Abstract
Heterotypic secondary dengue virus (DENV) infection is a risk factor for the development of severe disease. To assess the contribution of the developing polyclonal humoral immune response to the course of acute infection, we have determined anti-DENV IgG titers, neutralizing antibodies, percentages of antibodies binding to DENV-infected cells and antibody‑dependent enhancement (ADE) to the infecting serotype in DENV-infected Cambodian children (n = 58), ranging from asymptomatic dengue to severe disease. The results showed that ADE titers are highest against the infecting serotype during heterotypic secondary DENV-2 infection. Moreover, IgG titers, neutralizing antibodies and ADE titers against the infecting serotype peak at D10 and are maintained until D60 after laboratory-confirmed secondary DENV infection. Anti-DENV IgG titers and the magnitude of the functional antibody response were higher in secondary DENV-infected patients compared to primary infected patients. No differences in antibody titers, neutralizing or enhancing antibodies could be observed between asymptomatic or hospitalized patients between 6 and 8 days after laboratory-confirmed DENV-1 infection. However, at this time point, the level of IgG bound to DENV-infected cells was associated with disease severity in hospitalized patients. Taken together, our data offer insights for more comprehensive interpretation of antibody response profile to natural infection and its correlation to disease outcome.
Subject terms: Viral infection, Humoral immunity
Introduction
Dengue infections are the most widespread mosquito-borne viral infections in humans1. It has been estimated that around 390 million infections occur in the tropical and subtropical regions each year and approximately 96 million infections present clinically2. DENV is transmitted by mosquitoes of the Aedes species, such as Aedes aegypti and Aedes albopictus. DENV is a single positive-stranded RNA virus belonging to the Flavivirus genus. The group of DENVs consists of four serotypes, DENV-1 to DENV-4, which share 65-80% homology in their genetic sequence3. In fact, multiple dengue serotypes co-circulate in hyperendemic regions, which creates complications in the monitoring of dengue epidemiology and challenges the development of a vaccine to prevent infection against all four serotypes4–6.
Infections with DENV result in either asymptomatic or inapparent infection, self-limiting dengue fever (DF) or might result in life-threatening severe diseases, dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) in about 0.5% of the cases6. Primary infection with any DENV serotype induces antibodies with potent protective capacity against homotypic re-infection but also elicits cross-reactive antibodies against other serotypes. While protection against homotypic secondary infection is often long-lasting, cross-protecting antibodies against different serotypes are short-lived, with a half-life from several months to 3 years7–10.
Severe dengue, in which hemorrhage, thrombocytopenia, vascular leakage and shock are the major clinical signs and possible cause of death in those patients, occurs almost exclusively in patients infected with a different dengue serotype11–14. Moreover, infants born to mothers immune to dengue are likewise more susceptible to severe dengue in the first year after birth15. The one commercially available vaccine is recommended by WHO for people who had a confirmed DENV infection prior vaccination, since dengue-naïve individuals who receive the vaccine seem to be at risk of severe diseases and hospitalization when being naturally infected after vaccination16.
DENV-specific antibodies can neutralize the virus but also could possibly enhance dengue immunopathogenesis depending on the titer, affinity, avidity and targeted epitopes of the immunoglobulins. Indeed, ADE has been proposed as a mechanism to explain severe dengue disease in seropositive individuals. Here, low affinity antibodies, serotype cross-reactive antibodies or sub-neutralizing concentrations of antibodies acquired during primary infection can increase viral uptake via Fc receptors expressed on target cells such as monocytes, macrophages and dendritic cells. Dengue plasma with neutralizing capacity or neutralizing anti-dengue monoclonal antibodies, when highly diluted, can also have the potential to enhance DV infection in in vitro models17–20. Indeed, the ADE hypothesis was observed in studies showing that the presence of low to intermediate titers of pre-existing dengue antibodies correlated with increased risk of development of severe dengue in secondary infected children18,19. Specific antibodies targeting the fusion loop of the envelop protein (E) and the other surface protein (prM) have been identified to promote ADE in vitro and in animal models17,21,22 and structural determinants of the virus, such as its maturation status, also have an impact on ADE23,24.
Next to antibody dependent enhancement, cross-reactive T cells, the infecting serotype and timing between sequential infections can affect the outcome of infection7,9. At present, there are no good strategies or predictive markers to identify immune-enhanced dengue disease in general or antibody-dependent enhancement in particular. Antibody titers as well as their affinity and avidity vary greatly from person to person and over time. Therefore, the neutralizing versus enhancing properties of these antibodies also varies. In addition, during acute secondary infection, both pre-existing anti-DENV IgG and newly formed IgG co-circulate. Here, pre-existing IgG antibodies are directed against the previous infecting serotype. Newly formed IgGs are produced by plasmablasts which are mainly derived from memory B cells. In general, these memory B cells are either directly activated, and hence retain binding specificity to the previous infecting serotype, or have re-entered germinal center reactions for further maturation and selection and are hence directed against the current infecting serotype18,25,26. It is this polyclonal antibody response that will contribute to either protection through virus neutralization and antibody-dependent effector functions, or enhancement of infection through different ADE mechanisms27–30. Since antibodies can exert both protective and potentially detrimental functionalities, these need to be assessed side by side. Therefore, in this study, we aim to understand the kinetics of the anti-DENV antibody titers, neutralizing antibodies, antibodies binding to infected cells and enhancing antibodies during the acute phase of infection and their association to disease severity.
Methods
Ethics statement
Ethical approval for the study was obtained from the National Ethics Committee of Health Research of Cambodia. Written informed consent was obtained from all participants or the guardians of participants under 16 years of age prior to inclusion in the study. All experiments were performed in accordance with relevant guidelines and regulations.
Patient recruitment
Briefly, dengue cases were identified from hospitalized patients presenting with acute dengue-like illness at Kampong Cham Provincial hospital between June and October in 2018 (cohort 1) and at the same hospital and two district hospitals in Kampong Cham province in 2012–2013 (cohort 2). Blood samples were obtained at hospital entry (D0) for DENV detection by RT-qPCR. To identify asymptomatic dengue (ASD) cases, we collected biological specimen from individuals using a household investigation approach around the index cases identified in hospitals in 2018 (cohort 1) and 2012–2013 (cohort 2)31,32. Household members of DENV index cases tested positive for DENV by RT-qPCR (D0) but without any clinical symptoms were included. These individuals were classified as ASD at the end of a 10-day follow-up period. In detail, these individuals were questioned about history of symptoms four days before D0 and were followed up until ten days after inclusion for the occurrence of symptoms (including but not limited to fever, rash, headache, retro-orbital pain). For cohort 1, hospitalized patients were included between 1 and 6 days after onset of fever and two follow-up samples were collected at day 10 (D10) and day 60 (D60) after inclusion. ASD cases were sampled at D0, D10 and D60. For cohort 2, hospitalized patients were included at hospital entry, 2–6 days after onset of fever and an additional blood sample was obtained four days later (6–10 days after onset of fever). ASD cases were sampled at D0 and D7. Hospitalized patients were classified according to the WHO 1997 classification scheme in dengue fever (DF), dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS)33,34. Demographics and clinical parameters of patients included in both cohorts are summarized in Tables 1 and 2.
Table 1.
Demographic and clinical characteristics of cohort 1.
| Cohort 1 | ASD | DF | Overall |
|---|---|---|---|
| (N = 5) | (N = 13) | (N = 18) | |
| Age | |||
| Mean (SD) | 12.2 (1.9) | 11.4 (2.1) | 11.6 (2.6) |
| Gender | |||
| F | 1 (20%) | 6 (46%) | 7 (39%) |
| M | 4 (80%) | 7 (54%) | 11 (61%) |
| Serotype | |||
| DENV-2 | 5 (100%) | 13 (100%) | 18 (100%) |
| Immune status | |||
| Secondary | 5 (100%) | 13 (100%) | 18 (100%) |
| Day of Fever | |||
| Mean (SD) | NA | 2.62 (1.3) | 2.62 (1.326) |
| Viral load | |||
| Mean (SD) | 1.20E + 08 (2.67E + 08) | 1.00E + 08 (3.61E + 08) | 1.06E + 08 (3.30E + 08) |
| Day of Sampling | |||
| Mean (SD) | 0(0), 10 (0), 60 (0) | 0(0), 10 (0), 60 (0) | 0(0), 10 (0), 60 (0) |
| No of samples | |||
| D0 | 5 | 13 | 18* |
| D10 | 4 | 11 | 15* |
| D60 | 4 | 10 | 14* |
NA: not applicable.
*Among those, there are 12 paired-plasma samples across D0,D10 and D60.
Table 2.
Demographic and clinical characteristics of cohort 2.
| Cohort 2 | ASD | DF | DHF | DSS | Overall |
|---|---|---|---|---|---|
| (N = 8) | (N = 13) | (N = 13) | (N = 6) | (N = 40) | |
| Age | |||||
| Mean (SD) | 10.0 (4.1) | 8.68 (3.3) | 9.23 (3.1) | 10.7 (3.5) | 9.43 (3) |
| Gender | |||||
| Female | 3 (38%) | 7 (54%) | 6 (46%) | 2 (33%) | 18 (45%) |
| Male | 5 (62%) | 6 (46%) | 7 (53%) | 4 (66%) | 22 (55%) |
| Serotype | |||||
| DENV-1 | 8 (100%) | 13 (100%) | 12 (92.3%) | 4 (66.7%) | 37 (92.5%) |
| Undetermined | 0 (0%) | 0 (0%) | 1 (7.7%) | 2 (33.3%) | 3 (7.5%) |
| Immune Status | |||||
| Primary | 4 (50.0%) | 6 (46.2%) | 0 (0%) | 0 (0%) | 10 (25.0%) |
| Secondary | 4 (50.0%) | 7 (53.8%) | 13 (100%) | 6 (100%) | 30 (75.0%) |
| Day of Fever | |||||
| Mean (SD) | NA | 3.6 (1.3) | 4.5 (0.8) | 4.3 (0.5) | 4.1 (1.1) |
| Viral load | |||||
| Mean (SD) | 5.99E + 03 (1.06E + 03) | 5.75E + 07 (9.1E + 07) | 1.18E + 07 (7.27E + 04) | 2.28E + 04 (2.73E + 04) | 2.47E + 07 (6.13E + 07) |
| Day of Sampling | |||||
| Mean (SD) | 7.00 (0) | 7.00 (0) | 7.00 (0) | 7.00 (0) | 7.00 (0) |
NA: not applicable.
Laboratory diagnosis
Plasma specimens obtained from patients were tested for presence and serotype of DENV using a RT-qPCR35 at the Institut Pasteur in Cambodia (IPC), the reference laboratory for arboviral diseases in Cambodia. Only DENV RT-qPCR positive cases were included for further analysis. In 2012–2013, DENV-1 was the most prevalent serotypes circulating in Cambodia, whereas in 2018 DENV-2 was most prevalent. Anti-DENV IgM was measured with an in-house IgM-capture ELISA (MAC-ELISA)33,36 and total anti-DENV antibodies using hemagglutination inhibition (HI) assay for DENV, as previously described37, to determine primary/secondary DENV infection as per WHO criteria38. Primary infections were characterized by the presence or absence of HI antibodies in acute-phase samples (or first samples for inapparent infection) and by low titers with a fourfold rise of HI antibodies (≤ 1:1280) in serum from the convalescence phase (or second samples for inapparent infection) with an elapsed time of at least 7 days between the two collected samples. Conversely, secondary infections were defined by the presence of HI antibodies in acute-phase samples (or first samples for inapparent infection) and by high titers with a fourfold rise of HI antibodies (≥ 1:2560) in serum from the convalescence phase (or second samples for inapparent infection).
DENV IgG ELISA
DENV-specific IgG antibodies in patient plasma samples were measured with the commercially available Panbio Dengue IgG Indirect ELISA (PanBio) according to the manufacturer’s instructions. The antigen for this ELISA is unknown and proprietary information. Antibody titers were calculated from a dilution range of the provided positive control. Data is reported as Arbitrary Units/ml (AU/ml). In this assay, 12 paired-plasma samples collected from 12 individuals in cohort 1 and 40 plasma samples in cohort 2 were tested.
Virus production
Dengue virus, DENV-1 (Hawaii strain, GenBank: KM204119), DENV-2 (New Guinea C strain, GenBank: AF038403), DENV-3 (H87 strain, GenBank: M93130) and DENV-4 (H241 strain, Genbank: AY947539) are used as reference strains in this study and produced under BSL2 safety conditions. Briefly, 8×106 Aedes albopictus C6/36 cells were seeded in a 75 cm2 flask and grown overnight at 28℃, and infected with virus at an MOI of 0.1 and cultured for 5–7 days at 28 °C in Leibovitz 15 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 2% fetal bovine serum (FBS; Gibco, Waltham, MA, USA), 1% L glutamine (Gibco), 10% tryptose-phosphate (Gibco) and 100 U/ml penicillin, 100 µg/ml streptomycin (Gibco). At 5–7 days post infection, the virus culture supernatants were harvested and concentrated using 40% polyethylene glycol (PEG) 8000 solution (Sigma Aldrich) as described before39. The virus concentrate was resuspended in FBS and stored at − 80 °C.
Foci Reduction neutralization test (FRNT)
Vero cells (ATCC CCL-81) were cultured in Dulbecco’s modified Eagle medium (DMEM; Sigma-Aldrich) supplemented with 5% FBS, and were seeded in 96-well culture plates. Heat-inactivated plasma samples were serially diluted, mixed with equal volume of DENV and incubated for 1 h at 37 °C, 5% CO2. Afterwards, plasma-virus mixtures were transferred onto the Vero cells. After 1 h of incubation at 37 °C, the mixture was replaced with 1.8% carboxymethyl cellulose (Sigma-Aldrich) diluted in above-mentioned supplemented DMEM and incubated at 37 °C, 5% CO2. After 2–3 days, cells were fixed and stained using mouse anti-DENV polyclonal hyperimmune ascites fluids (IPC). Secondary staining to detect foci was performed using anti-mouse IgG antibody conjugated to horseradish peroxidase (Bio-Rad, München, Germany) and 3, 3′, 5, 5′-tetramethylbenzidine as substrate (Sure- Blue™TMB 1-component microwell peroxidase substrate, medac, Wedel, Germany). The neutralizing antibody titer was expressed as reciprocal of the highest plasma dilution showing ≥ 90% reduction in foci counts (FRNT90 titer) compared to conditions without plasma. FRNT90 curve was generated by non-linear regression analysis using the 4PL sigmoidal dose curve equation on Prism 8 (Graphpad Software). A valid FRNT90 curve required an R2 > 0.90, hill slope absolute value ≥ 0.7, and had to reach at least 90% relative infection within the range of the plasma dilutions in the assay. In this assay, 12 paired-plasma samples collected from 12 individuals in cohort 1 and 40 plasma samples in cohort 2 were tested. 1 secondary DF and 2 DHF samples in cohort 2 were excluded after quality control.
DENV binding IgG assay (DENflow)
A549 cells, a human epithelial carcinoma cell line, were grown in 75 cm2 flask of DMEM supplemented with 10% FBS (Gibco), 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco) and 1% L-glutamine (Gibco) at 37 °C and 5% CO2. When the cells reached 70–80% confluency they were inoculated with reference strain DENV-1 or DENV-2 (in 10 ml of RPMI supplemented with 2% FBS) for 90 min at a MOI of 5, depending on the infecting serotype of the patient. Uninfected cells were used as negative control. Afterwards, the cells were washed to remove residual virus and incubated overnight at 37 °C and 5% CO2. The cells were harvested and stained with anti-DENV E protein antibody (clone 4G2, ATCC HB-112) labelled with Alexa Fluor 488 (Molecular probes; Thermo Fisher) to confirm infection. For the binding assay, 100 µl of heat-inactivated plasma diluted 1:10 in RPMI were incubated with 50,000 DENV-infected cells/well. The plates were incubated for 30 min on ice. The cells were washed twice with PBS and stained with goat anti-human IgG labeled with Alexa Fluor 647 (Thermo Fisher) to detect IgG antibodies bound to DENV-infected cells. Cells were fixed and analyzed by flow cytometry (FACSCanto II, BD Bioscience). The amount of binding IgG were determined by subtracting percentage of IgG binding to non-infected cells from percentage of IgG binding to DENV-infected cells. Therefore, this assay measures the antibodies that bind to antigens of DENV-1 (cohort 2) or DENV-2 (cohort 1) presented on infected cells. In this assay, 12 paired-plasma samples collected from 12 individuals in cohort 1 and 40 plasma samples in cohort 2 were tested. 1 ASD sample at D10 in cohort 1 was excluded in the final data due to high background staining.
ADE assay
Human monoclonal antibody G10 (kind gift from Katja Fink, A*STAR, Singapore)21, specific for the fusion loop of DENV E protein, and plasma from patients were assessed for ADE activity in human myelomonocyte cell line U937 (ATCC CRL-1593.2). U937 cells were cultured in RPMI (Gibco) supplemented with 10% FBS (Gibco), 100 U/ml penicillin, 100 µg/ml streptomycin (Gibco) and 1% L-glutamine (Gibco). The G10 antibody and heat-inactivated dengue patient plasma was serially diluted fivefold (1:100 to 1:1,562,500) in RPMI with 2% FBS and incubated with each DENV serotype corresponding to MOI of 1 for one hour at 37 °C, 5% CO2. Immune complexes were transferred to 96-well round-bottom plates containing 80,000 U937 cells/well. The plates were incubated for 90 min at 37 °C, 5% CO2. Direct infection of U937 cells with DENV in the absence of G10 antibody or plasma was used as control. After infection, cells were washed and incubated for 72 h at 37 °C, 5% CO2. Cells were stained with Zombie Aqua viability dye (BioLegend) to distinguish live from dead cells and then fixed, permeabilized and stained for presence of DENV using anti-DENV E protein antibody (clone 4G2, ATCC HB-112) labelled with Alexa Fluor 488 and analyzed by flow cytometry (FACSCanto II, BD Biosience). Percentages of DENV-infected cells were measured and dose-dependent enhancement of infection curve was plotted according percentage of infected cells at each serial dilution. Peak of enhancement titer (PET) was identified as the titer occurring the highest percentage of infection. Area under the curve (AUC) was generated using GraphPad Prism 8 software. In this assay, 18 samples at D0, 15 samples at D10 and 14 samples at D60 collected in cohort 1 and 40 plasma samples in cohort 2 were tested.
Statistical analysis
Data were analyzed and plotted using GraphPad Prism, version 8.0. The data were tested for normality but did not pass the D’Agostino-Pearson normality test. Therefore, statistical analysis was done using a non-parametric Mann–Whitney test or Friedman test as indicated. Non-parametric Spearman’s method was used for correlation analyses. For all analyses, p < 0.05 was considered significant.
Results
Patient cohorts
In order to understand the kinetics of the polyclonal antibody response during primary and secondary infection in hospitalized and asymptomatic dengue infected individuals and their relative contribution to protection or severe disease we included 58 DENV-infected Cambodian children, in two separate cohorts. Cohort 1 (n = 18) consisted of 5 ASD and 13 hospitalized dengue fever (DF) cases and were all classified as secondary infected patients with DENV-2. Cohort 2 (n = 40) consisted of both primary and secondary dengue infected individuals, including ASD cases (n = 8) and dengue patients classified as classical DF (n = 13), DHF (n = 13) and DSS (n = 6)34. Thirty-eight infected-individuals (92.5%) had a confirmed DENV-1 infection while in 4 individuals (7.5%) the infecting serotype could not be determined. The plasma samples were collected at three different time points, at D0, the day of laboratory confirmation of infection and at D10 and D60 after laboratory confirmation. Hospitalized patients were included and sampled after 1–6 days of fever onset (D0). Demographic information and laboratory characteristics from the participants are summarized in Table 1 (cohort 1) and Table 2 (cohort 2).
Total ADE activity calculated as area under the curve (AUC) is the highest against the infecting serotype during secondary heterotypic infection
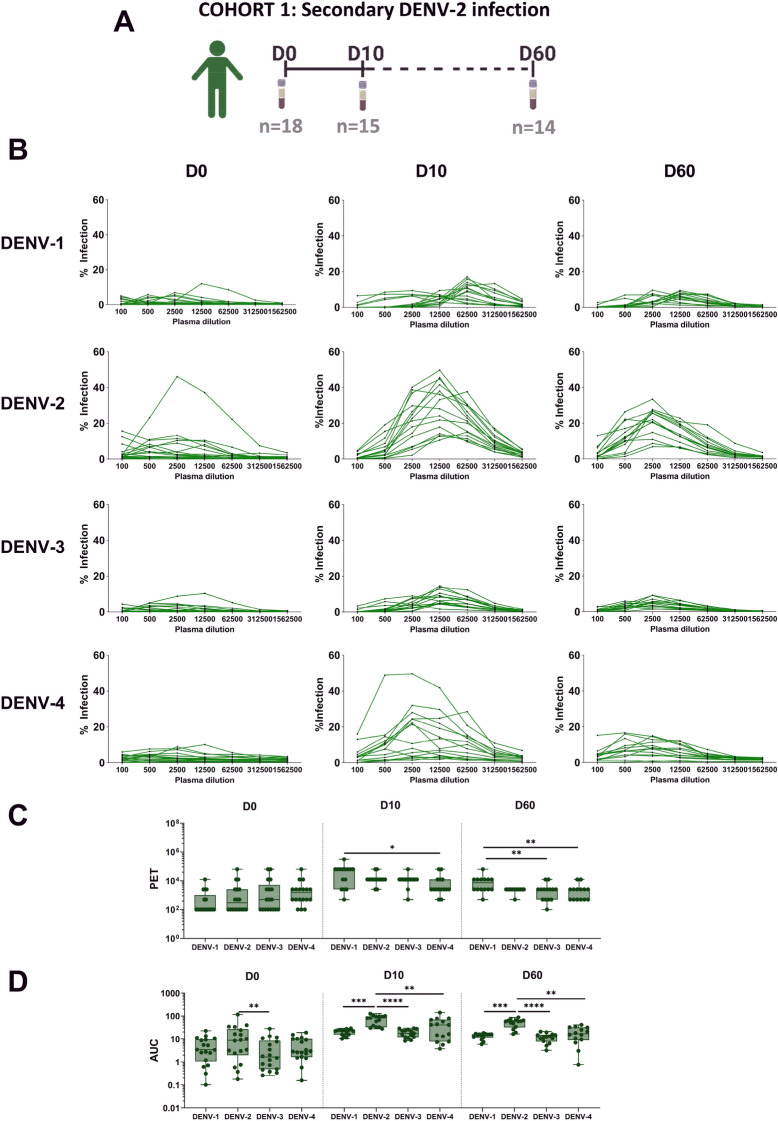
We assessed the impact of the polyclonal pool of pre-existing and newly formed anti-DENV IgG to ADE in the acute phase of infection. In order to understand if ADE is mainly directed against the infecting serotype or against other serotypes we performed in vitro ADE assays measuring infection of FcγRI and FcγRII expressing U937 cells40,41 in the presence of serially diluted plasma obtained at D0, D10 and D60 after PCR-confirmed DENV-2 infection in cohort 1 (Table 1, Fig. 1A, Figure S1). Each plasma was assessed for ADE activity against four serotypes (DENV-1 to 4). Dose-dependent enhancement of infection curves was obtained against the four DENV serotypes (Fig. 1B). No differences were observed in peak enhancement titer (PET) against four serotypes at the early phase of infection while PET was significantly higher against DENV-1 compared to DENV-4 at late time points, D10 and D60 (Fig. 1C). However, total of ADE activity calculated as area under the curve (AUC) was higher against DENV-2, which is the infecting serotype in the investigated cohort, compared to DENV-1, DENV-3 and DENV-4 at D10 and D60 (Fig. 1D). Overall, these data indicate that plasma from secondary infected patients gradually induced ADE activity against the infecting serotype.
Figure 1.
Kinetics of antibody-dependent enhancement (ADE) activity to all four DENV serotypes. Graphical summary of plasma samples collected in cohort 1 (A). 18 plasma samples were collected on the day at inclusion (D0, day of laboratory confirmed infection), D10 and D60. There are 12 paired-plasma samples across the 3 time points. Plasma was analyzed in vitro using U937 cells infected with one of the four DENV serotypes (DENV-1 to 4). The percentages of DENV-infected cells in the presence of serial diluted plasma is shown and each line represents an individual plasma sample (B). ADE titers are shown as peak of enhancement titer (PET) and area under the curve (AUC) generated from each plasma (C, D). Each point in the box plot represents an individual plasma sample. Whiskers show max, min, median values and the interquartile range beyond the 25th and 75th percentiles. Friedman test was used to compare multiple groups in each time point (P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Antibody titers against the infecting serotype peak at D10 following RT-PCR confirmed infection
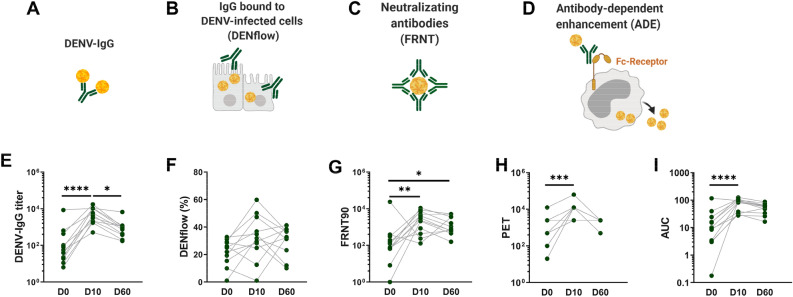
In order to assess the dynamics of the polyclonal antibody response following secondary DENV-2 infection, we measured anti-DENV IgG titers by ELISA, neutralizing antibodies to DENV-2 by FRNT90 and IgG bound to the surface of cells infected with DENV-2 by DENflow assay in patients from cohort 1, next to ADE as described above (Fig. 2A–D). Anti-DENV IgG titers in the plasma peaked at D10 and decreased by D60 (Fig. 2E). The DENflow assay measures the amount of IgG bound to surface of DENV-2 infected cells by flow cytometry, reflecting in vivo conditions of IgG attached to DENV-infected cells (Fig S2). This assay will therefore measure antibodies that are capable of inducing antibody-effector functions such as antibody-dependent cellular cytotoxicity or antibody-depended phagocytosis of infected cells, which are all important mechanisms contributing to clearance of virus-infected cells and innate immune activation42.
Figure 2.
Longitudinal assessment of the anti-DENV antibody responses in paired plasma after secondary DENV-2 infection. Graphical summary of the assays performed in the study including anti-DENV IgG by ELISA assay (A), amount of IgG bound to DENV-infected A549 cells by DENflow assay (B), neutralizing antibodies by FRNT assay on Vero cells (C) and ADE assay on U937 cells (D). Paired-plasma samples at different time points (D0, D10 and D60) from twelve individuals infected with DENV-2 in cohort 1 were analyzed for anti-DENV IgG titer (E), percentage of IgG binding to DENV-2-infected cells (DENflow) (F), neutralizing antibodies (FRNT90) to DENV-2 (G) and ADE activity to DENV-2 as PET and AUC (H, I). Each point in the graphs represents an individual sample. Friedman test was used to compare three different time points each other (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
However, no differences could be observed between the percentages of DENV-infected cells that bound anti-DENV IgG at D0, D10 and D60 post-infection (Fig. 2F). Neutralizing antibodies against DENV-2 as measured by the FRNT90 assay increased from D0 to D10 and remained stable until D60 post-infection. In parallel, ADE titers measured as PET and overall ADE activity as measured as AUC peaked at D10 and remained stable or decreased slightly until D60 post-infection (Fig. 2G–I). Taken together, these results suggest that the quantity and quality of antibody responses to DENV infection peaks at D10 and changes over time in individuals after secondary DENV-2 infection.
Comparison of anti-DENV antibody responses in infected individuals with different immune status and clinical outcome
As secondary infection results in an increase in anti-DENV IgG titers, we wanted to confirm that these antibodies result in an increase in functional antibodies as well as measured by their neutralizing, binding and enhancing capacities. Hence, we analyzed these features in a second cohort of DENV-1 infected patients close to the peak of antibody titer, between 6 and 8 days after laboratory-confirmed infection, in patients undergoing either primary or secondary DENV infection and in patients with different disease outcome (Fig. 3A). As expected, DENV-IgG titer, FRNT90 titers, IgG bound to infected cells and ADE activity shown as PET and AUC were significantly higher in secondary infected patients compared to primary infected patients. (Fig. 3B–F). Within secondary infected patients, no differences could be observed among ASD, DF, DHF and DSS cases in terms of DENV-IgG titer (Fig. 3G). However, the percentages of DENV-infected cells that bound anti-DENV IgG was decreased in patients with DF compared to patients with DHF, DSS or asymptomatic infected individuals (Fig. 3H). Moreover, both titers of neutralizing antibodies and ADE titers tended to be increased in more severe patients, albeit no significance could be reached (Fig. 3I–K). Taken together, the magnitude of the functional antibody response was associated with immune status, while only the level of IgG bound to DENV-infected cells was associated with disease severity in hospitalized patients between 6 and 8 days after laboratory-confirmed DENV-1 infection.
Figure 3.
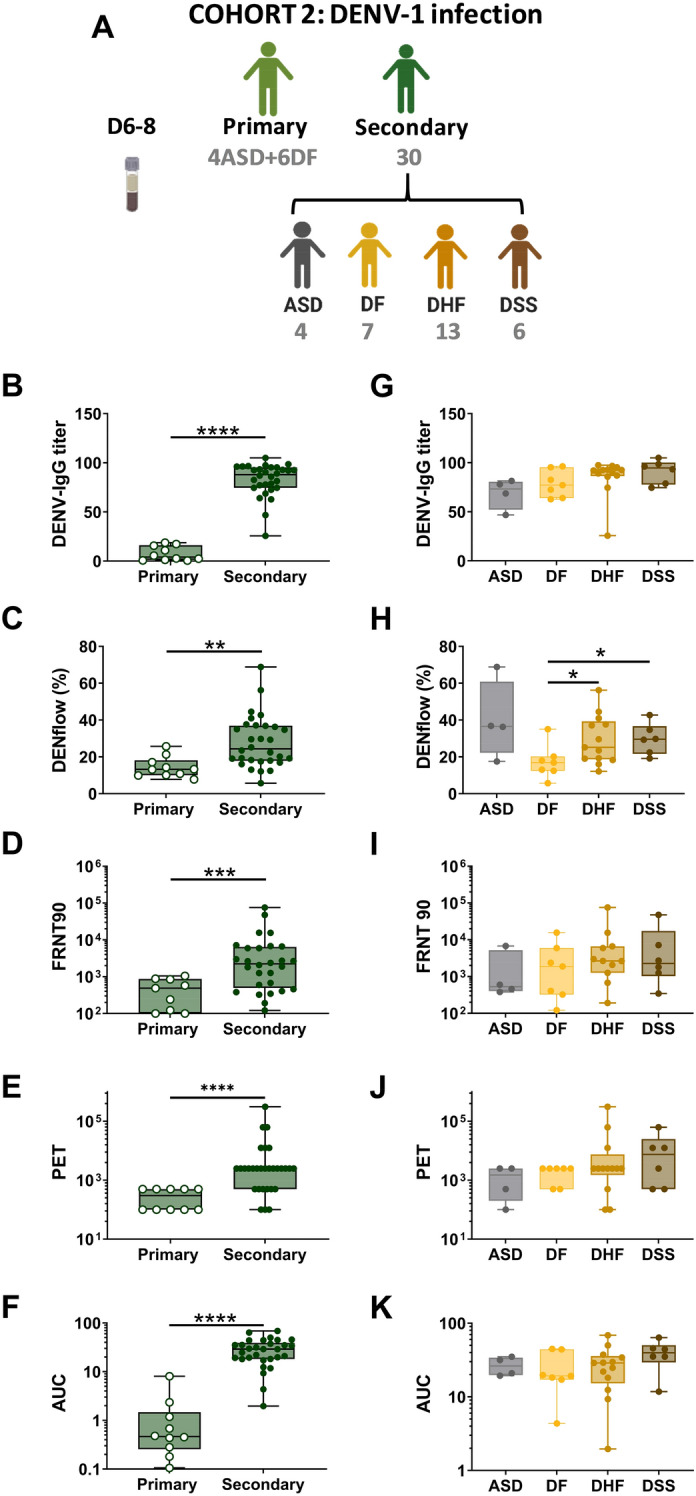
Comparison of anti-DENV antibody responses in infected individuals with different immune status and clinical outcome. Graphical summary of plasma samples collected in cohort 2 (A). Plasma samples were collected at day 6 to day 8 after the day after laboratory-confirmed infection. Plasma samples in cohort 2 were analyzed for DENV IgG titer (B, G), percentage of IgG binding to DENV-1-infected cells (DENflow) (C, H), neutralizing antibodies (FRNT90) to DENV-1 (D, I) and ADE activity to DENV-1 expressed as PET (E, J) and AUC (F, K). Each point in the box plot represents an individual plasma sample. Whiskers show max, min, median values and the interquartile range beyond the 25th and 75th percentiles. Mann–Whitney test was used to compare groups. (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
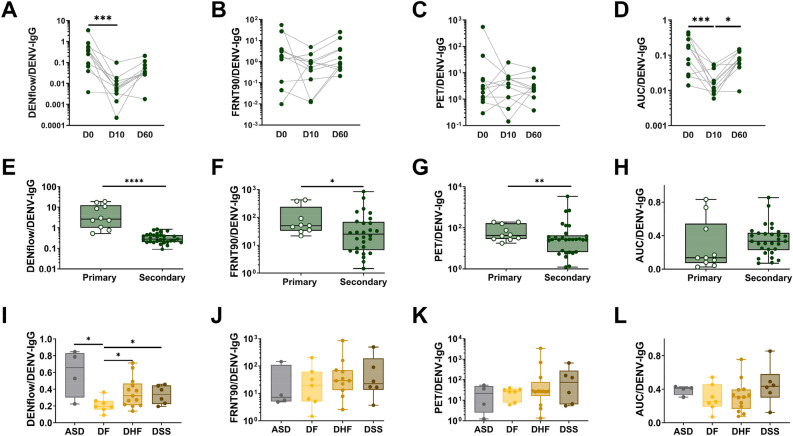
The proportion of binding, neutralizing and enhancing antibodies in total anti-DENV IgG is increased in patients with a primary DENV infection
Neutralization and ADE do not only depend on the quantity of anti-DENV antibodies, but also on the quality of these antibodies. Hence, we assessed if a difference in the proportion of binding, neutralizing or enhancing antibodies was associated with either protection or the development of severe disease after DENV infection. We calculated the ratio of IgG bound to infected cells, neutralizing or enhancing antibodies to total anti-DENV IgG. The amount of IgG bound to infected cells, and AUC (but not PET) titers within the total anti-DENV IgG were higher at D0 compared to D10 and D60 (Fig. 4A,C,D) while the proportions of FRNT90 titers within total DENV-IgG titer were not different among the time points (Fig. 4B). Interestingly, the proportions of IgG bound to infected cells, FRNT90 titers and PET titers within the total anti-DENV IgG were significantly higher in primary infection compared to secondary infection at day 6–9 post laboratory confirmation, (Fig. 4E–H). Within secondary dengue infection, the proportion of IgG bound to infected cells within total anti-DENV IgG was decreased in DF patients compared to other patient groups (Fig. 4I-L). Therefore, these data suggest that the fraction of neutralizing and enhancing antibodies in the total anti-DENV IgG antibodies in primary infected patients contains more neutralizing and enhancing antibodies against the infecting serotype compared to secondary infected patients.
Figure 4.
Proportions of neutralizing antibodies and enhancing antibodies within the total DENV-IgG titer. Proportions of DENflow, FRNT90, PET or AUC within the total anti-DENV IgG antibodies in plasma obtained from paired-secondary samples at different time points (D0, D10 and D10) from cohort 1 (A-D), in plasma obtained from primary infected patients compared to secondary infected patients from cohort 2 (6–8 days after laboratory-confirmed infection) (E–H); and in plasma obtained from secondary infected dengue cases with different disease outcome from cohort 2 (6–8 days after laboratory-confirmed infection) (I–L). Whiskers show max, min, median values and the interquartile range beyond the 25th and 75th percentiles. Mann–Whitney test was used to compare two patient groups (*P < 0.05; **P < 0.01, ***P < 0.00; ****P < 0.0001).
Discussion
In this study, we aimed to understand the dynamics of the polyclonal antibody responses during the acute phase of secondary infection. Whereas most studies have focused on understanding the role and function of pre-existing anti-DENV antibodies to the outcome of subsequent infection5,12,43,44, very few studies have investigated the role of the complex and developing polyclonal anti-DENV IgG antibodies in the outcome of the ongoing infection45,46. During acute secondary infection, both pre-existing anti-DENV IgG against the previous infecting serotype and newly formed IgG against either the previous serotype or against the new infecting serotype co-circulate47,48. Pre-existing memory B cells raised against the previous infecting serotype will be activated and differentiate to antibody secreting cells (a concept called original antigenic sin)26,47. Overall DENV antibody avidity shifts from the previous infecting serotype to the current infecting serotype over time46. For all investigated aspects of the humoral response, the functional antibody response has the trend to peak at day 10 after laboratory-confirmed infection, an observation also made in a Nicaraguan cohort46.
Serotype-specific neutralization assays remain the gold standard for antibody detection and neutralizing antibodies are used as a proxy for protection, either from infection or at least from clinical disease6,34. However, presence of neutralizing antibodies measured after primary infection or vaccination are not always associated with protection during subsequent natural infection in humans. Protective titers can vary according to the serotype, and the quality of the neutralizing response (serotype-specific versus serotype cross-neutralization) is important in defining disease outcome after infection4,44,49–53. At 6–8 days after onset of symptoms, which partly coincides with the critical phase of the disease, we do not observe an association between FRNT90 titers measured against the infecting serotype and disease severity in hospitalized patients. No differences were observed in neutralizing antibody titers between secondary infected DF and DHF/DSS patients. However, when comparing neutralizing antibody titers between primary and secondary infected patients we detected higher neutralizing titers after primary infection. Whether this could be one of the factors contributing to a general milder disease outcome after primary infection remains to be established.
We assessed DENV infection enhancing activity of anti-DENV antibodies by an in vitro assay using a FcγRI and FcγRIIa expressing cell line54–56. Both PET and AUC were used to evaluate ADE44,57–59. While PET provides information of antibody enhancement activity at a particular serum dilution, AUC assesses the magnitude of antibody enhancement activity. In the current study, the range of plasma dilution was appropriate for most plasma samples to observe the peaks of ADE, with a few exceptions coming from some plasma collected at the early time point of the infection. Factors including the choice of FcγR-expressing cell line, virus strains and the ratio between plasma dilution and amounts of virus are important determinants of outcome of the in vitro assay. Neat plasma and diluted plasma were used to observe the kinetics of ADE in previous studies20,44,60–67. However, no gold standard ADE assay has been described.
ADE is attributed to either low affinity, serotype cross-reactive antibodies, and/or sub-neutralizing antibody concentrations, or can be attributed to antibodies targeting specific epitopes such as the the prM surface protein or fusion loop of the envelope protein E17,20–22. The fraction of neutralizing and enhancing antibodies changes over time due to the evolution of the quality and quantity of anti-DENV antibodies. For example, the amount of afucosylated Fc-IgG increases at convalescence compared to acute infection in primary infection, which might impact antibody dependent enhancement30,68 and titers of dengue specific antibody tend to decrease overtime which might favor ADE18,19. In our results, ADE is the highest for DENV-2 after secondary DENV-2 infection at day 10, which is also the time point when high neutralization is expected. Indeed, most neutralizing antibodies are able to enhance infection at low concentrations17–20.
During an acute DENV infection, a very large population of plasmablasts has been observed, already at time of laboratory diagnosis48,69,70. These plasmablasts could be derived either from activated memory B cells and will retain specificy for the primary infection (original antigenic sin) or could be derived from memory B cells that underwent further affinity maturation and selection in a germinal center response and are more cross-reactive or specific for the secondary infection25,26,47. Whichever their origin, they could contribute to the increasing concentration of either low affinity or cross-reactive antibodies resulting in increased ADE at D10.
A study in Thailand showed no association of enhancing antibody activity in pre-illness plasma and subsequent disease severity in secondary DENV infection. In a Taiwanese cohort, ADE against the infecting serotype (DENV-2) was assessed during the acute phase and at convalescence. Plasma derived from DHF patients during the acute phase showed higher capacity to induce ADE in vitro than healthy donor plasma45. In Cambodia, since 2000 we had alternating outbreaks of DENV-1 (2011–2016), DENV-2 (2002–2004, 2009–2010, 2016–2017) and DENV-3 (2006, 2007)71. We report in this study that plasma derived from secondary infected patients compared to primary infection showed higher capacity to induce ADE activity in vitro presented as AUC and PET. In secondary infected patients, we observed that PET titers to the previously circulating serotype (DENV-1) in Cambodia were higher compared to DENV-3 and DENV-4, which had low circulation in Cambodia. Total ADE activity presented as AUC induced by secondary plasma was preferentially directed to the infecting serotype (DENV-2) and increased over time.
Beyond neutralization, antibodies bind to infected cells and exert a range of effector functions, mediated through the antibody Fc part, which aids in the elimination of virus-infected cells. Antibodies bound to infected cells can engage to effector cells expressing Fc-receptors such as monocytes and NK cells. We measured percentages of antibodies bound to DENV-infected cells with an easy to perform binding assay mimicking more closely in vivo antibody binding in patients compared to an ELISA format. The assay measures antibodies that bind to the native confirmation of envelop protein (E) and non-structural protein 1 (NS1) expressed on the surface of infected cells or cells binding NS172–77. These antibodies are important to initiate effector functions, such as antibody-dependent cellular cytotoxicity, antibody dependent complement activation and antibody dependent phagocytosis, which results in antibody-mediated killing of infected cells and activation of innate immune cells42. Viral surface proteins such as E and prM can be attached to the surface of infected cells at high concentration, together with NS172,74. Therefore, antibodies with lower avidity to prM and E, as well as anti-NS1 antibodies might be better detected using DENflow compared to an ELISA format. We observed that increased percentage of IgG bound to infected cells 6–8 days after laboratory-confirmed infection is associated with more severe disease outcome in hospitalized dengue patients. These data indicate that binding antibodies might contribute to protection or pathogenesis via other mechanisms than neutralization or antibody-dependent enhancement, such as antibody effector functions and activation of innate immune cells via engagement of FcγR42.
One caveat of the study design is that it is impossible to assess the exact timing of infection in asymptomatic dengue cases, which could influence the magnitude of the measured humoral immune response. However, comparable viral loads were detected in asymptomatic and hospitalized dengue cases at inclusion, even though kinetics of viral clearance might be different in asymptomatic cases compared to symptomatic patients78. We also acknowledge the small sample size for the patients, which may weaken the power in statistical tests and make the interpretation of these results difficult, especially in the asymptomatic cases. Even with extensive household investigations, it remains a challenge to identify these individuals31,32. Therefore, larger sample sizes are needed in further studies to validate the conclusions.
In conclusion, we have assessed the kinetics of the functional antibody response in infected hospitalized patients and asymptomatic infected individuals during the acute and early/late convalescent phase of DENV infection. Anti-DENV IgG titers, neutralization capacity and ADE activity are different between primary and secondary infected hospitalized cases but not between asymptomatic cases and hospitalized patients at the time of laboratory-confirmation of the DENV infection. Titers of neutralizing antibodies and ADE-mediating antibodies against the infecting serotype evolved over time and but were not correlated with disease severity. Total ADE activity induced by secondary plasma was preferentially directed to the infecting serotype (DENV-2) and increased over time. Percentages of IgG bound to DENV-infected cells as measured by DENflow are associated with severity in hospitalized patients. Taken together, these data suggest that binding antibodies might contribute to pathogenesis via other mechanisms than neutralization or enhancement of infection. Measurement of the totality of this response, including the proportion of enhancing antibodies within the total anti-DENV IgG response and determination of IgG binding to DENV-infected cells will give us more insight into humoral immune response to natural infection and vaccine candidates and can identify correlates of protection.
Data availability
All data are available in the main text or the supplementary materials. All materials, except for clinical specimens, are available on request after completion of a materials transfer agreement with Institut Pasteur du Cambodge.
Supplementary Information
Acknowledgements
We would like to thank all patients and legal guardians who participated in the study. We thank the doctors and nurses of the three hospitals in Kampong Cham province for patient enrollment and sample collection and H. Rekol and the team from the National Dengue Control Program. We would like to thank Katja Fink for providing the G10 anti-dengue E antibody.
Author contributions
H.T.M.V., V.U., S.L., S.S., S.K. and A.V. performed the experiments. H.T.M.V., V.U. and T.C. designed the experiments and interpreted the data. S.S., S.K. and S.L. enrolled patients and coordinated clinical data management. V.D., P.D. and T.C. coordinated experiments and interpreted the clinical data. T.C. designed the study. T.C. and H.T.M.V. wrote the original manuscript. T.C., H.T.M.V., H.A. and V.U. edited the manuscript.
Funding
The work was supported by The Pasteur Network (TC: PTR 212-19 and VU: Calmette-Yersin PhD scholarship), HHMI-Wellcome Trust (TC, 208710/Z/17/Z) and NIH (TC, U01AI151758 – 01). Sample collection was funded in part by the European Union Seventh Framework Programme (282 378).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21722-2.
References
- 1.Gubler DJ. The economic burden of dengue. Am. J. Trop. Med. Hyg. 2012;86:743–744. doi: 10.4269/ajtmh.2012.12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzelnick LC, et al. Dengue viruses cluster antigenically but not as discrete serotypes. Science. 2015;349:1338–1343. doi: 10.1126/science.aac5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anon Srikiatkhachorn I-KY. Immune correlates for dengue vaccine development. Expert Rev. Vaccines. 2016;176:139–148. doi: 10.1586/14760584.2016.1116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katzelnick LC, Harris E. Immune correlates of protection for dengue. Vaccine. 2017;176:4659–4669. doi: 10.1016/j.vaccine.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman MG, Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KB, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical Illness in a school-based cohort in Thailand. J. Infect. Dis. 2014;209:360–368. doi: 10.1093/infdis/jit436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olkowski S, et al. Reduced risk of disease during postsecondary dengue virus infections. J. Infect. Dis. 2013;208:1026–1033. doi: 10.1093/infdis/jit273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montoya M, et al. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl. Trop. Dis. 2013;7:1–10. doi: 10.1371/journal.pntd.0002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snow GE, Haaland B, Ooi EE, Gubler DJ. Review article: Research on dengue during world war II revisited. Am. J. Trop. Med. Hyg. 2014;91:1203–1217. doi: 10.4269/ajtmh.14-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman MG, Kouri G, Valdes L, et al. Epidemiologic Studies on Dengue in Santiago de Cuba, 1997. Stud. Financ. Adm. Athens. 2000;152:1–1. doi: 10.1093/aje/152.9.793. [DOI] [PubMed] [Google Scholar]
- 12.Halstead SB, O’Rourke EJ. Dengue viruses and mononuclear phagocytes: I. Infection enhancement by non-neutralizing antibody*. J. Exp. Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothman KJ. Risk factors in dengue shock syndrome: A prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Public Health. 1980;17:587–592. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 14.Halstead SB, Nimmannitya S, Cohen S. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J. Biol. Med. 1969;42:311–328. [PMC free article] [PubMed] [Google Scholar]
- 15.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 16.Sridhar S, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 2018;379:327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 17.Dejnirattisai W, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salje H, et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature. 2018 doi: 10.1038/s41586-018-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzelnick LC, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dejnirattisai W, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016;17:1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu M, et al. A potent neutralizing antibody with therapeutic potential against all four serotypes of dengue virus. npj Vaccines. 2017 doi: 10.1038/s41541-016-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodenhuis-Zybert IA, et al. A fusion-loop antibody enhances the infectious properties of immature flavivirus particles. J. Virol. 2011;85:11800–11808. doi: 10.1128/JVI.05237-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn RJ, Dowd KA, Post CB. Shake, rattle, and roll: Impact of the dynamics of flavivirus particles on their interactions with the host. Virol. J. 2015 doi: 10.1016/j.virol.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plevka P, et al. Maturation of flaviviruses starts from one or more icosahedrally independent nucleation centres. EMBO Rep. 2011;12:602–606. doi: 10.1038/embor.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouers A, et al. CD27hiCD38hi plasmablasts are activated B cells of mixed origin with distinct function. iScience. 2021;24:102482. doi: 10.1016/j.isci.2021.102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vatti A, et al. Original antigenic sin: A comprehensive review. J. Autoimmun. 2017;83:12–21. doi: 10.1016/j.jaut.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang TT, et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science. 2017;355:395–398. doi: 10.1126/science.aai8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott B Halstead, Suresh Mahalingam, Mary A Marovich, Sukathida Ubol, D. M. M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect. Dis. (2010). [DOI] [PMC free article] [PubMed]
- 29.Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020;20:633–643. doi: 10.1038/s41577-020-00410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bournazos, A. S., Thi, H., Vo, M., Duong, V. & Auerswald, H. Antibody fucosylation predicts disease severity in secondary dengue infection. Science (80-. ).1105, 1102–1105 (2021). [DOI] [PMC free article] [PubMed]
- 31.Ly S, et al. Asymptomatic dengue virus infections, cambodia, 2012–2013. Emerg. Infect. Dis. 2019;25:1354–1362. doi: 10.3201/eid2507.181794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon-Lorière E, et al. Increased adaptive immune responses and proper feedback regulation protect against clinical dengue. Sci. Transl. Med. 2017;9:eaal5088. doi: 10.1126/scitranslmed.aal5088. [DOI] [PubMed] [Google Scholar]
- 33.Dussart P, et al. Comparison of dengue case classification schemes and evaluation of biological changes in different dengue clinical patterns in a longitudinal follow-up of hospitalized children in Cambodia. PLoS Negl. Trop. Dis. 2020;14:1–23. doi: 10.1371/journal.pntd.0008603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization (WHO) Dengue hemorrhagic fever: Diagnosis, treatment, prevention and control, 2nd edition. World Heal. Organ. 1997;6:39–48. [Google Scholar]
- 35.Ou TP, et al. Improved detection of dengue and Zika viruses using multiplex RT-qPCR assays. J. Virol. Methods. 2020;282:113862. doi: 10.1016/j.jviromet.2020.113862. [DOI] [PubMed] [Google Scholar]
- 36.Andries AC, et al. Value of routine dengue diagnostic tests in urine and saliva specimens. PLoS Negl. Trop. Dis. 2015;9:e0004100. doi: 10.1371/journal.pntd.0004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duong V, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. 2015;112:14688–14693. doi: 10.1073/pnas.1508114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization (WHO) Dengue guidelines for diagnosis, treatment, prevention and control: New edition. World Heal. Organ. 2009;409:160. [PubMed] [Google Scholar]
- 39.Lewis GD, Metcalf TG. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol. 1988;54:1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai X, et al. Differential signal transduction, membrane trafficking, and immune effector functions mediated by Fc RI versus FcRIIa. Blood. 2009 doi: 10.1182/blood-2008-10-184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Looney RJ, Abraham GN, et al. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J. Immunol. 1986;136:1641–1647. [PubMed] [Google Scholar]
- 42.Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waggoner JJ, et al. Homotypic dengue virus reinfections in Nicaraguan children. J. Infect. Dis. 2016;214:986–993. doi: 10.1093/infdis/jiw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laoprasopwattana K, et al. Dengue Virus (DV) enhancing antibody activity in preillness plasma does not predict subsequent disease severity or viremia in secondary DV infection. J. Infect. Dis. 2005;192:510–519. doi: 10.1086/431520. [DOI] [PubMed] [Google Scholar]
- 45.Wang WH, et al. A clinical and epidemiological survey of the largest dengue outbreak in Southern Taiwan in 2015. Int. J. Infect. Dis. 2019;88:88–99. doi: 10.1016/j.ijid.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Puschnik A, et al. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS Negl. Trop. Dis. 2013;7:1–8. doi: 10.1371/journal.pntd.0002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong R, et al. Affinity-restricted memory B cells dominate recall responses to heterologous flaviviruses. Immunity. 2020;53:1078–1094.e7. doi: 10.1016/j.immuni.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balakrishnan T. Dengue virus activates polyreactive, natural IgG B cells after primary and secondary infection. PLoS ONE. 2011 doi: 10.1371/journal.pone.0029430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buddhari D, et al. Dengue virus neutralizing antibody levels associated with protection from infection in Thai cluster studies. PLoS Negl. Trop. Dis. 2014;8:e3230. doi: 10.1371/journal.pntd.0003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corbett KS, et al. Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J. Infect. Dis. 2015;211:590–599. doi: 10.1093/infdis/jiu481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc. Natl. Acad. Sci. 2016;113:728–733. doi: 10.1073/pnas.1522136113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halstead SB. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine. 2017;35:6355–6358. doi: 10.1016/j.vaccine.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 53.Sabchareon A, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 54.Moi ML, Lim CK, Chua KB, Takasaki T, Kurane I. Dengue virus infection-enhancing activity in serum samples with neutralizing activity as determined by using FcγR-expressing cells. PLoS Negl. Trop. Dis. 2012;6:e1536. doi: 10.1371/journal.pntd.0001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pierson TC, et al. The stoichiometry of antibody-mediated neutralization and enhancement of west Nile virus infection. Cell Host Microbe. 2007;1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chawla T, et al. Dengue virus neutralization in cells expressing Fc gamma receptors. PLoS One. 2013;8:e65231. doi: 10.1371/journal.pone.0065231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang B, Xiao Y, Sander B, Kulkarni MA, Wu J. Modelling the impact of antibody-dependent enhancement on disease severity of Zika virus and dengue virus sequential and co-infection. R. Soc. Open Sci. 2020;7:191749. doi: 10.1098/rsos.191749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borges MB, et al. Detection of post-vaccination enhanced dengue virus infection in macaques: An improved model for early assessment of dengue vaccines. PLoS Pathog. 2019;15:1–25. doi: 10.1371/journal.ppat.1007721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaughn DW, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 60.Yamanaka A, Imad HA, Phumratanaprapin W, Juthamas Phadungsombat E. Antibody-dependent enhancement representing in vitro infective progeny virus titer correlated with the viremia level in dengue patients. Sci. Rep. 2021 doi: 10.1038/s41598-021-91793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boonnak K, Dambach KM, Donofrio GC, Tassaneetrithep B, Marovich MA. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J. Virol. 2011;85:1671–1683. doi: 10.1128/JVI.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ly MHP, et al. Dengue virus infection-enhancement activity in neutralizing antibodies of healthy adults before dengue season as determined by using FcγR-expressing cells. BMC Infect. Dis. 2018 doi: 10.1186/s12879-017-2894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castanha PMS, et al. Placental transfer of dengue virus (DENV)-specific antibodies and kinetics of DENV infection-enhancing activity in Brazilian infants. J. Infect. Dis. 2016;214:265–272. doi: 10.1093/infdis/jiw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chau TNB, et al. Dengue in Vietnamese infants—Results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J. Infect. Dis. 2008;198:516–524. doi: 10.1086/590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Alwis R, et al. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog. 2014;10:e1004386. doi: 10.1371/journal.ppat.1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaichana P, et al. Low levels of antibody-dependent enhancement in vitro using viruses and plasma from dengue patients. PLoS One. 2014;9:e92173. doi: 10.1371/journal.pone.0092173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Libraty DH, et al. A prospective nested case-control study of dengue in infants: Rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thulin NK, et al. Maternal anti-dengue IgG Fucosylation predicts susceptibility to dengue disease in infants. Cell Rep. 2020;31:107642. doi: 10.1016/j.celrep.2020.107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wrammert J, et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J. Virol. 2012;86:2911–2918. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Upasani V, et al. Impaired antibody-independent immune response of b cells in patients with acute dengue infection. Front. Immunol. 2019;10:1–13. doi: 10.3389/fimmu.2019.02500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Connor O, et al. Potential role of vector-mediated natural selection in dengue virus genotype/lineage replacements in two epidemiologically contrasted settings. Emerg. Microbes Infect. 2021;10:1346–1357. doi: 10.1080/22221751.2021.1944789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noisakran S, et al. Association of dengue virus NS1 protein with lipid rafts. J. Gen. Virol. 2008;89:2492–2500. doi: 10.1099/vir.0.83620-0. [DOI] [PubMed] [Google Scholar]
- 73.Jacobs MG, Robinson PJ, Bletchly C, Mackenzie JM, Young PR. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J. 2000;14:1603–1610. doi: 10.1096/fj.14.11.1603. [DOI] [PubMed] [Google Scholar]
- 74.Avirutnan P, et al. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog. 2007;3:1798–1812. doi: 10.1371/journal.ppat.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puerta-Guardo H, Glasner DR, Harris E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016;12:e1005738. doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun P, et al. Infection and activation of human peripheral blood monocytes by dengue viruses through the mechanism of antibody-dependent enhancement. Virology. 2011;421:245–252. doi: 10.1016/j.virol.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 77.Sun P, et al. NK cell degranulation as a marker for measuring antibody-dependent cytotoxicity in neutralizing and non-neutralizing human sera from dengue patients. J. Immunol. Methods. 2017;441:24–30. doi: 10.1016/j.jim.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Matangkasombut P, et al. Dengue viremia kinetics in asymptomatic and symptomatic infection. Int. J. Infect. Dis. 2020;101:90–97. doi: 10.1016/j.ijid.2020.09.1446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials. All materials, except for clinical specimens, are available on request after completion of a materials transfer agreement with Institut Pasteur du Cambodge.