Abstract
Background
Insertable cardiac monitors (ICMs) are indicated for long-term monitoring of patients with unexplained syncope or who are at risk for cardiac arrhythmias. The volume of ICM-transmitted information may result in long data review times to identify true and clinically relevant arrhythmias.
Objective
The purpose of this study was to evaluate whether artificial intelligence (AI) may improve ICM detection accuracy.
Methods
We performed a retrospective analysis of consecutive patients implanted with the Confirm RxTM ICM (Abbott) and followed in a prospective observational study. This device continuously monitors subcutaneous electrocardiograms (SECGs) and transmits to clinicians information about detected arrhythmias and patient-activated symptomatic episodes. All SECGs were classified by expert electrophysiologists and by the WillemTM AI algorithm (IDOVEN).
Results
During mean follow-up of 23 months, of 20 ICM patients (mean age 68 ± 12 years; 50% women), 19 had 2261 SECGs recordings associated with cardiac arrhythmia detections or patient symptoms. True arrhythmias occurred in 11 patients: asystoles in 2, bradycardias in 3, ventricular tachycardias in 4, and atrial tachyarrhythmias (atrial tachycardia/atrial fibrillation [AT/AF]) in 10; with 6 patients having >1 arrhythmia type. AI algorithm overall accuracy for arrhythmia classification was 95.4%, with 97.19% sensitivity, 94.52% specificity, 89.74% positive predictive value, and 98.55% negative predictive value. Application of AI would have reduced the number of false-positive results by 98.0% overall: 94.0% for AT/AF, 87.5% for ventricular tachycardia, 99.5% for bradycardia, and 98.8% for asystole.
Conclusion
Application of AI to ICM-detected episodes is associated with high classification accuracy and may significantly reduce health care staff workload by triaging ICM data.
Keywords: Artificial intelligence, Detection accuracy, Insertable cardiac monitors
Key Findings.
-
•
An artificial intelligence (AI) algorithm that was developed and trained on electrocardiographic (ECG) and cardiac Holter data, when applied to data from an implantable cardiac monitor (ICM), classified arrhythmia subcutaneous electrocardiograms (SECGs) with 95.4% overall accuracy, 97.19% sensitivity, 94.52% specificity, 89.74% positive predictive value, and 98.55% negative predictive value.
-
•
The fact that AI algorithms may classify ICM-detected episodes with high accuracy suggests that AI algorithms can be used to triage ICM data with the aim of discarding ICM false-positive detections and reducing health care staff workload.
-
•
The AI algorithm applied in our research, if used to review SECGs collected in our patient population during the observation period, would have reduced the number of false-positive detections by 98.0% overall: 99.5% for bradycardia, 98.8% for asystole, 94.0% for atrial tachyarrhythmias, and 87.5% for ventricular tachyarrhythmias.
Introduction
Insertable cardiac monitors (ICMs) are indicated for detection of arrhythmias in patients with unexplained syncope.1,2 Moreover, they are established as a monitoring and diagnostic solution in patients with unexplained symptoms, such as dizziness, palpitations, chest pain, and shortness of breath. They also are used in patients who are at risk for cardiac arrhythmias, for example, to monitor atrial tachyarrhythmias (ie, atrial tachycardia/atrial fibrillation [AT/AF]) before and after ablation and in patients with cryptogenic stroke.3,4
ICMs may detect asystole events, bradycardia episodes, and both atrial and ventricular tachycardias. The detection logic is based on continuous ambulatory monitoring of the patient’s subcutaneous electrocardiograms (SECGs).
New-generation ICMs are connected via the patient’s smartphone and Bluetooth technology and can transmit data in a patient-activated mode or daily in an automatic mode.5 ICM detection yield is such that a large amount of data can be transmitted to clinicians. This is beneficial because it provides opportunities to associate patient symptoms with cardiac arrhythmias, to detect unknown cardiac conditions, and to derive clinical insight and relevant knowledge to guide medical action. However, false arrhythmia detections may occur6, 7, 8, 9, 10, 11, 12, 13 and result in unnecessary review workload for health care staff, which partially reduces broader ICM use in clinical practice and may entail high costs for health care systems.
Standard detection algorithms use fixed rules for cardiac electrical signal processing and have limited capacity to adapt to specific patient patterns. However, artificial intelligence (AI), which already is being applied to electrocardiographic and Holter data,14, 15, 16, 17, 18 offers the promise of performing efficient data triage by reducing false detections19 and identifying and using patient-specific rate and rhythm models to predict future clinical events.17,18,20
Within the framework of a pilot prospective observational study in patients implanted with an ICM, we performed a retrospective analysis to evaluate whether AI may significantly improve ICM detection accuracy, thus supporting clinicians in arrhythmia evaluation while reducing their workload.
Methods
Project design
We performed a retrospective analysis on consecutive ICM patients included in a pilot prospective observational study. The study protocol was approved by the hospital ethics committee. All patients provided consent for the use of their data.
Patient population
Patients were included in the study after they had undergone implantation of a Confirm RxTM ICM (Abbott, Sylmar, CA) according to standard indications.1, 2, 3, 4
ICM
The characteristics of the Confirm Rx ICM have been previously described.5,13 In brief, the Confirm Rx ICM is a subcutaneously implanted device that can continuously monitor patient heart rhythm for up to 2 years. Specific detection algorithms warrant accurate detection of arrhythmias. The ICM is capable of remote monitoring, connecting patients with their cardiologists via Bluetooth wireless technology and a dedicated smartphone application.
ICM implantation
ICM implantations were performed between April 2018 and January 2020. ICM devices were inserted near the left parasternal area over the fourth intercostal space at 45° to the sternum. At the end of the implantation procedure, SECGs were recorded to assess the quality of R-wave sensing.
ICM arrhythmia detection
The arrhythmia detection algorithms used in the Confirm Rx devices have undergone technological evolutions.5,10,13 In April 2019, an improved SharpSenseTM arrhythmia detection technology was implemented.13 Fifteen of 20 patients in our cohort were included in the study before April 2019; therefore, their ICMs, in the first observation period (average 11 months), used the standard detection algorithm. The SharpSense software then was uploaded to the devices of these patients during in-person patient follow-up visits, so in these patients the SharpSense technology was used in their second observation period (average 14 months). Five of 20 patients in our cohort were included in the study after April 2019; therefore, their ICMs used the SharpSense technology from the time of implant.
The details of the device detection algorithms have been previously described.5,10,13 Specifically, the SharpSense technology evaluates R-R intervals and uses (1) a Markov chain model to detect R-R interval irregularities; (2) a variance model to reject regularly irregular rhythms such as bigeminy and trigeminy; and (3) sudden-onset criteria to reject rhythms that are not sudden. For AT/AF detection, a p-wave detection algorithm is activated when the base algorithm triggers AT/AF detection. The p-wave detection algorithm analyzes the SECG signal before the trigger and rejects the initial detection if consistent p waves are found.
In our patient population, the ICMs were programmed to detect asystole episodes if a pause ≥4.5 seconds was observed; bradycardia episodes if heart rate was ≤40 bpm; ventricular tachyarrhythmia (VT) episodes if heart rate was ≥160 bpm; or AT/AF episodes if the device detected an AT/AF rhythm lasting at least 6 minutes.
Objectives
The main objective of this research was to evaluate AI arrhythmia detection accuracy in terms of positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity. Secondary objectives were to evaluate the percentage of false-positive detections that can be detected by AI.
Data collection and transmission
SECGs were collected according to established standards (ie, 25 mm on the x-axis corresponding to 1 second and 10 mm on the y-axis corresponding to 1 mV). SECG minimum duration was 10 seconds. The SECG signal was recorded at a sampling frequency of 128 Hz with standard bandpass filtering of 0.05–150 Hz and notch of electric current of 50 Hz. The dynamic range of the signal amplitude values was ±10 mV, and the storage analog-to-digital conversion factor was ≥8 bits. The device was programmed to store SECGs and other information on detected arrhythmic events. These data were transmitted daily to the Merlin.net™ patient care network (https://www.merlin.net/it/web/chakravyuh/login), with transmissions occurring just after midnight. Through the MyMerlin application, patients were able to manually initiate SECG recordings, which were immediately transmitted.
Episode classification
The Merlin.net system allowed extraction of the episode SECGs both as images for expert electrophysiologists to review and as anonymized digital data for the AI algorithm to be applied.
Episode classification was performed by expert cardiologists. At least 2 reviewers independently analyzed all episodes and classified each episode according to 5 types (asystole, bradycardia, ventricular tachycardia, AT/AF, or Other). In case of differences between the classifications of 2 reviewers, the episode classification was derived by the consensus of other expert electrophysiologists. Each episode was classified as either a true or false arrhythmia and according to arrhythmia type. In case of false episode classification, the following etiologies were identified: presence of premature atrial contractions, presence of premature ventricular contractions, sinus tachycardia, oversensing, undersensing, noise, or other causes.
AI algorithm
The AI algorithm used in this research is Willem™ software (IDOVEN, Madrid, Spain). Willem algorithms correspond to a set of data-driven AI models developed in a cloud platform based on deep learning methodologies trained with a database including 1,234,207 hours of electrocardiograms (ECGs) from 47,035 real anonymized patients. No simulated data have been used for these trainings. A supervised learning methodology has been used to train the AI algorithm using data labeled by cardiology experts. Specifically, Willem is composed of an improved Inception network,14 which maps ECG recordings to 84 diagnostic classes. The main model takes the raw ECG data of 1, 2, 3, or up to 18 different leads (sampled at a minimum of 128 Hz) as input; it also allows the inclusion of static hand-crafted features. The model corresponds with a series of convolutional networks layers (CNN) followed by inception blocks.21 Likewise, the final fully connected (FC) layer is modified to incorporate static hand-crafted features, and relatively large kernel sizes are used in CNNs. Finally, the training error was obtained as an average binary cross-entropy loss, whereas loss was optimized using the Adam optimizer with an initial learning rate 0.001. The models were trained with 50 epochs using a batch size of 128.
Statistical analysis
Continuous data are given as mean ± SD or median [interquartile range] as appropriate. All categorical data are given as proportions. Global performance of the AI detection algorithms is described according to standard estimations of accuracy, sensitivity, specificity, PPV, NPV, and the F1 score.22 Statistical analyses were performed using Stata software (StataCorp, College Station, TX).
Results
Our study cohort included 20 patients (mean age 68 ± 12 years; 50% women) with an ICM. Patient characteristics are given in Table 1. Indications for ICM implantation were unexplained syncope in 15 patients, AT/AF in 1, cryptogenic stroke in 1, and risk of cardiac arrhythmias in 3.
Table 1.
Baseline patient characteristics and ICM implant primary indications (N = 20)
| Age (y) | 68 ± 12 |
|---|---|
| Female | 10 (50) |
| Insertable cardiac monitor indication | |
| Unexplained syncope Risk for cardiac arrhythmias Atrial fibrillation Cryptogenic stroke |
15 (75)3 (15)1 (5)1 (5) |
| Hypertension | 10 (50) |
| Diabetes | 5 (25) |
| Stroke | 3 (15) |
| Heart failure | 1 (5) |
| Atrial fibrillation | 6 (30) |
| Ventricular tachycardia | 3 (15) |
| Coronary artery disease | 2 (10) |
| History of cerebral ischemia, vascular disease, previous myocardium infarction, peripheral arterial disease, or aortic plaque | 8 (40) |
| CHA2DS2VASc score | 3.0 ± 2.2 |
| Oral anticoagulation | 2 (10) |
| Platelet inhibitors | 10 (50) |
| Class III antiarrhythmic drugs | 2 (10) |
| Beta-blockers | 6 (30) |
| Calcium channel blockers | 2 (10) |
| Renin–angiotensin inhibitors | 9 (45) |
| Statins | 7 (35) |
| Diuretics | 5 (25) |
Values are given as mean ± SD or n (%) unless otherwise indicated.
ICM = insertable cardiac monitor.
During mean follow-up of 23 ± 4 months, 19 patients had 2261 SECGs recordings associated with cardiac arrhythmia detections or patient symptoms.
AI detection accuracy
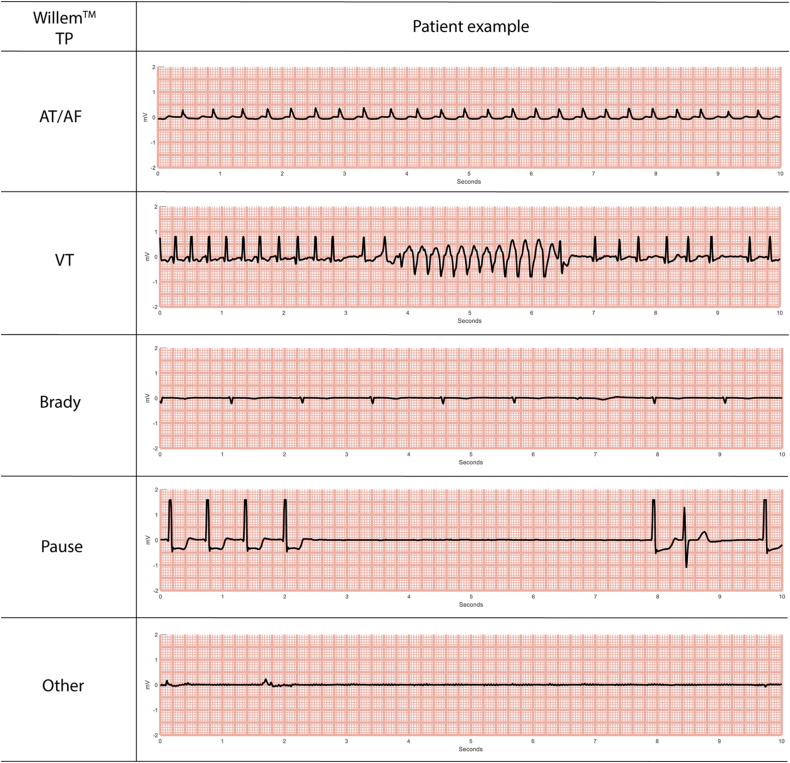
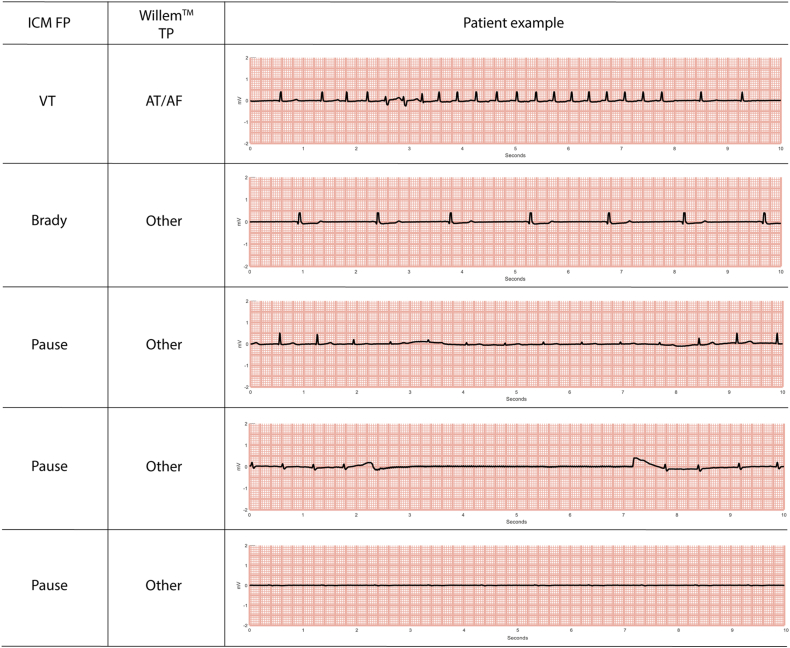
Expert electrophysiologist classifications, together with the AI algorithm classifications and their correspondences and discrepancies, are given in Table 2. AI classification overall accuracy, PPV, NPV, sensitivity, specificity, and F1 score are given in Table 3. The AI algorithm classified arrhythmia SECGs with 95.4% overall accuracy, with 97.19% sensitivity, 94.52% specificity, 89.74% PPV, and 98.55% NPV. Figure 1 shows patient ECG examples of cardiac events correctly classified by AI for each of the 5 types of episodes (AT/AF, VT, bradycardia, pause, or Other).
Table 2.
Comparison of episode classifications by expert cardiologists and AI algorithm
| Cardiologist classification |
|||||
|---|---|---|---|---|---|
| AI classification | AT/AF (n = 528) | VT (n = 18) | Brady (n = 199) | Pause (n = 2) | Other (n = 1514) |
| AT/AF (n = 589) | 510 | 0 | 0 | 1 | 78 |
| VT (n = 20) | 1 | 14 | 0 | 0 | 5 |
| Brady (n = 200) | 0 | 0 | 199 | 1 | 0 |
| Pause (n = 0) | 0 | 0 | 0 | 0 | 0 |
| Other (n = 1452) | 17 | 4 | 0 | 0 | 1431 |
AI = artificial intelligence; AT/AF = atrial tachycardia/atrial fibrillation (ie, atrial tachyarrhythmia); Brady = bradycardia; VT = ventricular tachyarrhythmia.
Table 3.
AI algorithm classification accuracy
| Class | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | F1 score (%) |
|---|---|---|---|---|---|
| AT/AF | 86.59 | 98.92 | 96.59 | 95.44 | 91.32 |
| Ventricular tachycardia | 70.00 | 99.82 | 77.78 | 99.73 | 73.68 |
| Bradycardia | 99.50 | 100.00 | 100.00 | 99.95 | 100 |
| Asystole | - | 99.91 | - | 100.00 | - |
| Any arrhythmia | 89.74 | 98.55 | 97.19 | 94.52 | 93.32 |
| Global accuracy = 95.27% | |||||
NPV = negative predictive value; PPV = positive predictive value; other abbreviations as in Table 2.
Figure 1.
Examples of electrocardiograms from different patients showing events that were correctly classified the WillemTM artificial intelligence algorithm according to the real cardiac alteration (WillemTM TP column). AT/AF = atrial tachycardia/atrial fibrillation (ie, atrial tachyarrhythmia); Brady = bradycardia; TP = true positive; VT = ventricular tachycardia.
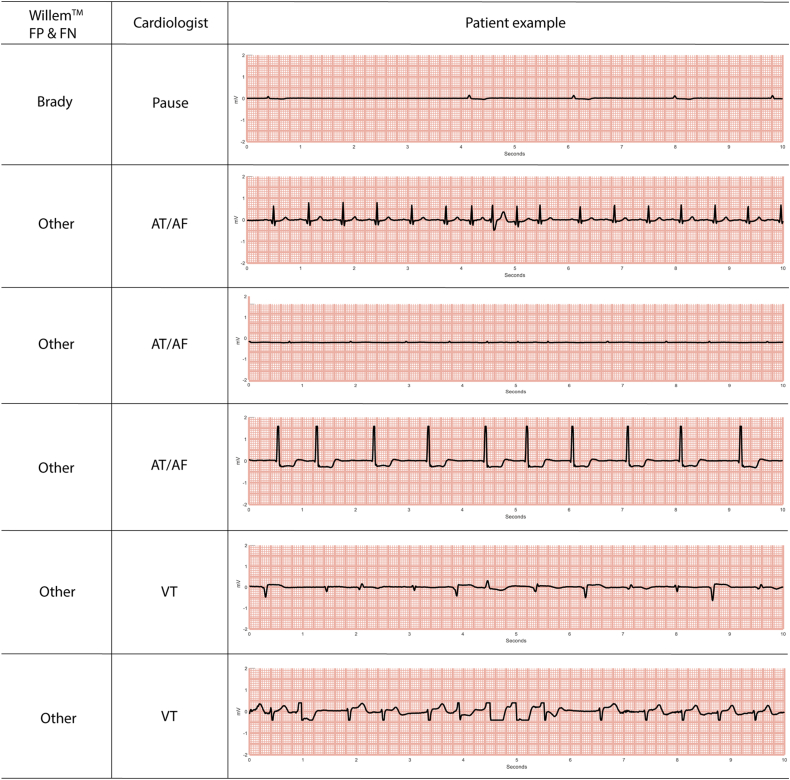
AI misclassified 24 true-positive arrhythmias (Table 2). In 3 cases, the AI algorithm classified episodes as true arrhythmias but assigned to them the wrong type. This occurred for 2 pauses, classified as bradycardia (ECG example shown in Figure 2) and AT/AF, respectively, and for 1 AT/AF classified as ventricular tachycardia. The other 21 cases, which were classified by expert cardiologists as 4 VT and 17 AT/AF, were classified by AI as nonmajor cardiac arrhythmias (ECG examples in Figure 2). All 4 AI false-negative VTs were very short (<4 seconds) in duration. AT/AF false-negatives were short (<4 seconds) atrial tachycardias or reinitiation of previously detected AF episodes with a controlled ventricular response. In addition, most of the AI misclassifications were nonsevere cardiac arrhythmias (Figure 3).
Figure 2.
Examples of electrocardiograms from different patients showing events that were misclassified (false-positive [FP]) or undetected (false-negative [FN]) by the WillemTM artificial intelligence algorithm (WillemTM FP & FN column). The annotations given by cardiologists also are shown (Cardiologist column). Abbreviations as in Figure 1.
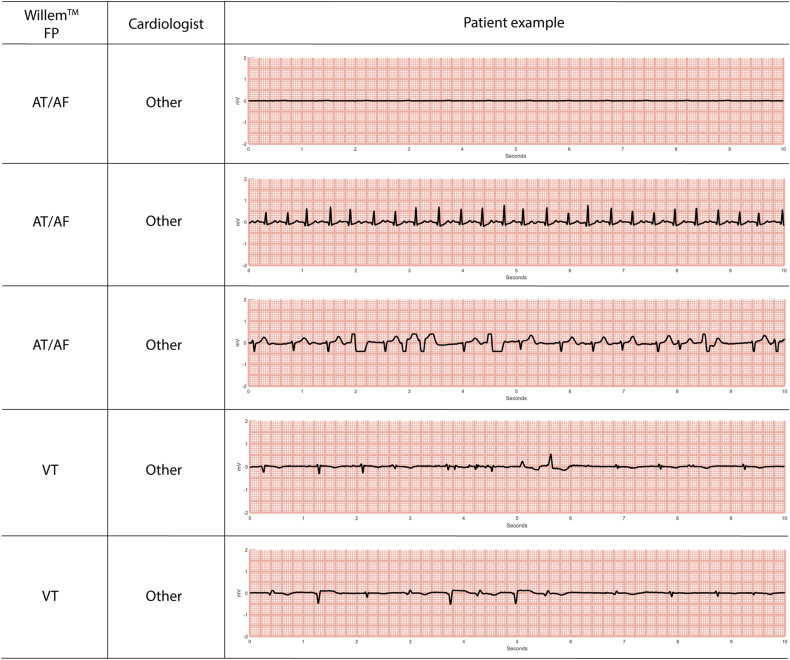
Figure 3.
Examples of electrocardiograms from different patients showing events that were misclassified by the WillemTM artificial intelligence algorithm (WillemTM FP column). The annotations of the cardiologists also are shown (Cardiologist column). Abbreviations as in Figure 1, Figure 2.
The only AI false-positive in VT detection was due to an AF episode with fast ventricular response at 129 bpm and aberrant ventricular conduction.
AI capability to reduce false-positive detections
Of the 1514 episodes classified by expert cardiologists as nonarrhythmic events, the AI algorithm identified 1431 episodes as nonarrhythmic; therefore, the application of AI would have reduced the number of false-positive detections by 94.52% overall (Table 2).
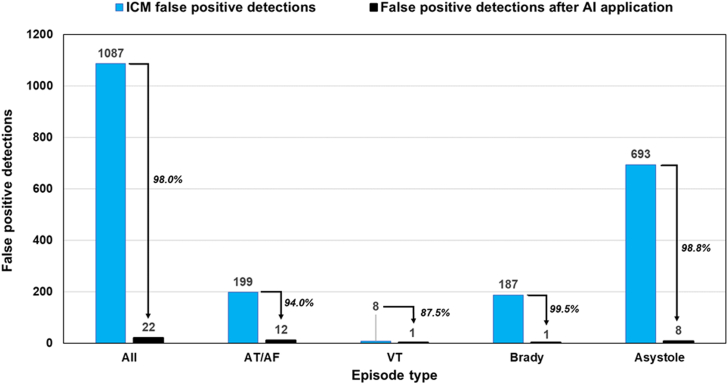
To evaluate AI capability to discard false-positives as a function of ICM-detected arrhythmia type, we had to discard SECGs transmitted by patients due to symptomatic episodes and to associate device classifications, expert electrophysiologist classifications, and AI algorithm classifications. Due to the data anonymization process, this association was possible for only 1464 episodes, which formed the dataset for analyses of the secondary objectives. The AI algorithm identified 1065 of 1087 false-positive arrhythmias; therefore, AI application to ICM data would have reduced false-positive detections by 98.0% (Figure 4). In particular, the AI algorithm identified 187 of 199 AT/AF false-positives (false-positive reduction = 94.0%); 7 of 8 VT false-positives (false-positive reduction = 87.5%); 186 of 187 bradycardia false-positives (false positive reduction = 99.5%); and 685 of 693 asystole false-positives (false-positive reduction = 98.8%). Figure 5 shows some ECG examples of device false-positive classifications corrected by AI.
Figure 4.
Histogram showing the absolute number of false-positive episodes detected by the insertable cardiac monitor (ICM) (blue bars) and after application of the WillemTM artificial intelligence algorithm (black bars). The reduction in false-positive detections is also expressed as percentage and indicated next to the arrows for each episode type. Abbreviations as in Figure 1.
Figure 5.
Examples of electrocardiograms from different patients showing events that were detected as arrhythmias by the insertable cardiac monitor (ICM FP column) and then correctly reclassified by the WillemTM artificial intelligence algorithm according to the real cardiac alteration made by the cardiology experts considered as the gold standard (WillemTM TP column). Abbreviations as in Figure 1, Figure 2.
SharpSense detection technology
Of 15 patients implanted before April 2019, 9 had observation periods >6 months both before and after upload of the SharpSense detection technology. Implementation of SharpSense detection technology reduced the number of ICM false-positive detections from 3331 false-positive detections in 2938 days to 685 false-positive detections in 3843 days. When normalized as a function of time, this reduced 7.9 false-positive detections per week to 1.24 false-positive detections per week (82.3% reduction of false-positive detections).
During SharpSense detection, there were 518 false-positive detections for which we could associate device classifications, expert electrophysiologists classifications, and AI algorithm classifications. In this sample of episodes, the AI algorithm identified 507 of 518 false-positives; therefore, AI would have reduced false-positive detections by 97.9%. In particular, the AI algorithm identified 71 of 78 AT/AF false-positives (false-positive reduction = 91.0%); 5 of 6 VT false-positive episodes (false-positive reduction = 83.3%); 38 of 38 bradycardia false-positives (false-positive reduction = 100%); and 393 and 396 asystole false-positives (false-positive reduction = 99.2%).
ICM-confirmed detections and clinical outcomes
Of the 20 patients (mean follow-up 23 months), 15 had cardiac arrhythmias detected by the ICM. True arrhythmias were confirmed in 11 patients, and 6 patients had confirmed device detections that were clinically relevant to improving diagnosis or treatment.
The ICM detected AT/AF episodes in 9 patients, and detections were confirmed as true AT/AF episodes in 8 of 9 patients (88.9%). Patient-averaged PPV was 49%. Device AT/AF classification accuracy improved as a function of AT/AF duration. For any AT/AF episode duration, the raw PPV of device detection on AT/AF episodes was 23%, whereas for episodes with AT/AF duration longer than the median (59 minutes) raw PPV was 58%; and for episodes with AT/AF duration longer than the third quartile (4.5 hours) raw PPV was 100%.
Oral anticoagulation therapy was initiated in 1 patient who received the ICM to detect cardiac arrhythmias after a stroke. This patient had a CHA2DS2-VASc score of 5. During the observation period, the ICM detected several AT/AF episodes with median duration of 10 minutes and longest duration of 66 minutes.
AF ablation was performed in a patient who received the ICM to characterize the burden and frequency of AT/AF. In this patient during an observation period of 19 months, 23 AT/AF episodes were detected, with median duration of 5 hours and longest duration of 27 hours.
Pacemaker implantation was indicated in a patient who received the ICM for unexplained syncope after the ICM detected 9 episodes of severe bradycardia, with a mean rate of 30 bpm, mean duration of 11 seconds, and a 5-second asystole.
In 2 other patients who received the ICM for unexplained syncope, true bradycardia episodes were detected, but the bradycardia rate was >40 bpm and no clinical actions were taken.
The ICM also detected a true asystole of 5-second duration in another patient who received the ICM for AF monitoring but had a presyncopal episode in his history.
The ICM detected true VT in 4 patients. In 2 of these patients, the ICM allowed an improved characterization of ventricular tachycardias because VT was already known in the patient history, and the ICM was implanted to monitor threatening arrhythmias in these patients. In the other 2 patients who received the ICM for unexplained syncope, the VT episodes had a duration of 5 and 7 seconds, respectively. No specific actions were taken in these 2 patients.
Patient-averaged PPV was not estimated for bradycardia, asystole, and VT episodes because of the limited number of true episodes per patient or of patients with those arrhythmia type.
Discussion
The main finding of this study was that the evaluated AI algorithm accurately classified episodes detected by ICM. This result confirms that AI algorithms can be used to triage ICM data with the aim of discarding ICM false-positive detections and reducing health care staff workload.
AI detection accuracy
The Willem AI algorithm, which was developed and trained on ECG and cardiac Holter data, when applied to our ICM data classified arrhythmia SECGs with 95.4% overall accuracy, 97.19% sensitivity, 94.52% specificity, 89.74% PPV, and 98.55% NPV.
To the best of our knowledge, before our analyses, only Mittal et al19 had evaluated AI algorithm application to improve accuracy of ICM detection of cardiac arrhythmias. Our data add important insight into the field of applying AI to ICM data, as Mittal et al19 evaluated a different AI algorithm and a different ICM. Furthermore, they only evaluated AI applied to AT/AF detections in patients with cryptogenic stroke, patients with known AT/AF, and patients who had undergone AT/AF ablation, whereas we also applied AI to bradycardia, asystole, and VT detections. Another important difference with our research is that Mittal et al19 applied the AI algorithm on ECG signals extracted from images of episodes stored in the ICM summary report, with the inherent possibility of data distortion, whereas we extracted digital data from the device diagnostics, which could have contributed to a better sampling frequency of our signals. Our findings for AT/AF detections (PPV 86.59%, NPV 98.86%, sensitivity 96.41%, specificity 95.44%) (Table 3) compare favorably with the results reported by Mittal et al19 (PPV 58.7%–81.1%; NPV 96.8%–98.6%; relative sensitivity 98.1%–99.4%; specificity 39.5%–66.4%).
The AI algorithm used in our research failed to classify 24 arrhythmias (Table 2). However, if we had applied the AI algorithm in our clinical practice, these classification failures would have not caused clinical issues. For 3 of those episodes, the AI algorithm classified them as true arrhythmias but assigned them to the wrong arrhythmia type, regardless of this, the AI would have appropriately selected those episodes for clinician review. When considering the other 21 arrhythmia episodes, which were classified by expert cardiologists as 4 VT and 17 AT/AF and were classified by AI as nonmajor cardiac arrhythmias (ECG examples in Figure 2), all of these episodes occurred in patients who had previously suffered those types of arrhythmias, so arrhythmia occurrence was already known to the cardiologists following those patients.
Impact of AI algorithm on false-positive arrhythmia reduction
The AI algorithm applied in our research, if used to review SECGs collected in our patient population during the complete observation period, would have reduced the number of false-positive detections by 98.0% overall: 99.5% for bradycardia, 98.8% for asystole, 94.0% for AT/AF, and 87.5% for VT. As discussed previously, our results on AI application can be compared only with those of the study by Mittal et al,19 which found that AI application decreased the number of false-positive AT/AF episodes by 66.4% for patients with cryptogenic stroke, by 66.2% for patients with known AT/AF, and by 39.5% for patients who had undergone AT/AF ablation. The finding that our false-positive percent reductions with AI were higher may depend on several potential factors, such as AI algorithm classification accuracy, ICM detection accuracy, ICM detection yield, and ICM programming in terms of how many detected episodes are stored in the device diagnostics. In a randomized study, Ip et al5 demonstrated that Confirm Rx devices, such as those used in our study, are associated with a faster and higher detection yield and a higher number of episodes stored in the device diagnostics compared with the devices used in the study by Mittal et al.19
SharpSense detection algorithm
After enabling the SharpSense detection algorithm, the number of false-positive episodes detected by the ICM was reduced by 82.3%. This result is in accordance with recent findings described by Gopinathannair et al,13 who showed that upgrade to the SharpSense detection algorithm significantly reduced false-positive bradycardia episodes by 91.5% and false-positive pause episodes by 82.8%. Despite the SharpSense detection algorithm being more accurate than the previous detection algorithm, false-positive detections, especially for bradycardia and asystoles, also occurred in the SharpSense detection period, likely due to R-wave undersensing and/or transient loss of electrode–tissue contact. Application of the Willem AI algorithm to triage SharpSense detections improved detection accuracy, with a 97.9% reduction of false-positives.
ICM PPV
ICM PPV is a quantity that characterizes ICM detection accuracy and usually is estimated as an average of each patient detection PPV for each arrhythmia type. Only for AT/AF episodes did our data, from 9 patients with AT/AF episodes, allow us to perform a good estimation of patient-averaged PPV (49%). This value can be compared with similar estimations reported by other investigators on the same ICM used in our research or on different ICMs. In a randomized study comparing the detection yield of 2 ICMs (Reveal LINQTM [Medtronic, Minneapolis, MN] and Confirm Rx), 92 AT/AF episodes were detected from 11 patients in the Reveal LINQ group, and 1597 AT/AF episodes were detected from 20 patients in the Confirm Rx group.5 Patient-averaged PPVs were 52% and 38%, respectively (P = .50).5
Mittal et al19 evaluated Reveal LINQ ICMs and found an overall PPV for AT/AF detections of 53.9%, which differed according to the patient population: 32.8% for patients with cryptogenic stroke, 59.5% for patients with known AF, and 69.4% for patients who had undergone AF ablation.
For our data, PPV depended on arrhythmia duration for AF episodes, with improved PPV being a function of AT/AF duration.
Accurate estimation of patient-averaged ICM PPV for arrhythmias other than AT/AF was not possible because of the limited number of patients having those arrhythmias and/or the limited number of episodes per patient for those arrhythmias.
Clinical implications
The clinical value of ICM depends on reliable arrhythmia detection and fast transmission of data to clinicians that enable them to take prompt medical action and improve patient care. According to current clinical practice, ICMs automatically transmit SECG data daily using Bluetooth and Wi-Fi to a Web-based platform, and clinicians can review data and classify arrhythmias. Our results show that the evaluated AI algorithm can improve this practice by providing accurate arrhythmia classification. We showed that AI algorithms applied to ICM SECG data have high sensitivity and specificity in detecting true-positive arrhythmias and discarding false-positive detections. In this way, AI can be used to (1) triage data, (2) reduce the workload of health care staff in adjudicating transmitted information and discarding false alerts, and (3) focus on clinically actionable data. When confirmed in a larger cohort of patients, our results will be pivotal for the future application of AI and its integration into the hardware of future ICMs or in remote monitoring solutions.
Improved AI algorithm performance and new possibilities for AI application likely will derive from attempts to train AI algorithms on large SECG datasets using reinforcement learning techniques.
Study limitations
Our results derive from a limited number of patients who received a specific ICM and from application of a specific AI algorithm. Therefore, our findings cannot be generalized to all ICMs or to different AI algorithms. Our analyses confirm and expand those previously reported for a different ICM and a different AI algorithm19; therefore, we believe they are important and strongly suggest the possibility of improving ICM detection accuracy through AI application. Our findings cannot be generalized to patients who receive an ICM for different indications. Further research on larger cohorts of patients is warranted to confirm our findings and to evaluate whether AI application may translate into better clinical outcomes compared with standard clinical practice.
Conclusion
Our research showed that AI algorithms, trained on ECG and cardiac Holter data to detect cardiac arrhythmias, accurately classify episodes detected by ICMs. The application of AI may improve ICM detection accuracy and significantly reduce health care staff workload by triaging ICM data. This finding has relevant clinical implications for the use of ICMs and more generally for remote care of patients implanted with insertable cardiac devices because AI may reduce the data review burden, which currently hampers the expansion of remote monitoring. In the future, it also may improve arrhythmia detection and prediction.
Funding Sources
IDOVEN and Abbott devoted resources to perform data extractions, data management, AI application, and data analyses.
Disclosures
Dr Fabio Quartieri is an advisor for Abbott, Boston Scientific, and Biotronik. Dr Manuel Marina-Breysse, Mr Carlos Lizcano, and Dr José María Lillo-Castellano are employees and part of the AI research team of IDOVEN. Ms Annalisa Pollastrelli and Mr Andrea Grammatico are employees of Abbott Medical Devices.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients provided consent for the use of their data.
Ethics Statement
The study protocol was approved by the hospital Ethics Committee.
Acknowledgments
We acknowledge Raquel Toribio-Fernandez for support with figures and Felicity Champney for the English grammatical review.
References
- 1.Shen W.K., Sheldon R.S., Benditt D.G., et al. 2017 ACC/AHA/HRS Guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;136:e60–e122. doi: 10.1161/CIR.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M., Moya A., de Lange F.J., et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883–1948. doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- 3.Tracy C.M., Epstein A.E., Darbar D., et al. 2012 ACCF/AHA/HRS Focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2012;9:1737–1753. doi: 10.1016/j.hrthm.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e73–e189. doi: 10.1016/j.hrthm.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Ip J., Jaffe B., Castellani M., Sheikh A., Castellani C., Ip R. Accuracy of arrhythmia detection in implantable cardiac monitors: a prospective randomized clinical trial comparing Reveal LINQ and Confirm Rx. Pacing Clin Electrophysiol. 2020;43:1344–1350. doi: 10.1111/pace.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hindricks G., Pokushalov E., Urban L., et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3:141–147. doi: 10.1161/CIRCEP.109.877852. [DOI] [PubMed] [Google Scholar]
- 7.Pürerfellner H., Pokushalov E., Sarkar S., et al. P-wave evidence as a method for improving algorithm to detect atrial fibrillation in insertable cardiac monitors. Heart Rhythm. 2014;11:1575–1583. doi: 10.1016/j.hrthm.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Sanders P., Pürerfellner H., Pokushalov E., et al. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: results from the Reveal LINQ Usability Study. Heart Rhythm. 2016;13:1425–1430. doi: 10.1016/j.hrthm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Mittal S., Rogers J., Sarkar S., et al. Real-world performance of an enhanced atrial fibrillation detection algorithm in an insertable cardiac monitor. Heart Rhythm. 2016;13:1624–1630. doi: 10.1016/j.hrthm.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Nolker G., Mayer J., Boldt L.H., et al. Performance of an implantable cardiac monitor to detect atrial fibrillation: results of the DETECT AF Study. J Cardiovasc Electrophysiol. 2016;27:1403–1410. doi: 10.1111/jce.13089. [DOI] [PubMed] [Google Scholar]
- 11.Pürerfellner H., Sanders P., Sarkar S., et al. Adapting detection sensitivity based on evidence of irregular sinus arrhythmia to improve atrial fibrillation detection in insertable cardiac monitors. Europace. 2018;20:321–328. doi: 10.1093/europace/eux272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afzal M.R., Mease J., Koppert T., et al. Incidence of false-positive transmissions during remote monitoring with implantable loop recorders. Heart Rhythm. 2020;17:75–80. doi: 10.1016/j.hrthm.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Gopinathannair R., Lakkireddy D., Afzal M.R., et al. Effectiveness of SharpSense™ algorithms in reducing bradycardia and pause detection: real-world performance in Confirm Rx™ insertable cardiac monitor. J Interv Card Electrophysiol. 2022;63:661–668. doi: 10.1007/s10840-021-01099-4. [DOI] [PubMed] [Google Scholar]
- 14.Hannun A.Y., Rajpurkar P., Haghpanahi M., et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65–69. doi: 10.1038/s41591-018-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith S.W., Rapin J., Li J., et al. A deep neural network for 12-lead electrocardiogram interpretation outperforms a conventional algorithm, and its physician overread, in the diagnosis of atrial fibrillation. Int J Cardiol Heart Vasc. 2019;25 doi: 10.1016/j.ijcha.2019.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attia Z.I., Kapa S., Lopez-Jimenez F., et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25:70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 17.Attia Z.I., Noseworthy P.A., Lopez-Jimenez F., et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 18.Raghunath S., Cerna A.E.U., Jing L., et al. Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network. Nat Med. 2020;26:886–891. doi: 10.1038/s41591-020-0870-z. [DOI] [PubMed] [Google Scholar]
- 19.Mittal S., Oliveros S., Li J., Barroyer T., Henry C., Gardella C. AI filter improves positive predictive value of atrial fibrillation detection by an implantable loop recorder. JACC Clin Electrophysiol. 2021;7:965–975. doi: 10.1016/j.jacep.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Lillo-Castellano J.M., González-Ferrer J.J., Marina-Breysse M., et al. Personalized monitoring of electrical remodelling during atrial fibrillation progression via remote transmissions from implantable devices. Europace. 2020;22:704–715. doi: 10.1093/europace/euz331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pouyanfar S., Chen Shu-Ching, ShyuAn Mei-Ling. Efficient deep residual-inception network for multimedia classification. IEEE International Conference on Multimedia and Expo Conference Paper. 2017:373–378. doi: 10.1109/ICME.2017.8019447. [DOI] [Google Scholar]
- 22.Ferri C., Hernández-Orallo J., Modroiu R. An experimental comparison of performance measures for classification. Pattern Recognition Letters. 2009;30:27–38. [Google Scholar]