitis (PAM) symptoms disease, caused by Naegleria fowleri; however, without reducing the number of deaths (mortality rate of 95%) [67]. In addition, there are amebicidal agents, among which, both diamidines and biguanides display the capacity to destroy cystic forms. Diamidines, propamidine isethionate, and hexamidine are all agents that have been disclosed for the management of Acanthamoeba keratitis or neomycin when signs of corneal toxicity were present [68]. Among the biguanides, polyhexamethyl biguanide (PHMB), generally used at 0.02%, and chlorhexidine at 0.02% stand out [69, 70]. These drugs were used when patients had toxicity problems with diamidines.
There are other groups of medications that are not used as first-line drugs as they present toxic effects on the corneal epithelium when used for prolonged periods; these are the aminoglycosides (neomycin) and the imidazole [71, 72]. Other treatment schemes reported with success rates are propamidine with 1% miconazole, propamidine with topical 1 or 2% clotrimazole, and 0.1% miconazole plus debridement with itraconazole or oral ketoconazole. All these regimens were accompanied by neomycin polymyxin B sulfate.
Treatments usually used with positive results for corneal infections by Acanthamoeba (corneal ulcers), a combination of 0.02% chlorhexidine is used topically every two hours and topical polymyxin B neomycin every six hours, added to ketoconazole 200 mg orally every 12 hours. A follow-up is performed at two weeks to see the evolution of the disease and to continue until six weeks exclusively with 0.02% topical chlorhexidine every two hours. However, they are slow growing but effective with long-term treatment [69, 73]). However, although apparently, the treatments could be effective in treating amoeba infection, recently, have been tested new treatments options to improve the response of the immune system, but has been only tested in preclinical models with promising results [74, 75].
1.4. Identification of Amoebae in Extreme Environments
FLA has been isolated in extreme environments, such as the desert, at different soil depths and times of the year. Soil characteristics differ in terms of humidity and organic matter availability. Of note, a relationship has been observed between amoeba abundance and the number of bacteria present in the soil [76, 77]. Regarding extreme environments, Rodriguez-Zaragosa et al. isolated amoebas from the Negev Desert in Israel, including Acanthamoeba, Vahlkampfia, Naegleria, Willaertia, Tetramitus, Paratetramitus, and Adelphamoeba. This same group isolated 57 strains from desert environments, of which 39% belonged to the genus Acanthamoeba (which is essential to human health because it causes amoebic keratitis and granulomatous encephalitis), 16% to Hartmannella, 9% to Vahlkampfia, and the remaining proportion was divided among six other genera. Another factor, such as a decrease in bacterial load during the winter, increases the presence of flagellated amoebas because they can move freely to reach the microorganisms and so be able to feed. All these findings demonstrate the remarkable adaptability of FLA to survive in different extreme environments, and a new question arises about the mechanism of FLA and how it responds to other abiotic, biotic, and predatory prey [29, 78–81].
One of the more adaptative FLA is the Acanthamoeba, one of nature's most abundant genera, having been isolated from a wide range of environments, including freshwater pools and desert soil samples. Similarly, Balamuthia mandrillaris has been found in several environments, including hot tropical climates and cold regions with heavy snowfalls in northern Japan, where it was discovered for the first time in this type of cold environment [82]. In addition, regarding temperature, the genus Willaertia magna is a thermophilic FLA with an optimal growth temperature of 43°C. In its vegetative form named trophozoic, it measures between 50 to 100 μm in diameter and around 18 to 21 μm when it is cystic [83]. The W. magna is neither pathogenic, toxic nor ecotoxic. Different strains have been isolated from different extreme environments, such as Z503 and Z504, which were isolated from feces taken directly from the bovine rectum; TS-9 from the soil in France, M-1 from samples of contaminated thermal water in France, and strain T5 (S) 44 of sediment in the thermal effluent of a nuclear power plant in Belgium [84, 85]. In addition, the genus Acanthamoeba castellanii has been found in samples taken from frozen waters for recreational use in Oslo and Norway, demonstrating the wide distribution and resistance of the genus [86].
Astorga reported the presence of Acanthamoeba spp. in all regions of Chile and during all seasons of the year (from the region I to region XV) using phenotypic and genotypic identification. Water from Easter Island's Rano Kao crater and thermal waters obtained throughout Chile were analyzed, yielding temperature records of 44°C and declaring the thermotolerance; the distribution found was of Acanthamoeba spp 38.4%, Varmamoeba vermiformis 53.8%, Naegleria spp 12.8%, Stenamoeba spp 15.4%, Filamoeba spp 5.1%, and Heterolobosea 2.6%.
Acanthamoeba grows in water, soil, and plant samples at average temperatures such as 42°C. Groups II (84%) and III (84%) contained isolated Acanthamoeba spp. The T4 genotype predominates in Group II, and the T5 genotype predominates in Group III, genotypes that rank first and second in environmental studies; thus, all are potentially pathogenic, according to the literature. Isolated Acanthamoeba spp. was found in all areas with very different characteristics in terms of climate, water availability, and soil type, demonstrating its ubiquity and potential risk and highlighting the predominance of the T4 genotype in waters, soils, and plants. The T4 genotype is mainly found in recreational waters. T2, T3, and T4 genotypes have been identified in nonrecreational environmental water samples [87]. Through morphological analysis and partial sequencing of 18S rDNA, researchers in Turkey discovered the presence of Acanthamoeba genotype T4 and Vermamoeba vermiformis in tap water samples [88].
Regarding geographical location, it has been shown that Khyber Pakhtunkhwa province, located in Pakistan, displays several health problems associated with the presence of pathogens, which have been related to access and quality of the water. In fact, through PCR, it was possible to identify amoebas of the genus Acanthamoeba using specific genes; sequencing results confirmed seven different pathogenic and nonpathogenic genotypes, including T2–T10, T4, T5, T7, T15, T16, and T17 [89]. In addition, new reports have shown the presence of Acanthamoeba spp. (65%), Balamuthia mandrillaris (5%), and Naegleria fowleri from water plants in Karachi City [90]. Rather worryingly, from drinking tap water was possible to identify Acanthamoeba spp. (35%), but without the presence of Balamuthia mandrillaris (5%) and Naegleria fowleri in this last type of sample [90].
1.5. Possible Mechanisms to Explain the Survival of FLA in Extreme Environment
FLA has displayed resistance to environmental adversities and germicides. However, some organisms have developed resistance to the intracellular milieu of amoebas, as in the case of Acanthamoeba, which has been functioning as excellent reservoirs for amoeba-resistant microorganisms (ARMs), such as bacteria, viruses, and fungi. Little is known about these relationships and interaction mechanisms. Still, it is speculated that the FLAs need a broad repertoire or universal class of receptors to bind and recognize these diverse species of microorganisms. Also, it has been demonstrated that the temperature, pH, concentration of sulfhydric acid, and salinity can affect the survival of FLA; these factors strongly influence the amoeba's structure [59, 91].
FLAs displayed resistance to adverse environments and germicides. Of note, amoebas have developed cysts in response to several stressor agents nearing favorable environmental conditions to return to their metabolic activity (trophozoite state). In addition, FLA serves as a reservoir to several microorganisms (bacterium, viruses, and fungi) which indeed have developed resistance to the intracellular milieu of amoebas, as in the case of Acanthamoeba, which has been acted as excellent carriers and reservoirs for amoeba-resistant microorganisms (ARMs). Notably, although a close relationship between FLA and ARM has been described, little is known about the interaction mechanisms. Still, it is speculated that FLA is being able to develop a vast repertoire or universal class of receptors to recognize and bind to these microorganisms. Notably, cyst formation in response to several stressor agents is one of the more important mechanisms of FLAs in extreme environments; however, the molecular mechanism of encystment is still to be elucidated. The cysts are double-walled and consist of ectocyst and endocyst. The ectocyst is formed during the initial stage of encystment. It appears to be an amorphous and discontinuous layer, while the endocyst has a fine granular texture and is uniformly thicker. In addition, ectocyst consists of a mixture of proteins and polysaccharides, and endocyst consists of polysaccharides, mainly cellulose [92]. Acanthamoeba cyst walls contain 33% proteins, 4–6% lipids, 35% carbohydrates (prominent cellulose), 8% ash, and 20% unidentified material [93]. The carbohydrate components of the cyst wall contained about 48% galactose and 44% glucose.
Furthermore, linkage analysis revealed the presence of 3-linked galactopyranose (1,3-linked galactose) as the highest constituent of the cyst wall (about 29%). At the same time, 4-linked glucopyranose (β-1,4-linked glucose, i.e., likely cellulose) was 22% as the second principal component [94]. Thus, since cyst formation is one of the primary mechanisms in response to extreme environments, it is possible to speculate that some cyst components could change in response to stressor agents. Indeed, it has been demonstrated that temperature, pH, the concentration of sulfhydryl acid, and salinity can affect the survival of FLA. These factors can strongly influence the structure of the amoeba, giving robust mechanisms to survive in several extreme environments [95].
Among carbohydrates, cellulose was identified as a significant constituent. The cellulose precursor is glucose incorporated into the cell wall as β(1 → 4)-glucans (i.e., cellulose). However, in N. fowleri expression studies, an increase in the expression of the cysteine protease inhibitor genes (belonging to the cystatin family) was evidenced in the encystment process and mature amoeba cysts in N. fowleri (NfCPI) [96]. These findings collectively suggest that NfCPI may play a critical role in N. fowleri cyst formation by regulating cysteine proteases that may be actively involved in mediating cyst formation or else in the encystment process of amoebae [97]. Figure 3 shows a summary of the cyst formation mechanism.
Figure 3.
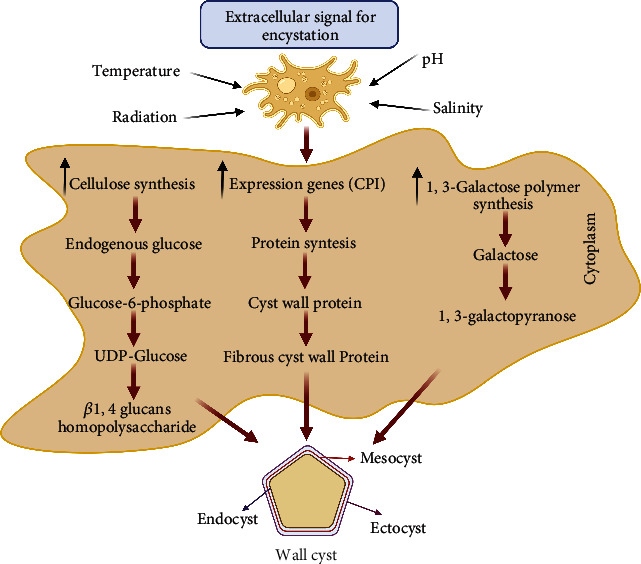
Mechanism cyst amoeba biosynthesis. The figure shows the formation encystment process mechanism of the cyst's wall. The formation of the cyst wall is characterized by three major components: protein synthesis, cellulose, and galactose polymers, which affect the amoeba wall formation, created with http://BioRender.com/.
1.6. Comparison between Two Best-Known Species of Free-Living Amoebae
Considering that Acanthamoeba and Naegleria are the more common FLA on our planet and both promote several pathophysiological conditions are very interesting genera of amoebas to compare them. Acanthamoeba spp. is free-living naked microbial predator, disseminators of opportunistic infections, and can survive in extreme environments by forming double-layered cysts [98]. It is characterized by two stages, trophozoite (ameboid) and its resistance form (Cysts) [99]. A. castillani contains locomotor pseudopods, spikelike “acanthopodia,” and microprojections from the cell surface formed by hyaline cytoplasm excluding formed elements of the cell and containing a fine fibrillar material [100]. In contrast, Naegleria fowleri presents three stages, including a flagellar that allows it to move in aquatic environments in search of nutrients primarily from bacteria [101]. To date, more than 40 different species of Naegleria have been identified [99]. In contrast, Acanthamoeba castellanii consists of pathogenic and nonpathogenic strains and has been classified into 17 different genotypes from T1 to T17 [8, 102]. Some species of FLA may be involved in opportunistic and nonopportunistic infections in humans. Pathogenic FLAs belong to five genera, Balamuthia, Acanthamoeba, Sappinia, Naegleria, and Vermamoeba [99, 103]. Using a transcriptome approach, the flagellation process of Naegleria shows that it takes about one hour to be completed and requires the transcription of a set of the basal body and flagellar apparatus genes [104].
Regarding morphological characteristics, Naegleria cysts are spherical, 8 to 12 μm in diameter, naturally resistant, and contain a single nucleus and a double wall with pores [105]. The cyst size of the Acanthamoeba can vary from 13 to 20 μm depending on the species. In their resistance stages, they are transported through dust and can enter through the nose, where they begin the invasion [35].
1.7. Perspectives
All this evidence suggests that FLAs could be an excellent candidate as an experimental model to explain mechanisms related to extreme environmental survival. The evidence showed that FLAs could survive harsh environments, so they are of great interest to study biological processes such as osmoregulation and their molecular mechanisms.
In addition, there is an evidence of meaningful participation in the increase of infectious outbreaks of pathogenic bacteria, the development of antibiotic resistance (AMR), and the recombination of virulence genes between bacteria.
2. Conclusions
FLAs are microorganisms that can be found in several environments, such as aquatic or terrestrial, also in extreme environments, which can present different physical features that drive these microorganisms to develop different survival strategies. It has born a great interest in studying physiological mechanisms from the point of view of understanding how FLAs have developed strategies of adaptation that allow survival in several extreme environments. Understanding the molecular mechanism of these microorganisms, it could enable the development of biotechnological applications to be used in different fields of science, or they can also be used as biological models.
Acknowledgments
Unfortunately, during the development of this work, Professor Dr. Jorge Araya-Rojas died; therefore, the present manuscript is in honor to the great work developed by Professor Araya for many years, forming outstanding professionals and scientists. This study was supported by the Agencia Nacional de Investigación y Desarrollo (ANID) through Anillo ACT210083. DCA was funded by grant Fondeyt de Iniciación #11220870 and by Minera Escondida Ltda. MEL2203.
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
C.S-A. contributed to the draft, preparation of the manuscript, and to the concept of the project. L.S.R. and D.C.A. contributed to the preparation of the manuscript. All authors approved the final version of the manuscript.
References
- 1.Gallegos-Neyra E. M., Lugo-Vázquez A., Calderón-Vega A., Sánchez-Rodríguez M. D. R., Mayén-Estrada R. Biodiversidad de protistas amébidos de vida libre en México. Revista Mexicana de Biodiversidad . 2014;85:10–25. doi: 10.7550/rmb.33691. [DOI] [Google Scholar]
- 2.Leidy J. Amoeba proteus. The American Naturalist . 1878;12(4):235–238. doi: 10.1086/272082. [DOI] [Google Scholar]
- 3.Campbell R. Microbial Ecology . 2nd. Oxford: Blackwell Scientific; 1983. [Google Scholar]
- 4.Nishihara E., Shimmen T., Sonobe S. New aspect of the membrane dynamics of the Amoeba Proteus contractile vacuole revealed by vital staining with FM4-64. Protoplasma . 2007;231(1-2):25–30. doi: 10.1007/s00709-007-0247-x. [DOI] [PubMed] [Google Scholar]
- 5.Clarke M., Kohler J., Arana Q., Liu T., Heuser J., Gerisch G. Dynamics of the vacuolar H+-ATPase in the contractile vacuole complex and the endosomal pathway of Dictyostelium cells. Journal of Cell Science . 2002;115(14):2893–2905. doi: 10.1242/jcs.115.14.2893. [DOI] [PubMed] [Google Scholar]
- 6.Nishihara E., Yokota E., Tazaki A., et al. Presence of aquaporin and V-ATPase on the contractile vacuole of Amoeba proteus. Biology of the Cell . 2008;100(3):179–188. doi: 10.1042/BC20070091. [DOI] [PubMed] [Google Scholar]
- 7.Saint-Vincent B., Lamarck J.-B. Essai d’une classification des animaux microscopiques / par M . Veuve Agasse: Bory de St-Vincent; 1826. [DOI] [Google Scholar]
- 8.Schuster F. L., Visvesvara G. S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. International Journal for Parasitology . 2004;34(9):1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Patterson D. J. The diversity of eukaryotes. The American Naturalist . 1999;154(S4):S96–S124. doi: 10.1086/303287. [DOI] [PubMed] [Google Scholar]
- 10.Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains archaea, bacteria, and eucarya. Proceedings of the National Academy of Sciences of the United States of America . 1990;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adl S. M., Bass D., Lane C. E., et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. The Journal of Eukaryotic Microbiology . 2019;66(1):4–119. doi: 10.1111/jeu.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosakyan A., Gomaa F., Lara E., Lahr D. J. G. Current and future perspectives on the systematics, taxonomy and nomenclature of testate amoebae. European journal of Protistology . 2016;55:105–117. doi: 10.1016/j.ejop.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Meisterfeld R., Kent O. A. An Illustrated Guide to the Protozoa . Allen Press; 1880. [Google Scholar]
- 14.Tsao H.-F., Scheikl U., Volland J.-M., et al. Candidatus Cochliophilus cryoturris’ (Coxiellaceae), a symbiont of the testate amoeba Cochliopodium minus. Scientific Reports . 2017;7(1):p. 3394. doi: 10.1038/s41598-017-03642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenchel T. Ecology of Protozoa: The Biology of Free-Living Phagotrophic Protists . Madison, Wis: Science Tech Publishers; 1987. [Google Scholar]
- 16.Anderson O. R. Berlin . New York: Springer-Verlag; 1988. Comparative protozoology: ecology, physiology, life history. [DOI] [Google Scholar]
- 17.Mazur T., Hadaś E., Iwanicka I. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Tropical Medicine and Parasitology: Official Organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft Fur Technische Zusammenarbeit (GTZ) . 1995;46(2):106–108. [PubMed] [Google Scholar]
- 18.Sriram R., Shoff M., Booton G., Fuerst P., Visvesvara G. S. Survival of Acanthamoeba cysts after desiccation for more than 20 years. Journal of Clinical Microbiology . 2008;46(12):4045–4048. doi: 10.1128/JCM.01903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page F. C. A New Key to Freshwater and Soil Gymnamoebae: With Instructions for Culture . Ambleside: Freshwater Biological Association; 1988. [Google Scholar]
- 20.Qvarnstrom Y., Nerad T. A., Visvesvara G. S. Characterization of a new pathogenic Acanthamoeba Species, A. byersi n. sp., isolated from a human with fatal amoebic encephalitis. The Journal of Eukaryotic Microbiology . 2013;60(6):626–633. doi: 10.1111/jeu.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonkowski M. Protozoa and plant growth: the microbial loop in soil revisited. New Phytologist . 2004;162(3):617–631. doi: 10.1111/j.1469-8137.2004.01066.x. [DOI] [PubMed] [Google Scholar]
- 22.Król-Turmińska K., Olender A. Human infections caused by free-living amoebae. Annals of Agricultural and Environmental Medicine . 2017;24(2):254–260. doi: 10.5604/12321966.1233568. [DOI] [PubMed] [Google Scholar]
- 23.Smirnov A. V., Chao E., Nassonova E. S., Cavalier-Smith T. A revised classification of naked lobose amoebae(Amoebozoa: Lobosa) Protist . 2011;162(4):545–570. doi: 10.1016/j.protis.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Visvesvara G. S., Moura H., Schuster F. L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunology & Medical Microbiology . 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 25.Szenasi Z., Endo T., Yagita K., Nagy E. Isolation, identification and increasing importance of free-living amoebae causing human disease. Journal of Medical Microbiology . 1998;47(1):5–16. doi: 10.1099/00222615-47-1-5. [DOI] [PubMed] [Google Scholar]
- 26.Tsvetkova N., Schild M., Panaiotov S., et al. The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitology Research . 2004;92(5):405–413. doi: 10.1007/s00436-003-1052-x. [DOI] [PubMed] [Google Scholar]
- 27.Chappell C. L., Wright J. A., Coletta M., Newsome A. L. Standardized method of measuring Acanthamoeba antibodies in sera from healthy human subjects. Clinical Diagnostic Laboratory Immunology . 2001;8(4):724–730. doi: 10.1128/CDLI.8.4.724-730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyun İ., Kolören Z., Karaman Ü., Tsiami A., Karanis P. Acanthamoeba spp. in river water samples from the Black Sea region, Turkey. Journal of Water and Health . 2020;18(2):186–199. doi: 10.2166/wh.2020.170. [DOI] [PubMed] [Google Scholar]
- 29.Juárez M. M., Tártara L. I., Cid A. G., et al. Acanthamoeba in the eye, can the parasite hide even more? Latest developments on the disease. Contact Lens and Anterior Eye . 2018;41(3):245–251. doi: 10.1016/j.clae.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo-Morales J., Lindo J. F., Martinez E., et al. Pathogenic Acanthamoeba strains from water sources in Jamaica, West Indies. Annals of Tropical Medicine & Parasitology . 2005;99(8):751–758. doi: 10.1179/136485905X65215. [DOI] [PubMed] [Google Scholar]
- 31.Visvesvara G. S., Sriram R., Qvarnstrom Y., et al. Paravahlkampfia francinae n. sp. masquerading as an agent of primary amoebic meningoencephalitis. Journal of Eukaryotic Microbiology . 2009;56(4):357–366. doi: 10.1111/j.1550-7408.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- 32.Abedkhojasteh H., Niyyati M., Rahimi F., Heidari M., Farnia S., Rezaeian M. First report of Hartmannella keratitis in a cosmetic soft contact lens wearer in Iran. Iranian Journal of Parasitology . 2013;8(3):481–485. [PMC free article] [PubMed] [Google Scholar]
- 33.Gelman B. B., Rauf S. J., Nader R., et al. Amoebic encephalitis due to Sappinia diploidea. Journal of the American Medical Association . 2001;285(19):2450–2451. doi: 10.1001/jama.285.19.2450. [DOI] [PubMed] [Google Scholar]
- 34.John D. T. En Topley & Wilson’s Microbiology and Microbial Infections . American Cancer Society; 2010. Opportunistic amebae. [DOI] [Google Scholar]
- 35.Martinez A. J., Visvesvara G. S. Free-living, amphizoic and opportunistic amebas. Brain Pathology . 1997;7(1):583–598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheid P. L., Balczun C. Failure of molecular diagnostics of a keratitis-inducing Acanthamoeba strain. Experimental Parasitology . 2017;183:236–239. doi: 10.1016/j.exppara.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Delafont V., Brouke A., Bouchon D., Moulin L., Héchard Y. Microbiome of free-living amoebae isolated from drinking water. Water Research . 2013;47(19):6958–6965. doi: 10.1016/j.watres.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 38.Scheid P. L., Schwarzenberger R. Free-living amoebae as vectors of cryptosporidia. Parasitology Research . 2011;109(2):499–504. doi: 10.1007/s00436-011-2287-6. [DOI] [PubMed] [Google Scholar]
- 39.Winiecka-Krusnell J., Dellacasa-Lindberg I., Dubey J. P., Barragan A. Toxoplasma gondii: uptake and survival of oocysts in free-living amoebae. Experimental Parasitology . 2009;121(2):124–131. doi: 10.1016/j.exppara.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 40.Cdc—Dpdx—Free living amebic infections. Recuperado 22 de octubre de 2020. 2019. https://www.cdc.gov/dpdx/freelivingamebic/index.html .
- 41.Bowers B. Comparison of pinocytosis and phagocytosis in Acanthamoeba castellanii. Experimental Cell Research . 1977;110(2):409–417. doi: 10.1016/0014-4827(77)90307-X. [DOI] [PubMed] [Google Scholar]
- 42.Bowers B., Olszewski T. E. Acanthamoeba discriminates internally between digestible and indigestible particles. Journal of Cell Biology . 1983;97(2):317–322. doi: 10.1083/jcb.97.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan N. A. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiology Reviews . 2006;30(4):564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 44.Kot K., Łanocha-Arendarczyk N. A., Kosik-Bogacka D. I. Amoebas from the genus Acanthamoeba and their pathogenic properties. Annals of Parasitology . 2018;64(4):299–308. doi: 10.17420/ap6404.164. [DOI] [PubMed] [Google Scholar]
- 45.Oddó D. Infecciones por amebas de vida libre.: comentarios históricos, taxonomía y nomenclatura, protozoología y cuadros anátomo-clínicos. Revista Chilena de Infectología . 2006;23(3) doi: 10.4067/S0716-10182006000300002. [DOI] [PubMed] [Google Scholar]
- 46.Adl S. M., Simpson A. G. B., Farmer M. A., et al. The new higher-level classification of eukaryotes with emphasis on the taxonomy of protists. The Journal of Eukaryotic Microbiology . 2005;52(5):399–451. doi: 10.1111/j.1550-7408.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- 47.Adl S. M., Simpson A. G. B., Lane C. E., et al. The revised classification of eukaryotes. Journal of Eukaryotic Microbiology . 2012;59(5):429–514. doi: 10.1111/j.1550-7408.2012.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasni I., Chelkha N., Baptiste E., et al. Investigation of potential pathogenicity of Willaertia magna by investigating the transfer of bacteria pathogenicity genes into its genome. Scientific Reports . 2019;9(1):p. 18318. doi: 10.1038/s41598-019-54580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Page F. C. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. The Journal of Protozoology . 1967;14(3):499–521. doi: 10.1111/j.1550-7408.1967.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 50.Page F. Nackte rhizopoda und heliozoea. Protozoenfauna Band . 1991;2:1–297. [Google Scholar]
- 51.Pussard M., Pons R. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida) Protistologica . 1977;13:557–598. [Google Scholar]
- 52.Alves J. M. P., Gusmão C. X., Teixeira M. M. G., Freitas D., Foronda A. S., Affonso H. T. Random amplified polymorphic DNA profiles as a tool for the characterization of Brazilian keratitis isolates of the genus Acanthamoeba. Brazilian Journal of Medical and Biological Research . 2000;33(1):19–26. doi: 10.1590/S0100-879X2000000100003. [DOI] [PubMed] [Google Scholar]
- 53.Stothard D. R., Schroeder-Diedrich J. M., Awwad M. H., et al. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18s rrna gene sequence types. Journal of Eukaryotic Microbiology . 1998;45(1):45–54. doi: 10.1111/j.1550-7408.1998.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schroeder J. M., Booton G. C., Hay J., et al. Use of subgenic 18s ribosomal dna pcr and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. Journal of Clinical Microbiology . 2001;39(5):1903–1911. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Booton G. C., Carmichael J. R., Visvesvara G. S., Byers T. J., Fuerst P. A. Identification of Balamuthia mandrillaris by PCR assay using the mitochondrial 16s rRNA gene as a target. Journal of Clinical Microbiology . 2003;41(1):453–455. doi: 10.1128/JCM.41.1.453-455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pélandakis M., Pernin P. Use of multiplex pcr and pcr restriction enzyme analysis for detection and exploration of the variability in the free-living amoeba Naegleria in the environment. Applied and Environmental Microbiology . 2002;68(4):2061–2065. doi: 10.1128/AEM.68.4.2061-2065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Jonckheere J. F., Brown S. Three different group I introns in the nuclear large subunit ribosomal DNA of the amoeboflagellate Naegleria. Nucleic Acids Research . 1998;26(2):456–461. doi: 10.1093/nar/26.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Jonckheere J. F., Brown S. The identification of vahlkampfiid amoebae by its sequencing. Protist . 2005;156(1):89–96. doi: 10.1016/j.protis.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Rodríguez-Zaragoza S. Ecology free-living amoebae. Critical Reviews in Microbiology . 2008;20(3):225–241. doi: 10.3109/10408419409114556. [DOI] [PubMed] [Google Scholar]
- 60.Gast R. J., Ledee D. R., Fuerst P. A., Byers T. J. Subgenus systematics of acanthamoeba: four nuclear 18s rdna sequence types. The Journal of Eukaryotic Microbiology . 1996;43(6):498–504. doi: 10.1111/j.1550-7408.1996.tb04510.x. [DOI] [PubMed] [Google Scholar]
- 61.Corsaro D., Venditti D. Detection of novel chlamydiae and Legionellales from human nasal samples of healthy volunteers. Folia Microbiologica . 2015;60(4):325–334. doi: 10.1007/s12223-015-0378-y. [DOI] [PubMed] [Google Scholar]
- 62.Fuerst P. A., Booton G. C. Species, sequence types and alleles: dissecting genetic variation in Acanthamoeba. Pathogens . 2020;9(7):p. 534. doi: 10.3390/pathogens9070534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuerst P. A., Booton G. C., Crary M. Phylogenetic analysis and the evolution of the 18s rrna gene typing system of Acanthamoeba. Journal of Eukaryotic Microbiology . 2015;62(1):69–84. doi: 10.1111/jeu.12186. [DOI] [PubMed] [Google Scholar]
- 64.Hewett M. K., Robinson B. S., Monis P. T., Saint C. P. Identification of a new Acanthamoeba 18S rRNA gene sequence type, corresponding to the species Acanthamoeba jacobsiSawyer, Nerad and Visvesvara, 1992 (Lobosea: Acanthamoebidae) Acta Protozoologica . 2003;42(4):325–330. [Google Scholar]
- 65.Lorenzo-Morales J., Khan N. A., Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite . 2015;22:p. 10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greub G., Raoult D. Microorganisms resistant to free-living amoebae. Clinical Microbiology Reviews . 2004;17(2):413–433. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Capewell L. G., Harris A. M., Yoder J. S., et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937-2013. Journal of the Pediatric Infectious Diseases Society . 2015;4(4):e68–e75. doi: 10.1093/jpids/piu103. [DOI] [PubMed] [Google Scholar]
- 68.Hargrave S. L., McCulley J. P., Husseini Z. Results of a trial of combined propamidine isethionate and neomycin therapy for Acanthamoeba keratitis. Brolene study group. Ophthalmology . 1999;106(5):952–957. doi: 10.1016/s0161-6420(99)00515-1. [DOI] [PubMed] [Google Scholar]
- 69.Kosrirukvongs P., Wanachiwanawin D., Visvesvara G. S. Treatment of Acanthamoeba keratitis with chlorhexidine. Ophthalmology . 1999;106(4):798–802. doi: 10.1016/S0161-6420(99)90169-0. [DOI] [PubMed] [Google Scholar]
- 70.Seal D., Hay J., Kirkness C., et al. Successful medical therapy of Acanthamoeba keratitis with topical chlorhexidine and propamidine. Eye . 1996;10(4):413–421. doi: 10.1038/eye.1996.92. [DOI] [PubMed] [Google Scholar]
- 71.Varga J. H., Wolf T. C., Jensen H. G., Parmley V. C., Rowsey J. J. Combined treatment of Acanthamoeba keratitis with propamidine, neomycin, and polyhexamethylene biguanide. American Journal of Ophthalmology . 1993;115(4):466–470. doi: 10.1016/s0002-9394(14)74448-4. [DOI] [PubMed] [Google Scholar]
- 72.Hay S., Kirkness C. Successful medical therapy of acanthamoeba keratitis with topical chlorhexidine and propamidine. Eye . 1997;10(4):413–421. doi: 10.1038/eye.1996.92. [DOI] [PubMed] [Google Scholar]
- 73.Brooks J. G., Jr., Coster D. J., Badenoch P. R. Acanthamoeba keratitis. Cornea . 1994;13(2):186–189. doi: 10.1097/00003226-199403000-00013. [DOI] [PubMed] [Google Scholar]
- 74.Kim J.-H., Sohn H.-J., Lee J., et al. Vaccination with lentiviral vector expressing the nfa1 gene confers a protective immune response to mice infected with Naegleria fowleri. Clinical and Vaccine Immunology: CVI . 2013;20(7):1055–1060. doi: 10.1128/CVI.00210-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J., Yoo J.-K., Sohn H.-J., et al. Protective immunity against Naegleria fowleri infection on mice immunized with the rNfa1 protein using mucosal adjuvants. Parasitology Research . 2015;114(4):1377–1385. doi: 10.1007/s00436-015-4316-3. [DOI] [PubMed] [Google Scholar]
- 76.Anderson O. R., Gorrell T., Bergen A., Kruzansky R., Levandowsky M. Naked amoebas and bacteria in an oil-impacted salt marsh community. Microbial Ecology . 2001;42(3):474–481. doi: 10.1007/s00248-001-0008-x. [DOI] [PubMed] [Google Scholar]
- 77.Rodríguez-Zaragoza S. Ecology free-living amoebae. Critical Reviews in Microbiology . 1994;20(3):225–241. doi: 10.3109/10408419409114556. [DOI] [PubMed] [Google Scholar]
- 78.Rodriguez-Zaragoza S., Mayzlish E., Steinberger Y. Seasonal changes in free-living amoeba species in the root canopy of Zygophyllum dumosum in the Negev desert, Israel. Microbial Ecology . 2005;49(1):134–141. doi: 10.1007/s00248-003-1056-1. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Zaragoza S., Rivera F., Bonilla P., et al. Amoebological study of the atmosphere of San Luis Potosi, slp, Mexico. Journal of Exposure Analysis and Environmental Epidemiology . 1993;3(Supplement 1):229–241. [PubMed] [Google Scholar]
- 80.Rodriguez-Zaragoza S., Mayzlish E., Steinberger Y. Vertical distribution of the free-living amoeba population in soil under desert shrubs in the Negev desert, Israel. Applied and Environmental Microbiology . 2005;71(4):2053–2060. doi: 10.1128/AEM.71.4.2053-2060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pérez-Juárez H., Serrano-Vázquez A., Lara E., et al. Population dynamics of amoeboid protists in a tropical desert: seasonal changes and effects of vegetation and soil conditions. Acta Protozoologica . 2018;57(4):231–242. doi: 10.4467/16890027AP.18.017.10093. [DOI] [Google Scholar]
- 82.Yamanouchi K., Arima H., Sakamoto Y., et al. First report of the isolation of Balamuthia mandrillaris in the northern region of Japan. Parasitology Research . 2018;117(9):2895–2900. doi: 10.1007/s00436-018-5980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pánek T., Silberman J. D., Yubuki N., Leander B. S., Cepicka I. Diversity, evolution and molecular systematics of the psalteriomonadidae, the main lineage of anaerobic/microaerophilic heteroloboseans (Excavata: Discoba) Protist . 2012;163(6):807–831. doi: 10.1016/j.protis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 84.De Jonckheere J. F., Dive D. G., Pussard M., Vickerman K. Willaertia magna gen. nov., sp. nov. (Vahlkampfiidae), a thermophilic amoeba found in different habitats. 1984. https://eurekamag.com/research/001/281/001281223.php .
- 85.Dey R., Bodennec J., Mameri M. O., Pernin P. Free-living freshwater amoebae differ in their susceptibility to the pathogenic bacterium Legionella pneumophila. FEMS Microbiology Letters . 2009;290(1):10–17. doi: 10.1111/j.1574-6968.2008.01387.x. [DOI] [PubMed] [Google Scholar]
- 86.Brown T. J., Cursons R. T. M. Pathogenic free-living amebae (Pfla) from frozen swimming areas in Oslo, Norway. Scandinavian Journal of Infectious Diseases . 1977;9(3):237–240. doi: 10.3109/inf.1977.9.issue-3.16. [DOI] [PubMed] [Google Scholar]
- 87.Astorga B. Chile. Recuperado de: Universidad de Zaragoza; 2016. Ecología de Acanthamoeba spp.En Chile: identificación fenotípica y genotípica en agua, suelos y vegetales. https://zaguan.unizar.es/record/47883/files/TESIS-2016-067.pdf . [Google Scholar]
- 88.Üstüntürk-Onan M., Walochnik J. Identification of free-living amoebae isolated from tap water in Istanbul, Turkey. Experimental Parasitology . 2018;195:34–37. doi: 10.1016/j.exppara.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Tanveer T., Hameed A., Muazzam A. G., Jung S.-Y., Gul A., Matin A. Isolation and molecular characterization of potentially pathogenic Acanthamoeba genotypes from diverse water resources including household drinking water from Khyber Pakhtunkhwa, Pakistan. Parasitology Research . 2013;112(8):2925–2932. doi: 10.1007/s00436-013-3465-5. [DOI] [PubMed] [Google Scholar]
- 90.Yousuf F. A., Siddiqui R., Khan N. A. Presence of rotavirus and free-living amoebae in the water supplies of Karachi, Pakistan. Revista Do Instituto de Medicina Tropical de Sao Paulo . 2017;59:p. e32. doi: 10.1590/S1678-9946201759032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guimaraes A. J., Gomes K. X., Cortines J. R., Peralta J. M., Saramago R. H., Peralta Acanthamoeba spp. as a universal host for pathogenic microorganisms: one bridge from environment to host virulence. Microbiological Research . 2016;193:30–38. doi: 10.1016/j.micres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Weisman R. A. Differentiation In Acanthamoeba castellanii. Annual Review of Microbiology . 1976;30(1):189–219. doi: 10.1146/annurev.mi.30.100176.001201. [DOI] [PubMed] [Google Scholar]
- 93.Neff R. J., Neff R. H. The biochemistry of amoebic encystment. Symposia of the Society for Experimental Biology . 1969;23:51–81. [PubMed] [Google Scholar]
- 94.Dudley R., Jarroll E. L., Khan N. A. Carbohydrate analysis of Acanthamoeba castellanii. Experimental Parasitology . 2009;122(4):338–343. doi: 10.1016/j.exppara.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 95.Rodríguez-Zaragoza S. Ecology of free-living amoebae. Critical Reviews in Microbiology . 1994;20(3):225–241. doi: 10.3109/10408419409114556. [DOI] [PubMed] [Google Scholar]
- 96.Lê H. G., Ham A.-J., Kang J.-M., et al. A novel cysteine protease inhibitor of Naegleria fowleri that is specifically expressed during encystation and at mature cysts. Pathogens . 2021;10(4):p. 388. doi: 10.3390/pathogens10040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anwar A., Khan N. A., Siddiqui R. Combating Acanthamoeba spp. cysts: what are the options. Parasites & Vectors . 2018;11(1) doi: 10.1186/s13071-017-2572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corsaro D. Update on Acanthamoeba phylogeny. Parasitology Research . 2020;119(10):3327–3338. doi: 10.1007/s00436-020-06843-9. [DOI] [PubMed] [Google Scholar]
- 99.Abdul Majid M. A., Mahboob T., Mong B. G. J., et al. Pathogenic waterborne free-living amoebae: an update from selected southeast Asian countries. PLoS One . 2017;12(2):p. e0169448. doi: 10.1371/journal.pone.0169448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bowers B., Korn E. D. The fine structure of Acanthamoeba castellanii (Neff strain) II. Encystment. The Journal of Cell Biology . 1969;41(3):786–805. doi: 10.1083/jcb.41.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Jonckheere J. F. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectiousm Diseases . 2011;11(7):1520–1528. doi: 10.1016/j.meegid.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 102.Nuprasert W., Putaporntip C., Pariyakanok L., Jongwutiwes S. Identification of a novel t17 genotype of acanthamoeba from environmental isolates and t10 genotype causing keratitis in Thailand. Journal of Clinical Microbiology . 2010;48(12):4636–4640. doi: 10.1128/JCM.01090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reyes-Batlle M., Niyyati M., Martín-Navarro C. M., et al. Unusual vermamoeba vermiformis strain isolated from snow in Mount Teide, Tenerife, Canary Islands, Spain. Novelty in Biomedicine . 2015;3(4):189–192. doi: 10.22037/nbm.v3i4.10386. [DOI] [Google Scholar]
- 104.Fritz-Laylin L. K., Cande W. Z. Ancestral centriole and flagella proteins identified by analysis of Naegleria differentiation. Journal of Cell Science . 2010;123(23):4024–4031. doi: 10.1242/jcs.077453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Visvesvara G. S., Shoff M. E., Sriram R., et al. Isolation, morphologic, serologic and molecular identification of Acanthamoeba T4 genotype from the liver of a Temminck’s tragopan (Tragopan temminckii) Veterinary Parasitology . 2010;170(3–4):197–200. doi: 10.1016/j.vetpar.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 106.Bertelli C., Greub G. Lateral gene exchanges shape the genomes of amoeba-resisting microorganisms. Frontiers in Cellular and Infection Microbiology . 2012;2 doi: 10.3389/fcimb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hampl V., Hug L., Leigh J. W., et al. Phylogenomic analyses support the monophyly of Excavata and resolve relationships among eukaryotic «supergroups». Proceedings of the National Academy of Sciences . 2009;106(10):3859–3864. doi: 10.1073/pnas.0807880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pawlowski J., Burki F. Untangling the phylogeny of amoeboid protists. Journal of Eukaryotic Microbiology . 2009;56(1):16–25. doi: 10.1111/j.1550-7408.2008.00379.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


