Abstract
We identified the human herpesvirus 6 (HHV-6)-dominant immunoglobulin M (IgM)-reactive virion protein as being the same 101-kDa protein (101K) previously identified as the major IgG immunoreactive protein and a specific serologic marker of HHV-6 infection. An immunoblot assay (IB) to detect HHV-6-specific IgM antibodies against the 101K protein in human serum samples was developed. The assay was validated by using acute- and convalescent-phase serum collected from children under 2 years of age in which we previously detected IgG seroconversion to the HHV-6 101K protein. Of 32 serum pairs which previously demonstrated IgG seroconversion to the 101K protein, 29 had IgM reactivity to the same protein in the acute-phase sample and the remaining 3 had reactivity in the convalescent-phase sample. We also detected HHV-6 IgM activity in sera collected from individuals ≥4 years of age who were also IgM seropositive to measles or rubella. Results of cross-adsorption studies using measles virus-, rubella virus-, and HHV-6-infected cells as the adsorbing antigen indicated no cross-reactivity between measles or rubella IgM and HHV-6 IgM in human serum samples. The IgM IB detected HHV-6-specific IgM antibody to the 101K protein in 78% (63 of 81) of tested acute-phase serum collected from young children with an undifferentiated rash illness by using a single serum dilution.
Human herpesvirus 6 variant B (HHV-6B) is a lymphotropic virus associated with fever and rash illness. HHV-6B is the etiologic agent of exanthem subitum (ES), a benign, self-limiting disease which is usually contracted by age 2 (32). Nearly 100% of the adult population is HHV-6 seropositive (19). A classic symptom of ES consists of fever of ≥38°C, which may last several days. Upon defervescence, the patient develops a macular-papular rash, usually on the trunk, which may also last several days. However, patients experiencing primary HHV-6 infection but presenting with only fever (26) or only rash (1) have been identified.
We have previously described the development of an immunoglobulin G (IgG)-based immunoblot assay (IB) which specifically detects the 101-kDa HHV-6 immunodominant virion protein (101K) and does not react with HHV-7 cross-reactive antibodies known to be present in human serum samples (4). The IgG IB was used to examine acute- and convalescent-phase sera collected from children with exanthem who were presumptively diagnosed with measles or rubella but were not laboratory confirmed (2). Twenty percent of the patients in that study had an IgG seroconversion to HHV-6, indicating that primary HHV-6 infection can be misdiagnosed as measles or rubella. Others have also reported significant numbers of children with acute HHV-6 infection misdiagnosed as measles or rubella (9, 28). HHV-6 has also been associated with neurologic symptoms and has been detected in the cerebral spinal fluid of children with febrile convulsions, encephalitis, and hemiplegia (14, 21, 30, 33, 34). These cases demonstrate the need to include primary or reactivated HHV-6 infection in the differential diagnosis of unexplained illness associated with rash, fever, or neurologic symptoms in very young children.
Various serologic methods have been used to diagnose acute HHV-6 infection, including immunofluorescence assays (IFA) and enzyme-linked immunoassays (EIA). The disadvantage associated with IgG-detecting immunoassays is the requirement for paired sera to demonstrate seroconversion or a rise in antibody titer; thus, they are not useful for rapid HHV-6 diagnostics. IgM is the predominant antibody produced during a primary immune response. It is usually detectable within 7 days of infection, reaches maximum titers within 2 to 3 weeks, and then declines to undetectable levels by 3 months. IFA, EIA, and neutralization assays (NT) have been used to detect HHV-6 IgM (11, 18, 25, 30). The NT requires approximately 1 week to complete. Many EIA and IFA using infected cells as antigen do not include measures to discriminate between HHV-6 and HHV-7 cross-reactive antibodies (3). Although these assays are generally highly sensitive, nonspecific activity can complicate the interpretation of results. IB assays based on reactivity with virus-encoded proteins are highly specific and yield easily interpretable results (2, 4, 15). In this paper, we first identified the HHV-6 IgM-reactive virion proteins and then developed an IB to detect IgM antibodies in human serum specimens reactive to the identified proteins.
MATERIALS AND METHODS
Sera.
Three sets of serum samples were tested for HHV-6 activity. (i) Acute- and convalescent-phase serum samples were collected from residents of Sao Paolo, Brazil, as previously described (2) who had been shown to seroconvert to HHV-6 by IgG. The subset of children tested in the present study were under 24 months of age with the exception of one 4 year old (ages 3 months to 4 years; mean age, 13.8 months). Acute-phase sera were collected between zero and 13 days after rash onset, and 73% (30 of 41) were collected between days zero and 4. (ii) Paired serum was collected from 37 Venezuelan children with a rash illness (kindly provided by Ed Maes, Centers for Disease Control and Prevention, Atlanta, Ga.) who had either a fourfold rise in IgG titer or an IgG seroconversion or equivocal activity to HHV-6 by EIA (age 3 to 33 months). The EIA was performed as previously described (4) using HHV-6-infected cells as antigen. Acute-phase sera were collected between zero and 3 days relative to rash onset except for one collected on day 22. Convalescent-phase sera were collected between days 17 and 34 with the exception of one collected on day 150. (iii) Sera were sent to the Washington State Public Health Laboratories for diagnosis of a rash illness for patients who were measles, rubella, or parvovirus IgM seropositive (age range, 4 to 45 years). Measles, rubella, and parvovirus B-19 IgM EIA were performed by the Washington State Public Health Laboratory. All sera used in this study were collected with consent.
IgM immunoblot assay.
The IB was performed as previously described for detection of IgG against the 101K protein (29). Briefly, proteins from filtered and pelleted HHV-6 infected-cell supernatants were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 9% polyacrylamide gel and then were transferred to nitrocellulose. Human sera were treated with an IgG adsorbent; to remove rheumatoid factor and immune complexes according to the manufacturer's instructions for a final dilution of 1:10. The nitrocellulose strips were then treated with the serum for 2 h at room temperature while rocking, washed, and then treated with alkaline phosphatase-conjugated goat anti-human IgM (diluted 1:2,000) for 2 h while rocking at room temperature. Bands were visualized by using an alkaline phosphatase substrate kit (Bio-Rad, Hercules, Calif.) according to the manufacturer's instructions. Color on the nitrocellulose was developed until the positive control became intense but stopped before background staining obscured any bands. Band intensity was scored while the nitrocellulose was wet. All acute-phase samples were tested. Convalescent-phase serum samples were tested only if no activity was detected in the acute-phase sample.
Adsorption protocol.
Lysates of HHV-6-infected cells, measles virus-infected cells (a gift of William Bellini, Centers for Disease Control and Prevention), and rubella virus-infected cells (a gift of Teryl Frey, Georgia State University, Atlanta) were prepared as described previously (4).
RESULTS
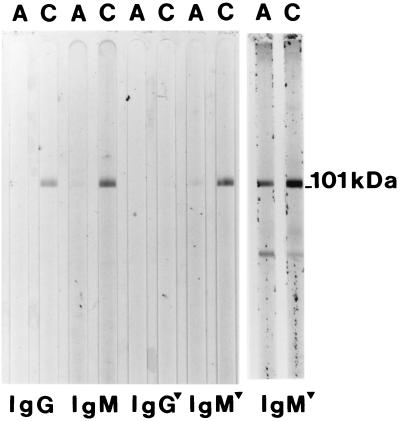
Acute- and convalescent-phase serum collected from an individual with a rash illness was evaluated for antibodies to HHV-6 by IB (2) (Fig. 1). IgG activity to the HHV-6 101K virion protein was detected only in the convalescent-phase serum, indicating that this individual had an IgG seroconversion to HHV-6 (Fig. 1, lanes 1 and 2). The same paired-serum sample was evaluated for IgM activity as described in Materials and Methods. Weak IgM activity to the 101K protein was detected in the acute-phase sera, and strong activity was detected in the convalescent-phase sera (Fig. 1, lanes 3 and 4). To circumvent false positive results due to rheumatoid factor or immune complexes, the samples were treated with an anti-IgG reagent. This reagent completely removed the IgG activity to the 101K protein (Fig. 1, lanes 5 and 6) but did not interfere with the weak IgM activity to the same protein (Fig. 1, lanes 7 and 8). Thus, the IgG adsorbant did not interfere with the detection of weak IgM-positive results by this assay. The last two lanes of Fig. 1 illustrate the results of a different acute- and convalescent-phase pair demonstrating strong IgM activity in the acute-phase sample. Paired sera collected from children who seroconverted to HHV-7 by IgG did not react in the HHV-6 IgM IB (not shown).
FIG. 1.
IgM immunoblot reactivity with the HHV-6 101K virion protein. Acute-phase (A) and convalescent-phase (C) sera from a single patient (lanes 1 to 8) were reacted with nitrocellulose containing HHV-6 virion proteins and tested for IgG and IgM activity with or without IgG depletion. The triangle indicates that the serum was treated with IgG adsorbent. The last two lanes represent results of an IgM serum antibody from a different child.
In our previous study of Brazilian patients with rash illness (2), we identified 37 serum pairs showing clear seroconversion by IgG to the HHV-6 101K protein by IB. An additional 13 serum pairs showed an increase in convalescent-phase signal intensity relative to the acute-phase reactivity by IB. Acute-phase serum with sufficient volumes from both the seroconversion and the increase groups were tested for IgM activity to the HHV-6 101K protein by IB (Table 1). IgM activity was detected in 90% of the acute-phase sera from the seroconversion group and in all 12 of the available acute-phase samples from the increase group. IgM activity was detected only in the convalescent-phase sample from the remaining three sera in the conversion group.
TABLE 1.
IgM activity in the acute- and convalescent-phase sera collected from Brazilian and Venezuelan children who were previously shown to seroconvert to HHV-6 by IgM
| Serum characteristic (origin) | No. of samples with HHV-6 IgM
|
||
|---|---|---|---|
| Total | Acute phase | Only conv phasea | |
| IgG seroconverted (Brazil) | 32 | 29 | 3 |
| IgG increase (Brazil)b | 12 | 12 | NT |
| IgG activity (Venezuela) | 37 | 22 | 12 |
Conv, convalescent-phase sera; NT, not tested.
HHV-6 IgG increase indicates an increase in band intensity of the convalescent-phase serum when compared to the acute-phase serum as previously described (2).
To further validate the HHV-6 IgM IB assay, we tested an additional 37 paired serum samples collected from Venezuelan children. These children presented with unexplained rash illness and were previously shown to have a either a fourfold rise in IgG antibody titer, a clear IgG seroconversion, or equivocal results to HHV-6 by using an EIA (J. Stewart and J. Patton, unpublished results). Thirty-four (92%) children had IgM activity to the HHV-6 101K protein (Table 1). Of the three that did not demonstrate IgM activity to the HHV-6 101K protein by IB, two showed evidence of HHV-7 seroconversion (an HHV-6 IgG-seronegative 7-month-old child and an HHV-6 IgG-seropositive 31-month-old child, data not shown); thus, the EIA activity was likely the result of cross-reactive antibodies. The third child clearly showed IgG seroconversion by EIA, an increase in IgG activity by IB, and IgM activity in the convalescent-phase sera by EIA (not shown). The HHV-6 IgM present in this sample was likely below the threshold of the IB sensitivity.
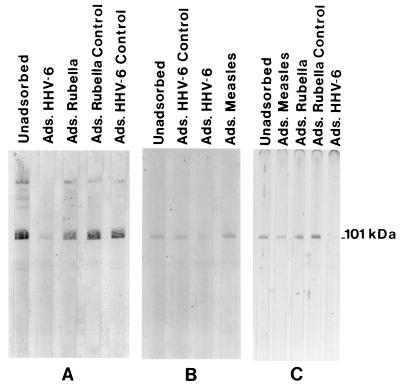
While evaluating cross-reactivity to the HHV-6 IgM reactive protein, we detected IgM activity to the HHV-6 101K band in measles, rubella, or parvovirus IgM-positive sera (Table 2). To determine if the 101K activity was specific or due to cross-reactivity, cross-adsorption studies were performed using measles-, rubella-, and HHV-6-infected cell antigen. Parvovirus studies were not done. Rubella and measles IgM+ sera that were also HHV-6 IgM+ were independently adsorbed with measles antigen, rubella antigen, and HHV-6 antigen, depleted of IgG, and then reacted with nitrocellulose containing HHV-6 virion proteins. Neither measles antigen (Fig. 2A) nor rubella antigen (Fig. 2B) adsorption had any effect on the 101K activity, but HHV-6 antigen adsorption greatly reduced the activity to the 101K band. An HHV-6 IgM-positive specimen that was measles and rubella IgM negative was also adsorbed with measles, rubella, and HHV-6 antigen (Fig. 2C). In this case, HHV-6-adsorbing antigen reduced the 101K IgM activity whereas the measles and rubella antigen had no effect on the HHV-6 IgM activity. The IB results show no cross-reactivity between measles IgM or rubella IgM and the HHV-6 101K protein. The 101K IgM activity observed in the measles and rubella IgM+ specimens was due to HHV-6-specific IgM antibody.
TABLE 2.
HHV-6 IgG activity in patients with confirmed measles, rubella, or parvovirus infections
| Infection | Total | No. HHV-6 IgM+ (%) |
|---|---|---|
| Measles | 9 | 4 (44) |
| Rubella | 8 | 6 (75) |
| Parvovirus | 7 | 4 (57) |
FIG. 2.
Adsorption studies for HHV-6 IgM specificity during the course of measles and rubella infection. (A) A dual measles and HHV-6 IgM+ serum sample was adsorbed with the indicated antigen as described in Materials and Methods and then tested for reactivity to the HHV-6 101K protein. (B) The same as panel A except that a dual HHV-6 and rubella IgM+ serum specimen was used. (C) An HHV-6 IgM+ serum that was IgM negative to both measles and rubella was adsorbed with the indicated antigen and tested as described above.
DISCUSSION
A utility of immunoassays for detecting IgM is the ability to detect IgM in the early days of illness and the lack of requirement for a convalescent-phase serum, which makes them useful as rapid diagnostic assays. HHV-6 serum IgM reacted with the 101-kDa IgG-reactive immunodominant virion protein, 101K. This protein is a structural virion tegument phosphoprotein encoded by open reading frame (ORF) U11 and is the homolog to the highly immunoreactive human cytomegalovirus and HHV-7 virion proteins p150 and 89K, respectively (10, 23) (Balada et al., unpublished data). In addition, ORFs U11 of both HHV-6A and HHV-6B encode nearly identical amino acid sequences and polyclonal antibody raised against HHV-6B 101K reacts with HHV-6A (20); thus, this protein is useful for detection of antibody to both HHV-6 variants. HHV-6 IgM against the 101K protein was detected in 63 of 81 (78%) of acute-phase samples tested, and of these, 51 (82%) were collected on days zero to 4. Insufficient numbers of samples were available from each day post-rash to determine the optimal sample collection time for IgM detection. Others have detected HHV-6 IgM in serum collected from ES patients on day 4 or 5 of the acute febrile phase and on day 1 relative to fever and rash by using IFA or NT (11, 25, 31). For comparison, IgM was detected in serum from 100% of children with measles virus infection by day 4 post-rash by using a highly sensitive IgM capture EIA with expressed measles virus nucleoprotein as antigen (13). A similar approach using recombinantly expressed 101K protein (20) in an IgM capture EIA-based assay may increase the sensitivity of reactivity to this protein. IgM antibody capture assays minimize competition from IgG antibodies and are more sensitive and specific than direct EIA, and commercially available methods to amplify signal are easily applicable.
HHV-6 IgM serologic assays described thus far used infected cells or infected cell lysates as antigen and none included steps to remove HHV-7 cross-reacting antibodies (5, 17, 18, 25), and thus they may lack specificity. Although HHV-6 is usually acquired prior to HHV-7, there have been many reports of HHV-7 acquisition first (3), which warrants the need for virus-specific assays. PCR methods for detecting HHV-6 DNA in blood cells cannot discriminate latent from active infection. In addition, inhibition of the PCR and lower sensitivities were reported with serum PCR assays (7, 8, 22). A sensitive and specific reverse transcription-PCR assay which detects active virus infection was recently reported (16). However, highly sensitive PCR assays may yield false positive results and the possibility of contamination makes these tests less attractive for a clinical laboratory setting. Comparisons of PCR DNA detection from whole blood to IgM detection by IFA using IgG-seronegative samples collected from symptomatic children (5, 17) have shown DNA positivity in both IgM-positive and -negative sera and IgM positivity in the presence and absence of viral DNA. Other methods for diagnosing HHV-6 infection include differential detection of DNA in blood but not saliva and detection of DNA in the absence of an IgG response (7, 8). These diagnostic methods are two-step algorithms. The 101K IB is more specific then currently used IFA and EIA (4) and has the potential of being configured into a commercially available “dipstick-like” assay, based on expressed 101K protein and signal amplification as discussed above, and may be useful in a clinical laboratory setting.
Hall et al. (12) have shown that 10% of emergency room visits for acute febrile illness in children under 2 years of age were due to primary HHV-6 infection. Of these children, only 17% exhibited the classic symptoms of ES. Twenty-one percent were hospitalized for the following reasons: (i) suspected sepsis and toxicity, (ii) diarrhea and dehydration, (iii) lower respiratory symptoms, and (iv) seizures. More recently, Chiu et al. (7) detected HHV-6 primary infection in 50% of hospital admissions in children under the age of 1 year. These studies clearly illustrate the high percentage of atypical clinical presentations associated with HHV-6 infection and the relationship of this virus to significant morbidity in young children. Simple, rapid diagnostic assays for primary HHV-6 infection would decrease the need for costly hospital evaluations and may help to deter inappropriate administration of antibiotics to febrile children.
We have previously shown that approximately 50% of cases clinically diagnosed as measles or rubella in measles- and rubella-seronegative children less than 2 years of age were actually primary HHV-6 infection (2). In the present study, we detected HHV-6 IgM in serum collected from patients with laboratory-confirmed measles, rubella, and parvovirus infections. Given the ages of the patients, the HHV-6 IgM activity was most likely the result of reactivation. Insufficient clinical information was available to assess any atypical clinical features resulting from dual infection in these cases. However, HHV-6 reactivation during the convalescent phase of measles (24), as well as dual primary infections (27), has been reported. In the cases of dual infections, a prolonged skin rash and lack of seroconversion to measles virus was described. Given the high percentage of apparent reactivation detected in this study during the course of other viral infections, and the fact that HHV-6 establishes latency in T lymphocytes and replicates in activated T cells (6), it is likely that the virus reactivates as a result of immune system activation. The frequency of dual infections or reactivation with other viruses and the contribution of HHV-6 to disease progression remain to be determined. The HHV-6 101K IgM assay maybe useful in studies of dual infections. It may also be useful in studies of viral reactivation and relationship to disease in older patients, perhaps in the transplant setting.
In conclusion, we demonstrated the specific HHV-6 IgM antibody reactivity of the 101K protein and the ability of the IB to diagnose primary HHV-6 infection in 80% of children <2 years of age with unexplained rash illness. The assay requires only a small volume (<100 μl) of a single acute-phase serum sample, and definitive results can usually be obtained using a single serum dilution. An advantage of IBs is that they can be configured as single strips convenient for individual tests. Assays based on detecting IgM against the HHV-6 101K protein are potentially clinically useful in the differential diagnosis of febrile and rash illness and neurologic manifestations in emergency room situations in children <2 years old. The rapid diagnosis of acute HHV-6 infection in these situations would help provide assurance to physicians and parents in distinguishing between a possibly life-threatening or contagious disease and a usually benign disease. In addition, this assay may be useful in monitoring measles and rubella vaccine efficacy in countries with vaccination programs in place to help determine the true etiology of diseases identified as measles or rubella in very young children (9).
ACKNOWLEDGMENTS
Special thanks are given to Michael Glass, who acted as mentor for the Emerging Infectious Disease Fellowship Program at the Washington State Public Health Laboratories during the development and course of the work, and to Philip Pellett for critical review of the manuscript. We also thank Kathleen Kite-Powell, Joanne Patton, and Janet Heath for their contribution to this work.
This research was supported in part by an appointment to the Emerging Infectious Diseases Fellowship administered by the Association of Public Health Laboratories and funded by the Centers for Disease Control and Prevention.
REFERENCES
- 1.Asano Y, Suga S, Yoshikawa T, Urisu A, Yazaki T. Human herpesvirus type 6 infection (exanthem subitum) without fever. J Pediatr. 1989;115:264–265. doi: 10.1016/s0022-3476(89)80078-2. [DOI] [PubMed] [Google Scholar]
- 2.Black J B, Durigon E, Kite-Powell K, de Souza L, Curli S P, Afonso Sardinha A M, Theobaldo M, Pellett P E. HHV-6 and HHV-7 seroconversion in children clinically diagnosed with measles or rubella. Clin Infect Dis. 1996;23:1156–1158. doi: 10.1093/clinids/23.5.1156. [DOI] [PubMed] [Google Scholar]
- 3.Black J B, Pellett P E. Human herpesvirus 7. Rev Med Virol. 1999;9:245–262. doi: 10.1002/(sici)1099-1654(199910/12)9:4<245::aid-rmv253>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Black J B, Schwarz T F, Patton J L, Kite-Powell K, Pellett P E, Wiersbitzky S, Bruns R, Muller C, Jager G, Stewart J. Evaluation of immunoassays for detection of antibodies to human herpesvirus 7. Clin Diagn Lab Immunol. 1996;3:79–83. doi: 10.1128/cdli.3.1.79-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bland R M, Mackie P L K, Shorts T, Pate S, Paton J Y. The rapid diagnosis and clinical features of human herpesvirus 6. J Infect. 1998;36:161–165. doi: 10.1016/s0163-4453(98)80006-6. [DOI] [PubMed] [Google Scholar]
- 6.Braun D K, Dominguez G, Pellett P E. Human herpesvirus 6. Clin Microbiol Rev. 1997;10:1–47. doi: 10.1128/cmr.10.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu S S, Cheung C Y, Tse C Y C, Peiris M. Early diagnosis of primary human herpesvirus 6 infection in childhood: serology, polymerase chain reaction, and virus load. J Infect Dis. 1998;178:1250–1256. doi: 10.1086/314432. [DOI] [PubMed] [Google Scholar]
- 8.Clark D A, Kidd I M, Collingham K E, Tarlow M, Aueni T, Riordan A, Griffiths P D, Emery V C, Pillay D. Diagnosis of primary human herpesvirus 6 and 7 infections in febrile infants by polymerase chain reaction. Arch Dis Child. 1997;77:42–45. doi: 10.1136/adc.77.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidkin I, Valle M, Peltola H, Hovi T, Paunio M, Roivainen M, Linnavuori K, Jokinen S, Leinikki P. Etiology of measles- and rubella-like illnesses in measles, mumps, and rubella-vaccinated children. J Infect Dis. 1998;178:1567–1570. doi: 10.1086/314513. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez G, Dambaugh T R, Stamey F R, Dewhurst S, Inoue N, Pellett P E. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J Virol. 1999;73:8040–8052. doi: 10.1128/jvi.73.10.8040-8052.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox J D, Ward P, Briggs M, Irving W, Stammers T G, Tedder R S. Production of IgM antibody to HHV6 in reactivation and primary infection. Epidemiology. 1990;104:289–296. doi: 10.1017/s095026880005946x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall C B, Long C E, Schnabel K C, Caserta M T, McIntyre K M, Costanzo M A, Knott A, Dewhurst S, Insel R A, Epstein L G. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331:432–438. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- 13.Helfand R F, Heath J L, Anderson L J, Maes E F, Guris D, Bellini W J. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J Infect Dis. 1997;175:195–199. doi: 10.1093/infdis/175.1.195. [DOI] [PubMed] [Google Scholar]
- 14.Kondo K, Nagafuji H, Hata A, Tomomori C, Yamanishi K. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J Infect Dis. 1993;167:1197–1200. doi: 10.1093/infdis/167.5.1197. [DOI] [PubMed] [Google Scholar]
- 15.Lazzarotto T, Brojanac S, Maine G T, Landini M P. Search for cytomegalovirus-specific immunoglobulin M: comparision between a new Western blot, conventional Western blot, and nine commercially available assays. Clin Diagn Lab Immunol. 1997;4:483–486. doi: 10.1128/cdli.4.4.483-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton R A, Caserta M T, Hall C B, Schnabel K, Hocknell P, Dewhurst S. Detection of human herpesvirus 6 by reverse transcription-PCR. J Clin Microbiol. 1999;37:3672–3675. doi: 10.1128/jcm.37.11.3672-3675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osiowy C, Prud'Homme I, Monette M, Zou S. Detection of human herpesvirus 6 DNA in serum by a microplate PCR-hybridization assay. J Clin Microbiol. 1998;36:68–72. doi: 10.1128/jcm.36.1.68-72.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker C A, Weber J M. An enzyme-linked immunosorbent assay for the detection of IgG and IgM antibodies to human herpesvirus type 6. J Virol Methods. 1993;41:265–276. doi: 10.1016/0166-0934(93)90017-l. [DOI] [PubMed] [Google Scholar]
- 19.Pellett P E, Black J B. Human herpesvirus 6. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2587–2608. [Google Scholar]
- 20.Pellett P E, Sanchez-Martinez D, Dominguez G, Black J B, Anton E, Greenamoyer C, Dambaugh T R. A strongly immunoreactive virion protein of human herpesvirus 6 variant B strain Z29: identification and characterization of the gene and mapping of a variant-specific monoclonal antibody reactive epitope. Virology. 1993;195:521–531. doi: 10.1006/viro.1993.1403. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Inoue T, Kajiwara M, Miyazaki C, Kusunoki K, Ueda K. Acute encephalopathy following exanthem subitum caused by human herpesvirus-6. J Jpn Assoc Infect Dis. 1992;66:551–554. doi: 10.11150/kansenshogakuzasshi1970.66.551. [DOI] [PubMed] [Google Scholar]
- 22.Secchiero P, Zella D, Crowley R W, Gallo R C, Lusso P. Quantitative PCR for human herpesviruses 6 and 7. J Clin Microbiol. 1995;33:2124–2130. doi: 10.1128/jcm.33.8.2124-2130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefan A, De Lillo M, Secchiero P, Neipal F, Campadelli-Fiume G. Development of recombinant diagnostic reagents based on pp85 (U14) and p86 (U11) proteins to detect the human immune response to human herpesvirus 7 infection. J Clin Microbiol. 1999;37:3980–3985. doi: 10.1128/jcm.37.12.3980-3985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suga S, Yoshikawa T, Asano Y, Nakashima T, Kobayashi I, Yazaki T. Activation of human herpesvirus-6 in children with acute measles. J Med Virol. 1992;38:278–282. doi: 10.1002/jmv.1890380409. [DOI] [PubMed] [Google Scholar]
- 25.Suga S, Yoshikawa T, Asano Y, Nakashima T, Yazaki T, Fukuda M, Kojima S, Matsuyama T, Ono Y, Oshima S. IgM neutralizing antibody responses to human herpesvirus-6 in patients with exanthem subitum or organ transplantation. Microbiol Immunol. 1992;36:495–506. doi: 10.1111/j.1348-0421.1992.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 26.Suga S, Yoshikawa T, Asano Y, Yazaki T, Hirata S. Human herpesvirus-6 infection (exanthem subitum) without rash. Pediatrics. 1989;83:1003–1006. [PubMed] [Google Scholar]
- 27.Suga S, Yoshikawa T, Asano Y, Yazaki T, Yoshida S. Simultaneous infection with human herpesvirus-6 and measles virus in infants. J Med Virol. 1990;31:306–311. doi: 10.1002/jmv.1890310412. [DOI] [PubMed] [Google Scholar]
- 28.Tait D R, Ward K N, Brown D W G, Miller E. Measles and rubella misdiagnosed in infants as exanthem subitum (roseola infantum) BMJ. 1996;312:101–102. doi: 10.1136/bmj.312.7023.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto M, Black J B, Stewart J A, Lopez C, Pellett P E. Identification of a nucleocapsid protein as a specific serological marker of human herpesvirus 6 infection. J Clin Microbiol. 1990;28:1957–1962. doi: 10.1128/jcm.28.9.1957-1962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanishi K, Kondo K, Mukai T, Kondo T, Nagafuji H, Kato T, Okuno T, Kurata T. Human herpesvirus 6 (HHV-6) infection in the central nervous system. Acta Paediatr Jpn. 1992;34:337–343. doi: 10.1111/j.1442-200x.1992.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamanishi K, Kondo T, Kondo K, Hayakawa Y, Kido S, Takahashi K, Takahashi M. Exanthem subitum and human herpesvirus 6 (HHV-6) infection. Adv Exp Med Biol. 1990;278:29–37. doi: 10.1007/978-1-4684-5853-4_4. [DOI] [PubMed] [Google Scholar]
- 32.Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;i:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 33.Yanagihara K, Tanaka-Taya K, Itagaki Y, Toribe Y, Arita K, Yamanishi K, Okada S. Human herpesvirus 6 meningoencephalitis with sequelae. Pediatr Infect Dis J. 1995;14:240–242. [PubMed] [Google Scholar]
- 34.Yoshikawa T, Nakashima T, Suga S, Asano Y, Yazaki T, Kimura H, Morishima T, Kondo K, Yamanishi K. Human herpesvirus-6 DNA in cerebrospinal fluid of a child with exanthem subitum and meningoencephalitis. Pediatrics. 1992;89:888–890. [PubMed] [Google Scholar]