Abstract
Difference of onset of increase of PRL content in the anterior pituitary gland and plasma PRL concentration during the late stage of chicken embryogenesis is well known. To investigate the disagreement, changes in PRL content and PRL mRNA levels, and the effects of vasoactive intestinal polypeptides (VIP) on PRL release and PRL mRNA expression were examined using western blot analysis and real-time PCR quantification. Changes in SPRL content were strongly correlated with PRL mRNA levels. The increase in PRL content on day 17 of incubation may be caused by the increase in PRL mRNA levels on day 16 of incubation. Additionally, the effects of VIP on PRL release from the embryonic anterior pituitary gland were not observed until day 18 of embryogenesis. These results suggest that increased levels of PRL mRNA and PRL content in the anterior pituitary gland are closely correlated. However, the increased expression of PRL mRNA observed on day 17 and the initiation of PRL release from the anterior pituitary gland on day 19 were differentially regulated. According to the results of western blot analysis, the proportion of glycosylated PRL (G-PRL) and non-glycosylated PRL (NG-PRL) in the anterior pituitary gland at the end stage of development differed from the proportion of PRL released from the anterior pituitary gland. According to the results of two-dimensional western blot analysis, no isoforms with different isoelectric points were detected in the culture medium on days 19 and 20. These data suggest that the peptide chains of G-PRL and NG-PRL were not modified. In conclusion, the differentiation of PRL-producing cells and the maturation of the hypothalamus and anterior pituitary gland were completed at the end stage of incubation, and that different factors regulated the initiation of PRL mRNA expression before day 18 of incubation.
Keywords: anterior pituitary gland, prolactin, vasoactive intestinal polypeptide
Introduction
Prolactin (PRL) is a peptide hormone primarily synthesized in the anterior pituitary gland. PRL primarily promotes the growth and development of the mammary glands and stimulates milk production in mammals. Additionally, PRL modulates various physiological activities. Many functions of PRL are supported by the expression of the PRL receptor gene in various tissues (Kelly et al., 1989; Bole-Feysot et al., 1998). Similarly, the mRNA expression of PRL receptor genes in various tissues supports multiple functions of PRL in birds (Zhou et al., 1996; Ohkubo et al., 1998). For example, cropsac development in Columbiformes and the induction of broody behavior in Galliformes are well-known PRL functions in birds (Riddle et al., 1935). Furthermore, PRL is associated with anti-gonadal effects (Zadworny et al., 1989), immune responsiveness (Skwarlo-Sonta et al., 1986), and osmoregulation (Doneen & Smith, 1982a, 1982b).
Changes in plasma PRL levels in chicken embryos were first identified by Harvey et al. (1979), who observed an increase in PRL levels between days 13 and 15 of embryo development. Ishida et al. (1991) measured both plasma levels and pituitary PRL content during chicken embryogenesis. The increases in PRL levels between days 18 and 19 were significantly positively correlated with PRL mRNA levels in the anterior pituitary gland. Kansaku et al. (1994) reported a similar PRL mRNA expression profile and PRL content in the cephalic lobe of the anterior pituitary gland during chicken embryogenesis. Additionally, increases in plasma PRL levels, pituitary PRL content, and PRL mRNA expression have been detected at the end of turkey embryogenesis (Bédécarrats et al., 1999c). These results indicate that increases in plasma PRL levels in chicken embryos originate from PRL mRNA expression in the anterior pituitary gland.
PRL exists in several isoforms. Using western blot analysis, Bédécarrats et al. (1999c) detected PRL of different sizes in the anterior pituitary gland of turkey embryos. The higher levels of PRL observed on day 26 of incubation than on day 22 were due to changes in the PRL content in the anterior pituitary gland. Two sizes of PRL molecules have also been detected at different reproductive stages (Bédécarrats et al., 1999a, b). In addition, different PRL isoforms have been detected in chickens using one- and two-dimensional SDS-polyacrylamide gel electrophoresis (PAGE); they differed slightly in molecular size and isoelectric points (Hiyama et al., 2009). Interestingly, treatment with N-glycosidase and neuraminidase resulted in PRL of a single molecular weight and PRL of two molecular weights, respectively. Thus, different molecular sizes and isoelectric points are produced by post-translational modifications, such as glycosylation and the addition of neuraminic acid to the end of glycans.
Plasma PRL levels and pituitary PRL content were previously detected on day 12 of chicken embryogenesis and day 18 of turkey embryogenesis (Harvey et al., 1979; Ishida et al., 1991; Bédécarrats et al., 1999c). In addition, PRL in the anterior pituitary gland has been reported on days 17 and 22 of chicken and turkey embryogenesis, respectively (Bédécarrats et al. 1999c; Hiyama et al., 2009). Thus, PRL was only detected in the late stages of embryogenesis. However, it is unclear whether PRL, including glycosylated PRL, exists in the anterior pituitary gland during the mid-stage of embryonic development. Moreover, a two-dimensional identification of the released PRL has not yet been reported.
In addition, vasoactive intestinal polypeptide (VIP) induces the release of PRL in adult birds; thus, active and passive immunization against VIP prevents the secretion of PRL in galliform species, interrupting the incubation behavior (Sharp et al., 1989; Youngren et al., 1994; El Halawani et al., 1995). Notably, hypothalamic VIP content and mRNA levels of the incubation phase were higher than those of the laying phase (Sharp et al., 1989; Talbot et al., 1995; Chaiseha et al., 2004). In addition, PRL secretion from the anterior pituitary gland of adult chickens and turkeys can be stimulated by VIP (Proudman and Opel, 1988; Kansaku et al., 1995; Bedecarratts et al., 1999b). The VIP receptor (VIPR) is expressed in the anterior pituitary gland of embryonic chickens (Kansaku et al., 2001). Moreover, changes in PRL mRNA levels during the late stage of embryonic development are stimulated by VIP (Kansaku et al., 2016). These data suggest that hypothalamic VIP induces PRL mRNA expression and increases plasma PRL levels during the late stage of embryogenesis. However, VIPR mRNA levels in the late stages of embryogenesis have not been reported. Thus, the details of the relationship between VIP and PRL release remain unknown.
Therefore, this study aimed to investigate the regulatory mechanisms of PRL synthesis and release from the anterior pituitary gland of chicken embryos. In addition, the molecular form of the released PRL at the late stage of chicken embryogenesis was compared with the PRL isoform ratio in the anterior pituitary gland.
Materials and Methods
Preparation of Embryonic Anterior Pituitary Glands and Ethical Guidelines
A total of 450 fertilized eggs from White Leghorn hens were obtained from a local hatchery (Chiba Hatchery Co., Togane, Chiba, Japan) and incubated at 37°C in a humidified incubator. Handling of the eggs and sampling of embryonic anterior pituitary glands were conducted according to the guidelines for animal experimentation at Azabu University (approval numbers 190701-2 and 210121-2).
Production of Chicken PRL Antibodies
The pCold-I plasmid (Takara Bio, Tokyo, Japan) was used as an expression vector to produce recombinant chicken PRL. The mature chicken PRL-coding region was amplified via PCR and ligated into the cloning site of pCold-I. The constructed vector was used to transform Escherichia coli (BL21) according to the protocol described by Hanahan (1983). Transformed cells were grown, and the synthesis of recombinant PRL was induced using cold shock. Recombinant PRL was purified as previously described (Ohkubo et al., 1993). The extracted PRL was then used as an immunogen to produce polyclonal chicken PRL antibodies. Western blot analysis of the homogenate of the chicken anterior pituitary gland revealed that the antibodies raised against recombinant PRL produced the same immunoreactive bands as the antisera of the PRL Radioimmunoassay system previously provided by the National Hormone and Pituitary Program (Kansaku et al., 1998) and the monoclonal anti-PRL antibody (provided by Prof. Luc. R. Berghman, Texas A&M University) raised against recombinant PRL (Ohkubo et al., 1993).
Detection of PRL in Embryonic Anterior Pituitary Glands
Anterior pituitary glands were harvested from developing embryos on days 14, 15, 16, 17, 18, 19, and 20 of embryogenesis. Between six and ten anterior pituitary glands were pooled and stored at −80°C until homogenization. The anterior pituitary glands were homogenized using a microhomogenizer (Wheaton Science, Millville, NJ, USA) in 50 µL of homogenizing buffer (10 mM Tris–HCl (pH 8.0), 1 mM EDTA, and 0.5% Tween 20). After centrifugation, aliquots were used for western blot analyses. Total protein content was measured using the Bradford method (Bradford, 1976). Then, the protein concentrations of the aliquots of the homogenate were adjusted to 500 ng protein/µL, as described by Hiyama et al. (2009), and 5 µg of protein was analyzed via SDS-PAGE using precast 12.5% polyacrylamide gels (e-Pagel E-T12.5L, ATTO, Tokyo, Japan). Electrophoresis was conducted under reducing conditions with 2% 2-mercaptoethanol. After electrophoresis, the proteins were electro-transferred onto a Hybond-P membrane (GE Healthcare UK Ltd., Little Chalfont, England) using a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad Laboratories, Hercules, CA, USA) at a constant voltage of 15 V for 1 h. Next, the membrane was incubated for 1 h with rabbit anti-chicken PRL antibody at a 1:6000 dilution in Tris-buffered saline (TBS) containing 0.5% Tween 20. The membrane was then washed thrice with TBS containing 0.5% Tween 20 and incubated for 1 h with horseradish peroxidase-linked goat anti-rabbit IgG antibody (GE Healthcare UK Ltd., UK) at a 1:15000 dilution in TBS with 0.5% Tween 20. The membrane was then washed three times (10 min each time) before PRL detection. PRL was detected using detection reagents (GE Healthcare). In addition, immunoreactive PRL band signals were detected using Chemi-Doc. (Bio-Rad Laboratories). Finally, PRL was quantified by measuring the signal strength of the immunoreactive bands using Image Lab. (Bio-Rad Laboratories).
Changes in PRL and VIPR mRNA Levels
Total RNA was extracted from the remaining homogenate using an extraction kit (NucleoSpin® RNA Plus, Takara Bio). The amount of total RNA was measured via spectrophotometry (DS-11/R DeNovix Inc., Wilmington, DE, USA), and the RNA was reverse-transcribed using a PrimeScript™ RT Master Mix (Takara Bio). Reverse transcription was performed in 10-µL reactions using a thermocycler (Takara MP, Takara Bio) at 37°C for 15 min and 85°C for 5 min. The cDNA was stored at −20°C until the gene expression levels of PRL and VIPR were measured. Quantitative polymerase chain reactions (PCRs) using specific primers (Table 1) were per-formed for PRL and VIPR using a Thermal Cycler Dice® Real-Time System (Takara Bio). The amplification profile comprised an initial denaturation step at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and at 60°C for 30 s. Each sample was run in duplicate. Lastly, the 2−ΔΔCt method was used to calculate the relative expression of the analyzed genes after normalization to the expression of the glyceraldehyde-3-phosphate dehydrogenase gene.
Table 1. Primers used in real-time PCR.
| Gene | Direction | Sequence | Size |
|---|---|---|---|
| GAPDH | Forward | 5′-CTCTGGCAAAGTCCAAGTGGTG-3′ | 103 bp |
| Reverse | 5′-GCCCTTGAAGTGTCCGTGTGTA-3′ | ||
| PRL | Forward | 5′-AAAGCTGTTAATGGCTGCCACAC-3′ | 122 bp |
| Reverse | 5′-TCATTCCAGGAACGCAGCAC-3′ | ||
| VIPR | Forward | 5′-GAGCTCGTGGTTGGGTCATTC-3′ | 87 bp |
| Reverse | 5′-TCGCTTGAGCTCAGCTTGGA-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PRL, prolactin; VIPR, vasoactive intestinal peptide receptor 1.
Effects of VIP on PRL Release and PRL mRNA Expression
Based on the signal strength of the one-dimensional SDS-PAGE analysis, 6–8 anterior pituitary glands from days 16 to 20 of incubation were pooled and used to investigate the effects of VIP stimulation on PRL release and PRL mRNA expression. The pooled anterior pituitary glands were incubated in 300 µL of M199 (Life Technologies Corporation, Carlsbad, CA, USA) supplemented with 10 mM HEPES (Wako Pure Chemical Industries Ltd., Osaka, Japan) and 0.1% bovine serum albumin (Sigma, St. Louis, MO, USA) for 60 min in a humidified incubator at 37°C with 5% CO2 and 95% air. Subsequently, the anterior pituitary glands were incubated with or without synthetic chicken VIP (Genscript, Tokyo, Japan) (10−7 M) at 37°C for 60 min. Next, the protein content was measured and total RNA was extracted as described above. The amount of culture medium was adjusted for one- and two-dimensional analyses based on the protein content of the homogenized anterior pituitary glands. For one-dimensional analysis, the culture medium (300 µL×10 µg of protein/total protein obtained from the pituitary gland) was prepared; whereas for two-dimensional analysis, the culture medium (300 µL×100 µg of protein/total protein obtained from the pituitary gland) was prepared. A one-dimensional analysis was conducted as described above. To investigate the presence of glycosylated PRL, two different exposure times (short exposure, 30 s; long exposure, 2 min) were used. Two-dimensional analysis was conducted according to the methods described by O'Farrell (1975) and Hochestrasser et al. (1988). Briefly, the culture medium incubated with chicken VIP was concentrated through vacuum drying and dissolved in 125 µL rehydration buffer (8 M urea, 50 mM dithiothreitol (DTT), 2% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, and 0.2% Bio-lyte 3/10 (Bio-Rad Laboratories). The samples and an immobilized 7-cm pH gradient (pH 4–7) strip (Bio-Rad Laboratories) were rehydrated at a constant voltage of 50 V at 20°C for 12 h. After rehydration, isoelectric focusing was conducted at 250 V for 15 min, 4000 V for 1 h, and at 10,000 V/h at 20°C using a Protean IEF cell (Bio-Rad Laboratories). Next, the isoelectric focused strips were equilibrated with 2.5 mL of equilibration buffer I (6 M urea, 2% SDS, 0.375 M Tris–HCl (pH 8.8), 20% glycerol, 130 mM DTT, and 0.002% bromophenol blue) for 20 min. The strips were then equilibrated with 2.5 mL of equilibration buffer II (6 M urea, 2% SDS, 0.375 M Tris–HCl (pH 8.8), 20% glycerol, 135 mM iodoacet-amide, 0.002% bromophenol blue) for 10 min. After equilibration, the strips were fixed on the flat surface of a precast gel (12% Mini-Protean TGX Protein Gel; Bio-Rad Laboratories) using agarose to perform a two-dimensional analysis.
Effects of Forskolin on PRL Release
The anterior pituitary glands from day 18 were pooled and used to investigate the effect of forskolin on PRL release. The experimental procedure of one-dimensional analysis was conducted as described above.
Statistical Analysis
Changes in PRL content, PRL and VIPR mRNA, and the effects of VIP treatment on PRL release and PRL mRNA were analyzed using one-way analysis of variance. The significance of the differences between the means was assessed using the Tukey-Kramer test. The effects of VIP on PRL mRNA expression were analyzed using Student's t-test. The significance threshold was set at p<0.05.
Results
Detection of PRL in Embryonic Anterior Pituitary Glands
PRL was not detected in embryonic chicken anterior pituitary glands on days 14 and 15 of embryogenesis. A very weak 23-kDa PRL band was detected on day 16. Protein bands of two different molecular sizes, 23 kDa and 25 kDa, were detected on day 17. In addition, 23-kDa and 25-kDa bands were observed on days 18, 19, and 20 (Fig. 1). Finally, a significant increase (p<0.05) in PRL levels was observed between days 17 and 18 and days 18 and 19 (Fig. 2).
Fig. 1.
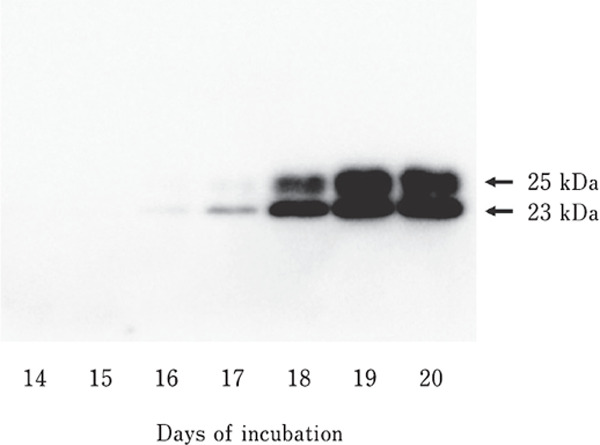
Example of SDS-PAGE and Western blot analyses of embryonic chicken PRL. Five micrograms of protein from the embryonic anterior pituitary glands were separated on a 12.5% SDS-PAGE gel, transferred to a mem brane, and visualized using antibodies against recombinant chicken prolactin.
Fig. 2.
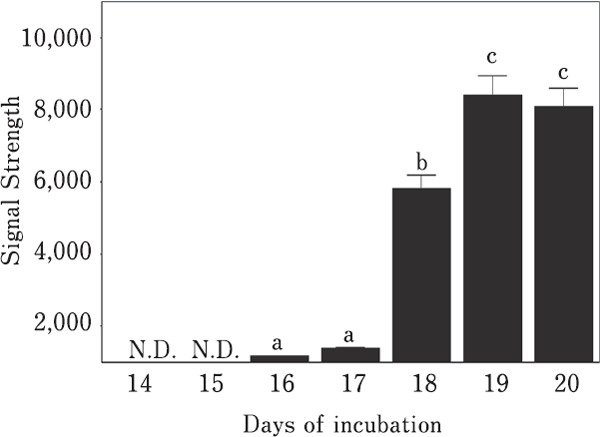
Changes in PRL levels as measured via densitometry. Means with different letters are significantly different from each other (p<0.05). The values are represented as the means±SEM (n=4).
Changes in PRL and VIPR mRNA Levels
PRL mRNA levels remained low until day 17 of embryogenesis. They began to increase on day 18 (p<0.05) and reached a maximum on day 20 (Fig. 3a). The levels of VIPR mRNA were generally low throughout embryogenesis. While low levels of VIPR mRNA were observed until day 19, a significant increase (p<0.05) was observed on day 20 of embryonic development (Fig. 3b).
Fig. 3.
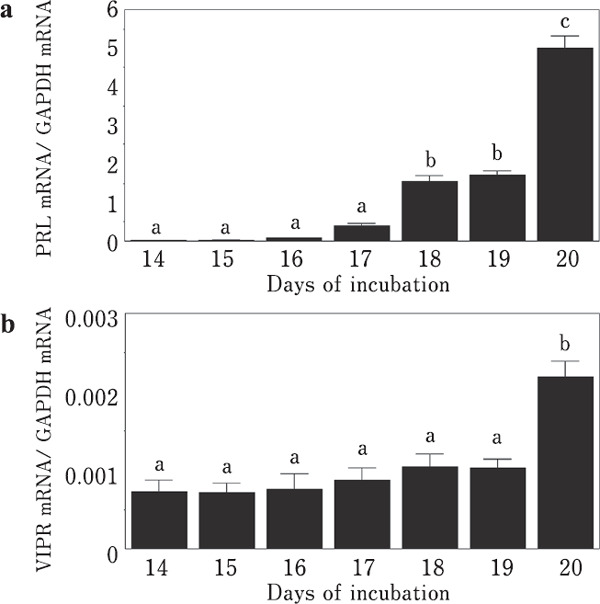
Changes in PRL and VIPR mRNA levels during embryogenesis. (a) PRL. (b) VIP receptor. Means with different letters are significantly different from each other (p<0.05). The values are represented as the means±SEM (n=4).
Effects of VIP on PRL Release and PRL mRNA Expression
PRL release from the embryonic anterior pituitary gland was not observed until day 18. VIP-induced PRL release was observed on days 19 and 20 (Fig. 4). Although the band signals were very weak, the 23-kDa PRL band was detected on days 19 and 20 without VIP stimulation (Fig. 4a). VIP-induced PRL release was observed on days 19 and 20 (p <0.05, Fig. 4b). Two spots of PRL molecules were detected using two-dimensional western blot analysis on days 19 (Fig. 5a) and 20 (Fig. 5b).
Fig. 4.
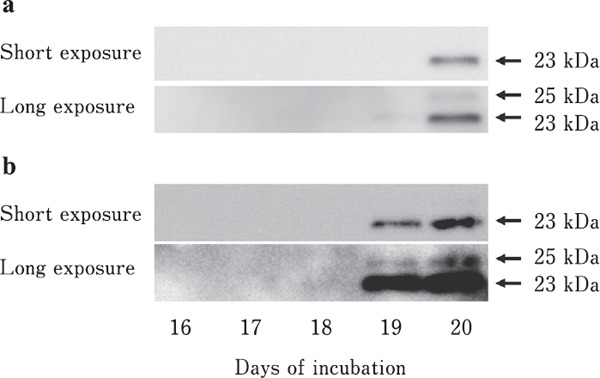
SDS-PAGE and Western blotting analyses of PRL released from the anterior pituitary gland. (a) Control. (b) VIP stimulation. Short and long exposure were conducted to identify G-PRL.
Fig. 5.
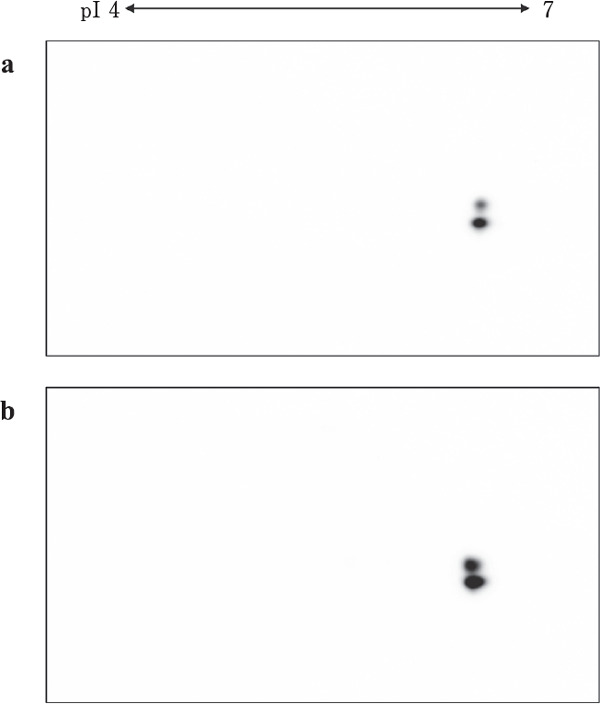
Two-dimensional analyses of the PRL released by the anterior pituitary gland on days 19 and 20 of incubation. (a) Day 19. (b) Day 20. Isoelectric points (pI) are indicated.
No significant difference in PRL mRNA levels was detected between VIP-stimulated and control conditions until day 18 of embryogenesis. In response to VIP-induced PRL release, significant differences (p<0.05) in PRL mRNA levels were detected on days 19 and 20 (Fig. 6).
Fig. 6.
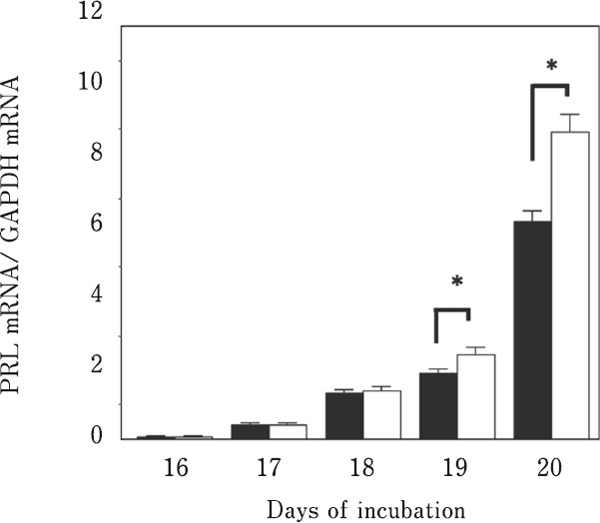
Effects of VIP stimulation on the expression of PRL mRNA. ■: control; □: vasoactive intestinal polypeptide stimulation. Means with asterisks are significantly different (p<0.05). The values are represented as the means±SEM (n=4).
Effects of Forskolin on PRL Release
PRL release from the embryonic anterior pituitary gland following VIP stimulation on day 18 was not observed. However, 23-kDa PRL was detected under forskolin-stimulated conditions (Fig. 7).
Fig. 7.
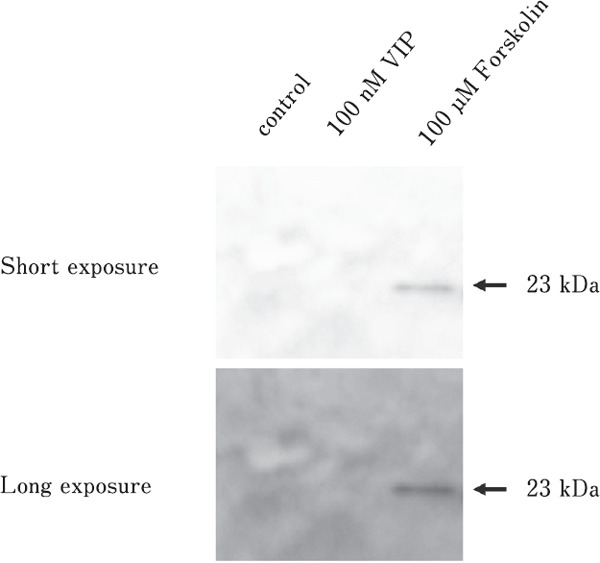
SDS-PAGE analyses of the PRL released from VIP- or forskolin-stimulated anterior pituitary glands. Short and long exposure were conducted to identify G-PRL.
Results and Discussion
PRL molecules in the anterior pituitary gland can be detected in turkey embryos five days before hatching (Bédécarrats et al., 1999c) and in chicken embryos four days before hatching (Hiyama et al., 2009). In this study, the anterior pituitary glands of chicken embryos from days 14 to 20 of embryogenesis were analyzed using western blot analysis to determine the presence of PRL. PRL was first detected on day 16, and a marked increase in PRL levels was observed between days 17 and 18 of incubation. In addition, significant increases in PRL mRNA levels were detected between days 17 and 18 of incubation. This increase in PRL mRNA levels strongly suggests increased translation of PRL mRNA and the production of PRL in the anterior pituitary gland.
Hiyama et al. (2015) found the increased change of in the mRNA levels of PREB, Pit-1, and PRL genes during chicken embryogenesis and proposed that these changes might be related to the regulatory mechanisms of PRL mRNA expression. Pit-1 is related to an initial increase in PRL mRNA levels; it then interacts with PREB to further increase PRL mRNA expression at the late stage of embryogenesis. In the present study, a significant increase in PRL mRNA levels was detected between days 17 and 18 of incubation. This increase in PRL mRNA expression may be related to Pit-1 mRNA and PREB mRNA expression.
This study examined the effect of VIP on PRL release and PRL mRNA expression. The initiation of a response to VIP is noteworthy. Strong PRL signals were observed in the embryonic anterior pituitary gland on day 18 (Fig. 1); however, VIP-induced PRL release was not initiated until day 18 (Fig. 4). Changes in plasma PRL levels during embryogenesis have previously been reported in chickens and turkeys (Harvey et al., 1979; Ishida et al., 1991, Bédécarrats et al., 1999c; Leclerc et al., 2007). In addition, Ishida et al. (1991) observed significant increases in plasma PRL levels two days before hatching in various breeds of chickens. The initiation of the response to VIP on day 19 strongly suggests an increase in plasma PRL concentration before hatching. Furthermore, these results suggest that the hypothalamic regulation of PRL release is initiated on day 19 of incubation.
The effects of VIP on PRL release were observed on days 19 and 20 of incubation. Similarly, the effect of VIP on PRL mRNA expression was observed on days 19 and 20. However, increases in PRL mRNA levels upon VIP stimulation were not detected until day 18 of incubation. In contrast, forskolin treatment induced PRL release from the embryonic anterior pituitary gland on day 18 (Fig. 7). Since the levels of VIPR mRNA were low until day 19, the expression of PRL mRNA and PRL content in the anterior pituitary gland before day 18 of incubation may be regulated independently of hypothalamic VIP. In contrast, VIP is critical for regulating PRL release and increasing PRL mRNA expression on days 19 and 20.
In this study, one- and two-dimensional analyses of released PRL were conducted. Differences in isoform components between pituitary PRL and released PRL were also noticeable. Hiyama et al. (2009) observed changes in the proportion of G-PRL to NG-PRL between days 17 and 20 of incubation, and similar proportions of G-PRL and NG-PRL in the anterior pituitary gland on days 19 and 20. However, the proportions of G-PRL and NG-PRL in the culture medium were different from those obtained from pituitary PRL. Two-dimensional western blot analysis also revealed a different isoform pattern. Hiyama et al. (2009) reported the presence of multiple G-PRL isoforms with different isoelectric points on day 20 of embryogenesis. In this study, a single G-PRL signal was detected in the culture medium using two-dimensional analyses for both days 19 and 20, whereas multiple G-PRL isoforms with different isoelectric points were detected via two-dimensional analyses using an aliquot of the anterior pituitary gland homogenate from days 19 and 20 (data not shown).
Phosphorylation, acetylation, and deamidation are among the post-translational modifications that reduce the isoelectric point of a protein. The post-translational modification of hormones occurs in the trans-Golgi network, which then become incorporated into immature vesicles. Subsequently, multiple immature vesicles fuse into mature secretory granules. Here, the difference in isoform composition between pituitary PRL and released PRL on days 19 and 20 of embryogenesis suggests that secretory granules located near the cell membrane contain only unmodified and glycosylated PRL. Therefore, PRL isoforms with different isoelectric points may be released after hatching.
In conclusion, the increased PRL content in chicken embryos is originating the PRL mRNA expression. However, PRL mRNA expression and PRL release from the anterior pituitary gland, may be regulated by different factors. The increased plasma PRL concentration before hatching is stimulated by hypothalamic VIP.
Acknowledgments
This work was supported, in part, by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (17K08055) to N. K.
Author Contributions
Norio Kansaku designed the experiment, performed the experiment, and wrote the manuscript; Shin Wakui codesigned the experiment and participated in the discussion of the results; Tomohiro Sasanami co-designed the experiment and produced the antibody; Takeshi Ohkubo discussed the results of the experiment and co-designed the experiment.
All authors share the experimental purpose, methods, re-sults, and conclusions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bédécarrats G, Guémené D, Kühnlein U and Zadworny D. Changes in levels of immunoreactive prolactin isoforms during a reproductive cycle in turkey hens. General and Comparative Endocrinology, 113: 96-104. 1999a. [DOI] [PubMed] [Google Scholar]
- Bédécarrats G, Guémené D, Morvan C, Crisóstomo-Pinto S, Kühnlein U and Zadworny D. In vitro release of isoforms of prolactin from pituitary glands of turkey hens at different physiological stages. General and Comparative Endocrinology, 113: 105-111. 1999b. [DOI] [PubMed] [Google Scholar]
- Bédécarrats G, Guémené D, Morvan C, Kühnlein U and Zadworny D. Quantification of prolactin messenger ribonucleic acid, pituitary content and plasma levels of prolactin, and detection of immunoreactive isoforms of prolactin in pituitaries from turkey embryos during ontogeny. Biology of Reproduction, 61: 757-763. 1999c. [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N and Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocrine Reviews, 19: 225-268. 1998. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248-254. 1976. [DOI] [PubMed] [Google Scholar]
- Chaiseha Y, Youngren OM and El Halawani ME. Expression of vasoactive intestinal peptide receptor messenger RNA in the hypothalamus and pituitary throughout the turkey reproductive cycle. Biology of Reproduction, 70: 593-599. 2004. [DOI] [PubMed] [Google Scholar]
- Doneen BA and Smith TE. Ontogeny of endocrine control of osmoregulation in chick embryo. I. Role of pituitary gland in distribution of water and ions among embryonic and extraembryonic compartments. General and Comparative Endocrinology, 48: 300-309. 1982a. [DOI] [PubMed] [Google Scholar]
- Doneen BA and Smith TE. Ontogeny of endocrine control of osmoregulation in chick embryo. II. Actions of prolactin, arginine vasopressin, and aldosterone. General and Comparative Endocrinology, 48: 310-318. 1982b. [DOI] [PubMed] [Google Scholar]
- El Halawani ME, Silsby JL, Rozenboim I and Pitts GR. Increased egg production by active immunization against vasoactive intestinal peptide in the turkey (Meleagris gallopavo). Biology of Reproduction, 52: 179-183. 1995. [DOI] [PubMed] [Google Scholar]
- Hanaham D. Studies on transformation of Escherichia coli with plasmids. Journal of Molecular Biology, 166: 557-580. 1983. [DOI] [PubMed] [Google Scholar]
- Harvey S, Davison TF and Chadwick A. Ontogeny of growth hormone and prolactin secretion in the domestic fowl (Gallus domesticus). General and Comparative Endocrinology, 39: 270-273. 1979. [DOI] [PubMed] [Google Scholar]
- Hiyama G, Kansaku N, Kinoshita M, Sasanami T, Nakamura A, Noda K, Tsukada A, Shimada K and Zadworny D. Changes in post-translational modifications of prolactin during development and reproductive cycles in the chicken. General and Comparative Endocrinology 161: 238-245. 2009. [DOI] [PubMed] [Google Scholar]
- Hiyama G, Kansaku N, Tanaka T, Wakui S and Zadworny D. Characterization of chicken prolactin regulatory element binding protein and its expression in the anterior pituitary gland during embryogenesis and different reproductive stages. Journal of Poultry Science, 52: 42-51. 2015. [Google Scholar]
- Hochstrasser DF, Harrington MG, Hochstrasser AC, Miller MJ and Merril CR. Methods for increasing the resolution of two-dimensional protein electrophoresis. Analytical Biochemistry, 173: 424-435. 1988. [DOI] [PubMed] [Google Scholar]
- Ishida H, Shimada K, Sato K, Seo H, Murata Y, Matsui N and Zadworny D. Developmental expression of the prolactin gene in the chicken. General and Comparative Endocrinology, 83: 463-467. 1991. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Shimada K, Terada O and Saito N. Gene expression of prolactin, growth hormone, and luteinizing hormone-b subunit gene expression in the cephalic and caudal lobes of the anterior pituitary gland during embryogenesis and different reproductive stages in the chicken. General and Comparative Endocrinology, 96: 197-205. 1994. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Shimada K and Saito N. Regionalized gene expression of prolactin and growth hormone in the chicken anterior pituitary gland. General and Comparative Endocrinology, 99: 60-68. 1995. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Shimada K, Saito N and Hidaka H. Effects of protein kinase A inhibitor (H-89) on VIP-and GRF-induced release and mRNA expression of prolactin and growth hormone in the chicken pituitary gland. Comparative Biochemistry and Physiology, 119C: 89-95. 1998. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Shimada K, Ohkubo T, Saito N, Suzuki T, Matsuda Y and Zadworny D. Molecular cloning of chicken vasoactive intestinal polypeptide receptor complementary DNA, tissue distribution and chromosomal localization. Biology of Reproduction, 64: 1575-1581. 2001. [DOI] [PubMed] [Google Scholar]
- Kansaku N, Tobari Y, Hiyama G, Wakui S, Minoguchi N, Numata M, Kino K and Zadworny D. Effects of vasoactive intestinal polypeptide and forskolin on mRNA expression of prolactin and prolactin regulatory element-binding protein in the anterior pituitary gland of chicken embryo and laying hens. Journal of Poultry Science, 53: 313-317. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, Boutin JM, Jolicoeur C, Okamura H, Shirota M, Edery M, Dusanter-Fourt I and Djiane J. Purification, cloning and expression of the prolactin receptor. Biology of Reproduction, 40: 27-32. 1989. [DOI] [PubMed] [Google Scholar]
- Leclerc B, Zadworny D, Bédécarrats G and Kühnlein U. Ontogenesis of the expression of prolactin receptor messenger ribonucleic acid during late embryogenesis in turkeys and chickens. Journal of Poultry Science, 86: 1174-1179. 2007. [DOI] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. Journal of Biological Chemistry, 250: 4007-40021. 1975. [PMC free article] [PubMed] [Google Scholar]
- Ohkubo T, Tanaka M, Nakashima K, Shimada K, Saito N and Sato K. High-level expression of biologically active chicken prolactin in E. coli. Comparative Biochemistry and Physiology, 105: 123-128. 1993. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Tanaka M, Nakashima K, Talbot RT and Sharp PJ. Prolactin receptor gene expression in the brain and peripheral tissues in broody and nonbroody breeds of domestic hen. General and Comparative Endocrinology, 109: 60-68. 1998. [DOI] [PubMed] [Google Scholar]
- Proudman JA and Opel H. Stimulation of prolactin secretion from turkey anterior pituitary cells in culture. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine, 187: 448-454. 1988. [DOI] [PubMed] [Google Scholar]
- Riddle O, Bates RW and Lahr EL. Prolactin induces broodiness in fowl. American Journal of Physiology-Legacy Content, 111: 352-360. 1935. [Google Scholar]
- Sharp PJ, Sterling RJ, Talbot RT and Huskisson NS. The role of hypothalamic vasoactive intestinal polypeptide in the maintenance of prolactin secretion in incubating bantam hens: observations using passive immunization, radioimmunoassay and immunohistochemistry. Journal of Endocrinology, 122: 5-13. 1989. [DOI] [PubMed] [Google Scholar]
- Skwarło-Sońta K, Rosołowska-Huszcz D, Sotowska-Brochocka J and Gajewska A. Daily variations in response of certain immunity indices to prolactin in White Leghorn chickens. Experimental and Clinical Endocrinology, 87: 195-200. 1986. [DOI] [PubMed] [Google Scholar]
- Talbot RT, Dunn IC, Wilson PW, Sang HM and Sharp PJ. Evidence for alternative splicing of the vasoactive intestinal polypeptide gene transcript. Journal of Molecular Endocrinology, 15: 81-91. 1995. [DOI] [PubMed] [Google Scholar]
- Youngren OM, Silsby JL, Rozenboim I, Philips RE and Halawani E. Active immunization with vasoactive intestinal peptide prevents the secretion of prolactin induced by electrical stimulation of the turkey hypothalamus. General and Comparative Endocrinology, 65: 330-336. 1994. [DOI] [PubMed] [Google Scholar]
- Zadworny D, Shimada K, Ishida H and Sato K. Gonadotropin-stimulated estradiol production in small ovarian follicles of the hen is suppressed by physiological concentrations of prolactin in vitro. General and Comparative Endocrinology, 74: 468-473. 1989. [DOI] [PubMed] [Google Scholar]
- Zhou JF, Zadworny D, Guémené D and Kuhnlein U. Molecular cloning, tissue distribution, and expression of the prolactin receptor during various reproductive states in Meleagris gallopavo. Biology of Reproduction, 55: 1081-1090. 1996. [DOI] [PubMed] [Google Scholar]


