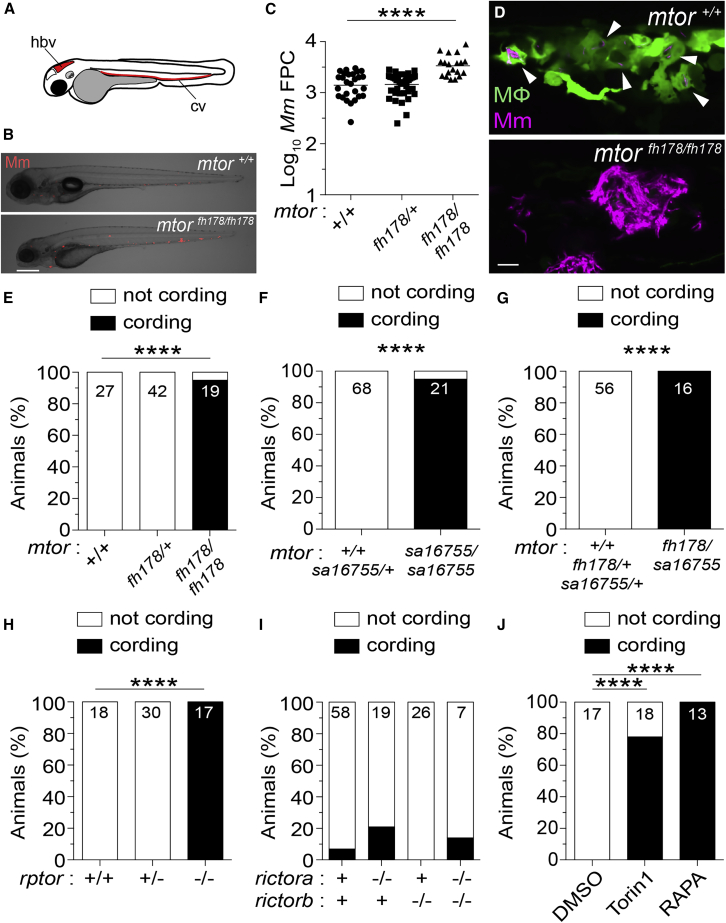
Figure 1.
mTORC1-deficient zebrafish are hypersusceptible to Mm infection
(A) Hindbrain ventricle (hbv) and caudal vein (cv) injection routes used in this study.
Larvae were infected with ∼150 Mm expressing tdTomato (B), (C), and (E–J) or tdKatushka2 (D) fluorescent proteins via the caudal vein 2 days post-fertilization (dpf).
(B) Overlaid micrographs of widefield mycobacterial fluorescence (Mm, red) and bright field in mtorfh178/fh178 or WT siblings (mtor+/+) 4 days post-infection (dpi).
(C) Quantification of bacterial fluorescence (fluorescent pixel counts [FPCs]) in animals from mtorfh178/+ incross 4 dpi. Symbols represent individual animals. Horizontal lines indicate mean values.
(D) Confocal micrograph optical sections of mtorfh178/fh178 and a WT sibling expressing Tg(mpeg1:YFP) 4 dpi, showing a granuloma in the WT animal and mycobacterial cording in the mtorfh178/fh178 animal. Mm (magenta) and macrophages (green) are shown. Arrowheads indicate intracellular Mm.
(E–J) Mycobacterial cording in animals from (E) mtorfh178/+ incross, (F) mtorsa16755/+ incross, (G) mtorfh178/+ × mtorsa16755/+ cross, and (H) rptorsa11537/+ incross at 4 dpi, and (I) rictorasa15967/+; rictorbsa18403/+ double heterozygote incross and (J) WT animals treated with torin1 (400 nM), rapamycin (400 nM), or 0.5% DMSO (vehicle control) 5 dpi.
(E–J) Numbers within columns indicate animals per group.
Scale bars: 300 μm in (B) and 25 μm in (D). Statistical analyses, (C) one-way ANOVA with Tukey’s post-test and (E–J) Fisher’s exact test. Data are representative of two or more independent experiments.