Summary
Background
Current osteoporosis guidelines do not identify individuals with intellectual disabilities (ID) as at risk of fracture, potentially missing opportunities for prevention. We aimed to assess the incidence of fractures in people with ID over the life course.
Methods
Descriptive analysis of open cohort study using anonymised electronic health records from the UK Clinical Practice Research Datalink, linked to the Hospital Episode Statistics database (Jan 1, 1998–Dec 31, 2017). All individuals with ID were matched on age and sex to five individuals without ID. We calculated the incidence rate (95% CI) per 10000 person-years (py) and incidence rate ratio (IRR, 95% CI) to compare fractures between individuals with and without ID (age 1–17 and ≥18 years) for any fracture, and in those aged 18–49 and ≥ 50 years for major osteoporotic fracture (vertebra, shoulder, wrist, hip), and for hip fracture.
Findings
43176 individuals with ID (15470 children aged 1–17 years; 27706 adults aged ≥ 18 years) were identified and included (40.4% females) along with 215733 matched control individuals. The median age at study entry was 24 (10th–90th centiles 3–54) years. Over a median (10th–90th centile) follow-up of 7.1 (0.9–17.6) and 6.5 (0.8–17.6) years, there were 5941 and 24363 incident fractures in the ID and non ID groups respectively. Incidence of any fracture was 143.5 (131.8–156.3) vs 120.7 (115.4–126.4)/10000 py (children), 174.2 (166.4–182.4)/10000 py vs 118.2 (115.3–121.2)/10000 py (adults) in females. In males it was 192.5 (182.4–203.2) vs 228.5 (223.0–234.1)/10000 py (children), 155.6 (149.3–162.1)/10000 py vs 128.4 (125.9–131.0)/10000 py (adults). IRR for major osteoporotic fracture was 1.81 (1.50–2.18) age 18–49 years, 1.69 (1.53–1.87) age ≥ 50 years in women. In men it was 1.56 (1.36–1.79) age 18–49 years, 2.45 (2.13–2.81) age ≥ 50 years. IRR for hip fracture was 7.79 (4.14–14.65) age 18–49 years, 2.28 (1.91–2.71) age ≥ 50 years in women. In men it was 6.04 (4.18–8.73) age 18–49 years, 3.91 (3.17–4.82) age ≥ 50 years. Comparable rates of major osteoporotic fracture and of hip fracture occurred approximately 15 and 20 years earlier respectively in women and 20 and 30 years earlier respectively in men with ID than without ID. Fracture distribution differed profoundly, hip fracture 9.9% vs 5.0% of any fracture in adults with ID vs without ID.
Interpretation
The incidence, type, and distribution of fractures in people with intellectual disabilities suggest early onset osteoporosis. Prevention and management strategies are urgently required, particularly to reduce the incidence of hip fracture.
Funding
National Institute for Health and Care Research.
Keywords: Intellectual disabilities, Fracture, Osteoporosis
Research in context.
Evidence before this study
We searched PubMed from inception until 29 March 2022, without language restrictions, using the terms *fracture*, *osteoporosis*, *bone density*, *skeletal dysplasia*, *bone dysplasia* AND *intellectual disability*, *mental retardation*, *learning disability*, *Down syndrome*, *Fragile X*, *Prader Willi*, *DiGeorge*, *Niemann-Pick*, *Williams*, *Rett*, *Cornelia de Lange* and other syndromes associated with an intellectual disability (ID). The literature review confirmed our background hypothesis of high fracture rates in adults with intellectual disabilities (ID). However, previous studies were limited in age range, characterisation of patients, comparison with control subjects, classification of fractures, and data sources.
Added value of this study
This is the first study to compare fracture incidence over the entire life span between age and sex matched individuals with and without ID and: 1) use linked primary and secondary care databases from a national health service with full coverage of the population 2) provide aetiological diagnoses of ID as recorded in these databases 3) investigate the incidence of major osteoporotic fractures, and of hip fracture, separately from other fractures 4) compare the distribution of fractures between individuals with and without ID. Main results showed higher rates of fracture and younger age at fracture, particularly of the hip, in adults with ID.
Implications of all the available evidence
The body of current evidence points to early-onset osteoporosis as a key determinant of the increased fracture rate, younger age at fracture, and strikingly higher incidence of hip fracture in adults with ID compared to their age and sex matched counterparts without ID. Results in children raise the possibility of impairments in bone acquisition and development.
Strategies for fall prevention and safe weight bearing exercise for bone health improvement should be promoted, further research should identify risk factors for fracture, and clinical guidelines should include individuals with intellectual disabilities in those at risk of osteoporotic fracture, particularly hip fracture.
Alt-text: Unlabelled box
Introduction
Intellectual disabilities (ID) are impairments in both intellectual functioning and in adaptive behaviour, which covers many everyday social and practical skills, originating in the developmental years.1 Globally, reported prevalence rates of intellectual disabilities vary between 0.5 and 1.6%.2 In England, where our study is based, prevalence rates vary between 0.5%, based on General Practitioners’ registers, and 2.1 %, based on a variety of other data sources.3,4 The most common genetic cause is Down syndrome, although many other syndromes are also associated with ID, and in most cases the cause is unknown.5
People with ID also suffer premature mortality and significant comorbidities, their health lagging behind the health of the general population.6,7 In this paper we focus on fractures, clinically significant problems with high health care costs (particularly in the case of hip fractures8), which are amenable to prevention.9, 10, 11, 12, 13, 14
A high rate of fractures or decreased bone mineral density has been consistently reported in people with ID for over thirty years.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Despite this, even the most recent osteoporosis guidelines28, 29, 30 do not identify people with ID as at increased risk of fracture, potentially missing opportunities for prevention.
To date, no studies have concomitantly reported the incidence of fractures in people with ID over the whole of the life span, distinguished between major osteoporotic, hip fractures, and other fractures, or described the clinical characteristics of the patients studied. We compared the incidence rate of any fractures, of major osteoporotic fractures (MOP: vertebra, shoulder, wrist, hip), and of hip fractures between individuals with ID and an age and sex matched sample representative of the general population, in one of the world's largest primary care databases, linked to a national secondary care database.
Methods
Study design and participants
The study was an open cohort study based on the Clinical Practice Research Datalink (CPRD, GOLD database).31 The CPRD, established in 1987, contains data from approximately 16 million people registered with General Practitioners in the UK. The data are generated from routine NHS care, and the CPRD population is broadly representative of the UK population.32 CPRD data are recorded in a standardised electronic format, and are particularly consistent in areas of care supported by disease registers under the Quality and Outcomes Framework. This is a performance-based remuneration system for general practices,33 including registers for Learning Disability and Osteoporosis.
The study protocol was approved by the Independent Scientific Advisory Committee of the Medicine and Healthcare Products Regulatory Agency.34 For CPRD studies, all data is anonymised and individual patient consent is not required. However, patients can opt out of data contribution to CPRD.
The anonymised records of all individuals with diagnoses indicative of ID available in CPRD between 1/1/1998 and 31/12/2017 and linked to the Hospital Episode Statistics (HES) Admitted Patient Care database35 were extracted.
To identify and characterise the population with ID within the CPRD we used the code list of the Quality and Outcomes Framework Learning Disability Register, defined in their Business Rules.33 We included Read diagnostic and service user codes.36,37 Service user codes included “referral to learning disability team”, “seen in learning disability clinic” and similar others.
For the diagnostic codes, we selected the population on the basis of the presence in the individual record of a general code and/or a specific code. As general codes we used those including “learning disability”, “mental retardation”, “learning difficulty” (following exclusion from the latter of records with codes for specific difficulties, e.g. dyslexia, dyscalculia). As specific codes we used those for malformations and for genetic syndromes associated with ID in the majority of the patients, based on published literature5,36 and our own clinical experience. These included microcephalus, Down syndrome, fragile X syndrome in males, Prader-Willi, Williams, Rett, Angelman and many other syndromes. Autism spectrum disorder and attention deficit disorder were only included if the patient's record also carried a code that clearly indicated the presence of a learning disability.
Once we selected the population, we characterised it by codes that fell into one or more of the following categories: 1) other congenital and hereditary syndromes, e.g. congenital ectodermal defect 2) perinatal complications, e.g. birth asphyxia 3) congenital malformations and other abnormalities, e.g. congenital nystagmus 4) central nervous system infections, e.g. congenital listeriosis 5) epilepsy 6) cerebral palsy 7) autism and attention deficit disorder 8) acquired post-natal brain lesion, e.g. brain injury.
The first step of this identification procedure allowed us to select the population with a high degree of certainty, by choosing codes that are definitely or in the majority of individuals associated with an intellectual disability. The second step allowed us to further characterise the selected population in order to improve our understanding of it. Code lists were agreed between three study clinicians (VF, TMA, TAH) and are presented in the supplementary material.
Individuals entered the study at the latest of 1 January 1998 and the date of recording one-year of data in their current GP practice,31 the one-year time lag allowing sufficient time for recording of all clinical and demographic data. Participants were followed up until the earliest of: 31 December 2017, the last date at which their practice contributed data to the CPRD GOLD database, or the date at which the participants died or left their practice. The exposed population for this study were patients with a code indicative of an ID at some time in their primary care records. The comparator group consisted of individuals without such an ID code. All qualifying records for individuals with ID were extracted from the CPRD GOLD database. Patients with ID were matched in a 1:5 ratio by sex and by age ± 1 year (at study entry) to people without ID. The study outcomes were identified using both CPRD GOLD data and linked HES data, which provided diagnostic codes for patients admitted to hospital. The final study population only included those participants who had linkage to HES inpatient data (approximately 58% of the CPRD population,32). A comprehensive list of fractures with their Primary Care (Read) terms and codes (for CPRD) and ICD-10 terms and codes (for HES) was compiled, discussed and agreed upon by three clinicians (VF, TAH, DPA). For Read codes, this included diagnostic codes (e.g. Fracture of thoracic vertebra), procedure codes (e.g. Vertebroplasty of fracture of spine), and service user codes (e.g. Seen in fracture clinic). Procedure codes and service user codes were used to identify the occurrence of a fracture in the absence of a diagnostic code for fracture. For ICD-10 the list included codes S02, S12, S22, S32, S42, S52, S62, S72, S82, S92, T02, T08, T10, T12, T14, and M80.
The list also included an inventory of fragility fractures. As it is very difficult to determine the mechanism of fracture from the patients’ clinical records, as customary in epidemiological studies, osteoporotic fractures were defined by the anatomical sites that are generally affected. Consistent with current definitions and previous studies,38 major osteoporotic fractures included hip, wrist, vertebra and shoulder. ICD-10 codes used for hip fracture were S72.0, S72.1, S72.2; for wrist S52.5, S52.6, S52.9; for vertebra S22.0, S32.0; for shoulder fracture S42.2, S42.9. Codes M80.0, M80.2, M80.5, M80.8, and M80.9 were used to identify osteoporotic fractures at unspecified sites.
Hip fracture is the fragility fracture with the most significant negative outcome at the population level, given its severity, frequency, associated costs, and poor clinical outcome. Therefore, hip fracture was investigated also independently of other osteoporotic fractures.
To avoid multiple counting of the same fracture event, when identical codes appeared within an individual record, only the first recorded event was considered. Each Read code was mapped to the corresponding ICD-10 code when records from CPRD and HES database were merged. Additionally, mutually exclusive codes were defined by anatomical site to minimise the risk of the same fracture being counted more than once simply because of appearing in the records under slightly different codes. In practice, fracture site was classified according to 3-digit ICD10 code. To avoid duplication of fracture events, we only considered the first fracture at each site.
Current epidemiological and clinical sources28,29,38,39 distinguish between childhood fractures, which are assumed to be mostly the result of significant trauma on normal bone, and fractures from age 50 years, which can be due to osteoporosis. Fractures in adults below age 50 are normally considered due to trauma unless there are strong risk factors for osteoporosis (e.g. glucocorticoid treatment). Hence, in order to be able to draw clearer clinical and public health implications and to make the study results easily comparable to other major epidemiological studies, for some of the statistical analyses, the study population was divided in three age bands (1–17, 18–49, ≥50 years).
Statistical analysis
We used descriptive analysis. We counted the number of fractures and the person time by sex and age at risk. 1-year age bands were used for children. For adults, age bands were 18–24, and then five-year age bands. The oldest age group included everyone beyond the age of 80 years. We calculated crude incidence rates (events/person time) stratified by ID, age band and sex. Age and sex specific fracture crude incidence rates and 95% confidence intervals were calculated for people with and without ID by dividing the number of fractures by the total person-years of follow-up within each age and sex band.
Crude sex-specific incidence rates of any fracture were also calculated for ID and non-ID groups within broader age bands (1–17, 18–49, ≥50 years). The incidence of major osteoporotic fractures, and of hip fractures alone were estimated in adults only. The incidence of fractures by wide anatomic region as defined by ICD-10 three-digit code were also estimated in both adults and children.
Crude incidence rate ratios (IRR) and 95% confidence intervals comparing rates of each type of fracture between ID and non-ID individuals were also calculated, stratified by the broader age bands and sex. All analyses were done using the strate command in Stata version 16.40
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study population
43,176 people (15,470 children, 27,706 adults) with ID were identified and 215,733 people (76,700 children, 139,033 adults) without ID were selected as described above. Demographic characteristics of the study population are given in Table 1.
Table 1.
Characteristics of the study population.
| Intellectual Disability(n = 43176) | No Intellectual Disability(n = 215733) | |
|---|---|---|
| Median age at entry (years)* | 24 (3–54) | 24 (3–54) |
| Female n (%) | 17429 (40.4) | 87836 (40.7) |
| Children (age 1–17 y at entry) | 15470 | 76700 |
| Median age at entry (years)* | 7.0 (1.0–15.0) | 7.0 (1.0–15.0) |
| Female n (%) | 5602 (36.2) | 27905 (36.4) |
| Adults (age ≥18y at entry) | 27706 | 139033 |
| Median age at entry (years)* | 35.0 (21.0–60.0) | 35.0 (21.0–60.0) |
| Female n (%) | 11827 (42.7) | 59931 (43.1) |
10th-90th centile
Table 2 shows the conditions associated with a diagnosis of ID. Down syndrome (DS) was the most frequent diagnosis (10.5% of those entering the study as children, 9% of those entering the study as adults). Genetic syndromes (DS and many others) and congenital syndromes (e.g. congenital malformation syndromes, foetal alcohol syndrome), were found in 2969 (19.2%) children and 3780 (13.6%) adults. Microcephalus and other individually recorded congenital malformations or abnormalities (e.g. cataract, deafness) were found in 10.0% of children and 2.8% of adults. Frequent comorbidities observed included epilepsy (15.1% of children, 22.1% of adults), autism and attention deficit disorder (17.7% of children, 6.7% of adults), and cerebral palsy (5.0% of children, 4.5% of adults). Perinatal complications, central nervous system infections and acquired post-natal brain lesions contributed to a minority of comorbid diagnoses (2.6% in children, 3.0% in adults). In 46.4% of the records for children and 57.3% of the records for adults we could not find a specific diagnosis term/code (e.g. Down Syndrome, Fragile X syndrome, perinatal complications etc.) accompanying the more general term/code indicating an intellectual disability (e.g. learning disability, mental retardation).
Table 2.
Diagnoses found in the study population with Intellectual Disability (some people may have more than one diagnosis).
| Children |
Adults |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Total study population with ID | 15470 | 100 | 27706 | 100 |
| Number (%) of people with ID with diagnosis | n | % | n | % |
| Down syndrome | 1631 | 10.54 | 2488 | 8.98 |
| Fragile X | 342 | 2.21 | 304 | 1.10 |
| Sturge-Weber | 56 | 0.36 | 113 | 0.41 |
| Tay-Sachs | 7 | 0.05 | 86 | 0.31 |
| Angelman | 64 | 0.41 | 55 | 0.20 |
| Williams | 33 | 0.21 | 45 | 0.16 |
| Rett | 77 | 0.50 | 42 | 0.16 |
| Gaucher | 14 | 0.09 | 42 | 0.16 |
| DiGeorge | 149 | 0.96 | 40 | 0.14 |
| Lennox-Gastaut | 43 | 0.28 | 40 | 0.14 |
| Cornelia de Lange | 33 | 0.21 | 33 | 0.12 |
| Laurence-Moon-Biedl | 7 | 0.05 | 32 | 0.12 |
| Sotos | 58 | 0.37 | 27 | 0.14 |
| Prader-Willi | 19 | 0.12 | 23 | 0.08 |
| Rubinstein-Taybi | 27 | 0.17 | 16 | 0.06 |
| Other genetic or congenital syndromes | 409 | 2.64 | 394 | 1.42 |
| Microcephalus | 910 | 5.88 | 418 | 1.51 |
| Other congenital malformations or abnormalities | 641 | 4.14 | 636 | 2.30 |
| Perinatal complications | 118 | 0.76 | 200 | 0.72 |
| Central nervous system infections | 263 | 1.70 | 582 | 2.10 |
| Acquired post-natal brain lesion | 16 | 0.10 | 62 | 0.22 |
| Epilepsy | 2329 | 15.05 | 6115 | 22.07 |
| Autism and Attention Deficit Disorder | 2739 | 17.71 | 1864 | 6.73 |
| Cerebral palsy | 776 | 5.02 | 1248 | 4.50 |
| None of the above | 7171 | 46.35 | 15865 | 57.26 |
Incidence of fractures
During the study period, there were 5941 and 24363 incident fractures in the ID and non ID groups respectively. A total of 4957 people with ID (11.5%) and 20915 people without ID (9.7%) had one or more recorded fractures over median 7.1 and 6.5 years of follow-up respectively (Table 3).
Table 3.
Incident fractures over study period.
| Intellectual Disability(n = 43716) | No Intellectual Disability(n = 215733) | |
|---|---|---|
| Number of incident fractures | 5941 | 24363 |
| Total follow-up time (1000s years) | 356 | 1727 |
| Individuals with 1 fracture (%) | 4176 (9.7) | 18139 (8.4) |
| Individuals with ≥ 2 fractures (%) | 781 (1.8) | 2776 (1.3) |
| Median follow-up (years)Δ | 7.1 (0.9–17.6) | 6.5 (0.8–17.6) |
10th–90th centile.
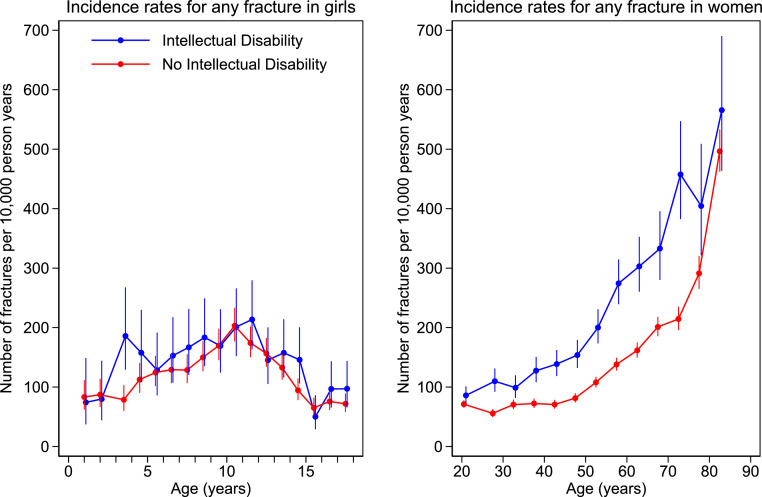
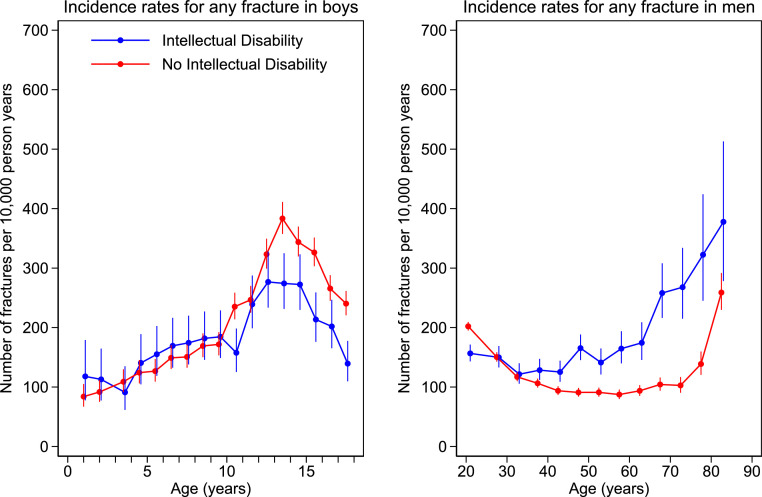
Figure 1, Figure 2 show the incidence rates of any fracture by age and sex from age 1 year. In females, incidence was higher over the whole life course in the ID compared with the non ID group. In males, incidence was lower in childhood and then consistently higher from age 40 years in ID vs non ID. Number of events, patient years of follow-up, and incidence rates (95%CI) by sex and age bands (1 year for children, 5 years for adults) are given in the supplementary material.
Figure 1.
Incidence rates by age band for any fracture in females with and without intellectual disability. Number of fractures per 10,000 person years by age. Filled circles represent age band specific incidence rates. Age bands are 1-year in children and 5-year in adults. The bars represent 95% CI.
Figure 2.
Incidence rates by age band for any fracture in males with and without intellectual disability. Number of fractures per 10,000 person years by age. Filled circles represent age band specific incidence rates. Age bands are 1-year in children and 5-year in adults. The bars represent 95% CI.
Table 4a & b shows incidence rates (95% CI), incidence rate ratios (95% CI) and distribution of fractures (number and percentage for age and sex group) by ICD10 three-digit codes in female and male children (1–17 years) and adults (≥ 18 years) with ID compared to those without ID.
Table 4a.
Incidence rate of fractures, Incidence Rate Ratio (IRR) and distribution of fractures by age and site as recorded in 3-digit ICD10 code in females.
| ID females |
Non ID females |
|||||
|---|---|---|---|---|---|---|
| ICD-10 Code and Term | Event N% | Rate* (95% CI) | Event N% | Rate* (95% CI) | IRRΔ (95% CI) | |
| 1-17 y | All fractures | 528 100% |
143.5 (131.8–156.3) |
1856 (100%) |
120.7 (115.4–126.4) |
1.19 (1.08–1.31) |
| S02 Skull and facial bone |
15 2.8% |
4.1 (2.5–6.8) |
58 3.1% |
3.8 (2.9–4.9) |
1.08 (0.61–1.91) |
|
| S12 Neck |
3 0.6% |
0.8 (0.3-2.5) |
1 0.05% |
0.1 (0.0–0.5) |
12.53 (1.30–120.50) |
|
| S22 Ribs, sternum and thoracic spine |
1 0.2% |
0.3 (0.0–1.9) |
7 0.4% |
0.5 (0.2–1.0) |
0.60 (0.07–4.85) |
|
| S32 Lumbar spine and pelvis |
4 0.8% |
1.1 (0.4–2.9) |
8 0.4% |
0.5 (0.3–1.0) |
2.09 (0.63–6.94) |
|
| S42 Shoulder and upper arm |
75 14.2% |
20.4 (16.3–25.6) |
198 10.7% |
12.9 (11.2–14.8) |
1.58 (1.21–2.06) |
|
| S52 Forearm§ |
114 21.6% |
31.0 (25.8–37.2) |
580 31.3% |
37.7 (34.8–40.9) |
0.82 (0.65–1.0) |
|
| S62 Wrist§ and hand level |
71 13.4% |
19.3 (15.3–24.4) |
300 16.2% |
19.5 (17.4–21.9) |
0.99 (0.76–1.28) |
|
| S72 Femur |
25 4.7% |
6.8 (4.6–10.1) |
15 0.8% |
1.0 (0.6–1.6) |
6.96 (3.67–13.21) |
|
| S82 Lower leg including ankle |
67 12.7% |
18.2 (14.3–23.1) |
143 7.7% |
9.3 (7.9–11.0) |
1.96 (1.46–2.62) |
|
| S92 Foot, except ankle |
40 7.6% |
10.9 (8.0–14.8) |
156 8.4% |
10.1 (8.7–11.9) |
1.07 (0.76–1.52) |
|
| T02 Multiple body regions |
0 | 0.0 |
2 0.1% |
0.1 (0.0–0.5) |
||
| T08 Spine, level unspecified |
3 0.6% |
0.8 (0.3–2.5) |
2 0.1% |
0.1 (0.0–0.5) |
6.27 (1.05–37.51) |
|
| T10 Upper limb, level unspecified |
11 2.1% |
3.0 (1.7–5.4) |
47 2.5% |
3.1 (2.3–4.1) |
0.98 (0.51–1.89) |
|
| T12 Lower limb, level unspecified |
3 0.6% |
0.8 (0.3–2.5) |
11 0.6% |
0.7 (0.4–1.3) |
1.14 (0.32–4.08) |
|
| M80, T14 Unspecified |
96 18.2% |
26.1 (21.4–31.9) |
328 17.7% |
21.3 (19.1–23.8) |
1.22 (0.97–1.54) |
|
| ≥ 18 y | All fractures | 1816 100% |
174.2 (166.4–182.4) |
6215 100% |
118.2 (115.3–121.2) |
1.47 (1.40–1.55) |
| S02 Skull and facial bone |
57 3.1% |
5.5 (4.2–7.1) |
184 3.0% |
3.5 (3.0–4.0) |
1.56 (1.16–2.10) |
|
| S12 Neck |
3 0.2% |
0.3 (0.1–0.9) |
30 0.5% |
0.6 (0.4–0.8) |
0.50 (0.15–1.65) |
|
| S22 Ribs, sternum and thoracic spine |
56 3.1% |
5.4 (4.1–7.0) |
294 4.7% |
5.6 (5.0–6.3) |
0.96 (0.72–1.28) |
|
| S32 Lumbar spine and pelvis |
64 3.5% |
6.1 (4.8–7.8) |
241 3.9% |
4.6 (4.0–5.2) |
1.34 (1.02–1.76) |
|
| S42 Shoulder and upper arm |
189 10.4% |
18.1 (15.7–20.9) |
493 7.9% |
9.4 (8.6–10.2) |
1.93 (1.64–2.29) |
|
| S52 Forearm§ |
246 13.5% |
23.6 (20.8–26.7) |
1186 19.1% |
22.6 (21.3–23.9) |
1.05 (0.91–1.20) |
|
| S62 Wrist§ and hand level |
220 12.1% |
21.1 (18.5–24.1) |
706 11.4% |
13.4 (12.5–14.5) |
1.57 (1.35–1.83) |
|
| S72 Femur |
228 12.6% |
21.9 (19.2–24.9) |
579 9.3% |
11.0 (10.2–11.9) |
1.99 (1.70–2.32) |
|
| S82 Lower leg including ankle |
325 17.9% |
31.2 (28.0–34.8) |
827 13.3% |
15.7 (14.7–16.8) |
1.98 (1.74–2.25) |
|
| S92 Foot, except ankle |
178 9.8% |
17.1 (14.7–19.8) |
683 11.0% |
13.0 (12.1–14.0) |
1.31 (1.11–1.55) |
|
| T02 Multiple body regions |
5 0.3% |
0.5 (0.2–1.2) |
10 0.2% |
0.2 (0.1–0.4) |
2.52 (0.86–7.38) |
|
| T08 Spine, level unspecified |
10 0.5% |
1.0 (0.5–1.8) |
30 0.5% |
0.6 (0.4–0.8) |
1.68 (0.82–3.44) |
|
| T10 Upper limb, level unspecified |
26 1.4% |
2.5 (1.7–3.7) |
85 1.4% |
1.6 (1.3–2.0) |
1.54 (0.99–2.39) |
|
| T12 Lower limb, level unspecified |
17 0.9% |
1.6 (1.0–2.6) |
53 0.9% |
1.0 (0.8–1.3) |
1.62 (0.94–2.79) |
|
| M80, T14 Unspecified |
192 10.6% |
18.4 (16.0–21.2) |
814 13.1% |
15.5 (14.5–16.6) |
1.19 (1.02–1.39) |
|
Number of fractures/10000 person years (95%CI).
Incidence rate ratio (95%CI) ID vs non ID group.
In ICD-10 the forearm codes include those of the distal forearm, which are those termed as “wrist fractures” in clinical practice and in the fracture risk calculators Frax and QFracture. In ICD-10 the wrist fracture codes relate to the carpal bones (S62.0 scaphoid, S62.1 other and unspecified), which on their own are not termed wrist fractures in clinical practice or included in the fracture risk calculators.
Table 4b.
Incidence rate of fractures, Incidence Rate Ratio (IRR) and distribution of fractures by age and site as recorded in 3-digit ICD10 code in males.
| ID males |
Non ID males |
|||||
|---|---|---|---|---|---|---|
| ICD-10 Code and Term | Events N% | Rate* (95% CI) | Events N% | Rate* (95% CI) | IRRΔ (95% CI) | |
| 1-17 y | All fractures | 1317 100% |
192.5 (182.4–203.2) |
6491 100% |
228.5 (223.0–234.1) |
0.84 (0.79–0.89) |
| S02 Skull and facial bone |
63 4.8% |
9.2 (7.2–11.8) |
347 5.3% |
12.2 (11.0–13.6) |
0.75 (0.58–0.99) |
|
| S12 Neck |
0 | 0.0 | 6 0.1% |
0.2 (0.1–0.5) |
||
| S22 Ribs, sternum and thoracic spine |
7 0.5% |
1.0 (0.5–2.1) |
48 0.7% |
1.7 (1.3–2.2) |
0.61 (0.27–1.34) |
|
| S32 Lumbar spine and pelvis |
6 0.5% |
0.9 (0.4–2.0) |
27 0.4% |
1.0 (0.7–1.4) |
0.92 (0.38–2.24) |
|
| S42 Shoulder and upper arm |
151 11.5% |
22.1 (18.8–25.9) |
704 10.8% |
24.8 (23.0–26.7) |
0.89 (0.75–1.06) |
|
| S52 Forearm§ |
294 22.3% |
43.0 (38.3–48.2) |
1705 26.3% |
60.0 (57.2–62.9) |
0.72 (0.63–0.81) |
|
| S62 Wrist§ and hand level |
266 20.2% |
38.9 (34.5–43.8) |
1523 23.5% |
53.6 (51.0–56.4) |
0.73 (0.64–0.83) |
|
| S72 Femur |
47 3.6% |
6.9 (5.2–9.1) |
95 1.5% |
3.3 (2.7–4.1) |
2.05 (1.45–2.91) |
|
| S82 Lower leg including ankle |
139 10.6% |
20.3 (17.2–24.0) |
583 9.0% |
20.5 (18.9–22.3) |
0.99 (0.82–1.19) |
|
| S92 Foot, except ankle |
113 8.5% |
16.5 (13.7–19.9) |
411 6.3% |
14.5 (13.1–15.9) |
1.14 (0.93–1.41) |
|
| T02 Multiple body regions |
2 0.2% |
0.3 (0.1–1.2) |
10 0.2% |
0.4 (0.2–0.7) |
0.83 (0.18–3.79) |
|
| T08 Spine, level unspecified |
0 | 0.0 | 6 0.1% |
0.2 (0.1–0.5) |
||
| T10 Upper limb, level unspecified |
25 1.9% |
3.7 (2.5–5.4) |
115 1.8% |
4.0 (3.4–4.9) |
0.90 (0.59–1.39) |
|
| T12 Lower limb, level unspecified |
7 0.5% |
1.0 (0.5–2.1) |
43 0.7% |
1.5 (1.1–2.0) |
0.68 (0.30–1.50) |
|
| M80, T14 Unspecified |
197 15.0% |
28.8 (25.0–33.1) |
868 13.4% |
30.6 (28.6–32.7) |
0.94 (0.81–1.10) |
|
| ≥ 18 y | All fractures | 2280 100% |
155.6 (149.3–162.1) |
9801 100% |
128.4 (125.9–131.0) |
1.21 (1.16–1.27) |
| S02 Skull and facial bone |
186 8.2% |
12.7 (11.0–14.7) |
910 9.3% |
11.9 (11.2–12.7) |
1.06 (0.91–1.25) |
|
| S12 Neck |
18 0.8% |
1.2 (0.8–1.9) |
61 0.62% |
0.8 (0.6–1.0) |
1.54 (0.91–2.60) |
|
| S22 Ribs, sternum and thoracic spine |
94 4.1% |
6.4 (5.2–7.9) |
550 5.6% |
7.2 (6.6–7.8) |
0.89 (0.72–1.11) |
|
| S32 Lumbar spine and pelvis |
50 2.2% |
3.4 (2.6–4.5) |
206 2.1% |
2.7 (2.4–3.1) |
1.26 (0.93–1.72) |
|
| S42 Shoulder and upper arm |
230 10.1% |
15.7 (13.8–17.9) |
746 7.6% |
9.8 (9.1–10.5) |
1.61 (1.39–1.86) |
|
| S52 Forearm§ |
242 10.6% |
16.5 (14.6–18.7) |
1025 10.5% |
13.4 (12.6–14.3) |
1.23 (1.07–1.41) |
|
| S62 Wrist§ and hand level |
387 17.0% |
26.4 (23.9–29.2) |
2401 24.5% |
31.5 (30.2–32.7) |
0.84 (0.75–0.93) |
|
| S72 Femur |
230 10.1% |
15.7 (13.8–17.9) |
370 3.8% |
4.8 (4.4–5.4) |
3.24 (2.75–3.82) |
|
| S82 Lower leg including ankle |
333 14.6% |
22.7 (20.4–25.3) |
1205 12.3% |
15.8 (14.9–16.7) |
1.44 (1.27–1.63) |
|
| S92 Foot, except ankle |
216 9.5% |
14.7 (12.9–16.8) |
846 8.6% |
11.1 (10.4–11.9) |
1.33 (1.15–1.54) |
|
| T02 Multiple body regions |
6 0.3% |
0.4 (0.2–0.9) |
23 0.2% |
0.3 (0.2–0.5) |
1.36 (0.55–3.34) |
|
| T08 Spine, level unspecified |
13 0.6% |
0.9 (0.5–1.5) |
34 0.3% |
0.4 (0.3–0.6) |
1.99 (1.05–3.77) |
|
| T10 Upper limb, level unspecified |
26 1.1% |
1.8 (1.8–2.6) |
104 1.1% |
1.4 (1.1–1.7) |
1.30 (0.85–2.00) |
|
| T12 Lower limb, level unspecified |
17 0.7% |
1.2 (0.7–1.9) |
95 1.0% |
1.2 (1.0–1.5) |
0.93 (0.56–1.56) |
|
| M80, T14 Unspecified |
232 10.2% |
15.8 (13.9–18.0) |
1225 12.5% |
16.0 (15.2–17.0) |
0.99 (0.86–1.14) |
|
Number of fractures/10000 person years (95%CI).
Incidence rate ratio (95%CI) ID vs non ID group.
In ICD-10 the forearm codes include those of the distal forearm, which are those termed as “wrist fractures” in clinical practice and in the fracture risk calculators Frax and QFracture. In ICD-10 the wrist fracture codes relate to the carpal bones (S62.0 scaphoid, S62.1 other and unspecified), which on their own are not termed wrist fractures in clinical practice or included in the fracture risk calculators.
Incidence of any fracture in the ID vs non ID group was 143.5 (131.8–156.3) vs 120.7 (115.4–126.4)/10000 py (female children); 174.2 (166.4–182.4)/10000 py vs 118.2 (115.3–121.2)/10000 py (female adults); 192.5 (182.4–203.2) vs 228.5 (223.0–234.1)/10000 py (male children); 155.6 (149.3–162.1)/10000 py vs 128.4 (125.9–131.0)/10000 py (male adults).
Differences in incidence rate ratios were seen for a variety of fractures, the largest being for fractures of the femur, with IRR 6.96 (3.67–13.21) and 1.99 (1.70–2.32) in ID female children and adults respectively and IRR 2.05 (1.45–2.91) and 3.24 (2.75–3.82) in ID male children and adults respectively.
The most frequent fractures in female children with and without ID were those of the upper limb (51.3% and 60.6% of all fractures respectively), followed by the lower limb (25.6% and 17.5% respectively). Femoral fractures were 4.7% of all fractures in girls with ID and 0.8% in girls without ID. In boys, upper limb fractures were also the most frequent both in the ID and non ID group (55.9% vs 62.3% respectively), followed by the lower limb (23.2% vs 17.4% respectively). Femoral fractures were 3.6% of all fractures in boys with ID and 1.5% in those without ID.
In women, upper limb fractures were 37.5% (ID), 39.7% (non ID) and lower limb fractures were 41.2% (ID) and 34.5% (non ID), with femoral fractures 12.6% (ID) and 9.3% (non ID) of total fractures.
In men, 38.8% of the fractures were in the upper and 34.9% in the lower limb in the ID group. In the non ID group, 43.6% of the fractures were in the upper and 25.7% in the lower limb. Femoral fractures were 10.1% and 4.8% of all fractures in the ID and non ID group respectively.
The incidence of any fracture, major osteoporotic (MOP) fracture by each site and combined, and the distribution of MOP fractures in adults are shown in Table 5a & b by age groups (18–49 and ≥ 50 years). Compared to people without ID, there was a higher incidence of any fracture in ID men in the older age group and in ID women at all ages. Rates of MOP fracture and of hip fracture alone were higher in both men and women with ID compared to their non ID counterparts. The differences in hip fracture rates were particularly high, especially in the 18–49 year age groups, with IRR (95% CI) of 7.79 (4.14–14.65) in females and 6.04 (4.18–8.73) in males. In the older age group, IRR for hip fracture was 2.28 (1.91–2.71) in females and 3.91 (3.17–4.82) in males. There was no difference in the rate of vertebral fracture, whilst IRR for fracture of the shoulder ranged between 2.02 (1.58–2.57) and 3.20 (2.09–4.90). Rates of wrist fracture were slightly higher in the older age group with ID in both sexes, with no difference observed in the 18–49 year groups.
Table 5a.
Incidence rates of major osteoporotic (MOP) fractures, Incidence Rate Ratio, and distribution of MOP fractures by age and site in females.
| ID females |
Non ID females |
|||||
|---|---|---|---|---|---|---|
| ICD-10 Code and Term | Events N% | Rate* (95% CI) | Events N% | Rate* (95% CI) | IRRΔ (95% CI) | |
| 18-49 y | S22.0, S32.0 Vertebra |
10 1.2% |
1.4 (0.7–2.6) |
24 1.1% |
0.8 (0.5–1.2) |
1.80 (0.86–3.77) |
| S42.2, S42.9 Shoulder |
37 4.4% |
5.2 (3.7–7.1) |
50 2.3% |
1.6 (1.2–2.1) |
3.20 (2.09–4.90) |
|
| S52.5, S52.6, S52.9 Wrist |
73 8.7% |
10.2 (8.1–12.8) |
256 11.7% |
8.2 (7.3–9.3) |
1.23 (0.95–1.60) |
|
| S72.0, S72.1, S72.2 Hip |
27 3.23% |
3.8 (2.6–5.5) |
15 0.7% |
0.5 (0.3–0.8) |
7.79 (4.14–14.65) |
|
| M80 Unspecified MOP |
5 0.6% |
0.7 (0.3–1.7) |
19 0.9% |
0.6 (0.4–1.0) |
1.14 (0.43–3.05) |
|
| Total MOP | 152 18.2% |
21.2 (18.1–24.8) |
364 16.6% |
11.7 (10.6–13.0) |
1.81 (1.50–2.18) |
|
|
All fractures |
835 100% |
116.4 (108.7–124.5) |
2192 100% |
70.6 (67.7–73.6) |
1.65 (1.52–1.79) |
|
| ≥50 y | Vertebra | 21 2.1% |
6.5 (4.2–9.9) |
109 2.7% |
5.1 (4.2–6.1) |
1.28 (0.80–2.04) |
| Shoulder | 85 8.7% |
26.2 (21.2–32.4) |
279 6.9% |
13.0 (11.5–14.6) |
2.02 (1.58–2.57) |
|
| Wrist | 137 14.0% |
42.2 (35.7–49.9) |
749 18.6% |
34.8 (32.4–37.4) |
1.21 (1.01–1.45) |
|
| Hip | 171 17.4% | 52.7 (45.3–61.2) |
498 12.4% |
23.1 (21.2–25.3) |
2.28 (1.91–2.71) |
|
| Unspecified MOP | 44 4.5% |
13.6 (10.1–18.2) |
159 4.0% |
7.4 (6.3–8.6) |
1.83 (1.31–2.56) |
|
| Total MOP | 458 46.7% | 141.1 (128.7–154.6) |
1794 44.6% |
83.4 (79.6–87.3) |
1.69 (1.53–1.87) |
|
| All fractures | 981 100% | 302.2 (283.8–321.7) |
4023 100% |
187.0 (181.3–192.8) |
1.62 (1.51–1.73) |
|
Number of fractures/10000 person years (95%CI).
Incidence rate ratio (95%CI) ID vs non ID group.
Table 5b.
Incidence rates of major osteoporotic (MOP) fractures, Incidence Rate Ratio, and distribution of MOP fractures by age and site in males.
| ID males |
Non ID males |
|||||
|---|---|---|---|---|---|---|
| ICD-10 Code and Term | Events N% | Rate* (95% CI) | Events N% | Rate* (95% CI) | IRRΔ (95% CI) | |
| 18-49 y | S22.0, S32.0 Vertebra |
19 1.2% |
1.7 (1.1–2.7) |
81 1.1% |
1.5 (1.2–1.9) |
1.13 (0.69–1.86) |
| S42.2, S42.9 Shoulder |
45 2.9% |
4.1 (3.1–5.5) |
105 1.4% |
2.0 (1.6–2.4) |
2.06 (1.46–2.93) |
|
| S52.5, S52.6, S52.9 Wrist |
134 8.6% |
12.3 (10.4–14.5) |
584 8.0% |
11.1 (10.2–12.0) |
1.10 (0.92–1.33) |
|
| S72.0, S72.1, S72.2 Hip |
64 4.1% |
5.9 (4.6–7.5) |
51 0.7% |
1.0 (0.7–1.3) |
6.04 (4.18–8.73) |
|
| M80 Unspecified MOP |
8 0.5% |
0.7 (0.4–1.5) |
13 0.2% |
0.2 (0.1–0.4) |
2.96 (1.23–7.15) |
|
| Total MOP | 270 17.3% | 24.7 (21.9–27.8) |
834 11.4% |
15.8 (14.8–17.0) |
1.56 (1.36–1.79) |
|
| All fractures | 1563 100% | 143.0 (136.1–150.3) |
7337 100% |
139.4 (136.3–142.7) |
1.03 (0.97–1.08) |
|
| ≥50 y | Vertebra | 16 2.2% |
4.3 (2.6–7.0) |
81 3.3% |
3.4 (2.7–4.2) |
1.26 (0.74–2.15) |
| Shoulder | 51 7.1% |
13.7 (10.4–18.0) |
134 5.4% |
5.6 (4.8–6.7) |
2.42 (1.75–3.34) |
|
| Wrist | 50 7.0% |
13.4 (10.2–17.7) |
221 9.0% |
9.3 (8.2–10.6) |
1.44 (1.06–1.96) |
|
| Hip | 142 19.8% | 38.1 (32.3–44.9) |
231 9.4% |
9.7 (8.6–11.1) |
3.91 (3.17–4.82) |
|
| Unspecified MOP | 21 2.9% |
5.6 (3.7–8.6) |
61 2.5% |
2.6 (2.0–3.3) |
2.19 (1.33–3.60) |
|
| Total MOP | 280 39.1% | 75.1 (66.8–84.4) |
728 29.5% |
30.7 (28.5–33.0) |
2.45 (2.13–2.81) |
|
| All fractures | 717 100% | 192.3 (178.7–206.9) |
2464 100% |
103.9 (99.9–108.1) |
1.85 (1.70–2.01) |
|
Number of fractures/10000 person years (95%CI).
Incidence rate ratio (95%CI) ID vs non ID group.
The distribution of MOP fractures as a percentage of total fractures was different between individuals with and without ID in both sexes and age groups. This was particularly evident in the 18–49 years group and in men more than in women. In particular, at age 18–49 years, hip fracture represented 3.2% and 0.7% of all fractures in ID and non ID women respectively and 4.1% and 0.7% in ID and non ID men respectively. At age ≥50 years, hip fracture represented 17.4% and 12.4% of all fractures in ID and non ID women respectively and 19.8% and 9.4% in ID and non ID men respectively.
As shown in Table 6, the percentage of femoral fractures which were fractures of the hip was similar and close to 90% in women with and without ID and in men with ID. It was lower, at 76% in men without ID. In children the percentage of femoral fractures localised at the hip was very low.
Table 6.
Hip fractures as a percentage of femoral fractures in females and males with and without ID.
| ID females | Non ID females | ID males | Non ID males | ||
|---|---|---|---|---|---|
| ICD-10 code and term | Events N % | Events N % | Events N % | Events N % | |
| 1-17 years | S72 Femur |
25 100% |
15 100% |
47 100% |
95 100% |
| S72.0, S72.1, S72.2 Hip |
4 16% |
0 | 12 (25.5%) |
16 (16.8%) |
|
| ≥ 18 years | S72 Femur |
228 100% |
579 100% |
230 100% |
370 100% |
| S72.0, S72.1, S72.2 Hip |
198 86.8% |
513 88.6% |
206 89.6% |
282 76.2% |
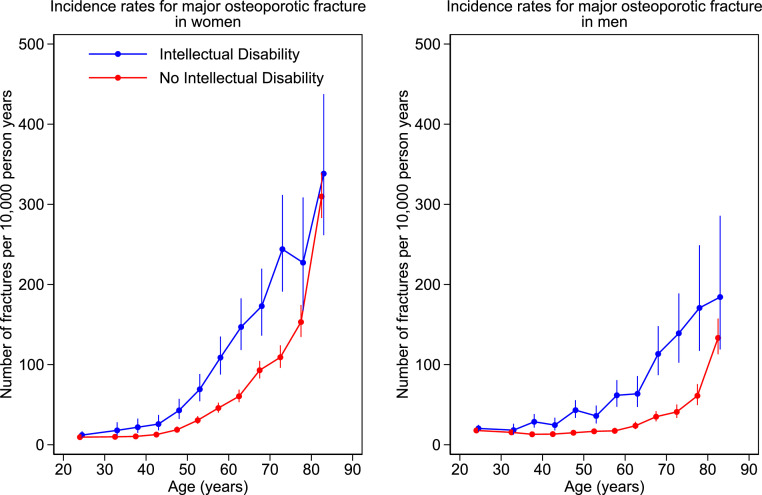
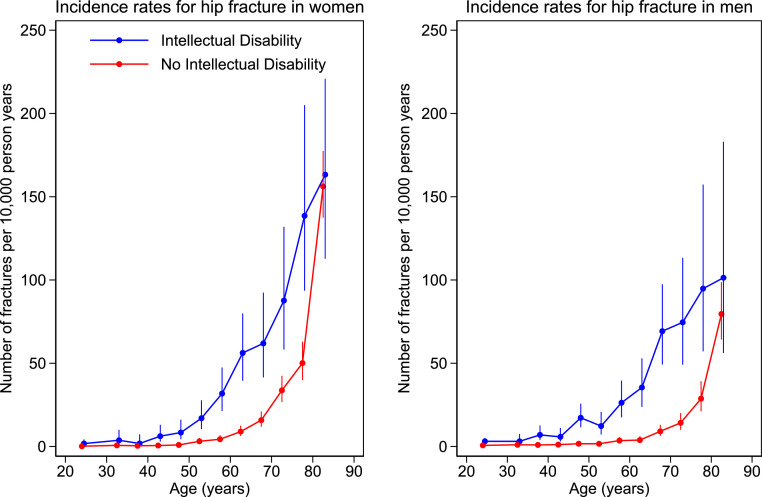
The incidence rates of combined MOP and of hip fracture alone in adults are shown in Figures 3 and 4. Number of events, patient years of follow-up, and incidence rates (95%CI) by sex and 5 year age bands are given in the supplementary material.
Figure 3.
Incidence rates by age band for major osteoporotic fracture in women and men with and without intellectual disability. Number of fractures per 10,000 person years by age. Filled circles represent age band specific incidence rates. Age bands are 1-year in children and 5-year in adults. The bars represent 95% CI.
Figure 4.
Incidence rates by age bands for hip fracture in women and men with and without intellectual disability. Number of fractures per 10,000 person years by age. Filled circles represent age band specific incidence rates. Age bands are 1-year in children and 5-year in adults. The bars represent 95% CI.
The incidence of MOP fracture was higher in the ID group in both men and women, from around age 35 years. Hip fracture incidence was higher in the ID group in both men and women, from around age 18 years. The confidence intervals were too wide in the older age groups (age ≥ 75 for MOP and ≥ 80 years for hip fracture), due to small number of people with ID in those groups, in order to detect any potential difference.
Analyses by 5-year age bands between 18 and 80 years shown in Figure 4 and tabulated in the supplementary material revealed incidence rates for hip fracture which were between two and ten times as high in ID vs non ID individuals, according to age band and sex. The largest differences were observed between age 35–60 years in men and 18–60 years in women. For instance, at age 45, the hip fracture incidence rate in ID vs non ID was 8.39 (4.37–16.13) vs 0.96 (0.40–2.31)/10000 py in women and 17.26 (11.57–25.76) vs 1.68 (0.93–3.03)/10000 py in men. Moreover, whilst hip fracture rates increase with age in both populations, the increase was observed approximately 15 years earlier in females and 20 years earlier in males in the ID compared to the non ID group. Comparable rates of MOP and of hip fracture occurred approximately 15 and 20 years earlier respectively in women with ID than without ID and 20 and 30 years earlier respectively in men with ID than men without ID.
Discussion
This study showed that people with ID have higher incidence of fractures than people without ID. In females, this is the case throughout the life course whilst in males this is true from approximately 35 years of age. Fracture rates increase with age in people with and without ID. However, this increase starts to be seen (for all fracture types combined) approximately 15 years earlier in women and 30 years earlier in men with ID compared to those without ID.
Analyses by type of fracture revealed that the largest difference in incidence rates are in fractures of the femur (mostly hip in adults, femoral shaft in children). Hip fracture rates were approximately two to ten times higher in adults with ID, with the greatest differences observed between age 35–60 years in men and 18–60 years in women. Rates of femoral fracture were approximately twice as high in boys and seven times as high in girls with ID compared to those without ID.
The incidence of fracture in childhood showed overall slightly higher rates in females but lower rates in males with ID compared to those without. This might partly be due to the presence among girls of patients with Rett syndrome, which can cause fractures at an early age.12
The increase in fracture rates normally seen in boys over the adolescent years, as shown in our study and in a major previous study,39 was blunted in those with ID compared to those without ID. This is likely due to the limited mobility and limited participation in sports of boys with ID. We believe this could explain our finding of overall lower fracture rates of male children with ID compared to those without ID.
Overall, MOP fracture rates in adults were 60% to 80% higher in those with ID. The largest differences were seen in hip fracture rates, and to a lesser extent in shoulder fracture rates, which were two to three fold higher in the ID group.
As in other major epidemiological studies,38 vertebral, shoulder (i.e. proximal humerus, shoulder unspecified), wrist (i.e. distal radius/ulna, forearm unspecified) or hip fracture in adults were assumed to represent major osteoporotic (MOP) fractures. Although this assumption might not always be correct, ours was a pragmatic decision due to the scarcity of details on the mechanism of fracture (e.g. height and type of fall, if any) recorded in the databases.
Vertebral, shoulder, wrist or hip fracture were classified as MOP in adults only, as no fracture can be assumed to be osteoporotic in nature in children in the absence of corroborating clinical and radiological information. Nevertheless, osteoporosis in the paediatric population is defined as a bone mineral density below 2.0 Z scores accompanied by history of two or more long bone fractures by ten years of age.41 Hence, osteoporosis cannot be excluded as a potential contributor to the seven-fold excess of femoral fractures and to the significant excess of shoulder and upper arm and of lower leg fracture (virtually all of which are long bone fractures) in girls with ID compared to those without ID. Moreover, the excess of femoral fractures was only two fold in ID compared to non ID boys, and the rate of shoulder and upper arm and of lower leg fracture was similar in the two groups of boys. Given the general lack of participation in sports and of the limited physical activity levels of ID compared to non ID boys, it is not inconceivable that osteoporosis may be at play also in boys with intellectual disabilities.
The rate of vertebral fracture was similar between people with and without ID. Vertebral fractures are largely clinically undiagnosed in the general population42 as symptoms may be poorly localised. In people with ID, whose ability to express and localise symptoms, including pain, is severely reduced, vertebral fractures could evade diagnosis even more easily. This can lead to spuriously low incidence rates of vertebral clinical fractures in comparison to radiologically detected vertebral fractures.43
The rates of wrist fracture were only slightly higher in people with ID, and in some age groups there was no difference between the ID and non ID group. This might be partly explained by the neurological impairment that often accompanies an intellectual disability. This could prevent the development of the postural reflex of protective extension, in which the arm is outstretched on falling, potentially leading to a fractured wrist but protecting more central structures of the body.
The distribution of fractures was profoundly different. In particular, in adults, hip fracture represented 9.9% of total fractures in the ID group and 5.0% of total fractures in the non ID group, with differences particularly marked in younger people and in men. Moreover, the percentage of femoral fractures which were fractures of the hip was similar and close to 90% in all adult groups except for men without ID, in whom it was only 76%. This reinforces the interpretation of our results as femoral fractures being due mostly to osteoporosis in people with ID, with ID men particularly affected.
The main strengths of this study include the size of the population, and the quality of the database, which originates from a national health system, links primary and secondary data, and has been used in numerous studies worldwide. To our knowledge, this is also the first study investigating fracture incidence in people with ID over the whole of the natural life course. Moreover, our study population is highly representative of people with ID, as shown by an excess of male individuals, by people with Down syndrome being the single largest diagnostic group, and by a high prevalence of epilepsy. This study is also the only one to provide a detailed diagnostic characterisation of individuals with ID, thus allowing a more informed and deeper interpretation of the findings. Another strength of the study lies in its large control population, and in the comparability of its results with those of the seminal studies by Curtis and Moon in all UK adults and children registered in CPRD experiencing a fracture over the years 1988–2012.38,39
The study's main weakness probably lies in the recording of clinical events in the data source, a common problem in all database studies. We mitigated this by linking the Clinical Practice Research Datalink to the Hospital Episode Statistics database, in order to capture fractures recorded in either source. Another limitation is a potential undercounting of second fractures due to our rigorous attempt to avoid multiple counting of the same fracture. Multiple counting is an ever present risk when the same event can be described by several terms, as in CPRD, and to a lesser extent in HES, and when combining databases. We took a conservative approach, viewing a potentially spurious inflation in the number of fractures as a serious threat to data quality.
In our study we did an age and sex specific analysis without controlling for any potential confounders beyond these factors, and this is another limitation. However, it would be difficult to select the variables for a multivariate analysis when the population of interest is already known to differ from the control population by many characteristics, which may influence the outcome. For instance, important co-morbidities known to be associated with fractures might be connatural to the condition of ID itself (e.g. epilepsy could be a symptom of the frequent accompanying neurological damage). Additionally, crucial clinical and lifestyle characteristics change enormously over the life course, and decisions would have to be made about the time points at which these variables should be extracted from the primary care records for meaningful report and analysis. Hence, the investigation of the potential reasons for the difference in fracture rates between the ID and the non ID population is a study in itself, which can only be done separately from the descriptive analysis we are reporting.
Our results confirm some of the findings of other important epidemiological studies, which consistently show a raised fracture rate in people with ID.23,24,27 However, our study's unique characteristics make its results particularly robust and novel. The high rate of fracture, the early increase in incidence, and the type and distribution of fractures we observed point to early-onset osteoporosis. This interpretation is also supported by the fact that people with ID are a highly sedentary population with very low levels of physical activity compared to the general population.44,45 Further research aiming to determine the risk factors for fracture in people with ID is ongoing, and should lead to the design of appropriate prevention programmes, based on the understanding of pathophysiological mechanisms. These could include a variety of co-morbid genetic or acquired conditions16,20,21,26,43, 44, 45, 46, 47, 48, 49, 50, 51, 52 impairing bone acquisition and development and/or inducing bone loss, and accompanying the intellectual disability as part of a multisystem disorder also affecting the skeleton. Meanwhile, as people with ID have a high rate of falls,53 strategies to reduce the risk of fall9,10 should be implemented, together with safe physical exercise to improve bone accrual and decrease bone loss.54 Finally, osteoporosis guidelines should be modified to include people with intellectual disabilities within the groups at high risk of fracture.
Contributors
All authors contributed to the design of the study, the acquisition of funding, the writing of the protocol, the conduct of the study, and critically reviewed and approved the final version of the manuscript. VF conceived of, led, supervised and administered the study, provided expertise in endocrinology and in intellectual disabilities, selected the medical terms and codes for the study population and the outcomes, searched the literature, wrote the original draft. MS provided expertise in epidemiology and in CPRD, extracted, curated and analysed the data, contributed to the writing of the original draft, produced the artwork. TMA provided expertise in intellectual disabilities and in neurology, selected the medical terms and codes for the study population, reviewed and edited the original draft. LC curated, analysed and produced a statistical report on a preliminary dataset, reviewed the original draft. GSC provided statistical expertise, reviewed and edited the original draft. AF provided CPRD expertise, extracted and curated the data, reviewed the original draft. JR gave advice throughout the study from a patient and career perspective, reviewed the original draft. TAH provided General Practice and CPRD expertise, selected the medical terms and codes for the study population and the outcomes, contributed to the supervision of the study, and to the writing of the original draft. MS and AF verified the underlying data. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Data sharing statement
This study is based on an anonymised dataset from the Clinical Practice Research Datalink. Access rules to CPRD prevent researchers from sharing CPRD datasets.
Declaration of interests
V.F., M.S., T.M.A., L.C., G.S.C., A.F., T.A.H. report grants from the National Institute for Health and Care Research (NIHR) during the conduct of this study. V.F., M.S., T.A.H. also report grants from The Baily Thomas Charitable Fund.
Acknowledgments
This project is funded by the National Institute for Health and Care Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-1216-20017). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. M Smith is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC) and by the NIHR Applied Research Collaborative Oxford and Thames Valley. This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. Copyright © 2022, re-used with the permission of The Health & Social Care Information Centre. All rights reserved. The authors acknowledge Professor Daniel Prieto Alhambra for his expertise in osteoporosis, contribution to funding acquisition, to the writing of the protocol, and for the selection of the outcomes medical terms and codes; the Royal Osteoporosis Society, the Royal Mencap Society, and the Down's Syndrome Association for their advisory collaboration in the research, and their forthcoming help in disseminating the results also beyond the scientific community. We also wish to thank Professor Nick Athanasou for expert comments on the manuscript and Dr Janet Finlayson for advice and encouragement throughout the project.
This work is dedicated to all the patients who inspired it.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101656.
Appendix. Supplementary materials
References
- 1.American Association of Intellectual and Developmental Disabilities. Definition of Intellectual Disability. https://www.aaidd.org/intellectual-disability/definition. Accessed 30 March 2022.
- 2.McKenzie K, Milton M, Smith G, Ouellette-Kuntz H. systematic review of the prevalence and incidence of intellectual disabilities: current trends and issues. Curr Dev Disord Rep. 2016;3:104–115. doi: 10.1007/s40474-016-0085-7. [DOI] [Google Scholar]
- 3.NHS Digital.https://digital.nhs.uk/data-and-information/publications/statistical/health-and-care-of-people-with-learning-disabilities/experimental-statistics-2020-to-2021. Accessed 30 March 2022.
- 4.Public Health England 2016 . 2015. Learning Disabilities Observatory. People with Learning Disabilities in England.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/613182/PWLDIE_2015_main_report_NB090517.pdf Main report. Accessed 30 March 2022. [Google Scholar]
- 5.Wallace RA. Genetic testing of aetiology of intellectual disability in a dedicated physical healthcare outpatient clinic for adults with intellectual disability. Intern Med J. 2016;46:177–185. doi: 10.1111/imj.12946. [DOI] [PubMed] [Google Scholar]
- 6.The LeDeR Team . 2020. The Learning Disabilities Mortality Review (LeDeR) Programme. Annual Report.https://leder.nhs.uk/images/annual_reports/LeDeR-bristol-annual-report-2020.pdf Accessed 30 March 2022. [Google Scholar]
- 7.Liao P, Vajdic C, Trollor J, Reppermund S. Prevalence and incidence of physical health conditions in people with intellectual disability - a systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leal J, Gray AM, Prieto-Alhambra D, et al. Impact of hip fracture on hospital care costs: a population-based study. Osteoporos Int. 2016;27:549–558. doi: 10.1007/s00198-015-3277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Public Health England . 2019. Preventing Falls in People with Learning Disabilities: Making Reasonable Adjustments.https://www.gov.uk/government/publications/preventing-falls-in-people-with-learning-disabilities/ Accessed 11 March 2022. [Google Scholar]
- 10.Finlayson J. Fall prevention for people with learning disabilities: key points and recommendations for practitioners and researchers. Tizard Learn Disabil Rev. 2018;23:91–99. doi: 10.1108/TLDR-06-2017-0026. [DOI] [Google Scholar]
- 11.May PB, Parker F. Oral bisphosphonates for osteoporosis in adult males with intellectual disabilities. J Appl Res Intellect Disabil. 2021;34:921–925. doi: 10.1111/jar.12862. [DOI] [PubMed] [Google Scholar]
- 12.Lambert AS, Rothenbuhler A, Charles P, et al. Lower incidence of fracture after IV bisphosphonates in girls with Rett syndrome and severe bone fragility. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaga Y, Ishii S, Kuroda I, et al. The efficacy of intravenous alendronate for osteoporosis in patients with severe motor intellectual disabilities. No To Hattatsu. 2017;49:113–119. [PubMed] [Google Scholar]
- 14.Uehara M, Nakamura Y, Suzuki T, Sakai N, Takahashi J. Efficacy of denosumab therapy for a 12-year-old female patient with Williams syndrome with osteoporosis and history of fractures: a case report. J Med Case Rep. 2021;15:594. doi: 10.1186/s13256-021-03175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tannenbaum TN, Lipworth L, Baker S. Risk of fractures in an intermediate care facility for persons with mental retardation. Am J Ment Retard. 1989;93:444–451. [PubMed] [Google Scholar]
- 16.Center J, Beange H, McElduff A. People with mental retardation have an increased prevalence of osteoporosis: a population study. Am J Mental Retardat. 1998;103:19–28. doi: 10.1352/0895-8017(1998). [DOI] [PubMed] [Google Scholar]
- 17.Lohiya GS, Crinella FM, Tan-Figueroa L, Caires S, Lohiya S. Fracture epidemiology and control in a developmental center. West J Med. 1999;170:203–209. [PMC free article] [PubMed] [Google Scholar]
- 18.Schrager S, Kloss C, Ju AW. Prevalence of fractures in women with intellectual disabilities: a chart review. J Intellect Disabil Res. 2007;51:253–259. doi: 10.1111/j.1365-2788.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 19.Jasien J, Daimon CM, Maudsley S, Shapiro BK, Martin B. Aging and bone health in individuals with developmental disabilities. Int J Endocrinol. 2012;2012 doi: 10.1155/2012/469235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srikanth R, Cassidy G, Joiner C, Teeluckdharry S. Osteoporosis in people with intellectual disabilities: a review and a brief study of risk factors for osteoporosis in a community sample of people with intellectual disabilities. J Intellect Disabil Res. 2011;55:53–62. doi: 10.1111/j.1365-2788.2010.01346.x. [DOI] [PubMed] [Google Scholar]
- 21.Hawli Y, Nasrallah M, El-Hajj Fuleihan G. Endocrine and musculoskeletal abnormalities in patients with Down syndrome. Nat Rev Endocrinol. 2009;5:327–334. doi: 10.1038/nrendo.2009.80. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Tian Z, Ye S, et al. Changes in bone mineral density in Down syndrome individuals: a systematic review and meta‑analysis. Osteoporos Int. 2022;33:27–37. doi: 10.1007/s00198-021-06070-7. [DOI] [PubMed] [Google Scholar]
- 23.Balogh R, Wood J, Dobranowski K, et al. Low-trauma fractures and bone mineral density testing in adults with and without intellectual and developmental disabilities: a population study. Osteoporos Int. 2017;28:727–732. doi: 10.1007/s00198-016-3740-2. [DOI] [PubMed] [Google Scholar]
- 24.Büchele G, Becker C, Cameron ID, et al. Fracture risk in people with developmental disabilities: results of a large claims data analysis. Osteoporos Int. 2017;28:369–375. doi: 10.1007/s00198-016-3733-1. [DOI] [PubMed] [Google Scholar]
- 25.Burke E, Carroll R, O'Dwyer M, Walsh JB, McCallion P, McCarron M. Quantitative examination of the bone health status of older adults with intellectual and developmental disability in Ireland: a cross-sectional nationwide study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frighi V, Morovat A, Andrews TM, et al. Vitamin D, bone mineral density and risk of fracture in people with intellectual disabilities. J Intellect Disabil Res. 2019;63:357–367. doi: 10.1111/jir.12581. [DOI] [PubMed] [Google Scholar]
- 27.Whitney DG, Caird MS, Jepsen KJ, et al. Elevated fracture risk for adults with neurodevelopmental disabilities. Bone. 2020;130 doi: 10.1016/j.bone.2019.115080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute of Health and care excellence (NICE). Osteoporosis- prevention of fragility fractures (last revised July 2021). https://cks.nice.org.uk/topics/osteoporosis-prevention-of-fragility-fractures/. Accessed 3 July 2022.
- 29.Scottish Intercollegiate Guidelines Network. Management of osteoporosis and the prevention of fragility fractures. Clinical guideline 142 (last updated January 2021) www.sign.ac.uk/our-guidelines/management-of-osteoporosis-and-the-prevention-of-fragility-fractures. Accessed 3 July 2022
- 30.National Osteoporosis Guideline Group (NOGG) UK. Clinical guideline for the prevention and treatment of osteoporosis (updated September 2021). https://www.nogg.org.uk/. Accessed 3 July 2022.
- 31.CPRD. https://www.cprd.com/. Accessed 3 July 2022
- 32.Herrett E, M Gallagher A, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD) Int J Epidemiol. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NHS Digital. Quality and Outcomes Framework. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/quality-and-outcomes-framework-qof. Accessed 30 March 2022.
- 34.Frighi V, Prieto-Alhambra D, Collins G, et al. https://www.cprd.com/protocol/fractures-people-intellectual-disabilities-comparison-general-population-and-development
- 35.NHS Digital. Hospital Episode Statistics (HES). https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics. Accessed 30 March 2022
- 36.Strategic Health Facilitation Team. Learning Disability Read Codes. https://www.england.nhs.uk/south/wp-content/uploads/sites/6/2018/06/LD-CODES-V4-March-2018-share.pdf. Accessed 30 March 2022.
- 37.Russell AM, Bryant L, House A. Identifying people with a learning disability: an advanced search for general practice. Br J Gen Pract. 2017;67:e842–e850. doi: 10.3399/bjgp17×693461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis EM, van der Velde R, Moon RJ, et al. Epidemiology of fractures in the United Kingdom 1988-2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. doi: 10.1016/j.bone.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon R, Harvey NC, Curtis EM, de Vries F, van Staa T, Cooper C. Ethnic and geographic variations in the epidemiology of childhood fractures in the United Kingdom. Bone. 2016;85:9–14. doi: 10.1016/j.bone.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.StataCorp . StataCorp LLC; College Station, TX: 2019. Stata Statistical Software: Release 16. [Google Scholar]
- 41.Weber DR, Boyce A, Gordon C, et al. The utility of DXA assessment at the forearm, proximal femur, and lateral distal femur, and vertebral fracture assessment in the pediatric population: 2019 ISCD official position. J Clin Densitom. 2019;22:567–589. doi: 10.1016/j.jocd.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int. 2000;11:577–582. doi: 10.1007/s001980070078. [DOI] [PubMed] [Google Scholar]
- 43.Mouillé M, Rio M, Breton S, et al. SATB2-associated syndrome: characterization of skeletal features and of bone fragility in a prospective cohort of 19 patients. Orphanet J Rare Dis. 2022;17:100. doi: 10.1186/s13023-022-02229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dairo YM, Collett J, Dawes H, Oskrochi GR. Physical activity levels in adults with intellectual disabilities: a systematic review. Prevent Med Rep. 2016;4:209–219. doi: 10.1016/j.pmedr.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melville CA, Oppewal A, Schäfer Elinder L, et al. Definitions, measurement and prevalence of sedentary behaviour in adults with intellectual disabilities – a systematic review. Prevent Med. 2017;97:62–71. doi: 10.1016/j.ypmed.2016.12.052. [DOI] [PubMed] [Google Scholar]
- 46.Thomas JR, Roper RJ. Current analysis of skeletal phenotypes in Down Syndrome. Curr Osteoporos Rep. 2021;19:338–346. doi: 10.1007/s11914-021-00674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostermaier KK, Weaver AL, Myers SM, Stoeckel RE, Katusic SK, Voigt RG. Incidence of celiac disease in Down Syndrome: a longitudinal, population-based birth cohort study. Clin Pediatr. 2020;59:1086–1091. doi: 10.1177/0009922820941247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tauber M, Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021;9:235–246. doi: 10.1016/S2213-8587(21)00002-4. [DOI] [PubMed] [Google Scholar]
- 49.Ohlsson Gotby V, Söder O, et al. Hypogonadotrophic hypogonadism, delayed puberty and risk for neurodevelopmental disorders. J Neuroendocrinol. 2019;31:e12803. doi: 10.1111/jne.12803. [DOI] [PubMed] [Google Scholar]
- 50.Ko A, Kong J, al Samadov Fet. Bone health in pediatric patients with neurological disorders. Ann Pediatr Endocrinol Metabol. 2020;25:15–23. doi: 10.6065/apem.2020.25.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bucerzan S, Miclea D, Lazea C, Asavoaie C, Kulcsar A, Grigorescu-Sido P. 16q24.3 Microduplication in a patient with developmental delay, intellectual disability, short stature, and nonspecific dysmorphic features: case report and review of the literature. Front Pediatr. 2020;8:390. doi: 10.3389/fped.2020.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marom R, Burrage LC, Venditti R, et al. COPB2 loss of function causes a coatopathy with osteoporosis and developmental delay. Am J Hum Genet. 2021;108:1710–1724. doi: 10.1016/j.ajhg.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finlayson J, Morrison J, Jackson A, Mantry D, Cooper SA. Injuries, falls and accidents among adults with intellectual disabilities. Prospective cohort study. J Intellect Disabil Res. 2010;54:966–980. doi: 10.1111/j.1365-2788.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- 54.Royal Osteoporosis Society. Exercise for bones. https://theros.org.uk/information-and-support/bone-health/exercise-for-bones/. Accessed 3 July 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.